Summary
Opposing sources of bone morphogenetic protein (BMP) and Nodal signaling molecules are sufficient to induce the formation of a full axis in zebrafish embryos. To address how these signals orchestrate patterning, we transplant sources of fluorescently tagged Nodal and BMP into zebrafish embryos, robustly inducing the formation of secondary axes. Nodal and BMP signal non-cell-autonomously and form similar protein gradients in this context, but the signaling range of Nodal (pSmad2) is shorter than the BMP range (pSmad5). This yields a localized region of pSmad2 activity around the Nodal source, overlapping with a broad domain of pSmad5 activity across the embryo. Cell fates induced in various regions stereotypically correlate with pSmad2-to-pSmad5 ratios and can even be induced BMP- and Nodal-independently with different ratios of constitutively active Smad2 and Smad5. Strikingly, we find that Smad2 and Smad5 antagonize each other for specific cell fates, providing a mechanism for how cells integrate and discriminate between overlapping signals during development.
Keywords: Nodal, BMP, zebrafish, embryo, development, Smad2, Smad5, gradients, axis induction
Graphical Abstract

Highlights
-
•
Nodal induces pSmad at a shorter range than BMP due to slower activation kinetics
-
•
Different ratios of active Smad2 and Smad5 can induce different embryonic structures
-
•
Smad2 and Smad5 inhibit each other or act synergistically to induce specific cell fates
Juxtaposed Nodal and BMP sources can induce the formation of a secondary axis in zebrafish embryos. Soh et al. analyze the input-output relationships in this patterning system and find that differential signaling kinetics lead to different activity ranges of Nodal and BMP, which are crucial for secondary axis formation.
Introduction
During development, cells need to know their location and fate in order to form an embryo. The required positional information can be conveyed by gradients of secreted signaling molecules that diffuse from a localized source to induce exposure-dependent cell responses (reviewed in Müller et al., 2013, Rogers and Schier, 2011). The earliest cell-fate decisions during vertebrate development are controlled by the signaling molecules Nodal and BMP, which form orthogonal overlapping activity gradients in zebrafish embryos (Figure 1A). Nodal induces the formation of the germ layers, which are subdivided into ventral and dorsal territories by BMP signaling (reviewed in Rogers and Müller, 2019). Nodal and BMP are secreted transforming growth factor β (TGF-β) superfamily ligands (Zhou et al., 1993, Wozney et al., 1988), which signal through a hetero-tetrameric complex composed of ligand-specific serine/threonine kinase receptors (Wrana et al., 1992) as well as co-receptors (Shen and Schier, 2000). Nodal signaling leads to the phosphorylation of the latent cytoplasmic signaling effectors Smad2/3, whereas BMP signaling causes the phosphorylation of Smad1/5/8. These pSmads then accumulate in the nucleus, where they regulate the expression of target genes (Heldin et al., 1997).
Figure 1.
Secondary Axis Inducing Nodal and BMP Double Clones Produce a Localized Region of pSmad2 Activity Overlapping with a Broad Domain of pSmad5 Activity
(A) Nodal and BMP form orthogonal overlapping gradients in zebrafish embryos. Transplanting ectopic sources of Nodal and BMP induces the formation of a secondary axis, which contains both anterior and posterior structures such as the hindbrain, otic vesicles, notochord, and tail.
(B) Double clones of Bmp2b/7-sfGFP and Squint-mVenus imaged 30 min and 180 min post-transplantation. The first row depicts confocal microscopy images of Bmp2b/7-sfGFP (red) and Squint-mVenus (green). The second row shows light-sheet microscopy images of embryos immunostained with anti-pSmad2 (green) or anti-pSmad5 (red) antibodies as well as a cross-reactive anti-GFP antibody to detect Bmp2b/7-sfGFP and Squint-mVenus (blue). The third row shows comparable wild-type embryos. Nodal clones are traced in cyan and BMP clones are traced in white. Scale bar, 150 μm.
(C) Higher magnification of images shown in (B) with separate fluorescent channels. Scale bar, 150 μm.
(D) Images showing Nodal/BMP double clones with different spacings of transplanted cells taken immediately after transplantation. Scale bar, 150 μm.
(E) Nodal/BMP double clones were transplanted with different spacings into blastula-stage zebrafish embryos: narrow (~0 μm between clones, n = 60), moderate (40–50 μm between clones, n = 44), wide (120–150 μm between clones, n = 29), and very wide (>170 μm between clones, n = 20). Narrow to wide spacings support the formation of secondary axes, whereas secondary axis formation fails with extremely wide spacing between Nodal and BMP clones. Quantification was performed at 24 h post-transplantation.
During zebrafish germ-layer patterning, the two Nodals Squint and Cyclops are produced at the embryonic margin and induce endoderm and mesoderm formation at a distance from the source (Bisgrove et al., 2017, Montague and Schier, 2017, Pelliccia et al., 2017, Chen and Schier, 2001, Feldman et al., 1998). Fgf8, a Nodal target gene, further extends the range of mesoderm (van Boxtel et al., 2018, van Boxtel et al., 2015, Mathieu et al., 2004, Rodaway et al., 1999). At the same time, the two BMPs Bmp2b and Bmp7 are produced predominantly on the ventral side to control dorsal-ventral patterning (Pomreinke et al., 2017, Zinski et al., 2017, Ramel and Hill, 2013). Bmp2b and Bmp7 form heterodimers, and homodimers of Bmp2b and Bmp7 alone do not elicit signaling (Little and Mullins, 2009). Additionally, Bmp2b is produced in the dorsal organizer to moderate the production of Chordin, a BMP inhibitory protein (Xue et al., 2014).
Strikingly, Nodal and BMP signaling together are sufficient to trigger all processes required to form an embryo. This was spectacularly demonstrated by generating ectopic juxtaposed sources of Nodal and BMP to induce a secondary embryonic axis in zebrafish (Figure 1A) (Xu et al., 2014). The ratio of Nodal to BMP signaling was suggested to be the determining factor in specifying the necessary cell fates for the embryonic axis. Nodal by itself creates axial structures, high Nodal-to-BMP ratios induce posterior head structures, low Nodal-to-BMP ratios organize the tail, and intermediate ratios generate the middle trunk (Fauny et al., 2009). However, it is unknown how Nodal and BMP gradients form, it is currently debated whether Nodal and BMP signal over long distances (Rogers and Müller, 2019, Pomreinke et al., 2017, Zinski et al., 2017, van Boxtel et al., 2015, Ramel and Hill, 2013, Müller et al., 2012, Chen and Schier, 2001), and the molecular mechanisms that allow cells to respond to different ratios of Nodal and BMP signaling are unclear.
To address these questions, we transplanted sources expressing fluorescently tagged Nodal and BMP into zebrafish embryos and generated secondary axes with high efficiency. Interestingly, Nodal and BMP formed protein gradients with similar shape and amplitude in these secondary axis formation assays, but BMP had a long signaling range whereas Nodal induced pSmad signaling only locally around the transplanted clone. We found that the difference in signaling ranges can be explained by differential signaling activities of BMP and Nodal. Strikingly, specific ratios of constitutively active Smad2 and Smad5 were also able to generate a variety of embryonic structures, showing that the organizing ability of different Nodal/BMP ratios is mediated by different ratios of Smad2 and Smad5. We discovered that Smad2 and Smad5 selectively antagonize each other for certain cell fates while acting synergistically for others, which allows cells to respond differently to varying Nodal/BMP ratios. This selective mutual antagonism might represent a general mechanism for how cells integrate and discriminate between two overlapping signals during development.
Results
BMP and Nodal Induce pSmad Signaling with Different Ranges
To visualize the organizing signaling gradients during secondary axis formation, we optimized a protocol to generate secondary axes by transplanting juxtaposed sources of fluorescently tagged Nodal and BMP into zebrafish embryos (Figure 1A). In contrast to the original blastomere injection approach (Xu et al., 2014), this method allows for precise control over the timing, placement, and spacing of Nodal- and BMP-producing sources. We tagged the signaling molecules with various fluorophores and tested different ratios of the fusion proteins to assess their efficiency in generating secondary axes after transplantation. Many combinations of fluorophores showed good activity in generating secondary axes (Table S1), similar to the previously reported efficiency using untagged Nodal and BMP (Xu et al., 2014). Interestingly, both zebrafish Nodals (Squint and Cyclops) were able to generate secondary axes (only Cyclops was used in the previous work of Xu et al., 2014), albeit at different amounts of the injected mRNAs. Although mCherry-tagged versions also induced secondary axes, the required relative molar amounts differed drastically from those of the untagged versions (Table S1). In contrast, mVenus- and sfGFP-labeled Nodal and BMP had similar activity as the untagged versions (Figure S1). Furthermore, immunoblots of extracellular extracts showed that the fusion proteins were properly processed without releasing free fluorophores (Figure S1). We therefore decided to use Squint-mVenus and a 1:1 mixture of Bmp2b-sfGFP plus Bmp7-sfGFP (Bmp2b/7-sfGFP) for all subsequent axis-induction assays.
By generating localized sources of Squint-mVenus and Bmp2b/7-sfGFP, we found that these signaling molecules formed extracellular protein gradients within 30 min (Figures 1B and 1C). The gradients remained largely unchanged over the following 2 h (Figures 1B and 1C), similar to previous descriptions of Bmp2b-sfGFP and Squint-GFP gradient formation (Pomreinke et al., 2017, Müller et al., 2012). Interestingly, at 30 min post-transplantation, Nodal signaling (as assessed by pSmad2 induction; Figures 1B and 1C) was mostly limited to regions near the Nodal source, whereas BMP signaling (as assessed by pSmad5 induction; Figures 1B and 1C) had already spread extensively across the embryo. At 180 min, the pSmad2 signal was extended but still largely restricted to regions near the Nodal source, whereas pSmad5 remained more widely distributed (Figures 1B and 1C).
The wide and flat distribution of BMP signaling implied that the previously postulated close juxtaposition of opposing Nodal and BMP clones (Xu et al., 2014) might not be necessary for secondary axis induction and that localized Nodal signaling might be sufficient as long as there is some additional BMP signaling in the embryo. To test this prediction, we varied the spacing between Nodal and BMP sources and found that secondary axes could be generated for a wide range of different spacings (Figures 1D and 1E). The formation of secondary axes only failed when we placed the Nodal source much farther away from the BMP source than the range spanned by the pSmad5 gradient (∼220 μm; Figure 1E). These results suggest that the BMP source generates a much more extensive signaling gradient than the Nodal source, even though the extracellular distributions of Nodal and BMP appear to be similar.
Secondary Axis Formation Does Not Depend on Relay Signaling
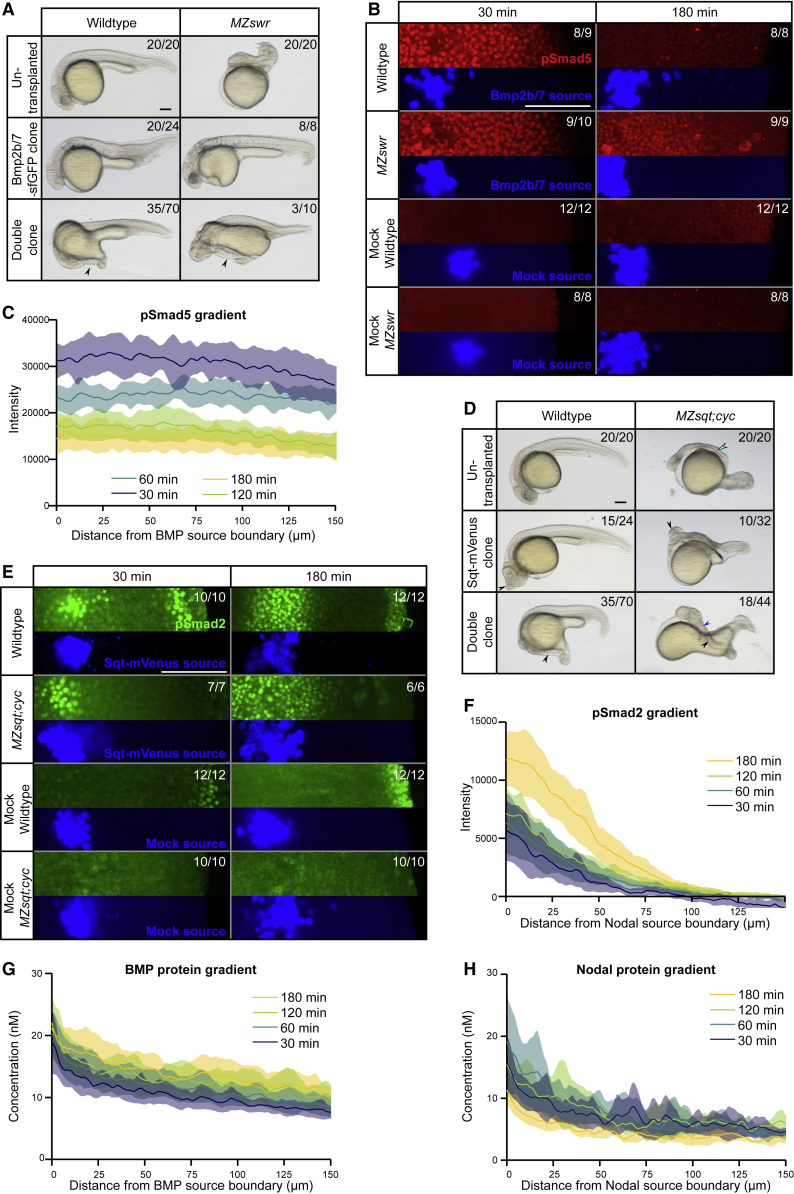
It had previously been suggested that secondary axis formation requires relay signaling through endogenous TGF-β superfamily ligands (de Olivera-Melo et al., 2018, Xu et al., 2014). To test this idea and measure signaling kinetics in the absence of endogenous signals, we transplanted clones secreting BMP and Nodal into wild-type and mutant zebrafish embryos that lack the endogenous signaling molecules. We first transplanted clones secreting zebrafish BMP into wild-type embryos and compared the induction kinetics of pSmad5 to MZswr (maternal-zygotic swirl−/− mutant) embryos that had received MZswr clones ectopically producing BMP. MZswr embryos lack functional Bmp2b (Schmid et al., 2000, Kishimoto et al., 1997) and are ideally suited to analyze the kinetics of pSmad5 induction in the absence of endogenous BMP signaling during embryonic development. Local BMP sources in wild-type embryos caused ventralization (Figure 2A, middle left), indicative of increased BMP activity in the entire embryo. The ubiquitous induction of BMP signaling was corroborated by the effects of small BMP clones in MZswr embryos (Figure 2A, middle right) that were well rescued except for tail defects, indicating that the BMP clone indeed induced long-range signaling. Consistent with these phenotypes, BMP clones induced signaling rapidly, and pSmad5 signal filled up the entire field in both wild-type and MZswr embryos (Figures 2B, 2C, S2, and S3A). The ability of the BMP source to induce pSmad5 signaling in MZswr embryos demonstrates that a relay through the induction of endogenous bmp2b is not required. Strikingly, local BMP juxtaposed to Nodal clones even induced secondary axes in MZswr embryos (Figure 2A, bottom right), indicating that BMP can work non-cell-autonomously and without relay signaling in this context.
Figure 2.
Nodal and BMP Form Similar Protein Gradients but Have Different Signaling Ranges during Secondary Axis Formation
(A) Bmp2b/7-sfGFP as well as Squint-mVenus and Bmp2b/7-sfGFP double clones in wild-type or maternal-zygotic swirl mutant (MZswr) embryos at 1 day post-transplantation, with untransplanted embryos for comparison. The arrowheads point to ectopic secondary axes. Scale bar, 150 μm.
(B) Bmp2b/7-sfGFP clones compared to uninjected mock clones 30 min and 180 min post-transplantation in wild-type or MZswr embryos. Embryos were immunostained with anti-pSmad5 (red) and anti-GFP (blue) antibodies. Mock sources were labeled with cascade blue-dextran (blue). Scale bar, 150 μm.
(C) pSmad5 distributions in embryos with single Bmp2b/7-sfGFP clones in MZswr embryos at 30 min (n = 9), 60 min (n = 8), 120 min (n = 10), and 180 min (n = 9) post-transplantation. Shaded regions indicate 95% confidence intervals around the mean (lines). Scale bar, 150 μm.
(D) Squint-mVenus as well as Squint-mVenus and Bmp2b/7-sfGFP double clones in wild-type or maternal-zygotic squint and cyclops double mutant (MZsqt;cyc) embryos 1 day post-transplantation, with untransplanted embryos for comparison. The arrowheads point to ectopic structures or secondary axes. Scale bar, 150 μm.
(E) Squint-mVenus clones compared to uninjected mock clones 30 min and 180 min post-transplantation in wild-type or MZsqt;cyc embryos. Embryos were immunostained with anti-pSmad2 (green) and anti-GFP (blue) antibodies. Mock sources were labeled with cascade blue-dextran (blue). Scale bar, 150 μm.
(F) pSmad2 distributions in embryos with single Squint-mVenus clones in wild-type embryos at 30, 60, 120, and 180 min post-transplantation (n = 11 each). Shaded regions indicate 95% confidence intervals around the mean (lines).
(G) BMP protein gradients in wild-type embryos with single Bmp2b/7-sfGFP clones at 30, 60, 120, and 180 min post-transplantation. The same embryos were imaged throughout the time course (n = 14). Fluorescence intensity was converted to concentration based on a calibration curve using recombinant sfGFP imaged with the same microscope settings. Shaded regions indicate 95% confidence intervals around the mean (lines).
(H) Nodal protein gradients in wild-type embryos with single Squint-mVenus clones at 30, 60, 120, and 180 min post-transplantation. The same embryos were imaged throughout the time course (n = 12). Fluorescence intensity was converted to concentration based on a calibration curve using recombinant mVenus imaged with the same microscope settings. Shaded regions indicate 95% confidence intervals around the mean (lines).
See also Figures S2–S4.
To test the role of potential relays and quantify the dynamics of Nodal signaling, we transplanted cells producing Squint-mVenus into wild-type or MZsqt;cyc mutant host embryos (clones transplanted into MZsqt;cyc host embryos were MZsqt;cyc mutant as well). MZsqt;cyc mutants lack all maternal and zygotic Nodal ligands (Feldman et al., 1998) and serve as a background to analyze Nodal signaling in the absence of endogenous Nodal signals. Squint-mVenus clones in wild-type and MZsqt;cyc embryos generated ectopic axial trunk structures (Figure 2D, middle). Strikingly, Nodal and BMP double clones were able to generate a secondary axis with anterior mesodermal structures such as anterior somites (Figure 2D, bottom right, blue arrowhead), which are normally absent in Nodal-deficient MZsqt;cyc embryos (Figure 2D, top right, cyan arrowhead). The primary axis in these embryos lacked anterior mesodermal structures (Figure 2D, bottom right), as is expected for MZsqt;cyc embryos (Feldman et al., 1998).
In agreement with the restricted effect of anterior mesoderm rescue, pSmad2 staining was found locally around the Nodal clones (Figure 2E). pSmad2 signal in MZsqt;cyc embryos extended up to several cell diameters away from the clone (Figures 2E and S3B), demonstrating that Nodal acts non-cell autonomously and without the need for a relay-based mechanism in this context as well. However, the range of Nodal-induced pSmad2 was significantly shorter than the range of BMP-induced pSmad5 (Figures 2C, 2F, and S3B). Importantly, untagged Squint and Bmp2b/7 generated a similarly large difference in the spatial ranges of Nodal and BMP signaling (Figures S2A and S2B), ruling out the possibility that the fluorescent tags are causal for the range differences.
Nodal and BMP Have Different Signaling Ranges despite Similar Ligand Distributions
Our finding that secondary axis formation is independent of relay signaling and purely relies on exogenously supplied signals provides an ideal system to test whether differences in signal gradient formation kinetics can explain the different signaling activity ranges. To relate the signal gradients to the signaling ranges, we developed a method to quantify the absolute concentrations of labeled Nodal and BMP in living zebrafish embryos based on their fluorescence intensities. We first purified recombinant sfGFP and mVenus proteins and established calibration curves relating the molar concentrations to their fluorescence intensities (Figure S4). We then used these calibration curves to determine the concentrations of fluorescently tagged Nodal and BMP expressed from local sources in zebrafish embryos. We found that the BMP gradient spanned a concentration range from 20 nM to 7 nM over 150 μm at 30 min post-transplantation (Figure 2G). At this time point, the pSmad5 gradient was similarly broad (Figure 2C), even when 5-fold less bmp2b/7-sfGFP mRNA was used (Figure S2C). Interestingly, although the concentration of BMP slightly increased over time (Figure 2G), most likely due to a larger effect of BMP production compared to its degradation, pSmad5 intensity concomitantly decreased (Figures 2C, S2A, and S2C). The decrease in pSmad5 intensity was also observed in chordin morphants (Figure S2D), arguing against the possibility of BMP signaling dampening by this major BMP antagonist (Fisher and Halpern, 1999, Blader et al., 1997, Schulte-Merker et al., 1997) in this context. In contrast, other Chordin-independent BMP-feedback inhibitors such as Bambia and Smad7 might be responsible for the downregulation of BMP signaling over time (Pogoda and Meyer, 2002, Tsang et al., 2000).
The Squint-mVenus gradient produced from a localized clone formed with similar concentration distributions and dynamics as the BMP protein gradient at early time points but sharply dropped by 180 min post-transplantation (Figure 2H), possibly due to unstable mRNA, decreased translation, reduced secretion, or rapid internalization. In contrast to the drop in the Nodal gradient amplitude (Figure 2H), the levels of pSmad2 increased over time (Figure 2F).
Together, these results show that although Nodal and BMP form similar proteins distributions, their respective pSmad gradients are radically different, similar to the distinct distributions of pSmads induced by endogenous signaling molecules (Figure S2E). Therefore, the drastic differences in the pSmad gradients cannot be explained by the small differences in the amount of secreted Nodal and BMP proteins or by their similar effective diffusion coefficients (∼3 μm2/s) (Bläßle et al., 2018, Pomreinke et al., 2017, Zinski et al., 2017, Müller et al., 2012).
Different Signaling Ranges Arise from Differences in Signaling Activity
Nodal signaling is antagonized by the feedback-induced Nodal inhibitors Lefty1 and Lefty2 during early zebrafish development (Rogers and Müller, 2019, Rogers et al., 2017, Agathon et al., 2001, Meno et al., 1999, Thisse and Thisse, 1999). To test whether the shorter signaling range of Nodal compared to BMP is due to inhibition by Lefty1 or Lefty2, we assessed signaling in Squint-mVenus clone experiments in which both the donor and the recipient embryo were MZlefty1;lefty2 double mutants lacking all maternal and zygotic Lefty activity (Rogers et al., 2017). Interestingly, in the absence of Lefty antagonism, the pSmad2 signal was extended (Figures S2F and S3C) but still much shorter than the range of pSmad5 induced by BMP clones (compare to Figure 2C). These results show that signaling range modulation by Lefty cannot explain the drastic difference between Nodal distribution and signaling in this context.
We therefore hypothesized that the different signaling ranges (pSmad5 and pSmad2) from similar input gradients (BMP and Nodal) might result from different signaling activation kinetics. According to the law of mass action and Hill kinetics, signals with higher sensitivity can induce activation faster, leading to a longer signaling range, whereas signals with low sensitivity might require extended exposure until activation is induced in a threshold-type manner, leading to a shorter signaling range (Michaelis et al., 2011). To test this idea, we took advantage of the recent discovery that a single source of mouse BMP4 can generate a secondary zebrafish axis (de Olivera-Melo et al., 2018), which suggested the possibility that mouse BMP4 might carry both BMP and Nodal signal activities with different ranges. Previous experiments were carried out with commercial preparations of recombinant mouse BMP4 (de Olivera-Melo et al., 2018), but we found that a single source of mRNA encoding mouse BMP4 can also induce secondary axes in zebrafish embryos (Figure 3A), ruling out the possibility that potential contaminations of commercial mouse BMP4 with other TGF-β superfamily ligands are responsible for secondary axis formation.
Figure 3.
Different BMP and Nodal Signaling Ranges Arise from Differential Signaling Activation Kinetics
(A) Single clones expressing mouse BMP4 (mBMP4) induce the formation of a secondary axis in zebrafish embryos (arrowhead). Scale bar, 150 μm.
(B) Mathematical modeling shows that a difference in signaling activation kinetics could explain how a single gradient of mBMP4 induces pSmad5 and pSmad2 at different ranges.
(C) Wild-type zebrafish embryos with clones expressing mBMP4 30 min and 180 min post-transplantation immunostained with anti-pSmad5 (red) and anti-pSmad2 (green) antibodies. The clones were labeled with cascade blue-dextran (blue). Scale bars, 150 μm.
(D) Higher magnification of images shown in (C) with separated fluorescent channels. Scale bar, 150 μm.
(E) MZsqt;cyc embryos with clones expressing mBMP4 30 min and 180 min post-transplantation immunostained with anti-pSmad5 (red) and anti-pSmad2 (green) antibodies. The clones were labeled with cascade blue-dextran (blue). Scale bars, 150 μm.
(F) Higher magnification of images shown in (E) with separated fluorescent channels. Scale bar, 150 μm.
(G) Zebrafish Bmp2b/7-sfGFP and Squint-mVenus clones in morphotrap-expressing wild-type embryos 30 min post-transplantation. Scale bar, 150 μm.
(H) Double clones with fluorescently tagged or untagged zebrafish Nodal and BMP and with narrow or wide spacing were generated in morphotrap-expressing embryos. The frequency of the different structures induced by the clones was assessed 24 h post-transplantation.
To assess the plausibility that a single source of mouse BMP4 might generate different pSmad distributions, we developed a mathematical model based on Hill kinetics that we parameterized with the diffusion coefficient and protein half-life previously measured for zebrafish BMP (Pomreinke et al., 2017, Zinski et al., 2017) (Figure 3B). In this model, the differential readout of the mouse BMP4 gradient by pSmad5 and pSmad2 is dependent on a single parameter, i.e., the steepness of the pSmad activation term (kd for pSmad5, ke for pSmad2) that convolves the affinity of the BMP4 ligand for the BMP and Nodal receptors as well as the pSmad activation kinetics (Figure 3B). Simulations with smaller kd compared to ke values predicted that pSmad2 should be activated close to the mouse BMP4 source, whereas pSmad5 should have a wider range (Figure 3B).
In agreement with this model prediction, we found that pSmad2 and pSmad5 were indeed activated at different ranges by mouse BMP4. Local sources of mouse BMP4 in zebrafish embryos induced locally restricted pSmad2 but widespread pSmad5 (Figures 3C and 3D), providing further support that opposing sources of Nodal and BMP are not strictly needed for secondary axis formation. To rule out the possibility that pSmad2 activation is due to indirect induction of endogenous Nodal signals (de Olivera-Melo et al., 2018), we generated clones expressing mouse bmp4 in Nodal-deficient MZsqt;cyc mutant zebrafish embryos. We found that mouse BMP4 can indeed directly induce both pSmad2 and pSmad5 non-cell-autonomously (Figures 3E and 3F). Together, these results support our model that the exact same signal gradient can induce signaling effector activation at different ranges solely due to differences in signaling activity.
Our model implies that the action range of a signaling molecule with high signaling activity should be limited by its diffusion coefficient, whereas the action range of a signaling molecule with low signaling activity should be limited by its signaling activity rather than its diffusivity. To test this prediction, we sought to artificially reduce the diffusion coefficients of Nodal and BMP and measure how this affects their signaling ranges. We perturbed the protein distributions of Nodal and BMP using morphotraps, transmembrane proteins with extracellularly facing anti-GFP nanobodies that can drastically reduce the diffusivity of extracellular proteins tagged with GFP derivatives (Mörsdorf and Müller, 2019, Almuedo-Castillo et al., 2018, Harmansa et al., 2017). The protein distributions of Bmp2b/7-sfGFP and Squint-mVenus expressed from localized sources were strongly restricted in the presence of the morphotrap (Figure 3G). The sharp Bmp2b/7-sfGFP distribution led to a strongly restricted pSmad5 signal around the clone, whereas the already narrow range of pSmad2 was only marginally affected when the Squint-mVenus distribution was perturbed (Figures 3G and S2G). These findings provide additional support for our model that BMP has a longer signaling range than Nodal due to its higher signaling activity.
Strikingly, morphotrap-mediated range-restricted Nodal and BMP still supported the formation of secondary axes when the clones were closely juxtaposed (∼0 μm between clones), whereas secondary axes could no longer be induced when the clones were far apart (>120–150 μm) from each other (Figure 3H, top). To test whether the long-range activity of Nodal or BMP is required in this context, we next perturbed the ranges of Nodal or BMP individually. Interestingly, morphotrap-mediated range restriction of Nodal was without consequence for narrowly or widely spaced clones (Figure 3H, middle), whereas secondary axis formation was abrogated when range-restricted BMP clones were placed far away from normal Nodal clones (Figure 3H, bottom). These results show that differences in the Nodal and BMP signaling ranges are functionally relevant for the formation of secondary axes. We note, however, that in normal embryos BMPs do not form a discrete source but are expressed in a broad domain, which gives rise to a broad signaling domain of pSmad5. Nodal by contrast is localized to the margin in a much more restricted domain, and pSmad2 is likewise activated in a much more restricted domain (reviewed in Rogers and Müller, 2019).
In conclusion, we showed that Nodal and BMP can induce signaling with different ranges, differential signaling activity can explain the differences in signaling ranges, and differential signaling ranges are relevant for secondary axis formation.
Different Structures Can Be Induced by Specific Amounts of Active Smad2 and Smad5
Our results suggest that the formation of a secondary axis requires a broad distribution of BMP and highly localized Nodal signaling. However, in addition to the spatial distributions, the relative signaling levels may also important for secondary axis formation (Fauny et al., 2009). By varying the relative levels of BMP and Nodal, we found that it is the ratio of Nodal to BMP rather than the absolute signaling level that determines whether a secondary axis can form. Lowering Nodal levels with respect to BMP levels precluded secondary axis induction (Figure S5A), whereas a commensurate reduction in both Nodal and BMP levels restored secondary axis formation (Figure S5A), correlating with a specific distribution of pSmad2 to pSmad5 ratios (Figures S5B and S5C).
To test whether the observed pSmad2-to-pSmad5 signaling effector ratio is causal for secondary axis induction, we generated embryos in which we activated specific ratios of Smads in a localized region independently of the extracellular signaling molecules. By exchanging the three C-terminal serines with aspartates, we generated constitutively active Smad2 (Smad2-CA) and Smad5 (Smad5-CA) signaling effectors, which can activate the transcription of their respective target genes (Figure S6). We found that expression of smad2-CA and smad5-CA in a localized region (Figure 4A) can generate various ectopic embryonic structures. Injecting smad2-CA alone generated an ectopic trunk structure containing axial tissues expressing the floorplate marker shha (Krauss et al., 1993) (Figure 4B, black arrowhead), just like in the case of an ectopic source of Nodal (Figure 4B) (Fauny et al., 2009). When we injected smad2-CA mixed with increasing amounts of smad5-CA mRNA (Figures 4C and 4D), structures expressing more ventral genes were induced, similar to previous findings in which the extracellular signaling molecules Nodal and BMP instead of the active signaling effectors were used (Fauny et al., 2009). Using Smad2-CA and Smad5-CA in a 1:1 ratio led to the induction of an ectopic structure with paired krox20 expression, similar to the secondary axis generated by Nodal and BMP double clones (Figure 4C). krox20 is expressed in rhombomeres 3 and 5 of the hindbrain (Ghosh et al., 2018), indicating that the ectopic structure represents an anterior trunk (Figure 4C). 4-fold more Smad5-CA over Smad2-CA can generate ectopic tail structures (Figure 4D) expressing the tail tip marker hoxc13b (Fauny et al., 2009). The induction of these structures was robust to the absence of endogenous Nodal and BMP signaling. Embryos expressing smad2-CA that were exposed to 40 μM of SB-505124, which fully inhibits signaling from the upstream Nodal receptor (Almuedo-Castillo et al., 2018, Rogers et al., 2017, Hagos and Dougan, 2007), were still able to generate ectopic trunk structures expressing shha (Figure 4E). Furthermore, embryos expressing smad2-CA and smad5-CA that were exposed to 40 μM of SB-505124 and 10 μM of the BMP receptor inhibitor Dorsomorphin were also able to generate anterior trunk structures expressing krox20 (Figure 4F).
Figure 4.
Ectopic Expression of Different Amounts of smad2-CA and smad5-CA mRNA Generates Distinct Embryonic Structures
(A) Ectopic structures were generated by injecting three adjacent blastomeres in 64- to 128-cell-stage embryos.
(B) The floorplate marker shha is expressed throughout the axis (gray arrowhead) of wild-type embryos 24 h post-fertilization (hpf). Injection of smad2-CA mRNA into animal pole blastomeres results in the formation of an ectopic axial structure (black arrowhead) that expresses shha, similar to the results with a squint-mVenus-expressing clone.
(C) krox20 is expressed as a pair in rhombomeres 3 and 5 in the hindbrain (gray arrowhead) of wild-type embryos at 24 hpf. Injection of smad2-CA and smad5-CA mRNA in animal pole blastomeres results in the formation of anterior trunk structures with paired krox20 expression (black arrowhead), similar to the outcome with a squint-mVenus and bmp2b/7-sfGFP-expressing double clone.
(D) hoxc13b is expressed in the tail tip (gray arrowhead) in wild-type embryos at 24 hpf. Injection of smad2-CA and four times more smad5-CA mRNA into animal pole blastomeres results in the formation of a tail structure expressing hoxc13b (black arrowhead), similar to the outcome with a double clone expressing low squint-mVenus and high bmp2b/7-sfGFP (Figure S5).
(E) shha is not expressed in embryos exposed to the Nodal receptor inhibitor SB-505124. Injection of smad2-CA mRNA into animal pole blastomeres results in ectopic shha-positive axial structures despite Nodal receptor inhibition.
(F) krox20 remains expressed (gray arrowheads) in embryos exposed to SB-505124. Injection of smad2-CA and smad5-CA mRNA into animal pole blastomeres stage results in the formation of anterior trunk structures with paired krox20 expression (black arrowheads). Nodal and BMP receptor inhibition by combined exposure to SB-505124 and Dorsomorphin generates embryos with reduced tails compared to the treatment with SB-505124 alone, but krox20 expression persists (gray arrowhead). Injection of smad2-CA and smad5-CA mRNA into animal pole blastomeres results in the formation of anterior trunk structures with paired krox20 expression (black arrowhead) despite Nodal and BMP receptor inhibition. Scale bar in all images, 150 μm.
Since the inductive capabilities of constitutively active Smad2 and Smad5 are similar to those of the upstream signaling molecules Nodal and BMP, we conclude that the organizing activities of Nodal and BMP are mediated by specific amounts and ratios of active Smad2 and Smad5.
Selective Mutual Antagonism of Active Smad2 and Smad5 Yields Specific Responses to Different Signaling Ratios
We found that different ectopic structures can be induced by specific ratios of Smad2 and Smad5. To determine how the induction of these structures is related to the activation of target genes, we injected embryos with different ratios of Smad2-CA and Smad5-CA and assessed the expression of Nodal and BMP target genes with representative endogenous expression domains (Figure 5A). gsc is induced by Nodal signaling and a marker of axial mesoderm (Bennett et al., 2007, Gritsman et al., 1999) but only expressed at the dorsal margin (despite pSmad2 activity throughout the margin), suggesting that it is induced by high pSmad2 and low pSmad5 levels; foxi1 is a BMP target gene and an epidermal marker that is expressed on the ventral side but appears to be excluded from the ventral margin at shield stage (Hans et al., 2007) (although pSmad5 signaling is also present at the ventral margin), suggesting that is induced by high pSmad5 and low pSmad2 levels; and eve1 is expressed in the ventral margin (where both pSmad2 and pSmad5 are active) and a marker for ventral mesoderm, suggesting that it is induced by high pSmad2 and high pSmad5 levels. Interestingly, we found that Smad2-CA and Smad5-CA antagonized each other for the induction of gsc and foxi1, whereas eve1 showed a biphasic sensitivity to these signaling effectors. Smad2-dependent gsc expression was suppressed by high levels of Smad5-CA compared to Smad2-CA (Figure 5B), whereas Smad5-dependent foxi1 expression was inhibited by high Smad2-CA levels (Figure 5C). Strikingly, eve1 was induced synergistically by both Smad2-CA and Smad5-CA at a moderate amount of Smad2-CA (Figure 5D). In contrast, high amounts of Smad2-CA led to reduced eve1 expression (Figure 5D), consistent with the absence of dorsal eve1 expression (Joly et al., 1993) where Nodal signaling is active over a long period of time (van Boxtel et al., 2018, Dubrulle et al., 2015). These results suggest that the selective mutual antagonism of Smad2 and Smad5 allows cells to respond specifically to different ratios of Smad2 and Smad5.
Figure 5.
Mutual Antagonism of Smad2 and Smad5 for Specific Cell Fates
(A) gsc is expressed at the dorsal margin (Stachel et al., 1993), while foxi1 is expressed on the ventral side but excluded from the margin (Dal-Pra et al., 2006), and eve1 is expressed at the ventral margin (Joly et al., 1993) where Nodal and BMP signaling overlap (Figure 1A).
(B) Average gsc fluorescence in situ hybridization (FISH) intensity in 6-hpf embryos that were injected with the indicated smad2-CA and smad5-CA mRNA amounts at the one-cell stage (n = 10, 7, and 7).
(C) Average foxi1 FISH intensity in 6-hpf embryos that were injected with the indicated smad2-CA and smad5-CA mRNA amounts at the one-cell stage (n = 7, 6, and 7).
(D) Average eve1 FISH intensity of 6-hpf embryos that were injected with the indicated smad2-CA and smad5-CA mRNA amounts at the one-cell stage (n = 5, 6, 7, and 8).
(E) Embryos with Nodal and BMP double clones subjected to FISH with gsc (left, blue), foxi1 (middle, blue), or eve1 (right, blue) probes followed by pSmad2 (green) and pSmad5 (red) immunostaining. Blue dotted lines trace Nodal clones, and white dotted lines trace BMP clones. Scale bar, 150 μm. Error bars indicate 95% confidence intervals around the mean (horizontal lines) in (B)–(D).
Our selective mutual antagonism mechanism predicted specific expression patterns of gsc, foxi1, and eve1 in the Nodal/BMP double clone secondary axis formation assay. gsc is induced by high Smad2 activity and suppressed by Smad5 activity (Figure 5B) and should therefore be expressed near the Nodal source opposite to the BMP clone, foxi1 is induced by high Smad5 activity and suppressed by Smad2 activity (Figure 5C) and should therefore be expressed near the BMP source opposite to the Nodal clone, and eve1 has a biphasic activation profile (Figure 5D) and should therefore be expressed in a complex pattern. To test these predictions, we subjected embryos carrying Nodal/BMP double clones to in situ hybridization with various probes followed by pSmad2 and pSmad5 immunostaining (Figure 5E). In agreement with the predictions of our selective mutual antagonism model, we found that gsc was expressed in the presence of pSmad2, but not when pSmad2 overlapped with pSmad5 (Figure 5E). In contrast, foxi1 was expressed in regions of pSmad5 activity, but expression was reduced when pSmad5 overlapped with pSmad2 (Figure 5E). Strikingly, eve1 was expressed in the predicted complex domain; eve1 was induced where pSmad2 overlapped with pSmad5, but it was not detected at the highest pSmad2 activity in the overlapping region (Figure 5E).
In conclusion, we found that the organizing activities of Nodal and BMP are mediated by specific amounts of active Smad2 and Smad5, whose selective mutual antagonism allows cells to respond specifically to different Nodal/BMP input ratios.
Discussion
Understanding the dynamics of axis formation during early vertebrate development has largely been hampered by the lack of tools to relate the input from signaling gradients to the patterning output in terms of signaling effector activation and target gene expression. Transgenic animals expressing fluorescent fusions of the relevant signaling molecules under the control of endogenous regulatory elements are currently not available, and the timing and amplitudes of signaling gradients cannot be easily manipulated with good spatiotemporal control. Here, we used our optimized secondary zebrafish axis induction assay as an experimentally tractable model system to understand signaling input-output relationships and to decipher how Nodal and BMP signaling are integrated to form a secondary embryo.
Using active fluorescent fusions of Nodal and BMP expressed from clonal sources, we found that the signaling molecules form similar protein gradients of comparable shape and amplitude in zebrafish embryos. The similar protein gradients are in stark contrast to the differential distributions of the signaling effectors. The Nodal source generates a localized pSmad2 gradient that is overlaid by a broader pSmad5 gradient induced by the BMP source. Taking advantage of the dual BMP/Nodal activity of mouse BMP4, we experimentally confirmed the prediction of our model that different signaling ranges of a single protein gradient can be explained by differences in signaling activity. In addition to differences in diffusion/clearance-based signal dispersal (Rogers and Müller, 2019), differences in signaling activity might therefore represent an additional knob to tune the ranges of signaling molecules and may play a role in restricting Nodal signaling to the margin. Consistent with this hypothesis, similar differences in TGF-β superfamily signaling dynamics were recently identified in cultured cells (Miller et al., 2019, Yoney et al., 2018). For example, Activin exogenously added to mouse embryonic stem cells was found to activate Smad2 rapidly and had a long signaling range, whereas BMP4 activated Smad1 more slowly and had a shorter signaling range (Yoney et al., 2018). In agreement with law of mass action considerations (Michaelis et al., 2011), it may thus be a general feature of developmental signaling systems that ligands that rapidly activate their effectors have a longer range, whereas ligands that slowly activate their effectors have a shorter range.
The difference in Nodal and BMP signaling ranges arises due to differences in signaling activation kinetics and yields a field of various positional information values in terms of pSmad2-to-pSmad5 ratios. Previous work has shown that ectopic expression of different amounts of Nodal and BMP induces the formation of specific embryonic structures (Fauny et al., 2009). Here, we found that ectopic expression of different amounts of constitutively active Smads is sufficient to generate these structures, indicating that varying ratios of Smads are the major factors that confer the inductive capabilities of Nodal and BMP. Mutual antagonism as well as limited synergism between activated Smad2 and Smad5 can lead to distinct combinations of target gene expression sets that correlate with their spatial expression domains (Figures 5 and 6), and an important future goal will be to decipher the molecular mechanism by which pSmad ratios are integrated at the level of signaling or at target gene promotors (Figure 6).
Figure 6.
Selective Mutual Antagonism of pSmad2 and pSmad5 Allows Cells to Respond to Different Ratios of Nodal and BMP Signaling
Schematic of a parsimonious model explaining the present findings. The antagonism of pSmad2 and pSmad5 to foxi1 and gsc induction, respectively, allows cells with both high pSmad2 and pSmad5 to express eve1 without expressing foxi1 or gsc. The activation and inhibition arrows are an abstraction, and the underlying mechanisms may be direct or indirect.
Similar cases of mutual antagonism also exist for other signaling pairs, such as Bicoid and Caudal (reviewed in Briscoe and Small, 2015). However, since Bicoid represses Caudal translation via direct binding to caudal mRNA (Niessing et al., 2002), their mutual antagonism is not selective. The selective antagonism mechanism might be needed for Nodal and BMP because they form overlapping orthogonal gradients (reviewed in Rogers and Müller, 2019) instead of anti-parallel gradients. The overlapping nature of the Nodal and BMP gradients leads to an area with both high pSmad2 and pSmad5 activity, areas with either high pSmad2 or high pSmad5 alone, as well as areas without pSmad2 or pSmad5. However, cell fates in areas with high pSmad2 alone or high pSmad5 alone are different from those in areas with both high pSmad2 and high pSmad5. Therefore, the selective antagonism mechanism not only allows cells to sense the ratio of Nodal and BMP but also can work when these gradients extensively overlap at the ventral margin. It is possible that similarly easily implemented mechanisms might generally be involved in the interpretation of other overlapping gradients.
In summary, we used the Nodal/BMP-mediated secondary axis formation assay as a model system to understand how the integration of signaling gradients leads to the activation of signaling effectors and subsequent patterning. In this context, we found that Nodal and BMP activate effector Smads non-cell autonomously and induce signaling at different spatial ranges due to differences in their signaling activities. This yields a field of positional information values in terms of differential signaling effector ratios. Varying ratios of constitutively active Smads can induce different embryonic structures, and selective mutual antagonism of activated Smad2 and Smad5 allows cells to respond to different ratios of Nodal and BMP signaling. It is tempting to speculate that, similar to the Yamanaka factors that can convert differentiated cells into pluripotent cells (Takahashi and Yamanaka, 2006), it might be possible in the future to use the inductive properties of different ratios of constitutively active Smads to induce the formation of desired embryonic structures from pluripotent stem cells for regenerative medicine.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-phospho-Smad2/Smad3 | Cell Signaling Technology | Cat#8828; RRID:AB_2631089 |
| Rabbit monoclonal anti-phospho-Smad1/Smad5/Smad9 | Cell Signaling Technology | Cat#13820S; RRID:AB_2493181 |
| Goat anti-rabbit horseradish peroxidase | Jackson ImmunoResearch | Cat#111-035-003; RRID:AB_2313567 |
| Anti-digoxigenin alkaline phosphatase Fab fragments | Roche Diagnostics | Cat#32871920 |
| Anti-digoxigenin horseradish peroxidase Fab fragments | Sigma-Aldrich | Cat#11207733910 |
| Chicken polyclonal anti-GFP | Aves Labs | Cat#GFP-1020; RRID:AB_10000240 |
| Goat anti-rabbit Alexa647 | Invitrogen | Cat#A21245; RRID:AB_141775 |
| Goat anti-chicken Alexa568 | Abcam | Cat#ab175477 |
| Goat anti-chicken DyLight405 | Agrisera | Cat#AS16 3624 |
| Rabbit anti-GFP | Life Technologies | Cat#A11122; RRID:AB_221569 |
| Bacterial Strains | ||
| One Shot TOP10 chemically competent E. coli | Life Technologies | Cat#C4040 |
| Chemicals and Recombinant Proteins | ||
| Dorsomorphin | Abcam | Cat#ab120843 |
| SB-505124 | Sigma-Aldrich | Cat#S4696-5MG |
| Cascade Blue dextran, 10 kDa, anionic, lysine fixable | Thermo Fisher Scientific | Cat#D1976 |
| Pronase | Roche | Cat#11459643001 |
| Critical Commercial Assays | ||
| TSA plus fluorescein system | Perkin Elmer | Cat#NEL741001KT |
| RNeasy kit | QIAGEN | Cat#74104 |
| Pierce protein concentrator PES, 10K MWCO, 5-20 ml | Thermo Fisher Scientific | Cat#88528 |
| SuperSignal West Dura extended duration substrate | Thermo Fisher Scientific | Cat#34075 |
| Q5® site-directed mutagenesis kit | New England Biolabs | Cat#E0554S |
| Zero Blunt TOPO PCR cloning kit | Thermo Fisher Scientific | Cat#K2800J10 |
| SP6 mMessage mMachine transcription kit | Thermo Fisher Scientific | Cat#AM1340 |
| DIG RNA labeling mix | Sigma-Aldrich | Cat#11277073910 |
| Experimental Models: Organisms/Strains | ||
| Zebrafish: sqtcz35 | Feldman et al., 1998 | N/A |
| Zebrafish: cycm294 | Sampath et al., 1998 | N/A |
| Zebrafish: swrtc300a | Mullins et al., 1996 | N/A |
| Zebrafish: lefty1a145 | Rogers et al., 2017 | N/A |
| Zebrafish: lefty2a146 | Rogers et al., 2017 | N/A |
| Oligonucleotides | ||
| eve1_For: CTGGTTCCAGAACCGGAGA | This paper | N/A |
| eve1_Rev: GGAAAGCATATGTACATGGGTTTGTAT | This paper | N/A |
| foxi1_For: GTCGAGCCAGCAGACCAG | This paper | N/A |
| foxi1_Rev: CTGTTGTTGTGCGATGCTG | This paper | N/A |
| shha_For: ATACTGGCGTCCTGTTACGC | This paper | N/A |
| shha_Rev: ACATTTCCTGACACCTTGCCT | This paper | N/A |
| krox20_For: CAAACCCTTCCAGTGTCGGA | This paper | N/A |
| krox20_Rev: GACCCGCGTTAGTCACTTCA | This paper | N/A |
| hoxc13b_For: AAGCCGAGATGAACGGCTAC | This paper | N/A |
| hoxc13b_Rev:ACACAAACAGTTTAATATTGGGGGA | This paper | N/A |
| smad2-CA_For: TCGATTCGAATTCGCCACCATGTCCTCCATCTTGCCTTTCAC | This paper | N/A |
| smad2-CA_Rev: ACTATAGTTCTAGATTAGTCCATGTCATCGCAGCGTACGGAGGG | This paper | N/A |
| smad5_For: GCGCGAATTCGCCACCATGACCTCCATGTCTAGTCTG | This paper | N/A |
| smad5_Rev: GCGCTCTAGATTACGAGACAGAAGAGATGGG | This paper | N/A |
| smad5-CA_For: GACGTCGACTAATCTAGAACTATAGTG | This paper | N/A |
| smad5-CA_Rev: ATCGATGGGGTTCAGAGG | This paper | N/A |
| Recombinant DNA | ||
| pCS2-Bmp2b-sfGFP | Pomreinke et al., 2017 | N/A |
| pCS2-Bmp7-sfGFP | This paper | N/A |
| pCS2-Bmp2b-mCherry | This paper | N/A |
| pCS2-Squint-mVenus | This paper | N/A |
| pCS2-Squint-mCherry | This paper | N/A |
| pCS2-Cyclops-mVenus | This paper | N/A |
| pCS2-Cyclops-mCherry | This paper | N/A |
| pCS2-Smad2-CA | This paper | N/A |
| pCS2-Smad5-CA | This paper | N/A |
| pCS2-mouseBMP4 | This paper | N/A |
| pCRII-eve1 | This paper | N/A |
| pCRII-foxi1 | This paper | N/A |
| pCRII-shha | This paper | N/A |
| pCRII-hoxc13b | This paper | N/A |
| pCRII-krox20 | This paper | N/A |
| pCS2-gsc | Müller et al., 2012 | N/A |
| pCS2-sox32 | Müller et al., 2012 | N/A |
| pBAD-sfGFP | Pédelacq et al., 2006 | Addgene Plasmid #54519; RRID:Addgene_54519 |
| pBAD-mVenus | Nagai et al., 2002 | Addgene Plasmid #54845; RRID:Addgene_54845 |
| Software and Algorithms | ||
| Fiji | Schindelin et al., 2012 | https://fiji.sc/ |
| Prism | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| COMSOL Multiphysics 3.5a | COMSOL, Inc. | https://www.comsol.com/ |
Lead Contact and Materials Availability
All reagents generated in this study are available without restriction from the Lead Contact, Patrick Müller (patrick.mueller@tuebingen.mpg.de).
Experimental Model and Subject Details
Zebrafish lines
Zebrafish husbandry was executed in accordance with the guidelines of the State of Baden-Württemberg (Germany) and approved by the Regierungspräsidium Tübingen (35/9185.46-5, 35/9185.81-5). The TE strain was used for experiments with wild-type zebrafish embryos. Maternal-zygotic double-homozygous sqtcz35 (Feldman et al., 1998) and cycm294 (Sampath et al., 1998) mutants (MZsqt;cyc) as well as maternal-zygotic swrtc300a (Mullins et al., 1996) (MZswr) mutants were generated by germline replacement (Ciruna et al., 2002). Maternal-zygotic double-homozygous lefty1a145;lefty2a146 mutants (MZlefty1;lefty2) were generated using a rescue approach with 4.8 μM of the Nodal inhibitor SB-505124 (Rogers et al., 2017).
Method Details
Plasmids and in vitro synthesis of RNA
All plasmids for in vitro synthesis of mRNA were generated by inserting the sequence of interest into the EcoRI and XhoI sites of the pCS2(+) vector, containing the consensus Kozak sequence GCCACC directly in front of the start codon (Müller et al., 2012).
To generate pCS2-Bmp7-sfGFP, sequences encoding sfGFP flanked by LGDPPVAT linkers were inserted two amino acids downstream of the RSVR Furin cleavage site (Hawley et al., 1995). pCS2-Bmp2b-sfGFP was described previously (Pomreinke et al., 2017). pCS2-Bmp2b-mCherry was derived from pCS2-Bmp2b-sfGFP by exchanging the sfGFP-coding sequence with mCherry-encoding sequences. pCS2-Squint-mVenus and pCS2-Squint-mCherry were derived from pCS2-Squint-GFP (Müller et al., 2012) by exchanging the GFP-coding sequence with mVenus- and mCherry-encoding sequences, respectively. pCS2-Cyclops-mVenus and pCS2-Cyclops-mCherry were derived from pCS2-Cyclops-GFP (Müller et al., 2012) by exchanging the GFP-coding sequence with mVenus- and mCherry-encoding sequences, respectively.
Constitutively active Smads were generated by replacing the three C-terminal serine codons with aspartate codons. Smad5 was cloned from zebrafish shield-stage cDNA into the pCS2(+) vector with the primers GCGCGAATTCGCCACCATGACCTCCATGTCTAGTCTG and GCGCTCTAGATTACGAGACAGAAGAGATGGG. The Q5 site-directed mutagenesis kit (New-England Biolabs) was then used to replace the C-terminal serine codons with the primers GACGTCGACTAATCTAGAACTATAGTG and ATCGATGGGGTTCAGAGG. Smad2-CA was directly amplified and modified from zebrafish shield-stage cDNA with the primers TCGATTCGAATTCGCCACCATGTCCTCCATCTTGCCTTTCAC and ACTATAGTTCTAGATTAGTCCATGTCATCGCAGCGTACGGAGGG. The amplicon was cloned into the pCS2(+) vector.
mRNA for microinjection was generated using the SP6 mMessage mMachine kit (Thermo Fisher Scientific) according to the manufacturer’s instructions after plasmid linearization with NotI-HF (New England Biolabs, Cat#R3189).
Plasmids containing sequence fragments of eve1, foxi1, shha, krox20 and hoxc13b were generated using Zero Blunt TOPO PCR Cloning (Invitrogen, Thermo Fisher Scientific) and the following primers: CTGGTTCCAGAACCGGAGA and GGAAAGCATATGTACATGGGTTTGTAT for eve1, GTCGAGCCAGCAGACCAG and CTGTTGTTGTGCGATGCTG for foxi1, ATACTGGCGTCCTGTTACGC and ACATTTCCTGACACCTTGCCT for shha, CAAACCCTTCCAGTGTCGGA and GACCCGCGTTAGTCACTTCA for krox20, AAGCCGAGATGAACGGCTAC and ACACAAACAGTTTAATATTGGGGGA for hoxc13b. For foxi1, shield-stage cDNA was used as a template, whereas genomic DNA was used as a template for eve1, shha, krox20 and hoxc13b. Linear fragments for eve1, foxi1, shha, krox20 and hoxc13b were produced by PCR with M13 forward and M13 reverse primers. To generate templates for gsc and sox32 probes, plasmids were linearized with EcoRI and NotI (New England Biolabs) respectively (Müller et al., 2012). RNA probes for in situ hybridization were synthesized from these linearized plasmids using SP6 or T7 polymerase and DIG-modified ribonucleotides (Roche). RNA probes were purified using RNeasy kits (QIAGEN).
Recombinant proteins
sfGFP and mVenus were expressed in One Shot TOP10 E. coli using the plasmids pBAD-sfGFP (Addgene plasmid #54519 (Pédelacq et al., 2006)) and pBAD-mVenus (Addgene plasmid #54845 (Nagai et al., 2002)) after overnight induction with 10 mg/ml arabinose at 16°C. The fluorescent proteins were then purified by ethanol extraction as previously described (Samarkina et al., 2009). Briefly, E. coli cells were lysed by sonication in 20 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA, pH 7.8, and sodium chloride and ammonium sulfate were added to the lysate to a final concentration of 0.41 M and 2.63 M, respectively. 1.2 volumes of 96% ethanol were then added to the lysate, and the mixture was vigorously shaken. After centrifugation, the fluorescent proteins became partitioned into the upper organic phase. The upper phase was recovered, and 0.25 volumes of n-Butanol were added. The mixture was then centrifuged, causing the fluorescent proteins to be partitioned into the lower aqueous phase from which they were recovered. The purified fluorescent proteins were then concentrated and buffer-exchanged into phosphate buffered saline (PBS) via ultrafiltration with Pierce protein concentrators (10K MWCO, Thermo Fisher Scientific). Fluorescent proteins were quantified using a Nanodrop 1000 (Thermo Fisher Scientific) by measuring their absorption spectra. The concentration was calculated according to the Beer–Lambert law using the measured peak absorption and the molar extinction coefficients taken from FPbase (Lambert, 2019).
Immunoblotting
Extra- and intracellularly enriched embryo extracts were prepared as described previously (Pomreinke et al., 2017, Müller et al., 2012). Protein samples were resolved on 12% polyacrylamide gels and transferred to polyvinylidene fluoride membranes using a semi-dry blotting system (Bio-Rad). The blots were blocked in 5% low fat milk in PBS containing 0.1% Tween 20 (PBST) for 1 hour, before being incubated overnight at 4°C with a dilution of 1:5000 rabbit anti-GFP antibody (Life Technologies) in 1% low fat milk in PBST. The blots were washed 3 times for 10 min each with PBST and then incubated for 1 h at room temperature with a dilution of 1:5000 goat anti-rabbit horseradish peroxidase (Jackson ImmunoResearch) in 1% low fat milk in PBST. Finally, the blots were washed 3 times for 10 min each with PBST, and the signal was developed with SuperSignal West Dura extended duration substrate (Thermo Fisher Scientific) for imaging with a chemiluminescence imaging system (Fusion 483 Solo, Vilber Lourmat).
Injections and transplantations
For transplantation experiments, embryos were dechorionated using 0.1 mg/ml Pronase (Roche) in 5 mL embryo medium and rinsed in embryo medium to remove the Pronase (Rogers et al., 2015). Embryos were then injected with 2 nL of injection mix at the 1- or 2-cell stage and incubated at 28°C until transplantation. Unfertilized or injured embryos were discarded. Transplantation was done when the embryos reached sphere stage. A cylinder of cells, approximately 80 μm in diameter and 100 μm in length, was transplanted from sphere-stage donor embryos expressing squint-mVenus or bmp2b-sfGFP + bmp7-sfGFP (termed bmp2b/7-sfGFP in the following) into uninjected sphere-stage sibling hosts. Combinations of 100 pg squint-mVenus mRNA or 100 pg + 100 pg bmp2b/7-sfGFP mRNA were used for most experiments, except for Figure S5, where 20 pg squint-mVenus mRNA along with 20 pg + 20 pg bmp2b/7-sfGFP mRNA and 20 pg squint-mVenus mRNA along with 100 pg + 100 pg bmp2b/7-sfGFP mRNA were additionally used. For transplantations with mouse BMP4, 40 pg of mouse bmp4 mRNA was used. Cells for mock transplantations and those with mouse BMP4 were additionally labeled by injecting 100 pg of 10 kDa cascade blue-dextran (Thermo Fisher Scientific) at the one-cell stage. Following transplantation, the embryos were placed in Ringer’s solution (116 mM NaCl, 2.8 mM KCl, 1 mM CaCl2, 5 mM HEPES) for 15 min to recover and then incubated at 28°C until further processing.
To test the activity of constitutively active Smads, different amounts of smad2-CA or smad5-CA mRNA as detailed in the figures were injected into one-cell stage embryos, which were dechorionated at shield stage before fixation.
For the generation of ectopic structures with constitutively active Smads, embryos were dechorionated. Three adjacent blastomeres in embryos at the 64-cell stage were then injected with 20 pg smad2-CA, 20 pg smad2-CA + 20 pg smad5-CA, or 20 pg smad2-CA + 80 pg smad5-CA mRNA per blastomere. For Nodal and BMP receptor inhibition, 40 μM of SB-505124 (Sigma-Aldrich) and 10 μM of Dorsomorphin (Abcam) were used, respectively.
Combined whole-mount immunofluorescence and in situ hybridization
Embryos were fixed in 4% formaldehyde in PBS overnight at 4°C, dehydrated in 100% methanol and stored at −20°C until further processing. Chromogenic in situ hybridization was carried out as described before (Thisse and Thisse, 2008). Fluorescent in situ hybridization (FISH) was executed as described before (Almuedo-Castillo et al., 2018). If immunostainings were subsequently done, embryos were also incubated with 20 mM HCl for 25 min to inactivate the horse radish peroxidase (Liu et al., 2006).
For whole-mount immunostainings, embryos were washed three times with PBST and then permeabilized with cold acetone at −20°C for 20 min. Blocking and antibody incubations were performed in 10% FBS in PBST, and all washes were done with PBST. To carry out the dual pSmad2 and pSmad5 stainings, embryos were first blocked and then incubated with a 1:5000 dilution of a rabbit anti-pSmad2 antibody (Cell Signaling Technology) at 4°C overnight followed by 8 washes for 15 min each. The samples were then blocked and incubated with 1:500 goat anti-rabbit horseradish peroxidase (Jackson ImmunoResearch) at 4°C overnight followed by 8 washes for 15 min each. The signal was detected with TSA fluorescein at a dilution of 1:75 in amplification buffer (Perkin Elmer) for 45 min at room temperature followed by three washes for 5 min each. The embryos were then incubated with methanol for 3 h, washed three times for 10 min, blocked and incubated with a 1:100 dilution of a rabbit anti-pSmad5 antibody (Cell Signaling Technology) and a 1:200 dilution of chicken anti-GFP antibody (Aves Labs) at 4°C overnight. The samples were washed 8 times for 15 min each, blocked and incubated with 1:100 anti-rabbit Alexa647 IgG and 1:200 anti-chicken Alexa 568 IgG (in cases where no in situ hybridization was carried out) or anti-chicken DyLight 405 IgG (for combined in situ hybridization) at 4°C overnight. The embryos were finally washed 8 times for 15 min each and imaged immediately afterward using a light-sheet microscope.
Light-sheet microscopy
Fluorescence images of fixed samples were obtained using a Lightsheet Z.1 microscope (ZEISS). Samples were mounted in 1% low-melting agarose (Lonza, Cat#50080) in embryo medium using a size 3 glass capillary sample holder (ZEISS) and a needle to orient the embryos. The samples were imaged as a series of z stacks with the following objectives and imaging conditions: W Plan-Apochromat 20 × objective, 0.5 × zoom, separate exposure, 80 ms exposure time, 6.4 μm average light-sheet thickness, 10 μm intervals between z-slices. For samples stained by in situ hybridization alone, a 488 nm laser (100 mW) was used at 6% power for foxi1, 1% power for gsc, 8% power for eve1. For samples with double pSmad staining, a 488 nm laser (100 mW) was used at 1% power, a 561 nm laser (20 mW) at 5% power, and a 638 nm laser (75 mW) at 8% power. For samples with in situ hybridization signals and double pSmad staining, a 561 nm laser (20 mW) was used at 5% power, and a 405 nm laser (20 mW) was used at 10% power in addition to the other three lasers. Images were acquired with 1920 pixels × 1920 pixels (877.13 μm × 811.13 μm) dimensions.
Confocal microscopy
Live imaging of embryos was executed on an LSM 780 NLO confocal microscope (ZEISS) using an LD C-Apochromat 40 × / 1.1 NA water immersion objective. Embryos were mounted in 1% low-melting point agarose in glass bottom Petri dishes (MatTek Corporation) and covered with embryo medium. Embryos were maintained at 28°C during the experiments using a heated chamber and imaged 30, 60, 120 and 180 min post-transplantation. The fluorophores were excited with a 50 mW argon laser. sfGFP was excited at 488 nm with 16% laser power, and mVenus was excited at 514 nm with 35% power. The emission was collected as a multispectral image using a 32-channel GaAsP QUASAR array. Images were acquired with 512 × 512 pixels (425.10 × 425.10 μm) dimensions. The multispectral image was then converted into a single channel image by linear unmixing using ZEN Black (ZEISS) (Figure S2H).
Quantification and Statistical Analysis
Image analysis
Fiji was used for all image analyses (Schindelin et al., 2012). Nodal and BMP gradients were quantified similar to previous approaches (Pomreinke et al., 2017, Müller et al., 2012). A median filter of 1 pixel radius was applied to the images for denoising. This was followed by a maximum intensity projection of 14 z-slices.
Nodal, BMP, pSmad2 and pSmad5 gradients were quantified in a rectangular 66 μm wide and 150 μm long region. The “plot profile” function in Fiji was used, which averages the data along the width. Background levels were determined by measuring the average intensity from untransplanted embryos, and the background was subtracted from the gradient profiles. Prism (GraphPad Software) was used for data plotting. All error bars indicate 95% confidence intervals.
The pSmad ratio images were generated by first subtracting background intensities from each channel separately followed by a Gaussian blur with a sigma of 10. The Gaussian blur was performed to visualize an area of pSmad2 or pSmad5 activity instead of specific nuclei and to minimize aberrantly high ratios resulting from division by very low pSmad5 intensities. After Gaussian blurring, the pSmad2 channel was divided by the pSmad5 channel to generate the final image.
To determine the target gene response with varying amounts of Smad2-CA + Smad5-CA, the average intensity in a central circular region with half of the embryo radius was measured.
Mathematical modeling
The finite element method was used for two-dimensional numerical simulations of differential signaling activation kinetics from a single input gradient. The zebrafish animal pole was modeled as a circle with a radius of 300 μm, and the mouse BMP4 (mBMP4) expressing clone was placed concentrically into the embryo disc with a radius of 10 μm. mBMP4 gradient formation was simulated using the following partial differentiation equation:
where D = 3 μm2/s represents the diffusion coefficient of mBMP4 (based on measurements of zebrafish Bmp2b (Pomreinke et al., 2017, Zinski et al., 2017)), k1 = 1/s represents the spatially restricted production rate constant of mBMP4 exclusively within the clone, and k2 = 10−4/s (based on measurements of zebrafish Bmp2b (Pomreinke et al., 2017)) represents the spatially uniform clearance rate constant.
The readout of the resulting mBMP4 gradient by pSmad5 and pSmad2 was modeled as
where k3 = k4 = 10−4/s represent the degradation rate constants of the pSmads and k5 = k6 = 1/s represent their production rate constants. kd = 100 and ke = 106 represent the different signaling thresholds that activate pSmad5 and pSmad2, respectively.
The solution at each time step in the discretized geometry was determined using a sparse LU factorization algorithm (UMFPACK), and the time stepping was computed using a backward Euler step method (COMSOL Multiphysics 3.5a). Simulations were executed for a total of 7200 s.
While D and k2 are based on directly measured quantities, the values for k1, k3, k4, k5, k6, kd and ke have not been experimentally determined. k1, k5 and k6 control the amplitude of the gradients – but not their shape – and were therefore set to an arbitrary value of 1/s. Equal values of k3 and k4 were chosen to reflect the timescale of gradient formation. A large difference between kd and ke values was chosen to illustrate that a single signaling molecule gradient can generate vastly different activity gradients based on differential signaling kinetics.
Data and Code Availability
The raw images and data used in this work are available from the Lead Contact upon request.
Acknowledgments
We thank Katherine Rogers and Daniel Čapek for helpful discussions and Jelena Raspopovic for providing the pCS2-mBMP4 plasmid. This work was supported by the Max Planck Society and the ERC Starting Grant QUANTPATTERN (Grant Agreement No. 637840).
Author Contributions
Conceptualization, G.H.S. and P.M.; Methodology, G.H.S. and P.M.; Investigation, G.H.S. and A.P.P.; Writing – Original Draft, G.H.S. and P.M.; Writing – Review & Editing, G.H.S., A.P.P., and P.M.; Funding Acquisition, P.M.; Resources, P.M.; Supervision, P.M.
Declaration of Interests
The authors declare no competing interests.
Published: April 7, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.03.051.
Supplemental Information
References
- Agathon A., Thisse B., Thisse C. Morpholino knock-down of antivin1 and antivin2 upregulates nodal signaling. Genesis. 2001;30:178–182. doi: 10.1002/gene.1059. [DOI] [PubMed] [Google Scholar]
- Almuedo-Castillo M., Bläßle A., Mörsdorf D., Marcon L., Soh G.H., Rogers K.W., Schier A.F., Müller P. Scale-invariant patterning by size-dependent inhibition of Nodal signalling. Nat. Cell Biol. 2018;20:1032–1042. doi: 10.1038/s41556-018-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.T., Joubin K., Cheng S., Aanstad P., Herwig R., Clark M., Lehrach H., Schier A.F. Nodal signaling activates differentiation genes during zebrafish gastrulation. Dev. Biol. 2007;304:525–540. doi: 10.1016/j.ydbio.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove B.W., Su Y.C., Yost H.J. Maternal Gdf3 is an obligatory cofactor in Nodal signaling for embryonic axis formation in zebrafish. eLife. 2017;6:e28534. doi: 10.7554/eLife.28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader P., Rastegar S., Fischer N., Strähle U. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science. 1997;278:1937–1940. doi: 10.1126/science.278.5345.1937. [DOI] [PubMed] [Google Scholar]
- Bläßle A., Soh G., Braun T., Mörsdorf D., Preiß H., Jordan B.M., Müller P. Quantitative diffusion measurements using the open-source software PyFRAP. Nat. Commun. 2018;9:1582. doi: 10.1038/s41467-018-03975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Small S. Morphogen rules: design principles of gradient-mediated embryo patterning. Development. 2015;142:3996–4009. doi: 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Schier A.F. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–610. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- Ciruna B., Weidinger G., Knaut H., Thisse B., Thisse C., Raz E., Schier A.F. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc. Natl. Acad. Sci. USA. 2002;99:14919–14924. doi: 10.1073/pnas.222459999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Pra S., Fürthauer M., Van-Celst J., Thisse B., Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev. Biol. 2006;298:514–526. doi: 10.1016/j.ydbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- de Olivera-Melo M., Xu P.F., Houssin N., Thisse B., Thisse C. Generation of ectopic morphogen gradients in the zebrafish blastula. Methods Mol. Biol. 2018;1863:125–141. doi: 10.1007/978-1-4939-8772-6_7. [DOI] [PubMed] [Google Scholar]
- Dubrulle J., Jordan B.M., Akhmetova L., Farrell J.A., Kim S.H., Solnica-Krezel L., Schier A.F. Response to Nodal morphogen gradient is determined by the kinetics of target gene induction. eLife. 2015;4:e05042. doi: 10.7554/eLife.05042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauny J.D., Thisse B., Thisse C. The entire zebrafish blastula-gastrula margin acts as an organizer dependent on the ratio of Nodal to BMP activity. Development. 2009;136:3811–3819. doi: 10.1242/dev.039693. [DOI] [PubMed] [Google Scholar]
- Feldman B., Gates M.A., Egan E.S., Dougan S.T., Rennebeck G., Sirotkin H.I., Schier A.F., Talbot W.S. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Fisher S., Halpern M.E. Patterning the zebrafish axial skeleton requires early chordin function. Nat. Genet. 1999;23:442–446. doi: 10.1038/70557. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Maurer J.M., Sagerström C.G. Analysis of novel caudal hindbrain genes reveals different regulatory logic for gene expression in rhombomere 4 versus 5/6 in embryonic zebrafish. Neural Dev. 2018;13:13. doi: 10.1186/s13064-018-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsman K., Zhang J., Cheng S., Heckscher E., Talbot W.S., Schier A.F. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Hagos E.G., Dougan S.T. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev. Biol. 2007;7:22. doi: 10.1186/1471-213X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S., Christison J., Liu D., Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev. Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmansa S., Alborelli I., Bieli D., Caussinus E., Affolter M. A nanobody-based toolset to investigate the role of protein localization and dispersal in Drosophila. eLife. 2017;6:e22549. doi: 10.7554/eLife.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S.H., Wünnenberg-Stapleton K., Hashimoto C., Laurent M.N., Watabe T., Blumberg B.W., Cho K.W. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Heldin C.H., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Joly J.S., Joly C., Schulte-Merker S., Boulekbache H., Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Lee K.H., Zon L., Hammerschmidt M., Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Krauss S., Concordet J.P., Ingham P.W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lambert T.J. FPbase: a community-editable fluorescent protein database. Nat. Methods. 2019;16:277–278. doi: 10.1038/s41592-019-0352-8. [DOI] [PubMed] [Google Scholar]
- Little S.C., Mullins M.C. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat. Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Amin S., Okuhama N.N., Liao G., Mingle L.A. A quantitative evaluation of peroxidase inhibitors for tyramide signal amplification mediated cytochemistry and histochemistry. Histochem. Cell Biol. 2006;126:283–291. doi: 10.1007/s00418-006-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J., Griffin K., Herbomel P., Dickmeis T., Strähle U., Kimelman D., Rosa F.M., Peyriéras N. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development. 2004;131:629–641. doi: 10.1242/dev.00964. [DOI] [PubMed] [Google Scholar]
- Meno C., Gritsman K., Ohishi S., Ohfuji Y., Heckscher E., Mochida K., Shimono A., Kondoh H., Talbot W.S., Robertson E.J. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol. Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Michaelis L., Menten M.L., Johnson K.A., Goody R.S. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.S.J., Schmierer B., Hill C.S. TGF-β family ligands exhibit distinct signalling dynamics that are driven by receptor localisation. J. Cell Sci. 2019;132:jcs234039. doi: 10.1242/jcs.234039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague T.G., Schier A.F. Vg1-Nodal heterodimers are the endogenous inducers of mesendoderm. eLife. 2017;6:e28183. doi: 10.7554/eLife.28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörsdorf D., Müller P. Tuning protein diffusivity with membrane tethers. Biochemistry. 2019;58:177–181. doi: 10.1021/acs.biochem.8b01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Rogers K.W., Jordan B.M., Lee J.S., Robson D., Ramanathan S., Schier A.F. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336:721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Rogers K.W., Yu S.R., Brand M., Schier A.F. Morphogen transport. Development. 2013;140:1621–1638. doi: 10.1242/dev.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins M.C., Hammerschmidt M., Kane D.A., Odenthal J., Brand M., van Eeden F.J., Furutani-Seiki M., Granato M., Haffter P., Heisenberg C.P. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K., Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Niessing D., Blanke S., Jäckle H. Bicoid associates with the 5′-cap-bound complex of caudal mRNA and represses translation. Genes Dev. 2002;16:2576–2582. doi: 10.1101/gad.240002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pédelacq J.D., Cabantous S., Tran T., Terwilliger T.C., Waldo G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- Pelliccia J.L., Jindal G.A., Burdine R.D. Gdf3 is required for robust Nodal signaling during germ layer formation and left-right patterning. eLife. 2017;6:e28635. doi: 10.7554/eLife.28635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda H.M., Meyer D. Zebrafish Smad7 is regulated by Smad3 and BMP signals. Dev. Dyn. 2002;224:334–349. doi: 10.1002/dvdy.10113. [DOI] [PubMed] [Google Scholar]
- Pomreinke A.P., Soh G.H., Rogers K.W., Bergmann J.K., Bläßle A.J., Müller P. Dynamics of BMP signaling and distribution during zebrafish dorsal-ventral patterning. eLife. 2017;6:25861. doi: 10.7554/eLife.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel M.C., Hill C.S. The ventral to dorsal BMP activity gradient in the early zebrafish embryo is determined by graded expression of BMP ligands. Dev. Biol. 2013;378:170–182. doi: 10.1016/j.ydbio.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A., Takeda H., Koshida S., Broadbent J., Price B., Smith J.C., Patient R., Holder N. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-β family signals and discrimination of mesoderm and endoderm by FGF. Development. 1999;126:3067–3078. doi: 10.1242/dev.126.14.3067. [DOI] [PubMed] [Google Scholar]
- Rogers K.W., Müller P. Nodal and BMP dispersal during early zebrafish development. Dev. Biol. 2019;447:14–23. doi: 10.1016/j.ydbio.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Rogers K.W., Schier A.F. Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- Rogers K.W., Blässle A., Schier A.F., Müller P. Measuring protein stability in living zebrafish embryos using fluorescence decay after photoconversion (FDAP) J. Vis. Exp. 2015;95:52266. doi: 10.3791/52266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K.W., Lord N.D., Gagnon J.A., Pauli A., Zimmerman S., Aksel D.C., Reyon D., Tsai S.Q., Joung J.K., Schier A.F. Nodal patterning without Lefty inhibitory feedback is functional but fragile. eLife. 2017;6:28785. doi: 10.7554/eLife.28785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarkina O.N., Popova A.G., Gvozdik E.Y., Chkalina A.V., Zvyagin I.V., Rylova Y.V., Rudenko N.V., Lusta K.A., Kelmanson I.V., Gorokhovatsky A.Y., Vinokurov L.M. Universal and rapid method for purification of GFP-like proteins by the ethanol extraction. Protein Expr. Purif. 2009;65:108–113. doi: 10.1016/j.pep.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Sampath K., Rubinstein A.L., Cheng A.M., Liang J.O., Fekany K., Solnica-Krezel L., Korzh V., Halpern M.E., Wright C.V. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B., Fürthauer M., Connors S.A., Trout J., Thisse B., Thisse C., Mullins M.C. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Lee K.J., McMahon A.P., Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Shen M.M., Schier A.F. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000;16:303–309. doi: 10.1016/s0168-9525(00)02006-0. [DOI] [PubMed] [Google Scholar]
- Stachel S.E., Grunwald D.J., Myers P.Z. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Tsang M., Kim R., de Caestecker M.P., Kudoh T., Roberts A.B., Dawid I.B. Zebrafish nma is involved in TGFbeta family signaling. Genesis. 2000;28:47–57. doi: 10.1002/1526-968x(200010)28:2<47::aid-gene20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- van Boxtel A.L., Chesebro J.E., Heliot C., Ramel M.C., Stone R.K., Hill C.S. A temporal window for signal activation dictates the dimensions of a Nodal signaling domain. Dev. Cell. 2015;35:175–185. doi: 10.1016/j.devcel.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel A.L., Economou A.D., Heliot C., Hill C.S. Long-range signaling activation and local inhibition separate the mesoderm and endoderm lineages. Dev. Cell. 2018;44:179–191.e5. doi: 10.1016/j.devcel.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J.M., Rosen V., Celeste A.J., Mitsock L.M., Whitters M.J., Kriz R.W., Hewick R.M., Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wrana J.L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X.F., Massagué J. TGF β signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Xu P.F., Houssin N., Ferri-Lagneau K.F., Thisse B., Thisse C. Construction of a vertebrate embryo from two opposing morphogen gradients. Science. 2014;344:87–89. doi: 10.1126/science.1248252. [DOI] [PubMed] [Google Scholar]
- Xue Y., Zheng X., Huang L., Xu P., Ma Y., Min Z., Tao Q., Tao Y., Meng A. Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat. Commun. 2014;5:3766. doi: 10.1038/ncomms4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoney A., Etoc F., Ruzo A., Carroll T., Metzger J.J., Martyn I., Li S., Kirst C., Siggia E.D., Brivanlou A.H. WNT signaling memory is required for ACTIVIN to function as a morphogen in human gastruloids. eLife. 2018;7:e38279. doi: 10.7554/eLife.38279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Sasaki H., Lowe L., Hogan B.L., Kuehn M.R. Nodal is a novel TGF-β-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- Zinski J., Bu Y., Wang X., Dou W., Umulis D., Mullins M.C. Systems biology derived source-sink mechanism of BMP gradient formation. eLife. 2017;6:e22199. doi: 10.7554/eLife.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw images and data used in this work are available from the Lead Contact upon request.