Abstract
Purpose
Chronic kidney disease (CKD) is a global nephrotic syndrome characterized by chronic inflammation, oxidative stress and fibrosis in the kidney. Isoliquiritigenin (ISL), a flavonoid from licorice, has historically been reported to inhibit innate immune responses to inflammation and fibrosis in vivo. However, the effect of ISL on CKD progression is largely unknown.
Materials and Methods
In this study, we employed the inflammatory and fibrotic models of LPS/TGF-β-induced bone marrow-derived macrophages (BMDM) in vitro and unilateral ureteral obstruction (UUO) model in vivo to explore the potential effects and mechanism of ISL on renal inflammation and fibrosis.
Results
Our results manifest that ISL improved UUO-induced renal dysfunction and reduced tubular damage with a significantly downregulated mRNA expression and secretion of IL-1β, IL-6, TNF-α and MCP-1 in vitro and in vivo. It is worth noting that ISL can strongly inhibit the mRNA and protein expression of Mincle (macrophage-induced c-type lectin) in BMDM and UUO. ISL inhibited the phosphorylation of Syk and NF-kappa B and simultaneously reduced the expression of α-SMA and Col III in vivo and in vitro. More interestingly, when dealing with TDB, a ligand of Mincle, it revealed significant reversal of protein expression levels as that observed with ISL. The expressions of IL-1β, IL-6, TNF-α, iNOS, p-Syk, p-NF-kappa B, α-SMA and FN in BMDM inflammatory model were significantly upregulated with TDB treatment. This confirms that ISL inhibits inflammation and fibrosis of macrophage by suppressing Mincle/Syk/NF-kappa B signaling pathway.
Conclusion
To conclude, ISL protects UUO-induced CKD by inhibiting Mincle-induced inflammation and suppressing renal fibrosis, which might be a specific renal protective mechanism of ISL, making it a novel drug to ameliorate CKD.
Keywords: Isoliquiritigenin, Mincle, macrophage, CKD, inflammation, fibrosis
Introduction
Chronic kidney disease is a life-threatening, global health problem with a prevalence between 8% and 16% of the world’s population.1,2 Patients with CKD have high rates of complications and comorbidities affecting their quality of life.3 CKD is associated with the development of secondary disorders such as diabetes, hypertension and high risk of autoimmune diseases, which are rarely diagnosed at an early stage, and once developed, their progress is unfortunately irreversible. The main pathological changes of CKD are characterized by progressive nephrotic inflammation and fibrosis where macrophages play an integral role.4 In the process of renal hypoxia and tissue repair, macrophages play a role in stabilizing the tissue environment by activating the immune response.5 Macrophage has many different phenotypes under normal physiological conditions.6 Macrophages are mainly divided into two subtypes M1, ie, pro-inflammatory and M2, ie, which should be present and function in a balanced manner to maintain homeostasis of renal microenvironment.7 Renal function markers, such as serum creatinine and blood urea nitrogen, gradually increase in CKD leading to persistent inflammation and subsequent increase in the M1:M2 ratio in the diseased kidney.8 At an early stage of CKD, immune cells are largely infiltrated into the kidney including M1 macrophages, which promotes Th1 and Th17 responses, and involved in the initiation and maintenance of inflammatory processes.9 Macrophage-inducible C-type lectin (Mincle) is a C-type lectin receptor (CLR) that was first identified in 1999 as a downstream target of the transcription factor NF-kappa B (p65).10 Mincle expression is strongly induced in response to several inflammatory stimulations and stresses such as LPS, TNF, IL-6.11 Mincle belongs to the Syk kinase-coupled C-type lectin receptors, and it is tightly regulated by TLR4/NF-kappa B signaling as evidenced by the binding of NF-kappa B to the promoter region of Mincle in LPS-primed macrophages. Notably, Mincle-Syk signaling promotes and maintains inflammatory M1 macrophage phenotypes. Studies have found that the Mincle-Syk signaling pathway is involved in abdominal adipose tissue inflammation and fibrosis.12,13 The early stages of kidney damage induced by UUO are followed by chronic inflammation and fibrosis, M1 macrophages are the predominant phenotypes expressing CD11c. These classically activated M1 macrophages activate and release pro-inflammatory mediators including ILs, TNFα, and reactive oxygen species during 7 days of UUO modeling. These pro-inflammatory mediators in turn stimulate inflammatory signaling pathways that cause tissue damage by release of large amounts of inflammatory cytokines and proteolytic enzymes. In the later stages of proliferative and remodeling phase or simply during repair phase, M1 switches to M2 phenotype to remove the necrotic tissue debris and dead cells such as neutrophils in order to repair the damaged tissue. This transition of M1 to M2 phenotype promotes fibrosis by producing cytokines, chemokines, and growth factors such as TGF-β and PDGF. These alternatively activated M2 macrophages modify extracellular matrix (ECM) by regulating the balance of matrix metalloprotease (MMP) and its tissue inhibitors, leading to the appearance of a pro-fibrotic phenotype in macrophages, and the macrophages derived from adult monocytes are irreversibly committed to the fibrosis process.14,15 Previous studies have confirmed that in a mouse unilateral ureteral obstruction (UUO) model, F4/80 + CD301-M1 type macrophages recruited in the early stages of kidney injury can be transformed into F4/80 + CD301 + M2 type in the later stage of injury. These later macrophage phenotypes are found to release TGF-β, angiotensin, PDGF, proliferation of fibroblasts; to stimulate the synthesis of interstitial collagen and adhesion proteins; to cause extracellular matrix deposition; and to induce renal interstitial fibrosis.16 In our in-vitro experiments, we used TGF-β to stimulate BMDM, causing it to exhibit fibrotic manifestations such as collagen and adhesion proteins. Consequently, preventing the transition of macrophage to fibroblasts can inhibit the development of fibrotic phenotype, which may improve chronic kidney disease.
Isoliquiritigenin (ISL) is a flavonoid with a structure of chalcone, which has been found in licorice and shallot. ISL is generally used in traditional Chinese medicine and known to possess anti-oxidant, anti-inflammatory, anti-fibrotic, estrogenic and anti-tumor effects.17,18 A recent report demonstrated that ISL can suppress IL-6 and TNF-α production induced by lipid A moiety of LPS in RAW264.7 cells and attenuated the formation of the LPS-TLR4/MD-2 complexes, resulting in inhibition of homodimerization of TLR4.19 Furthermore, studies have shown that ISL has significant anti-inflammatory effects on AKI20,21 and possesses the ability to inhibit innate immune responses to attenuate inflammation and fibrosis both in-vitro and in-vivo.22 However, the protective mechanism of ISL on chronic inflammation and fibrosis in early CKD is unclear.
In this study, we aimed to investigate the potential anti-inflammatory and anti-fibrotic role of ISL for the treatment of CKD by modulation of macrophage-maintained inflammation and fibrosis and reveal its potential therapeutic mechanism on CKD via Mincle/Syk/NF-kappa B signaling pathway.
Materials and Methods
Isoliquiritigenin
Isoliquiritigenin (purity >95%) was purchased from Chengdu Purify Technology Development Co, Ltd. It is an isoflavone compound extracted from licorice root with molecular formula C15H12O4 and structural formula shown in Figure 1A. In this study, ISL was used by dissolving in 10%DMSO.
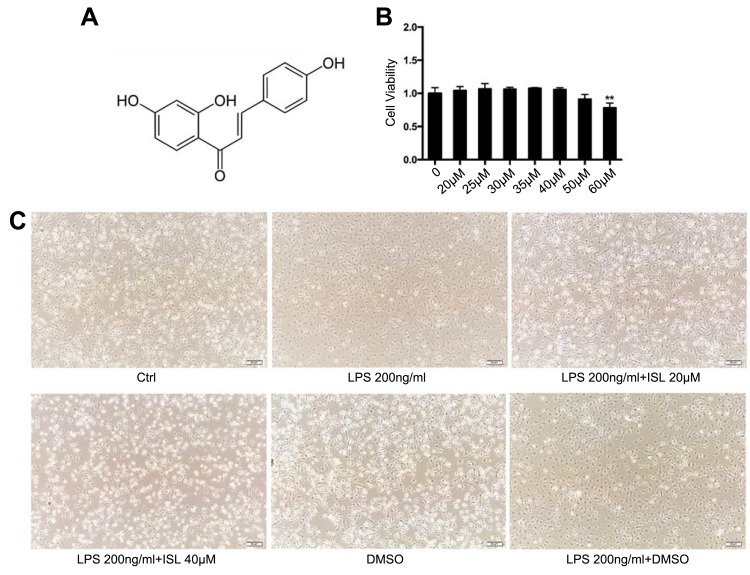
Figure 1.
Safe dose of Isoliquiritigenin to mouse bone marrow-derived macrophages and its effect on cellular morphology. (A) Chemical formula of Isoliquiritigenin (ISL); (B) cytotoxicity detection of ISL on BMDM. According to the results, we chose the therapeutic dose is of low dose (20 μM), high dose (40 μM); (C) morphological effects of low and high doses of ISL on LPS-stimulated BMDM.
Notes: **P<0.01, vs blank group.
BMDM Extraction
Male C57BL/6 mice were anesthetized with 1% sodium pentobarbital. The hind leg bones were carefully separated from femur bone and the attached muscles were removed. The hind leg bones were crushed using mortar and pestle in PBS as a solvent, until the color of the bones changed from red to white. The supernatant was collected, filtered through a 70 μm cell filter and centrifuged at 1500 rpm for 5 minutes. The resulting supernatant was discarded and broken red blood cells were removed using 10× PBS. Then, the remaining cells were passed through a 40 μm cell filter to obtain the bone marrow-derived cells which were cultured in BMDM complete medium for 5 days to differentiate macrophages.
Cell Culture
Mouse bone marrow-derived macrophage (BMDM) is a primary cell extracted from the bone marrow of the hind leg bone of male C57BL/6 mice. BMDM was cultured in Dulbecco’s Modified Eagle Medium (DMEM)/Low glucose (AD21573269, Hyclone) supplemented with 10% fetal bovine serum (Gibco, USA) and 30% L929 cell supernatant, 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C with 5% CO2 and 100% humidity. The L929 cells were cultured for 5 days after then the supernatant was collected and filtered through a 0.22 μm filter and added to the BMDM culture medium. The morphology of BMDM was observed using a light microscope (Olympus Corporation, Japan) at ×200 magnification.
Animal Experiments
The study was conducted in accordance with the National Institutes of Health guidelines on the use of experimental animals. The Animal Research Committee of Southwest Medical University reviewed and approved all animal experiments. The mice were fed in regular cages and allowed free access to food and water. All animals were kept in a temperature around 22 °C, humidity about 65% and maintained for 12 hours of light. Male C57BL/6 mice (8 weeks of age and 20–22 g body weight) were divided into five groups as follows: Sham, positive control (Irbesartan 20 mg/kg), UUO, ISL low (7.5 mg/kg) and ISL high (30 mg/kg) treatment groups with 7 mice in each group. Irbesartan belongs to angiotensin II receptor antagonist, angiotensin II (Ang II) mainly exerts the contractile function in the afferent arterioles, thereby increasing the fluid pressure in the glomeruli that can cause and aggravate kidney damage. Irbesartan can block the combination of AT1 receptor and Ang II, reducing the fluid pressure in the glomerulus and thus exert anti-fibrotic effect, therefore mainly utilized in chronic kidney disease.23 In a 5/6 rat nephrectomy model, Zhao found that the expression of TGF-β, smad2, and AT1R in the irbesartan-treated group was downregulated, while smad6 expression was upregulated, thereby playing a role in treating renal insufficiency.24 Mice in ISL treatment and Irbesartan group received intragastric administration 1 hour before modeling, and continuously received intragastric administration for 7 days. Control animals received a sham operation, in which the ureters were manipulated but not ligated and were killed under the same conditions as those receiving UUO.
Renal Function Test
Blood was taken immediately after the mice were sacrificed on the seventh day. The blood was centrifuged at 3000 rpm for 15 minutes at 4 °C to collect serum. The obtained serum was analyzed for levels of urea nitrogen (BUN) and creatinine (CREA), detected by BUN test kit (C013-2, Jian Cheng, Nanjing, China) and Creatinine test kit (C011-2, Jian Cheng, Nanjing, China) respectively, according to the standard method.
CCK8 Assay
One thousand and five hundred of BMDM cells were seeded in a well of 96-well plate. The next day, cells were treated with drug-containing medium for 24 hours. Then, 100 μL of serum-free medium containing 10% CCK8 stock solution (Promega, G3580) was applied to each well and was incubated at 37 °C for 2 hours, followed by measurement of the absorbance at 450 nm by BioTeK reader.
Real-Time PCR
The mRNA expression in both the kidney and BMDM was detected by Eastep qPCR Master Mix (Shanghai Promega, 0000206820). Total RNA was extracted from the cells and the kidney using the Total RNA Kit (Beijing Tian Gen, Q5905) according to the instructions. One microgram of total RNA of each sample was reverse transcribed into cDNA by using Eastep RT Master (5x) (shanghai Promega, 0000323749). Real-time PCR was performed using Light CyclerR 480II PCR (Roche). Primers for real-time PCR are listed in Table 1. The mRNA expression level of the target gene was normalized to GAPDH.
Table 1.
Specific Primers for Real-Time PCR
| Gene | Primer Sequence (5ʹ–3ʹ) |
|---|---|
| IL-1β | S: TGCCACCTTTTGACAGTGATG A: AAGGTCCACGGGAAAGACAC |
| IL-6 | S: AAAGAGTTGTGCAATGGCAATTCT A: AAGTGCATCATCGTTGTTCATACA |
| TNF-α | S: CATCTTCTCAAAATTCGAGTGACAA A: TGGGAGTAGACAAGGTACAACCC |
| MCP-1 | S: CTTCTGGGCCTGCTGTTCA A: CCAGCCTACTCATTGGGATCA |
| GAPDH | S: CGGAGTCAACGGATTTGGTCGTAT A: AGCCTTCTCCATGGTGGTGAAGAC |
Immunofluorescence Staining
For cellular immunofluorescence, the BMDM cells were seeded on round coverslips. After treatment with ISL for 1 day, the cover slips were washed twice with PBS for 5 minutes and then fixed in 10% formaldehyde for 15 minutes at room temperature. Subsequently, the cells were treated with 0.5% Triton X-100 for 10 min at room temperature to increase the permeation of cell membrane, followed by blocking with 5% BSA for 30 min and then incubated with mouse anti- IL-1β antibody (1:200, Santa Cruz) or mouse anti-IL-6 antibody (ab9324, 1:150, CST) overnight at 4 °C. For kidney immunofluorescence, after the kidney tissue was fixed with 10% formaldehyde for 24 hours, it was dehydrated with 7% sucrose for three times and cut into 4 μm with a slicer; then, it was washed three times with PBS for 5 minutes each time, as well as permeated with 0.5% Triton X-100 and blocked with 5% BSA for 1 h. Subsequently, tissues were incubated with mouse anti-Mincle antibody (sc161489, 1:150, Santa Cruz) at 4 °C over night. After incubation with the primary antibody, both tissue and cell sections were washed with PBS for 10 minutes, and then incubated with AlexaFluor® 488 (CST,1:150,4408S) secondary antibody for 1h. At last, the images were taken by a fluorescence microscope (OLYMPUS, Japan).
Western Blotting
The total proteins of kidney tissues and BMDM were extracted by using RIPA lysis buffer. The proteins were loaded onto 12% SDS-PAGE and transferred to nitrocellulose membranes. After 1h blocking with 5% BSA, the membranes were incubated with p-p65 (rabbit anti-mouse, 1:1000, CST, #3033L), Mincle (Mouse anti-mouse,1:500, Santa Cruz), iNOS (Rabbit anti-mouse,1:1000,CST), p-Syk (Rabbit anti-mouse 1:1000,CST), α-SMA (Rabbit anti-mouse, 1:1000, Invitrogen), Col III (anti-mouses, 1:1000, Santa Cruz) and GAPDH (Mouse anti-mouse, 1:5000) antibodies. After then, the membranes were incubated with HRP labeled goat anti-mouse IgG (1:3000) and goat anti-rabbit IgG (1:3000) for 1h at room temperature. The total protein was normalized to GAPDH. The bands’ gray intensity was analysed by image J software.
H&E, PAS and Masson Staining
Kidney tissues were fixed with 10% formaldehyde for 24 h and embedded in paraffin, and then cut into 4 μm sections and taken on slides. The prepared slides were deparaffinized twice in xylene and rehydrated in gradient ethanol, and then stained separately with hematoxylin-eosin, PAS (glycogen) and Masson's trichrome staining (fibrosis). PAS kit was purchased from Solarbio (Beijing, G1281) and Masson staining kit from BASO (Zhuhai, China, BA4079B). Sections were imaged at 200× magnification using an optical microscope.
Immunohistochemistry
Kidney tissues were fixed with 10% formaldehyde, embedded in paraffin, and cut into 4 μm. The sections were first subjected to antigen retrieval, and then performed immunohistochemical staining of the tissue according to the instruction in the kit. The primary antibodies were F4/80 (Bio-Rad antibodies, 1:150), α-SMA (Invitrogen, 1:100), Col III (Santa Cruz, 1:100). Sections were imaged at 200× magnification using an optical microscope.
Statistical Analysis
Results are shown as mean ± standard deviation. Data analysis was performed using the Student–Newman–Keul’s test. The Graph Pad Prism 6 software was used to analyze one-way ANOVA and post hoc tests. P <0.05 is considered as statistically significant.
Results
Safe Dose of Isoliquiritigenin to Mouse Bone Marrow-Derived Macrophages and Its Effect on Cellular Morphology
The chemical structure of ISL is shown in Figure 1A. To obtain the safety dose, the cytotoxicity of ISL in BMDM cells was determined by CCK8. When the cells were treated with a dose of ISL higher than 40 μM, a significant decrease in cell viability was noted (Figure 1B). Therefore, the dose of 40 μM was selected to perform the treatment in the cellular experiment. After LPS stimulation, BMDM cells were treated with ISL 20 μM and 40 μM, respectively, and the size and morphology of BMDM cells were largely changed, in which, the cells became round and the surrounding burrs increased, and gradually returned to the normal after treatment with ISL (20 μM, 40 μM) (Figure 1C).
Isoliquiritigenin Can Reduce the Inflammatory Response Induced by LPS in BMDM
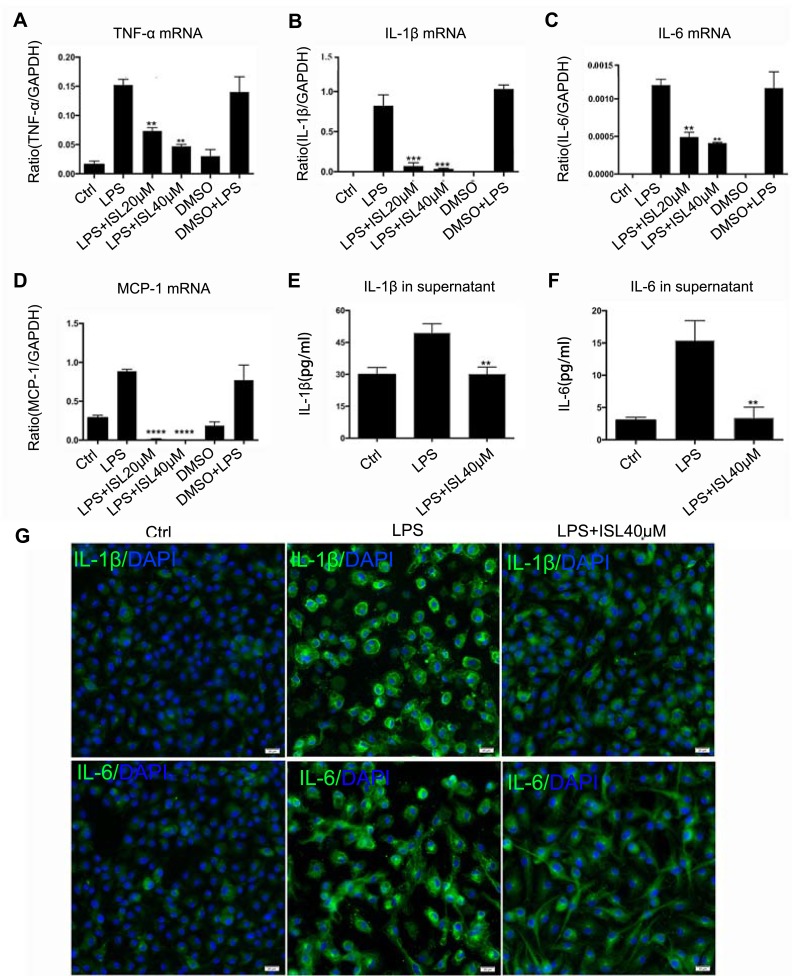
We next investigated the therapeutic effects of ISL in renal inflammation, an inflammatory cell model was established in BMDM cells using LPS at a dose of 200 ng/mL. Real-time PCR showed that inflammatory factors increased after 24 hours of LPS stimulation but ISL treatment at a dose of 20 μM and 40 μM significantly inhibited the LPS-induced mRNA expression of inflammatory factors (Figure 2A–D). In addition, we detected the secretion of these factors in the supernatant and found that ISL 40 μM significantly attenuated the secretion of IL-1β and IL-6 (Figure 2E–F). At the same time, it was found by immunofluorescence that ISL notably reduced LPS-induced protein level of IL-1β and IL-6 (Figure 2G). Thus, we can find that ISL can effectively reduce LPS-induced BMDM inflammation and secretion of inflammatory cytokines in vitro.
Figure 2.
Isoliquiritigenin reduced the inflammatory response induced by LPS in BMDM. (A–D) ISL can reduce the mRNA expression of inflammatory cytokines in LPS-induced BMDM, including IL-1β, IL-6, TNF-α and MCP-1. **P<0.01, vs LPS group. ***P<0.001, vs LPS group. ****P<0.0001, vs LPS group; (E, F) ISL reduced the secretion of IL-1β and IL-6 in supernatant of LPS-stimulated BMDM. **P<0.01, vs LPS group; (G) immunofluorescence results showed that ISL inhibited the protein level of IL-1β and IL-6 in LPS-stimulated BMDM.
Isoliquiritigenin Can Improve the Renal Function in the UUO Model Without Toxicity to the Main Organs
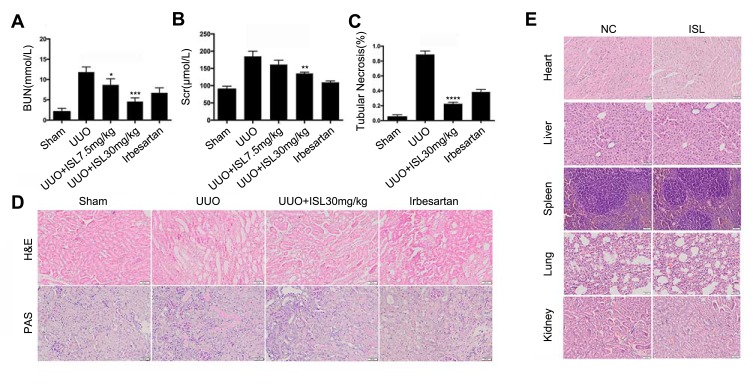
In order to examine the tubulointerstitial inflammation and fibrosis induced by UUO model in vivo, mice were randomly divided into control, UUO, ISL low dose (7.5 mg/kg), high dose of ISL (30 mg/kg), Irbesartan (20 mg/kg). The serum urea nitrogen (BUN) and Creatinine (CRE) were detected in serum. The levels of BUN and Scr were increased in the UUO model, which were downregulated in the low- and high-dose groups of ISL treatment (Figure 3A and B). Irbesartan can also effectively reduce urea nitrogen and creatinine in UUO, but the effect of reducing urea nitrogen is not as obvious as that of high-dose ISL. H&E and PAS staining showed UUO led to a serious renal histological damage, and the renal tubules were hollow. According to the H&E staining results, we found that the number of damaged renal tubulars was decreased in ISL-treated groups compared to the UUO group (Figure 3C), especially in ISL high-dose group (Figure 3D) Irbesartan can also reduce the number of damaged renal tubules and collagen deposition in UUO, but in repairing renal tubular tissue structure, its effect is not as obvious as that of ISL. In addition, ISL has no effects on the main organs in vivo (Figure 3E). This suggested that ISL significantly improves renal injury in the UUO model.
Figure 3.
Isoliquiritigenin improved the renal function in the UUO model without toxicity to the main organs. (A, B) In vivo, ISL can reduce creatinine and urea nitrogen in mouse UUO model. *P<0.05, vs UUO group. **P<0.01, vs UUO group. ***P<0.001, vs UUO group; (C) according to the H&E results, we calculated the renal tubular necrosis. ****P<0.0001, vs UUO group; (D) we also found that ISL can significantly improve tubular damage and inflammatory cell infiltration by H&E and PAS staining; (E) assessing the toxicity and damage of ISL to major organs in mice, and no damage was found to each organ by ISL.
Isoliquiritigenin Protects the Kidney by Inhibiting Mincle/Syk/NF-Kappa B Signaling Pathway and Reducing the Expression and Secretion of Inflammatory Cytokines in the Kidney and Blood of UUO Model
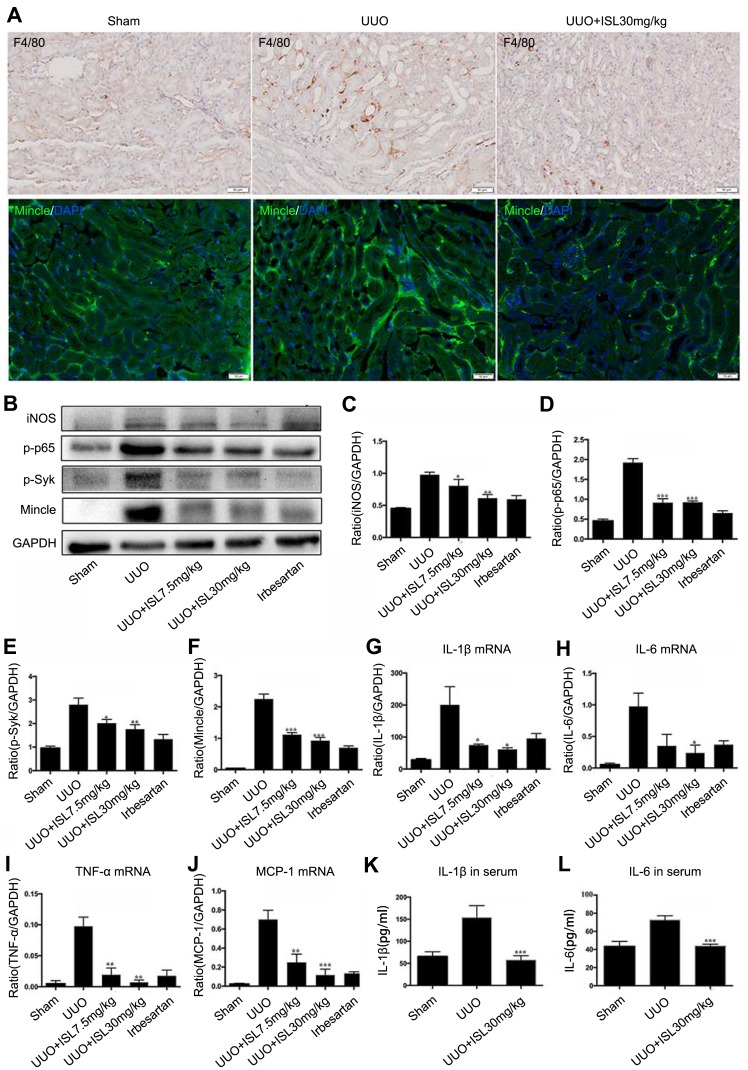
UUO model dominated kidney inflammation on the seventh day, which was observed as the increased expression of macrophage marker F4/80 in the UUO model by immunohistochemistry (Figure 4A). Mincle is a molecular pattern recognition receptor associated with injury and is involved in the initiation of innate immune responses, which is also increased in the UUO model with immunofluorescence (Figure 4A). Since Mincle induces an inflammatory response by signaling with downstream NF-kappa B and Syk, the potential effect of ISL on this signaling is further investigated. The Western blot results showed that ISL treatment reduced the phosphorylation of NF-kappa B and Syk in the kidney of UUO model. At the same time, the effect of ISL on Mincle was confirmed by Western blotting, which showed downregulation of Mincle protein level after ISL treatment, with consistent decrease in iNOS of the M1 macrophage marker (Figure 4B–F). Irbesartan can also reduce the expression of mincle/p-Syk/p-P65, and its effect is similar to the high dose of ISL. We also observed the expression of inflammatory cytokines in the kidney of UUO model. The level of inflammatory cytokines in the UUO model was significantly higher than that in the control group. After treatment with ISL, the expression of IL-1β, TNF-α, IL-6 and MCP-1 in the kidney was strongly inhibited compared with UUO (Figure 4G–J). The ELISA results showed that the secretion of inflammatory cytokines, IL-1β and IL-6 in serum was significantly decreased after ISL treatment in the UUO model (Figure 4K and L). Irbesartan also effectively reduced the expression of inflammatory factors in the kidney tissue of UUO, but the effect was not as obvious as that of high-dose ISL. These data indicated that ISL can significantly reduce chronic inflammation in the UUO mouse kidney by inhibiting Mincle/Syk/NF-kappa B signaling pathway.
Figure 4.
Isoliquiritigenin protects the kidney by inhibiting Mincle/Syk/NF-kappa B signaling pathway and reducing the expression and secretion of inflammatory cytokines in the kidney and blood of UUO model. (A) Immunohistochemistry results showed that the infiltration of macrophage was significantly increased in the kidney of UUO model and ISL reduced infiltrated macrophage; (B–F) immunofluorescence and Western blot results showed that ISL can effectively reduce the protein level of Mincle and iNOS as well as phosphorylated Syk and NF-kappa B in the kidney of UUO model; (G–J) ISL strongly reduced the mRNA expression of IL-1β, IL-6, TNF-α and MCP-1 in the kidney of UUO model; (K, L) ISL also inhibited the secretion of IL-1β and IL-6 in the serum of UUO mice.
Notes: *P<0.05, vs UUO group. **P<0.01, vs UUO group. ***P<0.001 vs UUO group.
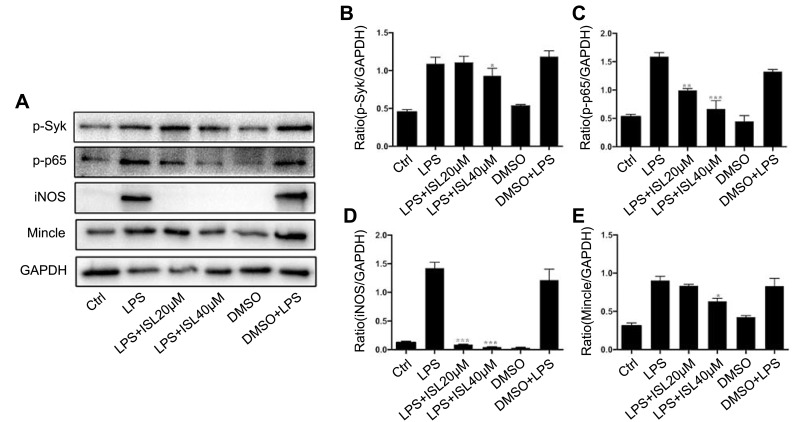
Isoliquiritigenin Also Inhibited Mincle/Syk/NF-Kappa B Signaling Pathway in BMDM
After the treatment of LPS-stimulated BMDM with ISL, we examined the renal expression of Mincle and downstream inflammatory factors among the four groups. The results showed that ISL treatment reduced the protein levels of p-NF-kappa B and p-Syk in LPS-stimulated BMDM (Figure 5A–C). Western blotting confirmed that the protein levels of Mincle and iNOS were downregulated after treatment with ISL in LPS-stimulated BMDM (Figure 5A, D and E). This indicates that ISL may inhibit inflammation through suppressing Mincle/Syk/NF-kappa B signaling pathway in macrophage.
Figure 5.
Isoliquiritigenin inhibited Mincle/Syk/NF-kappa B signaling pathway in BMDM. (A) ISL significantly reduced the protein level of key molecular of Mincle/Syk/NF-kappa B signaling pathway detecting by Western blot. The normalized data showed that the protein level of phosphorylated Syk (B), phosphorylated NF-kappa B (C), iNOS (D) and Mincle (E) were notably suppressed by ISL in LPS-stimulated BMDM.
Notes: *P<0.05, vs LPS group. **P<0.01, vs LPS group. ***P<0.001, vs LPS group.
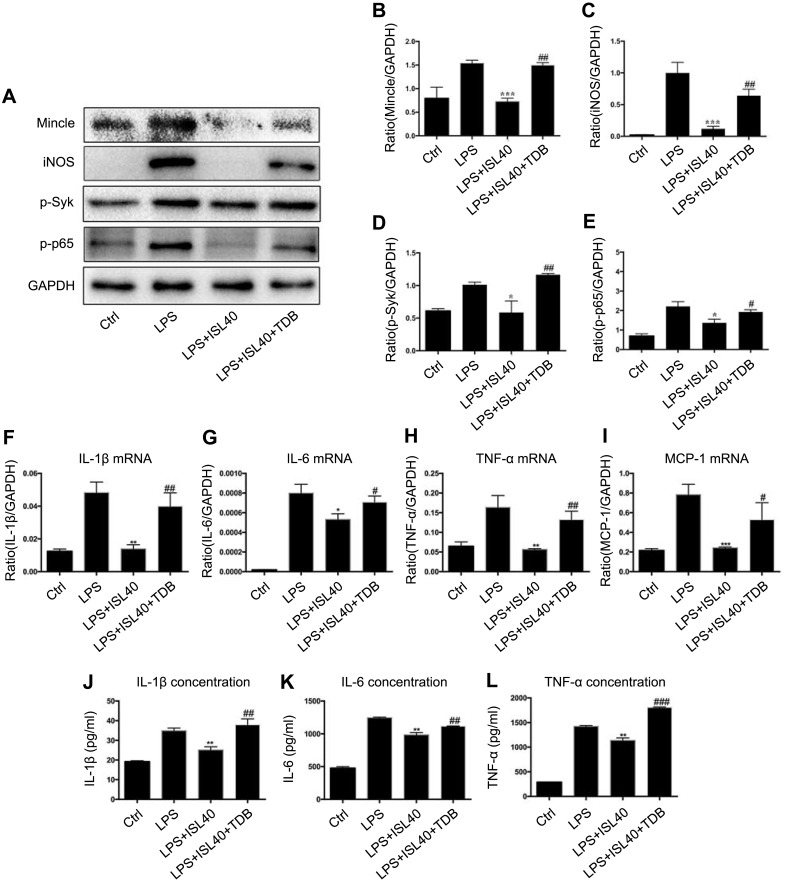
Isoliquiritigenin Inhibits Inflammation Through Mincle-Dependent Mechanism in BMDM Cells
From the above results, we can make a conclusion that ISL can alleviate the LPS-induced inflammatory microenvironment and it may exert its anti-inflammatory effect through inhibiting Mincle/Syk/NF-kappa B signaling pathway. To clarify the Mincle-dependent mechanism of ISL on inhibition of inflammation, we further revealed the relationship between Mincle and inflammatory molecular in BMDM. The results showed that ISL inhibited phosphorylation of NF-kappa B and Syk in LPS-stimulated BMDM. At the same time, LPS-induced Mincle and iNOS expression were also significantly inhibited by ISL in BMDM. However, upregulation of Mincle by its ligand TDB strongly reversed the inhibited inflammatory response in LPS-stimulated BMDM, by detecting the protein levels of iNOS and phosphorylated Syk and NF-kappa B (Figure 6A–E). Moreover, we also detected the expression and secretion of inflammatory cytokines after TDB administration, the data showed that after overexpression of Mincle by TDB administration, the expression and secretion of IL-1β, IL-6, TNF-α and MCP-1 were significantly increased (Figure 6F–L), indicating that ISL inhibits inflammation through downregulating Mincle.
Figure 6.
Isoliquiritigenin suppressed inflammation through Mincle-dependent mechanism in BMDM cells. (A) In the Mincle recovery experiment, we used TDB to upregulate Mincle in ISL-treated inflammatory BMDM. The results showed that the protein level of Mincle (B), iNOS (C), phosphorylated Syk (D) and phosphorylated NF-kappa B (E) were significantly increased in ISL-treated inflammatory BMDM, suggesting ISL inhibits inflammation by suppressing Mincle; Overexpression of Mincle by administration of TDB, upregulated the expression (F–I) and secretion (J–L) of IL-1β, IL-6 and TNF-α and MCP-1 in ISL-treated inflammatory BMDM.
Notes: *P<0.05, vs LPS group. **P<0.01, vs LPS group. ***P<0.001, vs LPS group. #P<0.05, vs LPS+ISL 40μM-treated group. ##P<0.01, vs LPS+ISL 40μM-treated group. ###P<0.001, vs LPS+ISL 40μM-treated group.
Isoliquiritigenin Relieved Fibrosis in vitro and in vivo by Inhibiting Mincle
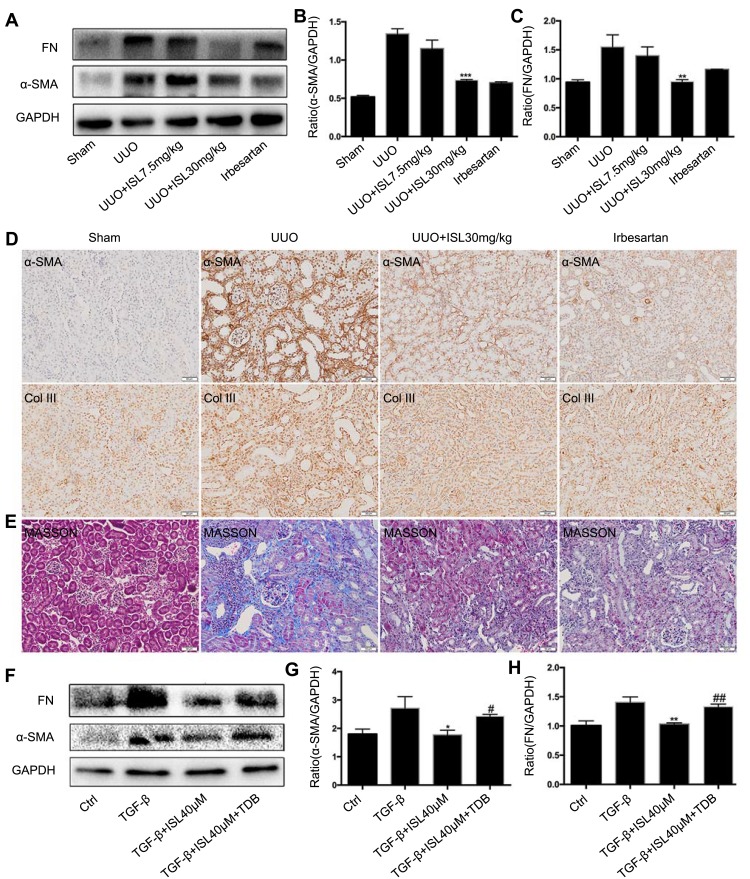
Kidney chronic fibrosis is a typical feature of the UUO model. The above results have shown that ISL reduced inflammation in the kidney of UUO model by inhibiting the Mincle signaling pathway, but whether it can reduce fibrosis in vivo or in vitro is unknown. We found that ISL significantly inhibited the protein level of fibrosis-related protein α-SMA and FN in the kidney of the UUO model by Western blot. Irbesartan can also effectively reduce fibrin expression in UUO (Figure 7A–C). The immunohistochemical results of α-SMA and Col III and Masson staining in the kidney of UUO model confirmed the effect of ISL on inhibition of fibrosis in vivo. Irbesartan can also effectively reduce the expression of α-SMA and Col III (Figure 7D–E). In vitro, we used TGF-β to stimulate BMDM cells to establish a fibrosis cell model. After treatment with ISL, we found a significant decrease in the protein level of fibrosis markers, α-SMA and FN, which were strongly upregulated by TDB, suggesting Mincle signaling plays a key role in macrophage-related fibrosis (Figure 7F–H).
Figure 7.
Isoliquiritigenin relieved fibrosis in vivo and in vitro by inhibiting Mincle. (A–D). According to the results of Western blot and Immunohistochemistry, ISL significantly inhibited the protein level of fibrosis markers α-SMA, Col III and FN in the kidney of UUO mice. **P<0.01, vs UUO group. ***P<0.001, vs UUO group; (E) Masson staining also showed a significant decrease in fiber deposition after ISL treatment; (F–H) after treating with TDB, reduction of α-SMA and FN by ISL in TGF-β-stimulated BMDM was significantly upregulated, suggesting the key role of Mincle in inhibition of fibrosis by ISL.
Notes: *P<0.05, vs TGF-β group. **P<0.01, vs TGF-β group. #P<0.05, vs TGF-β+ISL 40 μM-treated group. ##P<0.01, vs TGF-β+ISL 40 μM-treated group.
Discussion
As a widely used flavonoid for medical research, ISL has been studied for the treatment of AKI and presents positive therapeutic roles. However, the effects of ISL on anti-inflammation and anti-fibrosis in the UUO model, a common renal fibrosis model, have not been reported yet. Therefore, we try to explore the potential protective effect and mechanism of ISL on UUO. The results of the present study have revealed that the administration of ISL can alleviate UUO-induced chronic kidney injury and reduce renal inflammation and fibrosis in vivo, and inhibit the inflammatory response and fibrosis transformation in macrophage in vitro through downregulating the activation of Mincle/Syk/NF-kappa B signaling pathways. This may provide a new direction and new drug option for the treatment of obstructive nephropathy with ISL.
Chronic kidney disease (CKD) is characterized by persistent inflammation and progressive fibrosis.25 Numerous studies have shown an established consensus linking chronic inflammation and induction of profibrotic signaling.26 Therefore, the UUO model which initiates a sequence of events in the obstructed kidney, including interstitial inflammation and TEC death, ultimately leading to renal fibrosis is often used to evaluate the potential therapeutic effects of novel treatments on various kidney diseases.27,28 Previous studies have suggested that the renal fibrosis induced by obstructed kidneys is characterized by inflammatory cell recruitment, macrophage infiltration, and increased proinflammatory cytokines.29
ISL is a flavonoid with chalcone structure, which is extracted from the famous traditional Chinese herbal medicine licorice. In recent years, this flavonoid has attracted attention because it has a wide range of biological and pharmacological properties, such as antioxidant, anti-inflammatory, anti-platelet aggregation, antibacterial, antiviral, antitumor, hepatoprotective, neuroprotective, cardioprotective and detoxification effects. Furthermore, studies by Zhu Xiaodong found that ISL may prevent LPS-induced cognitive impairment and neuronal damage by promoting or maintaining antioxidant capacity and inhibiting neuroinflammation. This may be due to phosphorylation-dependent inactivation of GSK-3β, which enhances NRF2 reactive resistance.30 Watanabe Yasuharu found that ISL inhibited TLR4 or Mincle-stimulated expression of fibrosis-related genes from obese adipose tissue and macrophage stromal vascular parts in vitro. ISL can suppress adipose tissue inflammation through inflammatory body-dependent and non-independent ways, and reduce adipose tissue fibrosis by targeting innate immune sensors.22
The progression of renal injury is accompanied by increased infiltration of inflammatory cells, particularly macrophages under the influence of multiple chemokines. Macrophages are one of the most important cells which are recruited when the kidney is obstructed, a critical component during renal injury. M1 macrophages in host disease promote TH1 immune response and pathogen-associated molecular patterns (PAMP). Moreover, lipopolysaccharide (LPS) and proinflammatory cytokines such as IFNγ and tumor necrosis factor (TNF) can promote the M1 macrophage polarization.22,30 Therefore, we used LPS to stimulate macrophage and induce M1 polarization, an inflammatory phenotype. In recent studies, Mincle plays an important role in inflammation of the kidney and serves as a key factor for maintaining the M1 macrophage phenotype by TLR4/NF-kappa B signaling through the Syk dependency mechanism.31 Therefore, in this study, our goal was to investigate the regulatory mechanisms of ISL in macrophage-mediated renal inflammation and fibrosis through Mincle in UUO. We found that Mincle showed an increasing trend after LPS stimulation and in UUO models, indicating that Mincle plays a key role in inflammation. In the process of CKD progression, M1 macrophages appear in the early stage of chronic inflammation in CKD.32 Interestingly, our results showed that the treatment with ISL reduced the number of M1 macrophages (M1 marker: iNOS), partly by inhibiting the expression of Mincle in vivo and in vitro. On the other hand, NF-kappa B is an important transcription factor controlling the expression of proinflammatory cytokines.33,34 ISL can inhibit the expression of NF-kappa B signaling pathway and interfere with the hydrolysis and nuclear transposition of p-p65 protein, thus, playing an anti-inflammatory role. As a direct downstream effector of Mincle, Syk’s activation is also inhibited by ISL, which can be blocked by upregulated Mincle in the recovery experiment. The above results suggest that ISL protects the kidney from early inflammation of CKD by inhibiting the expression of Mincle, thereby further inhibiting the polarization of M1 macrophages and suppressing the activation of Syk and NF-kappa B.35,36,37 Previous studies have shown that ISL is an anti-inflammatory medicine monomer but in this study, we found that ISL not only reduces kidney inflammation but also has some anti-fibrotic effects. Irbesartan, as a positive control drug in this study, which can reduce the expression of inflammatory factors and fibrotic proteins in the UUO model to varying degrees, can provide a better control of anti-inflammatory and anti-fibrosis effect of ISL. Our results demonstrated that ISL inhibited fibrosis in the kidney of UUO model in vivo and downregulated the protein level of fibrotic markers, α-SMA, and FN in TGF-β-induced BMDM in vitro by inhibiting Mincle.
In conclusion, the present study showed that the treatment with ISL significantly reduced inflammation and fibrosis in the kidney of UUO model and inhibited inflammatory and fibrotic responses in BMDM by blocking Mincle/Syk/NF-kappa B signaling pathway. Together, these findings suggest that ISL may be a novel therapeutic monomer for chronic renal inflammation and fibrosis.
Acknowledgments
This work was supported by the Luzhou – Southwest Medical University Joint Project (2017LZXNYD-Z03 and 2018LZXNYD-PT03), the Southwest Medical University – Affiliated Traditional Medicine Hospital Joint Project (2018XYLH-034), the Innovation Team Project of Sichuan Provincial Education Department (17TD0046), and the Luzhou Municipal – Southwest Medical University Joint Special Grant for the Introduction of High-level Talents (Chen Chen Team and Lan Hui-Yao Team). We thank Dr Maryam Mazhar for revising this manuscript.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–2284. doi: 10.1681/ASN.2005121273 [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia GG, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 3.Santana MT, Cerini C, Burtey S. emerging roles of aryl hydrocarbon receptors in the altered clearance of drugs during chronic kidney disease. Toxins (Basel). 2019;11:undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glanz V, Myasoedova VA, Sukhorukov V, et al. Transcriptional characteristics of activated macrophages. Curr Pharm Des. 2019;25(3):213–217. doi: 10.2174/1381612825666190319120132 [DOI] [PubMed] [Google Scholar]
- 5.XL Z, Guo Y-F, Song Z-X, et al. Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats. Endocrinology. 2014;155(12):4939–4950. doi: 10.1210/en.2014-1020 [DOI] [PubMed] [Google Scholar]
- 6.Tang PM-K, Nikolic PDJ, Lan H-Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144–158. doi: 10.1038/s41581-019-0110-2 [DOI] [PubMed] [Google Scholar]
- 7.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118(11):3522–3530. doi: 10.1172/JCI36150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu S, Wang Y, Liu HT, et al. κThyroxine affects lipopolysaccharide-induced macrophage differentiation and myocardial cell apoptosis via the nf-b p65 pathway both in vitro and in vivo. Mediators Inflamm. 2019;2019:2098972. doi: 10.1155/2019/2098972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto M, Tanaka T, Kaisho T, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163(9):5039–5048. [PubMed] [Google Scholar]
- 11.chioka M, Suganami T, Tsuda N, et al. Increased expression of macrophage-inducible C-type lectin in adipose tissue of obese mice and humans. Diabetes. 2011;60(3):819–826. doi: 10.2337/db10-0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J-W, Roh Y-S, Jeong H, et al. Spliceosome-associated protein 130 exacerbates alcohol induced liver injury by inducing NLRP3 inflammasome-mediated IL-1β in mice. Am J Pathol. 2018;188(4):967–980. doi: 10.1016/j.ajpath.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 13.Drummond RA, Saijo S, Iwakura Y, et al. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41(2):276–281. doi: 10.1002/eji.201041252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios Francisco J, Zou Z-G, Harvey Adam P, et al. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc Res. 2020;116(3):721–735. doi: 10.1093/cvr/cvz164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simões Filipa C, Cahill Thomas J, Amy K, et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun. 2020;11(1):600. doi: 10.1038/s41467-019-14263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen B, Liu X, Fan Y, et al. Macrophages regulate renal fibrosis through modulating TGFβ superfamily signaling. Inflammation. 2014;37(6):2076–2084. doi: 10.1007/s10753-014-9941-y [DOI] [PubMed] [Google Scholar]
- 17.Chen HH, Zhu JF, Liu Y, et al. Lipopolysaccharide induces chronic kidney injury and fibrosis through activation of mTOR signaling in macrophages. Am J Nephrol. 2015;42(4):305–317. doi: 10.1159/000441506 [DOI] [PubMed] [Google Scholar]
- 18.Kim D-H, Park JE, Gyeong CI, et al. Isoliquiritigenin inhibits the proliferation of human renal carcinoma caki cells through the ROS-mediated regulation of the Jak2/STAT3 pathway. Oncol Rep. 2017;38(1):575–583. doi: 10.3892/or.2017.5677 [DOI] [PubMed] [Google Scholar]
- 19.Honda H, Nagai Y, Matsunaga T, et al. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukoc Biol. 2012;91(6):967–976. doi: 10.1189/jlb.0112038 [DOI] [PubMed] [Google Scholar]
- 20.Lee YK, Chin Y-W, Bae J-K, et al. Pharmacokinetics of isoliquiritigenin and its metabolites in rats: low bioavailability is primarily due to the hepatic and intestinal metabolism. Planta Med. 2013;79:1656–1665. doi: 10.1055/s-0033-1350924 [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Wang C, Wang YM, et al. Isoliquiritigenin attenuates LPS-induced AKI by suppression of inflammation involving NF-κB pathway. Am J Transl Res. 2018;10(12):4141–4151. [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe Y, Nagai Y, Honda H, et al. Isoliquiritigenin attenuates adipose tissue inflammation in vitro and adipose tissue fibrosis through inhibition of innate immune responses in mice. Sci Rep. 2016;6(1):23097. doi: 10.1038/srep23097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf G, Neilson E. Angiotensin 11 induces cellular hypertrophy in cultured murine proximal tubular cellS. Am J Physiol. 1990;259(5):768–777. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Zhao H, Tu L, et al. Effects and mechanism of irbesartan on tubulointerstitial fibrosis in 5/6 nephrectomized rats. J Huazhong Univ Sei Technolog Med Sci. 2010;30(1):48–54. doi: 10.1007/s11596-010-0109-1 [DOI] [PubMed] [Google Scholar]
- 25.Rayego MS, Rodrigues DR, Morgado PJL, et al. Role of Epidermal Growth Factor Receptor (EGFR) and its ligands in kidney inflammation and damage. Mediators Inflamm. 2018;2018:8739473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peddakkulappagari CS, Saifi MA, Khurana A, et al. Withaferin A ameliorates renal injury due to its potent effect on inflammatory signaling. Biofactors. 2019;undefined:undefined. [DOI] [PubMed] [Google Scholar]
- 27.Stroo I, Emal D, Butter LM, et al. No difference in renal injury and fibrosis between wild-type and NOD1/NOD2 double knockout mice with chronic kidney disease induced by ureteral obstruction. BMC Nephrol. 2018;19(1):78. doi: 10.1186/s12882-018-0867-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthur CK, Chung XR, Huang LZ, Heuchel R, Lai KN, Lan HY. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant. 2009;24(5):1443–1454. doi: 10.1093/ndt/gfn699 [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Sun F, Zhong XS, et al. Eplerenone-mediated aldosterone blockade prevents renal fibrosis by reducing renal inflammation, interstitial cell proliferation and oxidative stress. Kidney Blood Press Res. 2013;37(6):557–566. doi: 10.1159/000355736 [DOI] [PubMed] [Google Scholar]
- 30.Xiaobo Z, Jiankun L, Shaojie C, et al. Isoliquiritigenin attenuates lipopolysaccharide induced cognitive impairment through antioxidant and anti-inflammatory activity. BMC Neurosci. 2019;20(1):41. doi: 10.1186/s12868-019-0520-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Nakano Y, Mishiro K, et al. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep. 2013;3(1):3177. doi: 10.1038/srep03177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji LQ, Chen YF, Wang HQ, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol. 2019;55(1):103–115. doi: 10.3892/ijo.2019.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H, Cheng SB, Wu CZ, et al. Sedum sarmentosum Bunge extract alleviates inflammation and kidney injury via inhibition of M1-macrophage polarization. Phytomedicine. 2019;62:152976. doi: 10.1016/j.phymed.2019.152976 [DOI] [PubMed] [Google Scholar]
- 34.Lv LL, Tang PM-K, Li CJ, et al. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017;91(3):587–602. doi: 10.1016/j.kint.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 35.Li C, Ding XY, Xiang DM, et al. Enhanced M1 and impaired M2 macrophage polarization and reduced mitochondrial biogenesis via inhibition of AMP kinase in chronic kidney disease. Cell Physiol Biochem. 2015;36(1):358–372. doi: 10.1159/000430106 [DOI] [PubMed] [Google Scholar]
- 36.Tian X, Peng Z, Luo S, et.al. Aesculin protects against DSS-Induced colitis though activating PPARγ and inhibiting NF-кB pathway. Eur J Pharmacol. 2019;15(857):172453. [DOI] [PubMed] [Google Scholar]
- 37.Wang JX, Cao YM, Liu YQ, et al. PIM1 inhibitor SMI-4a attenuated lipopolysaccharide induced acute lung injury through suppressing macrophage inflammatory responses via modulating p65 phosphorylation. Int Immunopharmacol. 2019;73:568–574. doi: 10.1016/j.intimp.2019.05.040 [DOI] [PubMed] [Google Scholar]