Abstract
Background
Previous studies on coronavirus disease 2019 (COVID-19) have focused on populations with normal immunity, but lack data on immunocompromised populations.
Objective
To evaluate the clinical features and outcomes of COVID-19 pneumonia in kidney transplant recipients.
Design, setting, and participants
A total of 10 renal transplant recipients with laboratory-confirmed COVID-19 pneumonia were enrolled in this retrospective study. In addition, 10 of their family members diagnosed with COVID-19 pneumonia were included in the control group.
Intervention
Immunosuppressant reduction and low-dose methylprednisolone therapy.
Outcome measurements and statistical analysis
The clinical outcomes (the severity of pneumonia, recovery rate, time of virus shedding, and length of illness) were compared with the control group by statistical analysis.
Results and limitations
The clinical symptomatic, laboratory, and radiological characteristics of COVID-19 pneumonia in the renal transplant recipients were similar to those of severe COVID-19 pneumonia in the general population. The severity of COVID-19 pneumonia was greater in the transplant recipients than in the control group (five severe/three critical cases vs one severe case). Five patients developed transient renal allograft damage. After a longer time of virus shedding (28.4 ± 9.3 vs 12.2 ± 4.6 d in the control group) and a longer course of illness (35.3 ± 8.3 vs 18.8 ± 10.5 d in the control group), nine of the 10 transplant patients recovered successfully after treatment. One patient developed acute renal graft failure and died of progressive respiratory failure.
Conclusions
Kidney transplant recipients had more severe COVID-19 pneumonia than the general population, but most of them recovered after a prolonged clinical course and virus shedding. Findings from this small group of cases may have important implications for the treatment of COVID-19 pneumonia in immunosuppressed populations.
Patient summary
Immunosuppressed transplant recipients with coronavirus disease 2019 infection had more severe pneumonia, but most of them still achieved a good prognosis after appropriate treatment.
Keywords: Kidney transplantation, Coronavirus disease 2019, Immunosuppression, Pneumonia, Outcome
Take Home Message
Immunosuppressed renal transplant recipients with coronavirus disease 2019 infection had more severe pneumonia than the general population. Most patients could recover following a treatment regimen consisting of reduced immunosuppressant use, low-dose methylprednisolone therapy, and protection of renal graft function.
1. Introduction
As a newly recognized illness, coronavirus disease 2019 (COVID-19) has spread rapidly throughout Wuhan city to the rest of China and around the world. After more than a month of strenuous effort, the epidemic situation in China is gradually coming under control. However, the number of confirmed COVID-19 cases outside China is continuously growing. A better understanding of the clinical characteristics and outcomes of COVID-19 pneumonia is conducive to better prevention and treatment of the disease.
To date, some clinical studies have reported the demographic, epidemiological, clinical, laboratory, and radiological characteristics, as well as the treatment and prognosis of patients with COVID-19 pneumonia [1], [2], [3]. However, these reports are mainly aimed at general populations whose immunity was usually undisturbed before the disease occurred. Given that both innate and adaptive immunity play important roles in the clearance of viral infection, it is not clear whether COVID-19 pneumonia has its own specific effects in immunocompromised populations. The immune response, particularly the T-cell response, in these special populations is significantly suppressed because of the long-term use of immunosuppressive agents. Therefore, COVID-19 pneumonia in immunosuppressed populations may produce more severe clinical symptoms, a longer duration of illness, and a worse prognosis than in immunocompetent patients.
In this study, we enrolled 10 patients with laboratory-confirmed COVID-19 pneumonia from among the renal transplant recipients who had been followed up long term in Tongji Hospital, Wuhan, China. Detailed clinical data were collected and analyzed to evaluate the clinical features and outcomes of COVID-19 pneumonia in these immunosuppressed patients.
2. Patients and methods
2.1. Study design and patients
As the hospital with the largest volume of kidney transplants in China, our long-term follow-up recipients exceed 3000. During the COVID-19 epidemic, renal transplant recipients who developed susceptive symptoms were advised to contact the physicians at our transplant center. Before the COVID-19–diagnosed patients were admitted to the designated hospitals, our physicians instructed them to adjust the use of immunosuppressive agents and to take oral antiviral drugs. “Treatment after admission” will refer to the consultation advice of our kidney transplant specialists. A total of 10 renal transplant recipients with laboratory-confirmed COVID-19 pneumonia were enrolled in this study; they were hospitalized in five designated hospitals in Wuhan.
As the family members of transplant recipients infected with COVID-19 virus tend to exhibit concomitant cluster infections, we also collected clinical data from these immunocompetent family members with COVID-19 pneumonia as controls in this study.
This study was approved by the institutional review board (IRB) at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (IRB approval number: TJ-C20200120). Written informed consents were waived for emerging infectious diseases, but verbal informed consents were obtained.
2.2. Diagnosis and classification
All patients underwent chest computed tomography (CT) scans to determine pneumonia. Throat swab samples were subjected to reverse real-time polymerase chain reaction (RT-PCR) assay in order to test for the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4], [5]. A definitive diagnosis of COVID-19 pneumonia was made based on the following two criteria: (1) radiographic evidence of pneumonia and (2) positivity of respiratory specimens for SARS-CoV-2 by RT-PCR assay.
Clinical classification of COVID-19 pneumonia includes mild, severe, and critical types [6], [7]. The detail classification criteria, as well as the discharge criteria, are provided in the Supplementary material.
2.3. Principles of special treatment
For renal transplant recipients with COVID-19 pneumonia, the immunosuppressive agents were adjusted according to the clinical symptoms and severity of pneumonia. Intravenous corticosteroid therapy was given as an important adjunctive therapy. The details are described in the Supplementary material.
2.4. Data collection
Medical records of the patients were analyzed by the research team. Epidemiological, clinical, laboratory, and radiological characteristics, as well as treatment and outcome data, were obtained from electronic medical records. The data were reviewed by two senior transplant clinicians. The basic clinical information from infected family members was obtained mainly through telephone communication with the patients. The clinical outcomes (ie, discharge, mortality, time of virus shedding, and length of illness) were monitored up to March 16, 2020.
2.5. Statistical analysis
In our descriptive statistical analysis, results are expressed as numerical values for categorical variables. Continuous variables are presented as mean values with standard deviation if normally distributed, and as median and range otherwise. Normality was tested by Shapiro-Wilk test. Normally distributed continuous variables between groups were compared with Student t test. The ordinal variable (clinical condition) was compared by Mann–Whitney rank-sum test. For categorical covariates, p values were generated by Fisher’s exact test. Statistical analysis was performed using STATA (StataCorp LLC, Texas, USA), version 15.1.
3. Results
Among the 10 transplant patients with COVID-19 pneumonia, eight were male and two were female. The age range of the patients was 24–65 yr, and the time after renal transplantation was 6 mo–12 yr. Their kidney grafts were from living related donors (n = 3), donation after brain death (n = 3), or donation after circulatory death (n = 4). Six patients had one or more of the following underlying diseases: hypertension, coronary heart disease, hypertensive heart disease, chronic obstructive pulmonary disease, atrial fibrillation, and chronic heart failure. The maintenance immunosuppressive regimens included tacrolimus (Tac)/mycophenolate mofetil (MMF)/prednisone, Tac/MMF, and cyclosporine/mizoribine (Table 1 ).
Table 1.
Clinical and laboratory characteristics of 10 kidney transplant recipients infected with COVID-19.
| Patient |
n | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7a | 8 | 9 | 10 | ||
| Sex | Male | Male | Male | Male | Male | Female | Male | Male | Male | Female | – |
| Age | 24 | 55 | 29 | 30 | 50 | 65 | 52 | 49 | 59 | 37 | – |
| IS regimen | T/M/P | T/M/P | T/M/P | T/M/P | T/M/P | T/M | T/M/P | T/M | C/Mi | T/M/P | – |
| Comorbidities | No | CHD/AF/CHF | No | HTN | HTN | No | HTN/CHD | No | HTN/HHD/COPD | HTN | 6 |
| Contact Wuhan | Live | Live | Travel | Live | Live | Live | Travel | Live | Live | Live | 10 |
| Family cluster | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| No. of infections | 2 | 0 | 0 | 2 | 2 | 1 | 5 | 1 | 1 | 0 | 14 |
| Confirmed | 1 | – | – | 2 | 1 | 1 | 4 | 1 | – | – | 10 |
| Clinically diagnosed | 1 | – | – | – | 1 | – | 1 | – | 1 | – | 4 |
| Date of onset | Jan 17 | Jan 20 | Jan 20 | Jan 20 | Jan 25 | Jan 27 | Jan 28 | Jan 29 | Feb 1 | Feb 3 | – |
| Onset to diagnosis (d) | 26 | 2 | 7 | 12 | 11 | 4 | 5 | 9 | 4 | 2 | – |
| Onset to admission (d) | 27 | 5 | 7 | 21 | 8 | 4 | 7 | 9 | 8 | 10 | – |
| Fever | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Highest fever (°C) | 38 | – | 38.8 | 39 | 38.6 | 38 | 38.9 | 39.2 | 38.4 | 40 | – |
| Days of fever | 3 | – | 13 | 10 | 8 | 11 | 9 | 5 | 9 | 6 | – |
| Cough | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Short of breath | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Fatigue | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Diarrhea | No | No | Yes | No | No | Yes | No | Yes | No | No | 3 |
| SARS-CoC-2 positive | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Highest CRP (mg/l) | 30 | 80.5 | 118 | 42.6 | 40 | 40.1 | 54 | 49.7 | 100.5 | 34.1 | – |
| Lymphopenia | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 8 |
| Lowest Lym (×109/l) | – | 0.3 | 0.47 | 0.61 | 0.42 | 0.72 | 0.99 | – | 0.44 | 0.19 | – |
| Elevated Scr | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | 6 |
| Highest Scr () | 198 | 308 | 251 | 209 | – | – | – | – | 467 | 189 | – |
| Elevated ALT | No | No | No | No | Yes | No | Yes | Yes | Yes | Yes | 5 |
| Highest ALT (U/l) | – | – | – | – | 104 | – | 94 | 97 | 61 | 163 | – |
AF = atrial fibrillation; ALT = alanine aminotransferase; C = cyclosporine; CHD = chronic coronary disease; CHF = chronic heart failure; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; HHD = hypertensive heart disease; HTN = hypertension; IS = immunosuppression; Lym = lymphocyte; M = mycophenolate mofetil; Mi = mizoribine; n = total number of patients with available data; P = prednisolone; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; Scr = serum creatinine; T = tacrolimus.
A case report of this patient has recently been accepted for publication.
In terms of epidemiology, eight of 10 patients lived in Wuhan, and the other two visited Wuhan before the onset of the disease. Moreover, seven patients had other family members infected with SARS-CoV-2, of which 10 were laboratory confirmed and four were clinically diagnosed (Table 1).
The symptom onset time for the 10 patients was concentrated within 17 d (January 17–February 3, 2020). Nine patients presented with fever, with the highest body temperature ≥38 °C. In addition, nine patients had cough, shortness of breath, and fatigue; three had diarrhea (Table 1). The median time from onset of symptoms to the diagnosis of COVID-19 was 6 d (2–26 d); the median time to hospital admission was 8 d (4–27 d).
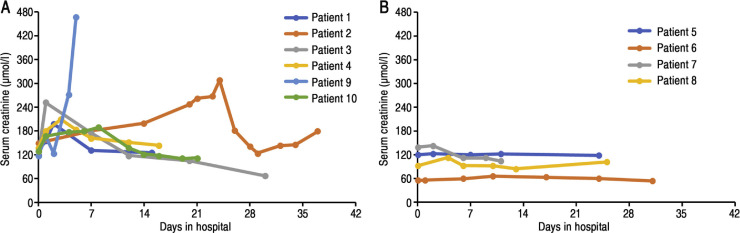
Laboratory results showed that eight of the 10 patients developed lymphopenia (<1.1 × 109/l), with the lowest average lymphocyte count being 0.52 ± 0.25 × 109/l (Table 1). C-reactive protein was significantly increased in all patients (30–118 mg/l), while procalcitonin was negative. During the course of the disease, the serum creatinine level of five patients showed a temporary increase and that of one patient showed a progressive increase (Fig. 1 A). In addition, five patients had a transient elevation in alanine aminotransferase levels (61–163 U/l).
Fig. 1.
Serum creatinine changes in 10 post-transplant patients during the course of illness. (A) Six patients had renal graft damage. (B) Four patients had stable renal graft function.
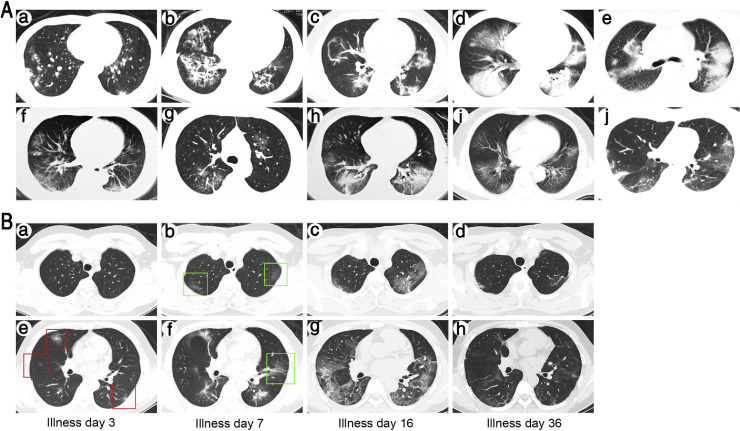
All patients had at least one chest CT scan, which showed abnormalities in both lungs in each case (Fig. 2 A). The chest CT findings of two patients with relatively mild pneumonia were multiple patchy ground-glass shadows in the lungs (Fig. 2Aa,g); the chest CT images of eight patients with relatively severe pneumonia showed bilateral multiple lobular and subsegmental areas of consolidation. Pulmonary lesions in nine patients exhibited a significant progression, followed by a marked absorption in the late stage of illness (Fig. 2B). Unfortunately, the remaining patient died during the progression of his pneumonia.
Fig. 2.
Chest CT scans of 10 post-transplant patients. (A) A representative CT image of each patient: (a) patient 1: multiple bilateral subpleural patchy consolidation, prominent on the right; (b) patient 2: multiple bilateral reticular patterns, prominent on the right; (c) patient 3: multiple patchy ground-glass opacities bilaterally; (d) patient 4: multiple bilateral consolidation and ground-glass opacities in the right; (e) patient 5: multiple bilateral patchy consolidation; (f) patient 6: multiple subpleural patchy ground-glass opacities bilaterally; (g) patient 7: bilateral patchy ground-glass opacities; (h) patient 8: bilateral consolidation and patchy ground-glass opacity in the right side; (i) patient 9: multiple bilateral ground-glass opacities; and (j) patient 10: multiple bilateral patchy consolidation and ground-glass opacities. (B) Changes of chest CT images during the course of disease in a typical case (patient 3): (a and e) on illness day 3, multiple subpleural patchy ground-glass opacities bilaterally; (b and f) on illness day 7, much more ground-glass opacities and patchy consolidation bilaterally; (c and g) on illness day 16, multiple ground-glass opacities bilaterally with enlarged ranges; (d and f) on illness day 36, the lesion was almost completely absorbed, leaving only a few blurs.
CT = computed tomography.
In terms of clinical classification, two cases were classified as mild, five cases were severe, and three cases were critical (Table 2 ). Regarding the treatment, all patients except one stopped using antimetabolic immunosuppressants, with an average discontinuation time of 18.0 ± 6.4 d. In seven patients, calcineurin inhibitors (CNIs) were completely discontinued for 6–14 d, after which the original doses before illness were gradually restored. Among the other three patients, one reduced the CNI dosage and in two the dosage remained unchanged during the whole course of their illness (Table 2). Eight patients were given intravenous methylprednisolone (MP) to control their high fever, with a daily dose of 20–40 mg and a median course of 9.5 d (2–19 d). Seven patients received intravenous immunoglobulin treatment (Table 2). The antiviral regimen included umifenovir (n = 6), oseltamivir (n = 2), ribavirin (n = 1), and ganciclovir (n = 1). All patients needed to receive oxygen via nasal catheter to maintain oxygen saturation >95%, and three of them required noninvasive mechanical ventilation for 1–3 d as the disease progressed. None of the patients underwent endotracheal intubation and invasive ventilation.
Table 2.
Treatments and outcomes of 10 kidney transplant recipients infected with COVID-19.
| Patient |
n | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Clinical condition | |||||||||||
| Mild | Yes | – | – | – | – | – | Yes | – | – | – | 2 |
| Severe | – | – | Yes | Yes | Yes | – | – | Yes | – | Yes | 5 |
| Critical | – | Yes | – | – | – | Yes | – | – | Yes | – | 3 |
| IS adjustment | |||||||||||
| M/Mi cessation | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Days of M/Mi cessation | – | 12 | 30 | 21 | 20 | 24 | 12 | 14 | 11 | 18 | – |
| CNI cessation | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Days of CNI cessation | – | – | – | 6 | 9 | 14 | 7 | 7 | 11 | 8 | – |
| CNI reduction | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Days of CNI reduction | – | 12 | – | 15 | 5 | 8 | 7 | 5 | 11 | 10 | – |
| Use of corticosteroid | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| MP i.v. dose (mg/d) | – | – | 20 | 40 | 20 | 40 | 40 | 40 | 40 | 20 | – |
| Days of MP i.v. | – | – | 2 | 9 | 9 | 19 | 12 | 10 | 5 | 10 | – |
| Use of IVIG | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Antiviral therapy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Oxygen support | |||||||||||
| Nasal cannula | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Noninvasive ventilation | No | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Outcomes | |||||||||||
| Discharge | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Death | Yes | 8 |
| Onset to shedding (d) | 37 | 45 | 25 | 29 | 30 | – | 15 | 22 | – | 24 | – |
| Hospital stay (d) | 16 | 43 | 30 | 16 | 26 | 45+ | 13 | 25 | 6 | 21 | – |
| Onset to discharge (d) | 43 | 48 | 37 | 37 | 34 | 49+ | 20 | 34 | – | 31 | – |
CNI = calcineurin inhibitors; COVID-19 = coronavirus disease 2019; IS = immunosuppression; i.v. = intravenous; IVIG = intravenous immunoglobulin; M = mycophenolate mofetil; Mi = mizoribine; MP = methylprednisolone; n = total number of patients with available data.
To date, eight of the 10 patients have recovered and been discharged from hospital, with a mean hospital stay of 23.8 ± 9.7 d and a mean illness course of 35.3 ± 8.3 d. The mean time from illness onset to SARS-CoV-2 virus shedding was 28.4 ± 9.3 d. After 45 d in the hospital, one showed significant improvement of the pneumonia but was still under hospital observation because of a recent positive result from a nucleic acid test for SARS-CoV-2.
Of the 10 patients, one died during hospitalization. He was a 59-yr-old man who had complex underlying diseases, including hypertension, hypertensive heart disease, and chronic obstructive pulmonary disease. He was admitted to the hospital on day 8 of illness. On hospital day 2, noninvasive mechanical ventilation was required because of a significant decrease in blood oxygen saturation (to 83%). Meanwhile, the patient showed a significant decrease in urine volume (<500 ml/d) and a significant increase in serum creatinine (467 μmol/l), indicating an acute renal allograft failure. On hospital day 6, the patient developed a sudden acute respiratory failure and died.
Serving as control patients without immunosuppression, the 10 family members with confirmed COVID-19 pneumonia had a mean age of 42.3 ± 14.4 yr, which was similar to that of the infected transplant recipients (45.0 ± 14.0 yr, p > 0.05). Of the 10 control patients, nine were admitted to hospitals and seven had fever with an average duration of 6.4 ± 4.3 d (Table 3 ). Most control patients’ (nine of 10) disease was classified as mild, and only one case was severe. By contrast, the transplant recipients had significantly more severe COVID-19 pneumonia (five severe and three critical cases) than the control family members (p < 0.01). Furthermore, these transplant patients had a longer course of the disease (35.3 ± 8.3 vs 18.8 ± 10.5 d, p < 0.01) and a longer time of virus shedding (28.4 ± 9.3 vs 12.2 ± 4.6 d, p < 0.01; Table 3).
Table 3.
Comparison of clinical features and outcomes of COVID-19 pneumonia in kidney transplant recipients and their family members.
| KTx recipients (n = 10) | Family members (n = 10) | p value | |
|---|---|---|---|
| Age (yr), mean (SD) | 45.0 (14.0) | 42.3 (14.4) | 0.68 |
| Sex (male), n | 8 | 5 | 0.35 |
| Fever, n | 9 | 7 | 0.58 |
| Days of fever, mean (SD) | 8.2 (3.1) | 6.4 (4.3) | 0.35 |
| Positive chest CT findings, n | 10 | 10 | – |
| Admission, n | 10 | 9 | 1.0 |
| Clinical condition, n | <0.01 | ||
| Mild, | 2 | 9 | |
| Severe | 5 | 1 | |
| Critical | 3 | 0 | |
| Recovery, n | 9 | 10 | 1.0 |
| Onset to shedding (d), mean (SD) | 28.4 (9.3) a | 12.2 (4.6) | <0.01 |
| Onset to discharge (d), mean (SD) | 35.3 (8.3) a | 18.8 (10.5) | <0.01 |
COVID-19 = coronavirus disease 2019; CT = computed tomography; KTx = kidney transplant; SD = standard deviation.
n = 8.
4. Discussion
We report here the clinical features and outcomes of 10 renal transplant recipients with laboratory-confirmed SARS-CoV-2 infection. All the patients had significant progressive pneumonia and were admitted to designated hospitals in Wuhan, China. The overall severity of COVID-19 pneumonia in this group of patients was greater than that in their infected family members and that reported in the general population [7], [8]. Nevertheless, all patients except one eventually recovered after treatment.
The clinical manifestations of COVID-19 pneumonia in post-transplant patients may be atypical as a result of long-term immunosuppression [9]. In this study, nine of the 10 patients had fever, cough, shortness of breath, and fatigue; three had diarrhea; eight had lymphopenia; six had renal injury; and six had liver function damage, all of which were similar to the manifestations of severe COVID-19 pneumonia in the general population [1], [3], [10]. In addition, these patients also had typical chest CT findings of COVID-19 pneumonia that were similar to those previously reported [5].
It is important to verify whether COVID-19 pneumonia in immunosuppressed people is more severe than that in nonimmunosuppressed general population. A report of 72 314 COVID-19 cases in the general population from China's Infectious Disease Information System showed that most confirmed cases (81%) were classified as mild, while only 19% were either severe or critical [7]. In the present study, the proportion of severe cases (80%) was significantly higher than that of their infected family members (10%) and that reported in the general population (19%) [7]. In addition, with the exception of one patient who died and one who has not yet been discharged, the average duration of illness was approximately 35 d, nearly twice as long as that of the control family member group (18.8 d) and of the general population, as reported in the literature (about 17–20 d) [3], [7]. Moreover, the post-transplant patients needed a much longer time to become negative for the SARS-COV-2 virus than their infected family members. These results suggest that because of their immunosuppressive status, transplant recipients may experience more severe COVID-19 pneumonia and a more difficult recovery than those in the general population.
In Guidance on Influenza A/H1N1 in solid organ transplant recipients, a reduction in immunosuppression is recommended in patients with significant disease [11]. However, it should be noted that the timing must be well controlled in terms of reducing or stopping CNIs. Once the symptoms improve, CNIs should be restored gradually to avoid acute graft rejection. In our study, the use of CNIs in seven patients was completely halted for about 9 d, and the dosage was halved for one patient for 12 d. None of the eight patients developed acute renal graft rejection.
In the treatment of COVID-19 pneumonia, the use of low to moderate doses of corticosteroids (0.5–1 mg/kg/d for ≤7 d) has been recommended [12]. This regimen may be able to suppress the overwhelming inflammation mediated by the hyperimmune response, thereby preventing the development of rocketing progressive pneumonia in patients. However, when it comes to steroid doses and treatments, it is important to find a delicate balance that maximizes the benefits and minimizes the potential harm. In the current study, eight patients used intravenous MP (20–40 mg/d) for an average of 9.5 d; this steroid may play an important role in preventing cytokine storm syndrome and facilitating recovery.
Although the pneumonia was more severe in the 10 transplant recipients, and most patients had different underlying diseases, all except one still achieved a good prognosis after treatment. To explain this phenomenon, we hypothesize that long-term immunosuppression might, on the one hand, delay virus clearance and prolong the course of disease, and on the other hand, avoid the occurrence of fatal critical pneumonia caused by the hyperimmune response. It is worth noting that the protection of renal graft function during the course of the disease is particularly important for these special patients. Elevated serum creatinine has been reported in approximately 4–10% of hospitalized patients with COVID-19 pneumonia [2], [13]. However, six of the 10 transplant patients in our study had elevated serum creatinine. Possible reasons why renal transplant recipients are more likely to develop kidney damage during the treatment of COVID-19 pneumonia include a potential lack of renal function, poor tolerance to therapeutic drugs, and possible immunological damage.
This study is limited by its small sample size and retrospective method. The following considerations should be taken into account when interpreting the findings: First, the health resources in Wuhan were in relatively short supply because of the rapid growth in the number of COVID-19 patients, and therefore some of our patients failed to be admitted to the hospital in a timely manner. Second, since our patients were sent to five different hospitals, individual differences in treatment regimens may have affected the process of recovery from the disease.
5. Conclusions
To the best of our knowledge, this is the first case series report on COVID-19 pneumonia in immunosuppressed patients. We conclude that immunosuppression may have two opposing effects on COVID-19: on the one hand, making the early course of the disease more severe and prolonging the virus shedding time; on the other hand, reducing the occurrence of fatal severe pneumonia by suppressing the hyperimmune response. We believe that the findings reported here provide an important reference for the future treatment of immunosuppressed patients with COVID-19 pneumonia.
Author contributions: Gang Chen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Z. Chen, G. Chen, Gong, Zhang.
Acquisition of data: Liu, X. Lu, Dong Chen, Guo.
Analysis and interpretation of data: G. Chen, Shu, Ma.
Drafting of the manuscript: Zhu, G. Chen.
Critical revision of the manuscript for important intellectual content: Cai.
Statistical analysis: Zhu.
Obtaining funding: Z. Chen.
Administrative, technical, or material support: Chen, Xu, Lu, Dongrui Chen, Ge, Jiang, Wei.
Supervision: Z. Chen, G. Chen.
Other: None.
Financial disclosures: Gang Chen certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The study was supported by Hubei Science and Technology Plan, China (grant number 2017ACA096).
Acknowledgments: We thank Dr. Deborah McClellan for editorial assistance. The clinical activities being reported are consistent with the principles of the declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Associate Editor: James Catto
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.03.039.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. In press. 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 6.China NHCo. New coronavirus pneumonia prevention and control program (7th). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf.
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. In press. 10.1001/jama.2020.2648. [DOI] [PubMed]
- 8.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 9.Michaels M.G., La Hoz R.M., Danziger Isakov L., et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. In press. 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed]
- 10.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. In press. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 11.Kumar D., Morris M.I., Kotton C.N. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010;10:18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 12.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.