Abstract
Introduction
An ongoing outbreak of a novel coronavirus disease (coronavirus disease 2019, COVID-19) has become a global threat. While clinical reports from China to date demonstrate that the majority of cases remain relatively mild and recover with supportive care, it is also crucial to be well prepared for severe cases warranting intensive care. Initiating appropriate infection control measures may not always be achievable in primary care or in acute-care settings.
Case
A 45-year-old man was admitted to the intensive care unit due to severe pneumonia, later confirmed as COVID-19. His initial evaluation in the resuscitation room and treatments in the intensive care unit was performed under droplet and contact precaution with additional airborne protection using the N95 respirator mask. He was successfully treated in the intensive care unit with mechanical ventilation and extracorporeal membrane oxygenation for respiratory support; and antiretroviral treatment with lopinavir/ritonavir. His total intensive care unit stay was 15 days and was discharged on hospital day 24.
Conclusions
Strict infection control precautions are not always an easy task, especially under urgent care in an intensive care unit. However, severe cases of COVID-19 pneumonia, or another novel infectious disease, could present at any moment and would be a continuing challenge to pursue appropriate measures. We need to be well prepared to secure healthcare workers from exposure to infectious diseases and nosocomial spread, as well as to provide necessary intensive care.
Keywords: Coronavirus disease 2019 (COVID-19), Extracorporeal membrane oxygenation (ECMO), Lopinavir/ritonavir, Infection control, Intensive care, Respiratory failure
1. Introduction
Since the emergence of a novel coronavirus (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19), in Wuhan, China [[1], [2], [3], [4], [5]], the number of patients and deaths is rising globally. Since diagnosing the first COVID-19 patient in Japan on January 16th, 2020, all patients identified during January had either travel history to an affected area in China, or known close contact with a COVID-19 patient. Health checks and testing for COVID-19 during this early phase were mainly performed based on epidemiological information such as travel histories and sick contacts. However, during mid-February, sporadic cases without significant travel histories or sick contacts have been publicized in Japan, leading to community recognition of the infection. Although the disease severity and case fatality ratio are currently not fully understood and cannot be determined during the ongoing outbreak, it is apparent that intensive care will be necessary for severe COVID-19 patients [[6], [7], [8]]. Preparations to prevent healthcare workers from infection and nosocomial spread from unexpected exposure to the disease could prove challenging when providing urgent and intensive care.
Our institution is an acute care medical center located in an urban setting in the eastern part of Tokyo and manages approximately 5000 emergent ambulant transfers and 2200 admissions to our emergency and critical care center each year. We are not a dedicated extracorporeal membrane oxygenation (ECMO) center but manage approximately 50 ECMO cases each year, with indications including extracorporeal cardiopulmonary resuscitation, respiratory failure, and cardiac failure. We have good access to infectious disease specialists and infection control teams are available for consultations. To our knowledge at this time, this is one of only a few COVID-19 pneumonia cases warranting intensive care for respiratory failure, where the patient has no relevant travel history or apparent sick contact. The main purpose of this case report is to describe the clinical course and intensive care management as well as how we implemented infection control measures, of a patient with severe COVID-19 pneumonia that emerged in the community at the time when sporadic cases in Japan were regarded as rare or limited.
2. Case report
A 45-year-old man was transferred to our emergency and critical care center from a nearby general hospital with a diagnosis of severe pneumonia. He had been on medication for diabetes mellitus, hypertension, and asthma, which had all been well controlled.
His onset of symptoms started with a cough at the beginning of February, followed by a fever of 38.5 °C on his 5th day of illness. His fever persisted and he developed dyspnea, which worsened further despite several visits to local medical clinics. When he visited a local medical facility on his 11th day of illness, a diagnosis of pneumonia was made, followed by immediate transfer to the Tokyo Metropolitan Bokutoh Hospital due to severe respiratory failure. Epidemiological information provided at this time, with no suspicious travel history or obvious sick contact, was insignificant to suggest COVID-19.
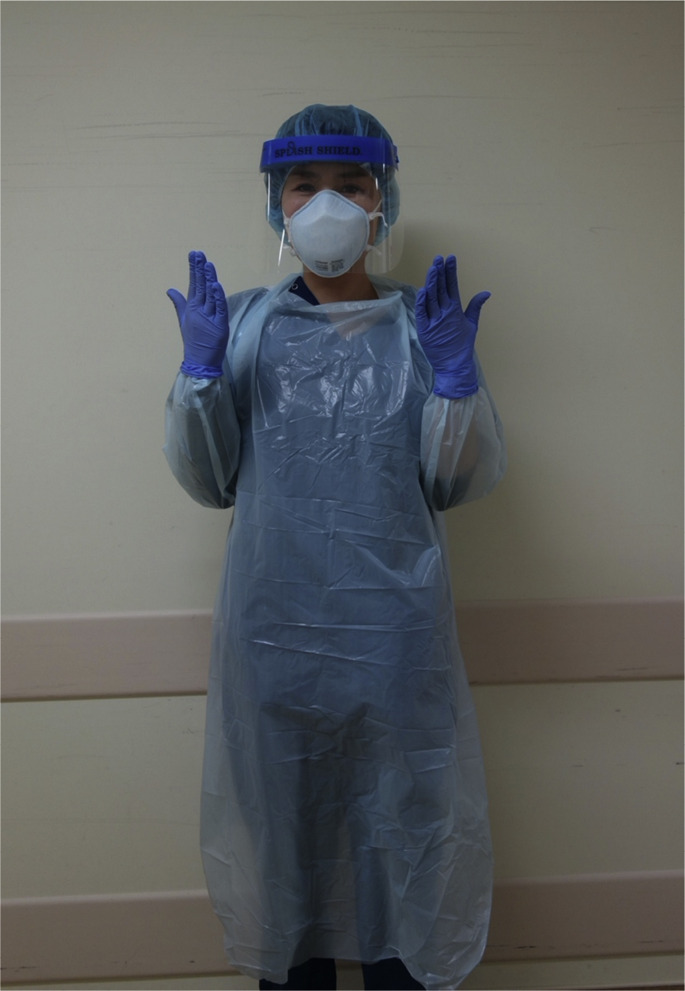
Since the emergence of COVID-19 in Wuhan, China, the emergency department in our hospital has organized infection precautions. These precautions include triage criteria and personal protective equipment (PPE) for medical staff, in order to safely manage unexpected COVID-19-positive ambulant transfers. All patients with respiratory failure, regardless of travel history and sick contact, were served by staff wearing PPE (N95 respirator mask, long sleeved gown, gloves, goggles, or face shields) (Fig. 1 ). They were isolated in a room with environmental cleaning and sterilization processes, until sufficient medical history could be taken and initial investigations were performed and evaluated. On his arrival, he was taken directly to an isolated resuscitation room in our emergency department where above-mentioned precautions were performed by medical staff equipped with PPE. The initial physical examination revealed consciousness oriented with a Glasgow Coma Scale of 15, body temperature of 38.5 °C, blood pressure of 117/83 mmHg, pulse rate of 120 beats/min, respiratory rate of 36 breaths/min, and oxygen saturation of 79% with a 10 L/min oxygen reservoir mask. His initial arterial blood gas analysis with 10 L/min oxygen showed: pH 7.490, PaCO2 36.4 mmHg, PaO2 43.4 mmHg, HCO3 − 27.5 mmol/L, SaO2 80.9%, and lactate 1.5 mmol/L, which revealed profound hypoxemia. He was unable to maintain oxygenation with 10 L/min oxygen and was intubated in the resuscitation room and placed on a mechanical ventilator.
Fig. 1.
An example of our staff wearing personal protective equipment.
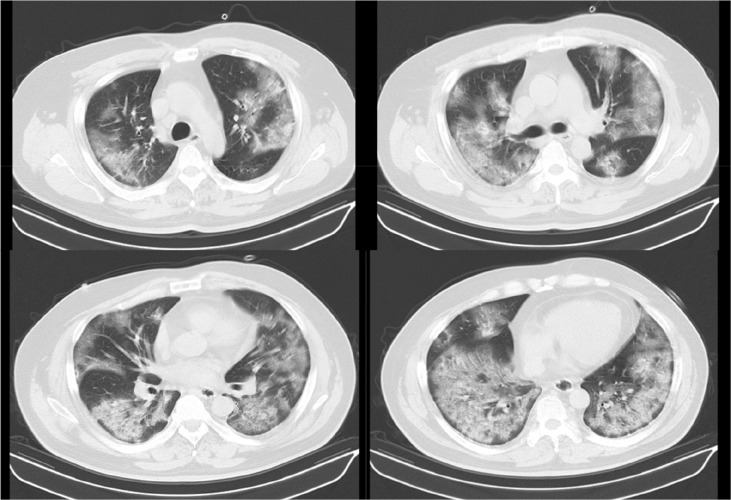
Initial and serial laboratory results are shown in Table 1 . A computed tomography (CT) scan of the chest showed diffuse infiltrate and ground-glass opacities bilaterally with no pleural effusion (Fig. 2 ). Transthoracic echocardiography revealed a normal left ventricular ejection fraction, size, and no abnormality of its valve. A rapid influenza test with a nasal swab taken on the day of admission was negative, and the gram-stain of his sputum specimen was insignificant. Both pneumococcal and legionella urinary antigen tests were negative, as was a loop-mediated isothermal amplification test for Mycoplasma pneumoniae. Sequential organ failure assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores on hospital day 1 were 4 and 13, respectively.
Table 1.
Clinical laboratory results.
| Variables | Reference range | HD1 |
HD3 | HD6 | HD9 | HD12 | HD15 |
|---|---|---|---|---|---|---|---|
| Illness day 11 | |||||||
| Complete blood cell count | |||||||
| White blood cells (/μl) | 3300–8600 | 5300 | 6500 | 7400 | 11,600 | 104,000 | 6300 |
| Segment neutrophil (%) | 37–80 | 78.6 | 84.4 | 78.8 | 76.7 | 78.8 | 69.4 |
| Lymphocyte (%) | 11–50 | 11.5 | 9.8 | 12.7 | 14.4 | 10.8 | 14.9 |
| Hemoglobin (g/dl) | 13.7–16.8 | 14.1 | 12.3 | 11.4 | 12.4 | 11.4 | 8.9 |
| Platelets (×104/μl) | 13.0–35.0 | 21.9 | 18.7 | 17.6 | 17.1 | 14.7 | 23.7 |
| Blood chemistry | |||||||
| Total protein (g/dl) | 6.6–8.1 | 5.8 | – | – | – | – | – |
| Albumin (g/dl) | 4.1–5.1 | 2.8 | 2.1 | 1.5 | 2 | 2.4 | 2 |
| Urea nitrogen (mg/dl) | 8.0–20.0 | 15.9 | 13.5 | 22.8 | 21.8 | 33.6 | 15.9 |
| Creatinine (mg/dl) | 0.65–1.07 | 0.82 | 0.55 | 0.65 | 0.71 | 0.62 | 0.49 |
| Total bilirubin (mg/dl) | 0.40–1.50 | 0.26 | 0.73 | 0.55 | 0.37 | 0.62 | 0.36 |
| Aspartate aminotransferase (U/l) | 13–30 | 50 | 65 | 48 | 28 | 22 | 15 |
| Alanine aminotransferase (U/l) | 10–42 | 48 | 66 | 56 | 30 | 20 | 16 |
| Alkaline phosphatase (U/l) | 106–322 | 149 | – | 238 | 242 | – | – |
| Lactate dehydrogenase (U/l) | 124–222 | 598 | 571 | 410 | 452 | 438 | 221 |
| Creatinine kinase (U/l) | 59–248 | 38 | 73 | 18 | – | 59 | – |
| Sodium (mmol/l) | 138–145 | 134 | 145 | 147 | 142 | 138 | 139 |
| Potassium (mmol/l) | 3.6–4.8 | 3.3 | 3.9 | 3.7 | 4 | 4.3 | 3.7 |
| Glucose (mg/dl) | 73–109 | 178 | 157 | 147 | 157 | 213 | 97 |
| C-reactive protein (mg/dl) | 0.00–0.14 | 20.28 | 26.24 | 16.77 | 2.58 | 13.82 | 6.78 |
| Procalcitonin (ng/ml) | 0.00–0.05 | 0.14 | – | – | – | – | – |
| Blood coagulation | |||||||
| Prothrombin time (%) | 75–120 | 110 | 94.5 | 99.2 | 104.2 | 97.6 | 97.4 |
| Activated partial thrombin time (sec) | 24.0–39.0 | 35.4 | 41.5 | 64.6 | 62.8 | 60.6 | 35.1 |
| Fibrinogen (mg/dl) | 200–400 | 536 | 629 | – | – | 423 | – |
| D-dimer (μg/ml) | <1.0 | 1.5 | 2.3 | 3.8 | 3.3 | 13.4 | – |
Abbreviations: HD, Hospital day.
Fig. 2.
Initial CT images of the patient (Hospital day 1, illness day 11).
In line with the information provided by the previous hospital, no relevant travel history or sick contact to suggest COVID-19 was identified through medical history taken from the patient and his family. As we could not identify microbiological or other possible non-infectious etiology from the initial investigations, concern for COVID-19 pneumonia was raised as one of the differentials. We decided to admit our patient to an isolated room under droplet and contact precaution with additional airborne protection using the N95 respirator mask. After admission, lung-protective mechanical ventilation and neuromuscular blockade (vecuronium) were initiated under sedation. His initial mechanical ventilator setting was volume-controlled mode, tidal volume of 400 mL (7 mL/kg predicted body weight), respiratory rate of 18 breaths/min, positive end-expiratory pressure (PEEP) of 14, and FIO2 of 45%. Since the etiology of his pneumonia remained unknown on the first day of admission, we performed bronchoalveolar lavage with measures above precaution, and empirical treatment was started with ceftriaxone 2 g every 24 hours, azithromycin 500 mg every 24 hours, and peramivir 600 mg every 24 hours. We did not administer corticosteroids systemically.
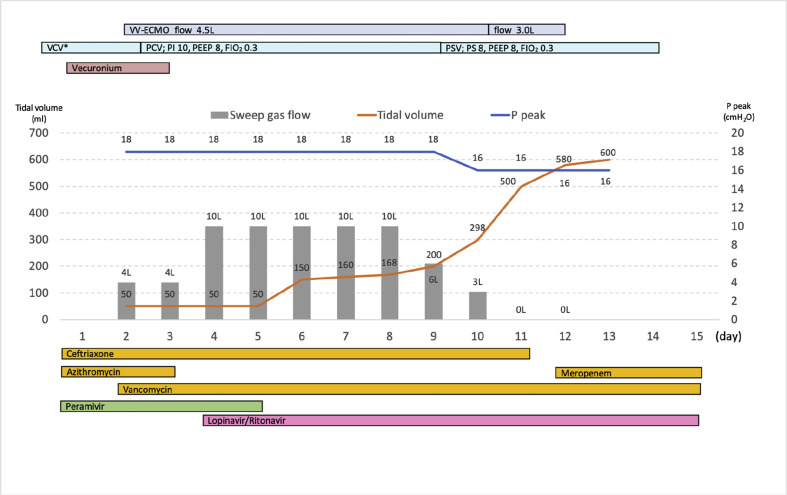
His oxygenation gradually worsened after 24 hours, arterial blood gas showed pH 7.394, PaO2 of 52.6 mmHg, PaCO2 of 51.7 mmHg and the mechanical ventilator plateau pressure increased 38 cmH2O under the mechanical ventilator setting; a tidal volume of 360 mL (6.3 mL/kg predicted body weight), respiratory rate of 22 breaths/min, PEEP of 18, and FIO2 of 80% (Murray score 4). Since his respiratory failure was incompletely treated with optimal mechanical ventilation and medication, venovenous extracorporeal membrane oxygenation (VV-ECMO) was introduced thirty hours after hospitalization (hospital day 2, illness day 12). The femoral-jugular VV-ECMO was initiated via the right femoral vein for access (25 Fr cannula, inflow) and via the internal jugular vein for return (20 Fr cannula, outflow) with an initial blood flow of 4.5 L/min and sweep gas of 4.0 L (MERA centrifugal blood pump system HCS-CFP, Senko Medical Instrument). The cannulation was performed percutaneously with ultrasound and fluoroscopic imaging guidance, and the procedures were completed under the above precautions. The mechanical ventilator was set on a pressure-controlled mode with an inspiratory pressure of 10 cmH2O (above PEEP), respiratory rate of 12 breaths/min, PEEP of 8, and FIO2 of 30%. After initiation of VV-ECMO, ECMO blood flow was maintained at 4.5 L until the day before removal. The sweep gas flow was maintained at 4 L/min of 100% oxygen (ECMO day 1–2), 10 L/min of 100% oxygen (ECMO day 3–7), and tapering was started from ECMO day 8. During VV-ECMO therapy, anticoagulation with continuous heparin infusion was administered, activated clotting time was controlled at approximately 180 s, and activated partial thromboplastin time about twice the reference value.
On the 4th day of hospitalization, polymerase chain reaction (PCR) tests for SARS-CoV-2 were performed on sputum specimens collected on the 1st and 3rd day of hospitalization, and throat swabs collected on the 3rd day. All tests returned positive results for COVID-19. Since lopinavir 400 mg/ritonavir 100 mg (LPV/RTV) was available in our hospital, and contraindications and adverse effects were not a major concern in this case, especially considering the patient's worsening clinical course, LPV/RTV was administered (two tablets, twice daily) via a nasogastric tube for 14 days. Bradycardia with atrioventricular block was noted on the 2nd day of LPV/RTV treatment. At this time, other medications that may cause bradycardia, such as dexmedetomidine and azithromycin, were discontinued and no further intervention was required. Other than this event, no significant adverse effects were noted that seemed to be related to LPV/RTV.
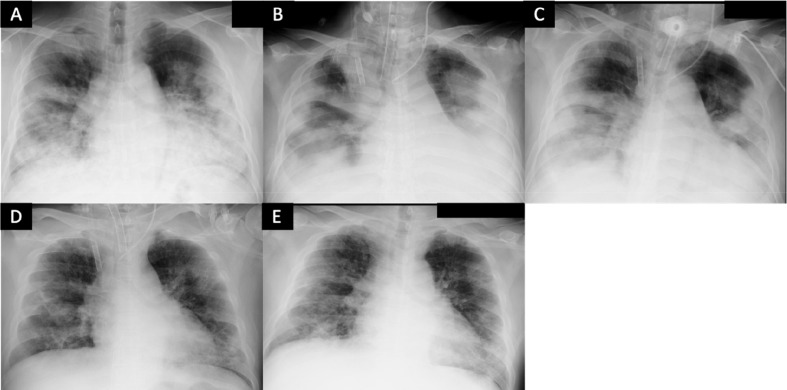
Since surgical tracheostomy under mechanical ventilation pose greater risks of droplet and aerosol exposure for operators, the procedure was performed under neuromuscular blockade and the patient was temporarily removed from the ventilator to prevent the generation of droplets and airborne particles. After surgical tracheostomy on the 6th day of hospitalization, improvement in lung compliance was observed, and tapering of sweep gas flow was started from the 9th day (ECMO day 8). On hospital day 12 (ECMO day 11), decannulation and removal of VV-ECMO was successfully performed, and he was freed from mechanical ventilation and oxygen on hospital days 14 and 18, respectively. His total intensive care unit stay was 15 days, and PCR tests performed on hospital days 13 and 15 were both negative. Serial chest X-rays and his clinical course in the intensive care unit are shown in Fig. 3, Fig. 4 , respectively. He was discharged to home on hospital day 24. We did not experience any nosocomial spread or infection to medical staff after caring the patient.
Fig. 3.
Serial chest-X-ray of the patient. (A) Hospital day 1; chest-X-ray of the patient before intubation. (B) Hospital day 2 (ECMO day 1); 12 hours after initiating VV-ECMO. (C) Hospital day 7 (ECMO day 6). (D) Hospital day 12 (ECMO day 11); removal of VV-ECMO. (E) Hospital day 17.
Fig. 4.
Clinical course of the patient in intensive care unit. Grey bar chart demonstrates sweep gas flow of VV-ECMO (L/min); orange line shows tidal volume (mL); blue line shows peak pressure of ventilator (cmH2O). ∗ Mechanical ventilation settings before initiating VV-ECMO are described in the Case presentation. VV-ECMO, venovenous-extracorporeal membrane oxygenation; VCV, volume-controlled ventilation; PEEP, positive end-expiratory pressure; FIO2, fraction of inspiratory oxygen; PCV, pressure-controlled ventilation; PI, inspiratory pressure; P peak, peak pressure of ventilator
3. Discussion
We present a case of COVID-19 pneumonia that originated from the community during the phase when patients without relevant travel history or sick contact were considered limited in Japan. All procedures, treatments, and nursing care in the resuscitation room and in the intensive care unit where performed under necessary infection control measures utilizing resources we had available. The patient was successfully treated with intensive care, including mechanical ventilation and VV-ECMO, with no major short-term complications that likely affected his daily life or work.
The present case developed respiratory symptoms as an onset and noticed fever on the 5th day of illness. After these non-specific symptoms, that could easily be explained as by common cold, his condition deteriorated with worsening dyspnea and fatigue. By the 11th day of illness, the diagnosis of pneumonia was already in a severe state. These initial clinical features were consistent with previous reports from China [[1], [2], [3], [4], [5]]. Chest CT findings from our patient were typical of those reported, showing ground-glass opacity and patchy shadowing involving bilaterally [1,2,4]. While our case presented with clinical and radiological findings consistent with that reported from China, it is difficult to differentiate COVID-19 pneumonia from other etiologies. Therefore, it is understandable for patients to visit medical clinics and be treated for the common cold in the early phase of illness. Laboratory findings were also non-specific, except for increased C-reactive protein and profound hypoxemia. Although severe COVID-19 patients may present with multi-organ failure and a higher APACHE II score [6], in this case organ function was maintained, apart from respiratory failure.
It is a usual concern to prevent healthcare workers from unexpected infectious diseases of any sort in acute care settings, and would be of the highest priority to prepare, train, and educate medical staff to achieve appropriate infection protection measures [7,8], especially during the global outbreak of a novel disease. Since the emergence of COVID-19 in Wuhan, China, the emergency department in our hospital has organized infection precautions described in the case presentation to all respiratory failure cases. Due to this precaution, under the circumstances that not many clinicians at the time recognized or expected the emergence of COVID-19 cases from the community, we were able to serve initial medical care without unexpected exposure.
In addition, some COVID-19 patients in China are also treated with ECMO [[2], [3], [4],6]. As of writing, it is challenging and could be premature to discuss the exact candidate of ECMO for COVID-19 patients in this phase of an outbreak, where the systematic collection of data is non-existent [9]. Since admission of our case, our most likely diagnosis was viral pneumonia, which we recognized as a reversible condition. When the respiratory condition deteriorated on hospital day 2, he did not suffer from multi-organ failure except for sole respiratory failure with Murray Score of 4, which we thought fulfilled the main indication of ECMO. These patient characteristics and presumptive conditions are likely to have been the important factors that benefited ECMO use and resulted in a favorable outcome. Other interventions such as prone positioning [6] could have been one of the choices for severe respiratory failure before initiating ECMO. However, because it requires additional staff to implement, and accidental extubation or disconnection of the ventilator tube may increase droplet exposure, we considered that the efficacy of prone positioning would not outweigh the safety of medical staff.
No antiviral treatment has been proven effective for COVID-19, and no randomized controlled studies have been completed yet. A report from Korea presented a case treated with LPV/RTV with improved clinical symptoms and decreased viral load [10]. We are unable to scientifically prove the association between LPV/RTV treatment and clinical improvement. The patient was already on mechanical ventilation and ECMO for respiratory support when LPV/RTV was started, and his clinical improvement may well be explained as the natural course of the disease under supportive care. We need more well-controlled studies and evidence to support the benefit of the treatment as well as an understanding of who most benefits and when. Additionally, close monitoring and usage under specialist consultation would be required for adverse effects and drug interactions with medications commonly used for critically ill patients.
We still do not fully understand the natural history of this emerging disease or how the ongoing spread of the outbreak will culminate. Medical facilities in Japan may not be equipped with enough negatively pressured isolation rooms, which could also be suitable for intensive care. There needs to be a well-organized and practical scheme to arrange critically ill patients according to disease severity, contagiousness, and size of the outbreak. The distribution and provision of critical care may well be determined by the phase of pandemic, available resources, and mostly when the clinical spectrum of this disease becomes more evident [11].
In conclusion, although this case report describes the successful treatment of severe COVID-19 pneumonia due to the above-mentioned intensive care, our intentions are not to promote extensive treatment options as standardized care for COVID-19, but to raise and reconsider how to provide critical care for unknown disease, while securing the safety of medical personnel. Except for the additional consideration of infection control precautions, the clinical course and intensive care management we described here were not different from those of severe pneumonia with other etiologies. Strict infection control precautions are not always an easy task, especially under urgent care in an intensive care unit. However, we could encounter severe COVID-19 pneumonia, or any other novel infectious disease at any moment, and it would be a continuing challenge to pursue appropriate measures.
Authorship statement
All authors meet the International Committee of Medical Journal Editors (ICMJE) authorship criteria. NK, MH, HS, YK, MT, MK, TI, KS, TW, NS, and YH were involved in the treatment and clinical management decision-making of the patient. NK and MH wrote the manuscript, and MH revised and edited the manuscript. HS, YK, MT, MK, TI, KS, TW, NS, and YH critically revised the manuscript for important intellectual content. All the authors approved the final version of the manuscript to be published.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent for publication
The administration of lopinavir/ritonavir was approved by the Ethics Committee of the Tokyo Metropolitan Bokutoh Hospital. Written informed consent was obtained from the patient for publication of this case report.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouadma L., Lescure F.X., Lucet J.C., Yazdanpanah Y., Timsit J.F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLaren G., Fisher D., Brodie D. Preparing for the most critically Ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 10.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020 doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu H., Tong Z., Ma P., Hu M., Peng Z., Wu W., et al. Intensive care during the coronavirus epidemic. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]