Abstract
Purpose
The purpose of this study was to report the clinical evaluation of a 3D-printed protective face shield designed to protect interventional radiologists from droplet transmission of the SARS-Cov-2.
Materials and methods
A protective face shield consisting in a standard transparent polymerizing vinyl chloride (PVC) sheet was built using commercially available 3D printers. The 3D-printed face shield was evaluated in 31 interventional procedures in terms of ability to perform the assigned intervention as usual, quality of visual comfort and tolerance using a Likert scale (from 1, as very good to 5, as extremely poor).
Results
The mean rating for ability to perform the assigned intervention as usual was 1.7 ± 0.8 (SD) (range: 1–4). The mean visual tolerance rating was 1.6 ± 0.7 (SD) (range: 1–4). The mean tolerability rating was 1.4 ± 0.7 (SD) (range: 1–3).
Conclusion
The 3D-printed protective face shield is well accepted in various interventions. It may become an additional option for protection of interventional radiologists.
Keywords: COVID-19, Interventional radiology, Three-dimensional printing, Protective face shield
Abbreviations: ABS, acrylonitrile butadiene styrene; IR, interventional radiology; PLA, polylactic acid; PPE, personnal protection equipment; PVC, polymerizing vinyl chloride; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation
1. Introduction
In the context of the current pandemic, the risk of human-to-human transmission of COVID-19 during patient interaction has dramatically increased [1], [2]. Interventional radiologists, nurses and technicians are at high risk of direct contact with COVID-19 patients while performing interventions. In a substantial number of patients, the COVID-19 status is unknown at the time of intervention. Scientific societies have defined a list of high-risk interventions, either because of risk of aerosolization or because the operator is close to the face of the patients [3].
Several countries and healthcare institutions are facing shortage of personal protection equipment (PPE) because of insufficient anticipation, poor manufacturing capacity or other local reasons. The need for additional options to limit dissemination between healthcare providers and patients, who are both potential sources of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) dissemination, must be addressed with an urgent, practical and efficient answer.
The purpose of this technical note was to report the clinical evaluation of a new protective face shield designed to protect caregivers from droplet transmission of the SARS-Cov-2 in interventional radiology.
2. Materials and methods
3D4care.org is a consortium of physicians, academics, MDs, PhDs, engineers and students from Paris, France. This consortium conceived a reusable frontal headband equipped with a disposable transparent sheet. The headband was adapted from the open source PRUSA RC2 model in order to be easily and rapidly custom-built in polylactic acid (PLA) or acrylonitrile butadiene styrene (ABS) using commercially available three-dimensional (3D) printers. The face shield itself consisted in a standard transparent polymerizing vinyl chloride (PVC) sheet for overhead projectors, 200-300 μm-thick, perforated to be plugged easily on the 4 spikes of the headband, which was secured on the head by a simple rubber elastic band (Fig. 1 ).
Fig. 1.
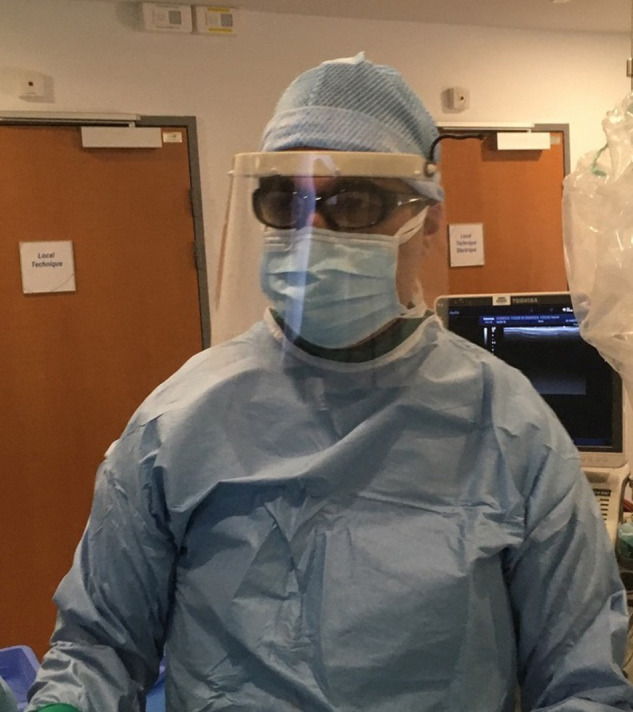
Photograph shows interventional radiologist wearing the 3D-printed protective face shield.
Before use, headbands were disinfected using standard anti-viral decontamination procedures approved by our hospital's hygiene department. The transparent sheet was changed on demand and between each patient. The complete process was approved by the 3D4care consortium and the University Hospitals of Paris. All the technical details are in open-access on the website of the consortium at www.3D4care.org.
We conducted a prospective evaluation in our IR unit aiming at evaluating the acceptability of the protective face shield in real conditions. IR personnel with different levels of experience were equipped with the 3D4care face-shield. Immediately after the intervention, they were asked to complete a standardized questionnaire based on a Likert scale (rated from 1 as very good to 5 as extremely poor) evaluation. They quoted on the scale the 3 following items:
-
•
ability to perform the assigned intervention as usual;
-
•
quality of visual comfort;
-
•
musculo-skeletal tolerance.
To evaluate the feasibility of re-using the PVC sheet we assessed the potential reduction in visual quality of the shield after cleaning in a subset of interventions. The cleaning was performed according to the recommendation of the manufacturer with a detergent/disinfectant (didecyldimethylammonium chlorure and polyhexamethylene biguanide chloride) using a soft sponge for one minute and allowing for spontaneous drying before using.
3. Results
The evaluation was conducted by a total of 38 operators in 31 consecutive interventions performed over a 7-day period. Interventions included central venous access placement (n = 12), percutaneous peripheral angioplasty (n = 10), percutaneous urinary intervention (n = 3), arterial embolization for acute bleeding (n = 3), radiofrequency ablation of lung tumor (n = 1), transjugular liver biopsy (n = 1), and adrenal vein sampling (n = 1). In some interventions, two interventional radiologists working together participated to the evaluation, being both equipped with the protective face shield.
The training level of the interventional radiologists were attending physicians (n = 21), fellows (n = 6) and residents (n = 11). The mean duration of the interventions was 59 ± 58 (SD) min (range: 15-240 min). Each protective shield was used 2 ± 1.7 (SD) times (range: 1-8 times). The mean ability to perform the assigned intervention as usual was 1.7 ± 0.8 (SD) (range: 1-4). The mean visual tolerance rating was 1.6 ± 0.7 (SD) (range: 1-4). The mean tolerability rating was 1.4 ± 0.7 (SD) (range: 1-3). In 3 procedures, the interventional radiologist commented for the need to reduce the size of the PVC sheet to gain a better degree of freedom of the head during rotation and to avoid touching his sterile surgical gown. For reused PVC shields after careful cleaning as recommended, the operators did not report any decrease in quality of visual comfort.
4. Discussion
Our evaluation demonstrates that the face shield designed by 3D4care can be used to perform various interventions without alterations by comparison with the usual working conditions. The ability to perform interventions as usual was not hampered by the use of the device, the visual tolerance was good and we did not observe any discomfort, even during long intervention. The various types of interventions that we monitored as well as the diverse level of experience of participating interventional radiologists allows to foresee that this experience could be easily reproduced in other countries/teams. This validation re-enforces the potential value of using this additional PPE, in order to contribute to fill in the gap of PPE for interventional radiologists during the COVID-19 pandemic.
In the setting of the COVID-19 pandemic, IR procedures can be performed both for complication of the disease and for usual interventions especially for oncologic patients (i. e., tumor ablation, implantable ports, intra-arterial treatment and supportive care) [4], [5], [6]. A subset of intervention have been defined as high-risk interventions because of close proximity to the patient's face or high-risk of aerosolization [3].
Because SARS-Cov-2 dissemination is recognized to be related to droplets of saliva or discharge from nose from patients when coughing, speaking, breezing or sneezing, the use of surgical face masks is recommended in association with goggles. Face shields provide protection to other facial areas in addition to eyes and better protection from splash or spray of any respiratory secretion from the patient. In the current situation of emergency the need for a readily available solution is of utmost importance.
Due to the acute and unforeseen spreading of COVID-19 pandemic as well as to the unpreparedness of several healthcare systems, our consortium designed the 3D4care face shield. The actual production of this mask requires less than 2 hours for a complete mask. A small farm of commercial 3D printers working 24 hours a day and 7 days a week can produce a large number of face shield protections. In our hospital, the design, printing and initial testing of the face shield was conducted in a approximately 48 hours, allowing to start the present study on the third day after the initial decision had been taken.
We acknowledge that a limitation of this study is the absence of evaluation of protection offered by the face shield in terms of viral count exposure. However, this would have required a controlled and specific environment non feasible in an emergency setting [7]. Accordingly, the action of the 3D4care consortium was focused on immediate access to this PPE in relation to COVID-19 pandemic.
In conclusion, the 3D4care face shield is well accepted in various interventions. It could become an additional option for protection of interventional radiologists. It is hoped that its rapid diffusion will confirm our preliminary findings.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Gabutti G., d’Anchera E., Sandri F., Savio M., Stefanati A. Coronavirus: update related to the current outbreak of COVID-19. Infect Dis Ther. 2020;8:1–13. doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pua U., Wong D. What is needed to make interventional radiology ready for COVID-19? Lessons learned from SARS-Cov epidemic. Korean J Radiol. 2020 doi: 10.3348/kjr.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapoval M, Vernhet-Kovacsik H. Activité de radiologie interventionnelle en phase d’épidémie COVID-19 + recommandations de la Fédération de Radiologie Interventionnelle pour la Société Française de radiologie (FRI-SFR). https://ebulletin.radiologie.fr/actualites-covid-19/activite-radiologie-interventionnelle-phase-depidemie-covid-19-recommandations.
- 4.Cornelis F.H., Bernhard J.C. Diagnostic and interventional radiology is a milestone in the management of renal tumors in Birt-Hugg-Dubé syndrome. Diagn Interv Imaging. 2019;100:657–658. doi: 10.1016/j.diii.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Aufranc V., Farouil G., Abdel-Rehim M., Smadja P., Tardieu M., Aptel S. Percutaneous thermal ablation of primary and secondary lung tumors: comparison between microwave and radiofrequency ablation. Diagn Interv Imaging. 2019;100:781–791. doi: 10.1016/j.diii.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee N.J., Shin J.H., Lee S.S., Park D.H., Lee S.K., Yoon H.K. Transcatheter arterial embolization for iatrogenic bleeding after endoscopic ultrasound-guided pancreaticobiliary drainage. Diagn Interv Imaging. 2018;99:717–724. doi: 10.1016/j.diii.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 7.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]


