Abstract
Care for patients during COVID-19 poses challenges that require the protection of staff with recommendations that health care workers wear at minimum, an N95 mask or equivalent while performing an aerosol-generating procedure with a face shield. The United States faces shortages of personal protective equipment (PPE), and surgeons who use loupes and headlights have difficulty using these in conjunction with face shields. Most arthroplasty surgeons use surgical helmet systems, but in the current pandemic, many hospitals have delayed elective arthroplasty surgeries and the helmet systems are going unused. As a result, the authors have begun retrofitting these arthroplasty helmets to serve as PPE. The purpose of this article is to outline the conception, design, donning technique, and safety testing of these arthroplasty helmets being repurposed as PPE.
Keywords: total joint arthroplasty, COVID-19, 3D printing, personal protective equipment, surgical helmet systems
Care for patients during COVID-19 poses challenges that require the protection and safety of staff. High risk of exposure to health care workers occurs with direct contact with respiratory droplets. Formal recommendations have been issued by the Anesthesia Patient Safety Foundation recommending that health care workers wear, at minimum, an N95 mask or equivalent while performing an aerosol-generating procedure with a face shield. A powered air-purifying respirator (PAPR) provides superior protection and may be warranted in patients with known or suspected COVID-19. PAPRs pull air through a HEPA filter (N95 grade or better) into a suit to create positive pressure with air always flowing out so that the person in the suit only receives filtered air, thus minimizing exposure to the person in the suit. Risk of aerosolization is increased during intubation. In addition, specific surgeries and procedures that involve the nasopharynx/oropharynx may carry similar risk to surgeons and other staff in the operating room. Amid this, the United States faces shortages of personal protective equipment (PPE) [1]. In addition, surgeons who use loupes and headlights have difficulty using these in conjunction with face shields. Most hospitals have a limited number of dedicated PAPR units posing a significant constraint when caring for multiple patients with suspected/confirmed COVID-19. Most arthroplasty surgeons use surgical helmet systems in efforts to decrease the patient’s risk of surgical infection and to protect surgical staff from infectious blood splashes/debris, with some studies questioning the capacity of surgical helmet systems to decrease contamination or deep infection [[2], [3], [4]]. In the current pandemic, many hospitals have delayed elective arthroplasty surgeries and the helmet systems are going unused.
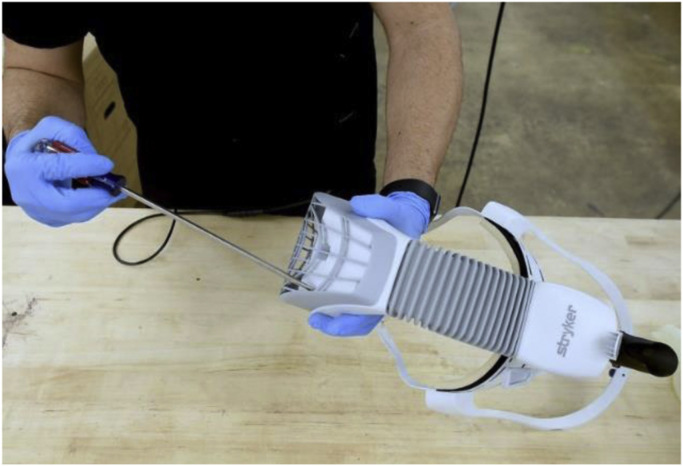
This scenario of need for PPE and helmets not being used led the authors to initiate an effort to retrofit the Stryker Flyte helmets (Stryker, Mahwah, NJ) at our institution. This helmet system covers the entire head and neck. The system is designed to pull air in through the top of the hood through a white section of AAMI class 3 material. The air then passes through the blue AAMI class 4 material on the sides of the hood. A fan mounted in the top of the helmet drives this flow. While class 4 material provides the highest level of protection against pathogens [5], the class 3 material may allow some pathogens to pass through. Helmet systems were used in conjunction with goggles and N95 masks during the 2003 SARS outbreak as using the helmet systems alone did not filter enough particles (0.02-1 μm diameter) to meet the standard for PAPRs [6]. Although this system is not intended as a respirator, a novel solution has been proposed with adaptation of the standard helmet system given the shortage of PAPRs and potential shortage of N95 masks. The concept developed at our institution was to use a 3D printing approach to create an adaptor for the Flyte helmet to allow conversion to PPE.
Methods
Initial Concept and Development
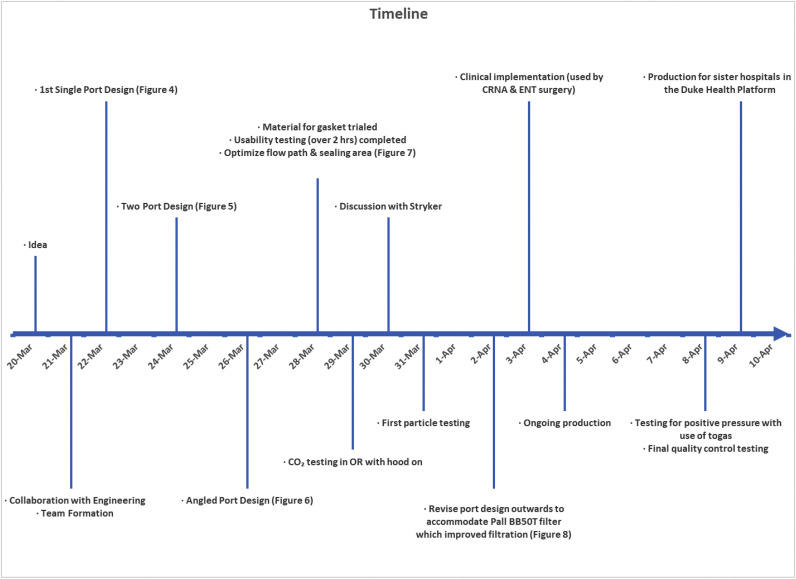
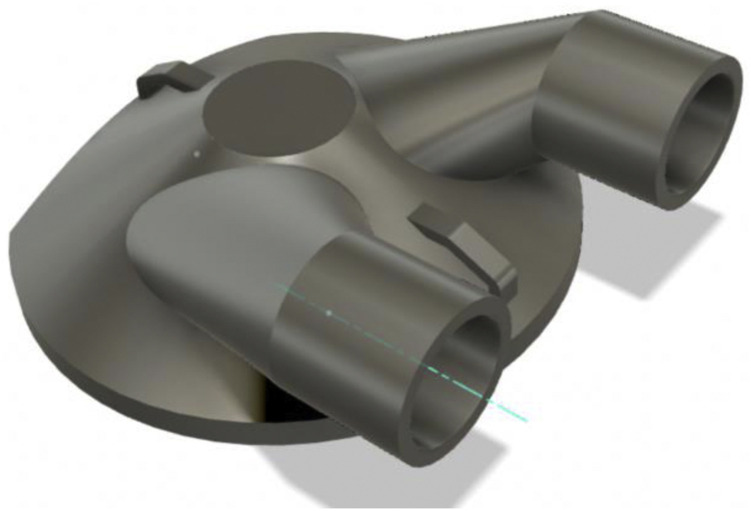
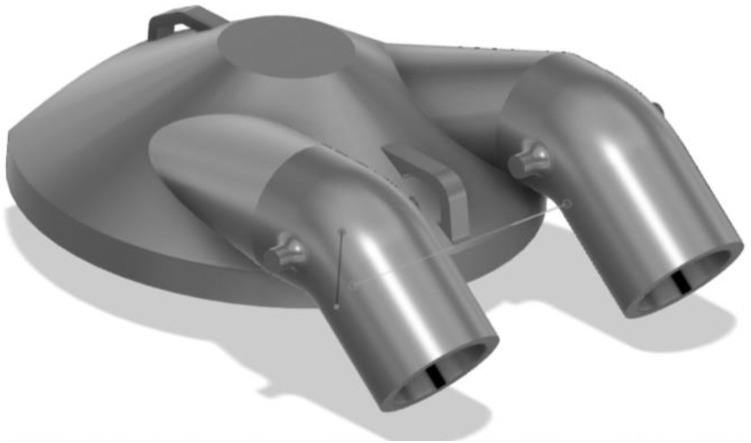
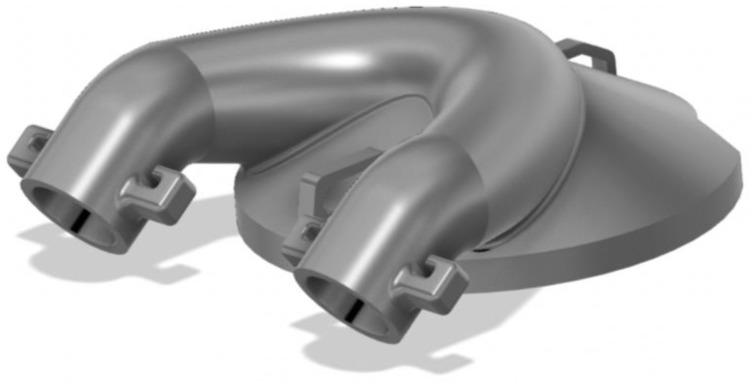
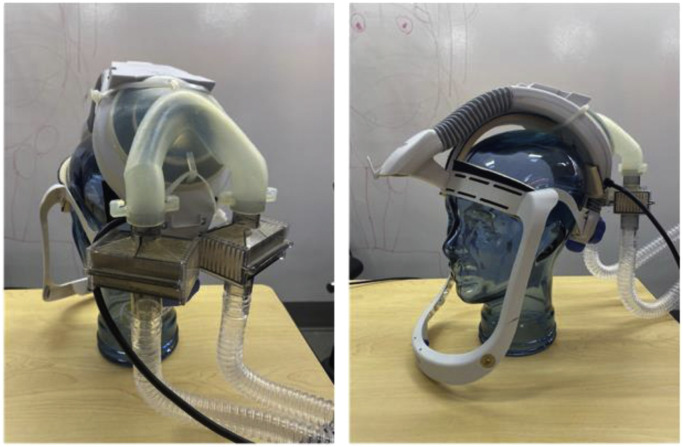
This effort was initiated on 03/20/2020 after the lead author (MME) noted that these unused helmets represented a possible solution to PPE shortage for high-risk scenarios (Fig. 1 ). Helmet modification, assembly, and quality testing was performed in collaboration with the Duke University Pratt School of Engineering. At the core of this modification is a single new component, the manifold. The manifold sits over the (previously open) intake fan and creates a sealed air path directly to the two mounted filters. The manifold is created from a Duke design that is then 3D printed (additive manufacturing) to arrive at the final component. We used Formlabs printers and their “durable” material for this new part. The engineering team worked to quickly design a robust manifold by using 3D printing (Fig. 2 ). The first single-port design was created on 3/22/2020 (Fig. 3 ). The single-port design had insufficient flow and choking at the fan but proved the concept, so we went to a two-port design on 3/24/2020 (Fig. 4 ). After some user testing in the hospital, we realized we needed to angle the ports down to better accommodate the hood and the design was updated on 3/26/2020 (Fig. 5 ). Then we optimized the flow path and sealing area on 3/28/2020 (Fig. 6 ). Next, we moved the ports outward to allow for the rectangular Pall BB50T filter which provided enhanced performance on 4/2/2020 (Fig. 7 ).
Fig. 1.
Timeline.
Fig. 2.
3D-printed manifold was designed to mate to where the fan cover can be removed from the Flyte helmet.
Fig. 3.
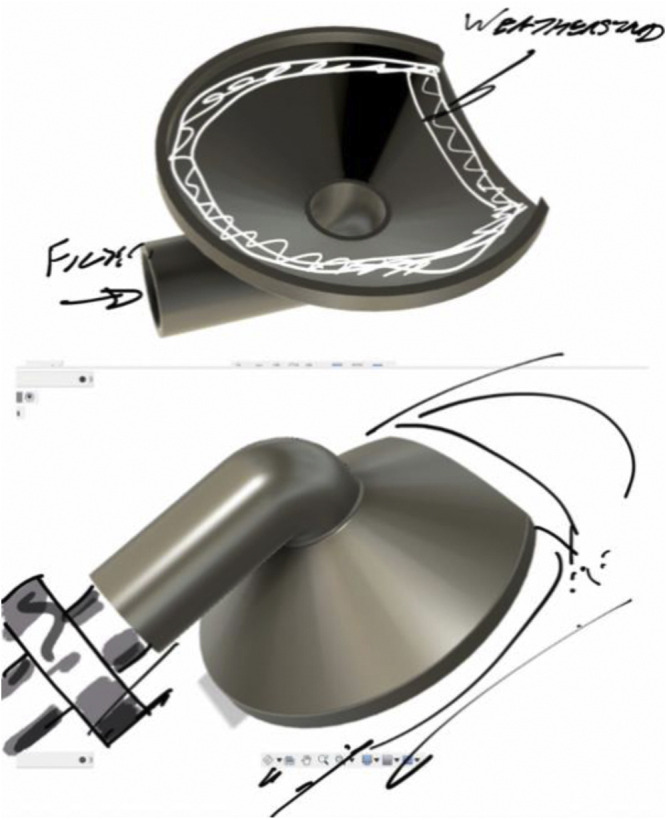
First single-port design was created on 3/22/2020.
Fig. 4.
Two-port design on 3/24/2020.
Fig. 5.
Angled the ports down to better accommodate the hood on 3/26/2020.
Fig. 6.
Optimized flow path and sealing area on 3/28/2020.
Fig. 7.
Moved the ports outward to allow for the rectangular Pall BB50T filter on 4/2/2020.
Modifying the Helmet
The fan cover is removed by placing pressure on the four push tabs with a screwdriver (Fig. 8 ). This is fitted with a 3-mm silicone piece and is cut to fit the diameters of the manifold and helmet, creating a seal. The helmet is attached to the manifold and secured with zip ties that loop through the eyelets in the front and back of the manifold (Fig. 9 ). Two PALL BB50T breathing circuit filters [7] are attached to the manifold. These are additionally secured with rubber bands. Anesthesia tubing is attached with a standard Hudson multiadapter (Fig. 10 ).
Fig. 8.
Removal of fan cover.
Fig. 9.
A 3 mm silicone piece is fit to the manifold and helmet.
Fig. 10.
Breathing circuit filters are attached to the manifold, secured, anesthesia tubing is attached.
Donning Technique for Helmet and Cover After Placement of Adapter
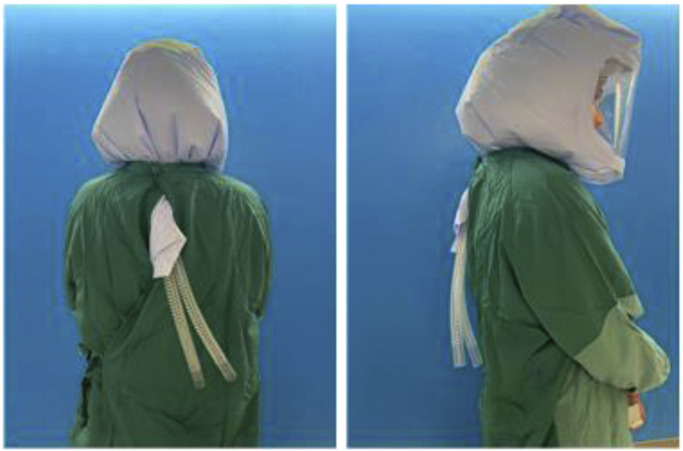
To prepare the hood, a seal is created against the white class 3 material on top of the hood with 3M Tegaderm. If needed, this can be done with sterile technique if a surgeon plans to wear the helmet system. It is important to create the seal on the inside of the hood which can be confirmed by locating the exposed seam side (Fig. 11 ). Two 8 × 12 inch 3M Tegaderm strips or multiple 4 × 10 inch strips can be used for this. The strips must cover the seams and overlap with each other to ensure an adequate seal. In addition, this is done on the inside as a safety measure so that positive air flow inside the hood pushes the strips up against the class 3 material.
Fig. 11.
Locating the exposed seam side to create the seal.
Standard donning of the hood is performed. It is recommended to wear a standard surgical mask under the helmet system throughout the procedure. When gowning, it is important to create as snug a fit as tolerated around the neck as this helps create the positive pressure. The tubing should be pulled out of the gown to ensure that unobstructed room air is brought into the filtering system (Fig. 12 ). These modifications will allow the user to have protection equivalent to or potentially greater than the protection offered by an N95 mask and face shield, as confirmed by particle flow testing.
Fig. 12.
Tubing should be pulled out of the gown to ensure that unobstructed room air is brought into the filtering system.
Safety Testing of Materials and Components
Testing was performed to monitor CO2 accumulation inside the hood using a GE Datex-Ohmeda anesthesia machine for 30 minutes (no significant accumulation noted). Independent consultants from Precision Air (Morrisville, NC) that certify clean rooms, HEPA filters, and PPE at Duke University were engaged to perform particle flow testing. Testing was performed according to a modified protocol previously established at our institution for PAPRs and showed that the system met HEPA standards [8]. There are no patient-related risks identified. In consideration of circumstances under which the device should not be used, users should be knowledgeable in how to use/don/doff the surgical hood.
Discussion
SARS-CoV-2 is a novel virus that can be spread through respiratory droplets. We created a novel design that modifies a Stryker Flyte helmet to filter intake air into the helmet to reduce the likelihood of SARS-CoV-2 transmission to health care providers. This product is especially useful for health care providers involved in airway management or higher risk surgeries/procedures in COVID-19 suspected or positive patients. We recognize that these modifications constitute off-label use of the device and the introduction of new elements/features. Given the emergent circumstances of the COVID-19 pandemic and shortage of PAPR devices, we propose that the risk of using this device are small in comparison with the risk of COVID-19 transmission. We encourage physicians and other health care workers to weigh these risks before use of the device.
The design team has engaged with health care workers in anesthesiology, surgery, and respiratory therapy to get feedback on the proposed design. Their collective thoughts are positive. They have expressed that the design is comfortable, and they are relieved that additional PPE are available. With the shortage of PAPRs and the knowledge that this design is off-label this device has been used by some anesthesiology staff and surgeons at our institution. We have consulted with PPE at Duke’s Biocontainment Laboratory to understand how PAPRs are tested and serviced at Duke, and subsequently appropriate quality control check has been performed on each unit before use. We have had technical conversations with engineers at Stryker who work on both the hood and the helmet.
Conclusions
In the current pandemic, we have learned that many hospitals are using these helmet systems as PPE without any of the proposed modifications. We believe that this design utilizing unused helmets, a novel 3D-printed manifold, and other modifications provides a possible solution to the shortage of PAPRs. We currently have ongoing production of these manifolds to use with this helmet. The product has had final quality control testing on 4/8/2020, and we are implementing in other hospitals within our health system.
Acknowledgments
The authors would like to acknowledge Dr. Melanie Hollidge MD, PhD, FRCPC, Scott Alderman MS, Matt Stiegel PhD, and this study received funding from Duke University Department of Surgery, Duke University Pratt School of Engineering, Duke University Office of Information Technology, and Duke Co-Lab.
Design files and supplemental videos have been made available to other institutions through Duke’s Office of Licensing and Ventures.
Footnotes
This article is published as part of a supplement supported by the American Association of Hip and Knee Surgeons and by an educational grant from Aerobiotix.
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.arth.2020.04.035.
Appendix A. Supplementary Data
References
- 1.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020 doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 2.McGovern P.D., Albrecht M., Khan S.K., Muller S.D., Reed M.R. The influence of surgical hoods and togas on airborne particle concentration at the surgical site: an experimental study. J Orthop Sci. 2013;18:1027. doi: 10.1007/s00776-013-0445-7. [DOI] [PubMed] [Google Scholar]
- 3.Vijaysegaran P., Knibbs L.D., Morawska L., Crawford R.W. Surgical space suits increase particle and microbiological emission rates in a simulated surgical environment. J Arthroplasty. 2018;33:1524. doi: 10.1016/j.arth.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Young S.W., Zhu M., Shirley O.C., Wu Q., Spangehl M.J. Do ‘surgical helmet systems’ or ‘body exhaust suits’ affect contamination and deep infection rates in arthroplasty? A systematic review. J Arthroplasty. 2016;31:225. doi: 10.1016/j.arth.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Administration USFD Medical Gowns. https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns [accessed 04.02.20]
- 6.Derrick J.L., Gomersall C.D. Surgical helmets and SARS infection. Emerg Infect Dis. 2004;10:277. doi: 10.3201/eid1002.030764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PALL PALL BB50T breathing circuit filter. https://shop.pall.com/us/en/medical/mechanical-ventilation/pall-bb50t-breathing-circuit-filter-zidgri78lyp [accessed 04.02.20]
- 8.Alderman T.S., Stiegel M.A., Estes R.A., Thomann W.R., Sempowski G.D. Field-testing method for loose-fitting powered air-purifying respirators equipped with HEPA filters. Appl Biosafety. 2016;21:71–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.