Abstract
Deep brain stimulation (DBS) is a successful treatment for patients with Parkinson’s disease. In adaptive DBS, stimulation is titrated according to feedback about clinical state and underlying pathophysiology. This contrasts with conventional stimulation, which is fixed and continuous. In acute trials, adaptive stimulation matches the efficacy of conventional stimulation while delivering about half the electrical energy. The latter means potentially fewer side-effects. The next step is to determine the long-term efficacy, efficiency, and side-effect profile of adaptive stimulation, and chronic trials are currently being considered by the medical devices industry. However, there are several different approaches to adaptive DBS, and several possible limitations have been highlighted. Here we review the findings to date to ascertain how and who to stimulate in chronic trials designed to establish the long-term utility of adaptive DBS.
Adaptive deep brain stimulation (DBS) uses feedback indicative of the clinical state and underlying pathophysiology to adjust stimulation, save energy, and limit side-effects. With several medical device companies considering trials of chronic adaptive DBS for Parkinson’s disease (PD), it is time to take stock and consider the approaches most likely to be fruitful and the potential limitations that should be anticipated. Hitherto, most research has focused on the use of beta local field potential activity to provide feedback control of DBS of the subthalamic nucleus (STN), and several publications have contrasted acute adaptive DBS with either no stimulation or conventional fixed stimulation. Here we consider the motivation behind these trials, what they have highlighted, and what remains to be understood.
Why Use Beta Activity for Feedback Control?
Multiple studies have demonstrated a correlation between the power of STN beta activity averaged over many seconds or minutes with bradykinesia-rigidity and between treatment-induced reductions in mean beta activity and improvement in bradykinesia-rigidity across patients.1–11 These early studies raised the possibility of using beta as a potential feedback signal and provided evidence that this signal could be recorded over many years.11–13 They also served to highlight that correlations between beta activity and motor symptoms did not extend to parkinsonian rest tremor.
However, only a handful of these early studies provided evidence that fluctuations in beta power related to variation in the severity of bradykinesia-rigidity within patients, a requisite should this activity provide the basis for adaptive DBS.9,14 In retrospect, the sparseness of these accounts probably relates to the lack of awareness of the bursting nature of beta activity and hence the optimal time resolution for correlative studies within patients. The bursting nature of beta activity has only recently been highlighted in PD,15–18 and within-subject correlations between bursts and motor state have been reported in the face of weaker or absent correlations with overall beta power.19–21
The aforementioned studies have concentrated on local field potential activity recorded in the STN, and it remains to be seen whether comparable correlations are to be found at other circuit levels, particularly the globus pallidus interna, which is also a common target for surgery in PD. In addition, it should be mentioned that there are a number of other local field potential activity features that have been linked to bradykinesiarigidity, most notably phase amplitude coupling between the cortex and the STN and within the STN.22–28 That said, it remains to be determined whether these other data features have more correlative value than beta in so far as they are themselves related to beta activity either partially, as a consequence of asymmetries in the beta waveform,29 or through their confinement to beta bursts.30 Moreover, the use of phase amplitude coupling involving high-frequency cortical activity for feedback control necessitates extra instrumentation and involving the very low amplitude, high-frequency signals in the STN might require significant changes to the design of the amplifiers in implantable bidirectional devices to increase sensitivity. One possible workaround might be to focus on the shape of the beta waveform rather than the modulation of high-frequency activity, but this remains to be explored as a marker for adaptive DBS.
Clinical Results
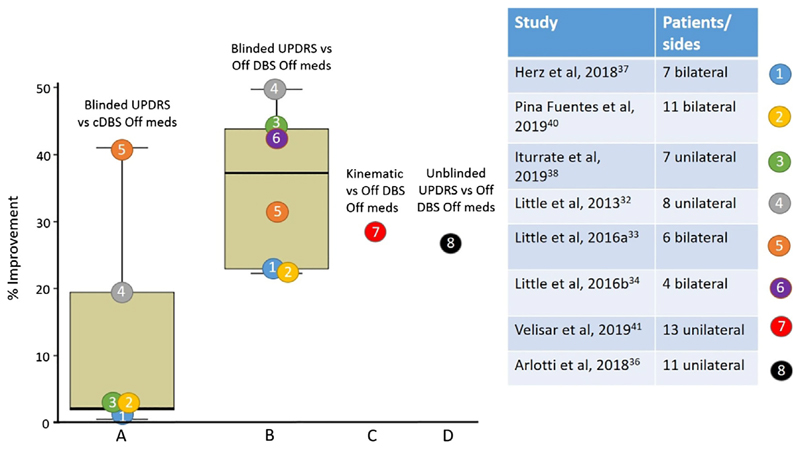
Correlations between changes in beta activity, most notably in the form of bursts, and bradykinesia-rigidity within and between patients have helped motivate acute trials of adaptive DBS.15,31 Until recently these were confined to patients in whom leads connecting to DBS electrodes were externalized as part of a 2-stage surgical procedure in which DBS electrodes are implanted and connected to an internal pulse generator a few days later.32–38 This experimental constraint is important as the range for possible improvement with DBS is limited because of the postoperative stun effect, which can be appreciable.39 As such, the contrast with conventional DBS may be more informative than the percentage improvement relative to no stimulation in these studies. Recently, a study was performed using the leads temporarily externalized at battery change,40 and a further study was performed using a combination of the Activa PC + S and Nexus D3 from Medtronic, Inc. (Dublin, Ireland).41 These 2 studies investigated chronically implanted patients who were unaffected by the stun effect. In most studies, the principal outcome measure has been the rating of performance on the motor United Parkinson’s Disease Rating Scale (UPDRS) by examiners who are blinded to the experimental conditions, an important step where novel treatment approaches are being tried. Figure 1 summarizes the results and suggests 2 immediate conclusions. First, the 5 acute trials of adaptive DBS where comparison with conventional, continuous DBS is possible show superiority of adaptive DBS in the reversal of motor deficits. The range is large, and it is important to note that in all of these studies, conventional DBS was delivered in the same way as adaptive DBS. This means that stimulation was restricted to monopolar stimulation of 1 or the other of the middle 2 contacts of each DBS electrode. Hence, the delivery of conventional DBS may differ from the optimal contact selection in the chronic state that can use any of the 4 contacts and be bipolar if necessary. In reality therefore, optimal conventional stimulation might be slightly more effective than in these studies, and perhaps a more conservative conclusion is that the studies have hitherto shown that acute adaptive DBS is not inferior to conventional DBS in efficacy. The intermittent nature of stimulation during adaptive DBS was reported not to cause significant paraesthesia.
Fig. 1.
The percentages of improvements in UPDRS motor scores during adaptive DBS in comparison with no stimulation (A) or conventional, continuous stimulation (B), and in kinematic measures in comparison to no stimulation (C). All are recorded in the off medication state, use variations of the control policy responding to fast beta dynamics, and are objective assessments (A–C). (D) Percentage improvement in unblinded UPDRS scores with the control policy responding to slow beta dynamics recorded in the off medication state. cDBS, conventional continuous deep brain stimulation; DBS, deep brain stimulation; meds, medications; UPDRS, United Parkinson’s Disease Rating Scale. [Color figure can be viewed at wileyonlinelibrary.com]
Second, in blinded assessments the median efficacy of stimulation assessed as percentage improvement over the unstimulated, off medication state is about 35%. This is within the range of improvement seen in other blinded assessments of the efficacy of chronic conventional DBS, which consistently report less change than unblinded assessments.32,42–44
The aforementioned considered studies use essentially the same simple control algorithm that extracts the beta signal from the STN local field potential, rectifies this, smooths the resulting signal with a moving average of 400 to 500 milliseconds, and sets a threshold. The threshold is set such that whenever the processed signal exceeds this level, high-frequency stimulation is triggered, and whenever it falls below this level, stimulation is aborted. The threshold is set so that stimulation is delivered about half the time in the off drug state. Careful analyses of the treatment of beta during such adaptive DBS demonstrates that beta bursts with a minimum duration of about 500 milliseconds are relatively selectively targeted, which reinforces the recent hypothesis that these long bursts play a central role in disturbing motor function in PD.15,17 However, 2 other control algorithms have also been used in studies. The first used 2 thresholds, whereby beta falling below the lower threshold triggered an incremental decrease in stimulation voltage and beta increasing above an upper threshold triggered an incremental increase in stimulation voltage.41 The beta power was also computed over 800-millisecond blocks, and therefore the effective temporal resolution was relatively close to that used in the majority of studies where beta amplitude was smoothed with a moving average of 400 to 500 milliseconds. Another major advance in this study was the demonstration of efficacy through objective kinematic measures in chronically implanted patients. Such quantitative and continuous measures may allow for more rapid prototyping and optimization of control algorithms in the future.
The second control algorithm to be used treats the beta signal in a fundamentally different manner.35,36 Instead of tracking bursts of beta activity, the beta signal is exponentially smoothed with a time constant of 50 seconds so that bursting is lost and the processed beta feedback signal instead tracks much slower dynamics in the original signal, particularly those related to medication. In addition, a proportional control policy was used whereby stimulation voltage linearly scaled with the feedback signal. With this slow beta adaptive DBS, the investigators were able to demonstrate that dyskinesias were reduced during adaptive DBS in the on drug state.35 In the off drug state, adaptive stimulation reduced the mean stimulation voltage by about 50%, but the improvement in UPDRS scores was relatively modest. Scores improved by 27% relative to OFF stimulation in unblinded assessments.36 This compares with, for example, a 66% improvement in UPDRS in an unblinded assessment made with a more dynamic control policy that aborted prolonged beta bursts.32 It is also possible that this slow algorithm will be too slow to improve mobility during night-time arousals from deep sleep, thereby compromising mobility in and out of bed.45 Nevertheless, the use of smoothing over a long time window makes this slow algorithm more resistant to artefacts, such as those related to rapid changes in stimulation. It should also be stressed that hitherto slow beta adaptive DBS (state change reactive) and dynamic beta adaptive DBS (burst reactive) have not as yet been contrasted head to head in the same trial.
In summary, the studies to date suggest approximately equivalent efficacy with respect to conventional, continuous stimulation, but these studies have been acute and involve only small numbers of patients. Efficacy has been achieved with around half the stimulation delivered in the off medication state. Savings are even greater in the on drug state34,36 and may remain appreciable even when allowing for the extra processing necessary to implement adaptive DBS.46 Nevertheless, it is doubtful that the conservation of battery life or of the prolongation of intervals between battery recharges is of itself a major benefit given advances in battery technology. Rather, the primary aim of adaptive DBS is to reduce the side-effects associated with conventional DBS given that the stimulation delivered is less overall. What is the evidence that a reduction in side effects might also be achieved? Stimulation-induced dysarthria has a prevalence of around 10%,47 and 2 studies have suggested that acute adaptive DBS may lead to significantly less reduction in speech intelligibility. 33,40 This is promising, but the results are preliminary, and again it should be borne in mind that the conventional DBS might not be fully optimized in these studies. Brittle dyskinesias are another potential side-effect of subthalamic stimulation and might be expected to be attenuated by adaptive DBS given that beta activity is suppressed on drug. Both the reduction in stimulation on drug and the parallel reduction in dyskinesias have been reported with beta-based adaptive DBS.34,35
Outside of the STN, cortical signals can also provide a gamma band feedback that may enable subthalamic stimulation to be reduced during dyskinesias, but this approach entails the additional implantation of an electrode strip over the motor cortex, and does not modulate stimulation outside of dyskinetic periods unless beta activity is also evaluated at the cortical level.48 Cortical signals are of interest as they are of larger amplitude than in the STN and allow a dissociation between sensing and stimulation, removing the constraint that STN stimulation can only be monopolar and at 1 or the other of the middle 2 contacts of the DBS electrode if standard 4-ring electrodes are used. However, this also means that a single implantable pulse generator can no longer control both sides (as the 2 channels of the implantable pulse generator have to accommodate the cortical strip and DBS electrode).
The effects of adaptive DBS on dyskinesias may also prove to indirectly impact on the motor efficacy of this approach. In conventional DBS, the amount of current that can be delivered may be limited by dyskinesias, particularly on medication. Consequently, this can result in patients being effectively understimulated when off medications (as the clinical stimulation amplitude is titrated against the on medication dyskinesia threshold). Adaptive DBS, either burst or slow tracking of beta, or tracking of cortical gamma, could potentially result in improved primary motor outcome in the off medication state through the facilitation of higher current delivery, specific to that medication state. To date, comparisons of adaptive and conventional DBS have had matched amplitudes potentially masking the potential for further motor improvement with adaptive control.
What About Tremor?
Earlier it was mentioned that although beta activity positively correlates with bradykinesia-rigidity, it does not positively correlate with tremor. Indeed, several studies report a decrease in beta activity during parkinsonian tremor for reasons that are obscure.49–52 Accordingly, it might be supposed that adaptive DBS should fail to treat parkinsonian tremor. The surprise is that this is only true in a minority of cases. At present, the explanation for this remains unclear. One possibility is that hitherto studies have been biased in their recruitment toward the akinetic-rigid phenotype and that the stun effect has obscured the presence of tremor where it would otherwise have been seen. However, in 2 trials testing the performance of adaptive DBS in chronically implanted patients, less than 1 in 5 of the cases were reported to fail in the control of tremor, and this only rose to 1 in 4 cases when only those patients with a history of dominant tremor were considered.40,41
Another possibility is that intermittent stimulation is by itself sufficient to control tremor in the majority of patients (irrespective of stimulation locking to beta bursts), at least acutely. One study used kinematic measures of tremor instead of neural signals to provide feedback control of stimulation for parkinsonian tremor,53 but this approach is likely to remain problematic as stimulation can only be adjusted once tremor changes, and yet tremor responds only once stimulation has been started.
Concerns That Adaptive DBS May Interact with Movement
It has been suggested that dynamic beta control algorithms might fail during voluntary movements.54 This concern stems from the well-known suppression of the average level of beta activity during movement planning, execution, and repetitive tasks seen in the basal ganglia in untreated patients with PD, although to a lesser degree than in the treated state.55–57 Given this, it is reasonable to suspect that adaptive DBS that preferentially responds to beta bursts will fail to react during such periods. However, evidence is accruing that although beta bursts appear less frequently after cues instructing movement, they do still occur in some responses, and it is these responses that have slower peak velocities in PD patients.19,21 Equally important, beta bursts can reoccur during self-paced repetitive upper limb movements and during gait and do so in association with bradykinesia and gait freezing, respectively. 20,58 The peak velocities of voluntary movements in PD patients form a distribution that is shifted to the left but usually overlaps with that of movements executed by healthy subjects,59 and it may be that the subset of slower movements are those with concurrent beta bursts. If correct, then dynamic beta feedback control may serve to improve bradykinetic movements while sparing those that are made with normal speed. This outcome seems likely as kinematic assessments of repeated tapping and wrist movements do still improve during dynamic beta feedback control of DBS.40,41
Conversely, it has been suggested that beta feedback–driven adaptive DBS may compromise voluntary movement because it is triggered by the physiological subthalamic beta rebound that occurs around the time of termination of a movement.38 In line with this hypothesis, it has been reported that beta feedback–driven adaptive DBS compromises behavior during the return phase of reaching movements and delays movement termination.38,54 The beta rebound is also linked to trial-to-trial learning, and this might potentially be compromised by adaptive DBS, although this has not as yet been tested.60 Another instance in which relative increases in subthalamic beta activity may be physiological is when responses should be delayed in the face of conflicting evidence.37 Here too behavior is compromised by beta feedback–driven adaptive DBS. However, it is to be expected that these subtle motor disturbances will likewise be elicited by conventional continuous DBS, which also serves to suppress beta,2,61,62 and this does indeed also reduce the ability to withhold responses in the setting of conflicting evidence.31 These subclinical deficits may be the price one has to pay for the amelioration of symptomatic, pathological disturbances by both conventional and adaptive DBS in PD.
Implications for Future Trials
Adaptive DBS for PD has arguably reached a point where further major development may depend on the demonstration that efficacy can be maintained over time in chronically implanted patients. It makes sense for these trials to implant systems that are capable of both conventional and adaptive DBS, allowing both within-subject contrasts and rescue if adaptive DBS proves inferior. With this proviso, there seems no reason to exclude parkinsonian patients with prominent tremor from trials on the understanding that tremor may not be adequately controlled in about a quarter of tremor dominant patients. In these patients, it may be that limiting the range of stimulation intensities used in adaptive DBS, so that periods of no stimulation are avoided, or restricting the duration of periods without stimulation, may serve to control refractory tremor. In addition, acute trials have hitherto been constrained in contact selection to monopolar stimulation at the middle 2 contacts of each electrode. To address this limitation, the surgery for chronic trials should aim to have these 2 middle levels within the STN, or alternatively, implant electrodes with 8 instead of 4 contact levels. Note that, at least in theory, there is no reason why adaptive DBS cannot be delivered through segmented electrodes.
Critical in any trial design will be the incorporation of an initial phase allowing for the relative optimization of control parameters in each patient. Key among these will be the thresholds, or gains, linking feedback to stimulation, and the operating limits before failing or unsafe adaptive stimulation is rescued with conventional stimulation. The decision as to whether to pursue slow beta adaptive DBS (state change reactive) or dynamic beta control algorithms (burst reactive) in clinical trials will be determined by both theoretical and practical considerations. Burst-reactive adaptive DBS is potentially modestly more complex to implement, requiring sufficient suppression of artefacts related to the termination of stimulus trains and ramping to avoid paraesthesias. However, if recent evidence that prolonged beta bursts are pathological is true, burst-reactive adaptive DBS may well be more efficacious as it gets closer to the underlying disease mechanism. Slow beta-adaptive DBS, which treats beta more as a biomarker of medication state, is likely to be slightly simpler to implement, but at the risk of potentially having less overall benefit relative to more dynamic beta control algorithms. As such, and given the greater experience with the latter, dynamic beta control algorithms using a single or dual threshold seem a good starting point for chronic trials.
Once chronic trials have provided the motivation for further development and a cohort of patients is available for additional chronic assessments, we can then further improve adaptive DBS, finessing the processing of feedback signals and optimising control policies, cognizant of power demands. Indeed, the literature is already replete with suggestions for different biomarkers and their combinations, different algorithms, multiple control loops, and different surgical targets for adaptive DBS. Best to walk before we can run, though, and first prove that simple adaptive DBS of the STN retains its efficacy, efficiency, and beneficial side-effect profile over time.
Acknowledgements
The work was supported by the Medical Research Council (MC_UU_12024/1) and Rosetrees Trust. Neither had a role in the writing or submission of the manuscript. No pharmaceutical or medical devices company or other agency were involved at any point.
Footnotes
Relevant conflicts of interests/financial disclosures: PB became a consultant for Medtronic after submission of the revised manuscript.
Author Roles
(1) Manuscript: A. Writing of the first draft, B. Review and Critique.
S.L.: 1A, 1B
P.B.: 1A, 1B
Financial Disclosures of all authors (for the preceding 12 months)
Dr. Brown reports personal fees from Medtronic, outside the submitted work, and became a consultant for Medtronic after submission of the revised manuscript. In addition, Dr. Brown has a patent “Measurement of Electrophysiological Signals During Stimulation of a Target Area of a Body” pending and a patent “Modelling and Emulating Brain Signals for Intelligent Deep-Brain Stimulation (iDBS)” pending. Dr. Little has nothing to disclose.
References
- 1.Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- 2.Kühn AA, Kempf F, Brücke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray NJ, Jenkinson N, Wang S, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Kühn AA, Tsui A, Aziz T, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Ozkurt TE, Butz M, Homburger M, et al. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol. 2011;229:324–331. doi: 10.1016/j.expneurol.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Neumann WJ, Degen K, Schneider GH, et al. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord. 2016;31:1748–1751. doi: 10.1002/mds.26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oswal A, Beudel M, Zrinzo L, et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain. 2016;139:1482–1496. doi: 10.1093/brain/aww048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trager MH, Koop MM, Velisar A, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol Dis. 2016;96:22–30. doi: 10.1016/j.nbd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Steiner LA, Neumann WJ, Staub-Bartelt F, et al. Subthalamic beta dynamics mirror parkinsonian bradykinesia months after neurostimulator implantation. Mov Disord. 2017;32:1183–1190. doi: 10.1002/mds.27068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beudel M, Oswal A, Jha A, et al. Oscillatory beta power correlates with akinesia-rigidity in the parkinsonian subthalamic nucleus. Mov Disord. 2017;32:174–175. doi: 10.1002/mds.26860. [DOI] [PubMed] [Google Scholar]
- 11.Neumann W-J, Staub-Bartelt F, Horn A, et al. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson’s dis-ease. Clin Neurophysiol. 2017;128:2286–2291. doi: 10.1016/j.clinph.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery. 2012;71:804–814. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- 13.Giannicola G, Rosa M, Servello D, et al. Subthalamic local field potentials after seven-year deep brain stimulation in Parkinson’s disease. Exp Neurol. 2012;237:312–317. doi: 10.1016/j.expneurol.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Androulidakis AG, Brucke C, Kempf F, et al. Amplitude modulation of oscillatory activity in the subthalamic nucleus during movement. Eur J Neurosci. 2008;27:1277–1284. doi: 10.1111/j.1460-9568.2008.06085.x. [DOI] [PubMed] [Google Scholar]
- 15.Tinkhauser G, Pogosyan A, Little S, et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain. 2017;140:1053–1067. doi: 10.1093/brain/awx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tinkhauser G, Pogosyan A, Tan H, Herz DM, Kühn AA, Brown P. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain. 2017;140:2968–2981. doi: 10.1093/brain/awx252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deffains M, Iskhakova L, Katabi S, Israel Z, Bergman H. Longer β oscillatory episodes reliably identify pathological subthalamic activity in parkinsonism. Mov Disord. 2018;33:1609–1618. doi: 10.1002/mds.27418. [DOI] [PubMed] [Google Scholar]
- 18.Deffains M, Bergman H. Parkinsonism-related β oscillations in the primate basal ganglia networks–recent advances and clinical implications. Parkinson Rel Disord. 2019;59:2–8. doi: 10.1016/j.parkreldis.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Torrecillos F, Tinkhauser G, Fischer P, et al. Modulation of beta bursts in the subthalamic nucleus predicts motor performance. J Neurosci. 2018;38:8905–8917. doi: 10.1523/JNEUROSCI.1314-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lofredi R, Tan H, Neumann WJ, et al. Beta bursts during continuous movements accompany the velocity decrement in Parkinson’s disease patients. Neurobiol Dis. 2019;127:462–471. doi: 10.1016/j.nbd.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinkhauser G, Torrecillos F, Pogosyan A, et al. The cumulative effect of transient synchrony states on motor performance in Parkinson’s disease. J Neurosci. 2019 doi: 10.1523/JNEUROSCI.1975-19.2019. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18:779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shreve LA, Velisar A, Malekmohammadi M, et al. Subthalamic oscillations and phase amplitude coupling are greater in the more affected hemisphere in Parkinson’s disease. Clin Neurophysiol. 2017;128:128–137. doi: 10.1016/j.clinph.2016.10.095. [DOI] [PubMed] [Google Scholar]
- 25.Wang DD, de Hemptinne C, Miocinovic S, et al. Subthalamic local field potentials in Parkinson’s disease and isolated dystonia: an evaluation of potential biomarkers. Neurobiol Dis. 2016;89:213–222. doi: 10.1016/j.nbd.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Wijk BCM, Pogosyan A, Hariz MI, et al. Localization of beta and high-frequency oscillations within the subthalamic nucleus region. Neuroimage Clin. 2017;16:175–183. doi: 10.1016/j.nicl.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Wijk BCM, Beudel M, Jha A, et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson’s disease. Clin Neurophysiol. 2016;127:2010–2019. doi: 10.1016/j.clinph.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Azcárate J, Tainta M, Rodríguez-Oroz MC, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, van der Meij R, Peterson EJ, de Hemptinne C, Starr PA, Voytek B. Nonsinusoidal beta oscillations reflect cortical pathophysiology in Parkinson’s disease. J Neurosci. 2017;37:4830–4840. doi: 10.1523/JNEUROSCI.2208-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meidahl AC, Moll CK, van Wijk BC, et al. Synchronised spiking activity underlies phase amplitude coupling in the subthalamic nucleus of Parkinson’s disease patients. Neurobiol Dis. 2019;127:101–113. doi: 10.1016/j.nbd.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little S, Pogosyan A, Kuhn AA, Brown P. Beta band stability over time correlates with parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236:383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little SJ, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson’s disease. Ann Neurol. 2013;74:449–457. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little S, Tripoliti E, Beudel M, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016;87:1388–1389. doi: 10.1136/jnnp-2016-313518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:717–721. doi: 10.1136/jnnp-2015-310972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosa M, Arlotti M, Marceglia S, et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov Disord. 2017;32:628–629. doi: 10.1002/mds.26953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arlotti M, Marceglia S, Foffani G, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology. 2018;90:e971–e976. doi: 10.1212/WNL.0000000000005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herz DM, Little S, Pedrosa DJ, et al. Mechanisms underlying decision-making as revealed by deep-brain stimulation in patients with Parkinson’s disease. Curr Biol. 2018;28:1169–1178. doi: 10.1016/j.cub.2018.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iturrate I, Martin S, Chavarriaga R, et al. Beta-driven closed-loop deep brain stimulation can compromise human motor behavior in Parkinson’s disease. Biorxiv. 2019 doi: 10.1101/696385. [published online ahead of print] [DOI] [Google Scholar]
- 39.Chen CC, Pogosyan A, Zrinzo LU, et al. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’s disease surgery. Exp Neurol. 2006;198:214–221. doi: 10.1016/j.expneurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Piña-Fuentes D, van Dijk JMC, de Vries RT, et al. Adaptive Deep Brain Stimulation as an Advanced Parkinson’s Disease Treatment (ADAPT): a pseudorandomised clinical trial. Biorxiv. 2019 doi: 10.1101/749903. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velisar A, Syrkin-Nikolau J, Blumenfeld Z, et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019;12:868–876. doi: 10.1016/j.brs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Ford B, Winfield L, Pullman SL, et al. Subthalamic nucleus stimulation in advanced Parkinson’s disease: blinded assessments at one year follow up. J Neurol Neurosurg Psychiatry. 2004;75:1255–1259. doi: 10.1136/jnnp.2003.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castrioto A, Lozano AM, Poon YY, et al. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol Psychiatry. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 44.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 45.Thompson JA, Tekriwal A, Felsen G, et al. Sleep patterns in Parkinson’s disease: direct recordings from the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2018;89:95–104. doi: 10.1136/jnnp-2017-316115. [DOI] [PubMed] [Google Scholar]
- 46.Afshar P, Khambhati A, Stanslaski S, et al. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2013;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: Summary and meta-analysis of outcomes. Mov Disord. 2006;21:S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 48.Swann NC, de Hemptinne C, Thompson MC, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018;15 doi: 10.1088/1741-2552/aabc9b. 046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qasim SE, de Hemptinne C, Swann NC, Miocinovic S, Ostrem JL, Starr PA. Electrocorticography reveals beta desynchronization in the basal ganglia-cortical loop during rest tremor in Parkinson’s disease. Neurobiol Dis. 2016;86:177–186. doi: 10.1016/j.nbd.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirschmann J, Baillet S, Woolrich M, Schnitzler A, Vidaurre D, Florin E. Spontaneous network activity < 35 Hz accounts for variability in stimulus-induced gamma responses. Neuroimage. 2019;20 doi: 10.1016/j.neuroimage.2019.116374. 116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang SY, Aziz TZ, Stein JF, Liu X. Time-frequency analysis of transient neuromuscular events: dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J Neurosci Meth. 2005;145:151–158. doi: 10.1016/j.jneumeth.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Hirschmann J, Abbasi O, Storzer L, et al. Longitudinal recordings reveal transient increase of alpha/low-beta power in the subthalamic nucleus associated with the onset of parkinsonian rest tremor. Front Neurol. 2019;10:145. doi: 10.3389/fneur.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malekmohammadi M, Herron J, Velisar A, et al. Kinematic adaptive deep brain stimulation for resting tremor in Parkinson’s disease. Mov Disord. 2016;31:426–428. doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]
- 54.Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB, Vitek JL. Closed-loop deep brain stimulation effects on parkinsonian motor symptoms in a non-human primate—is beta enough? Brain Stimul. 2016;9:892–896. doi: 10.1016/j.brs.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doyle LMF, Kühn AA, Hariz M, Kupsch A, Schneider G-H, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s disease. Eur J Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- 56.Devos D, Szurhaj W, Reyns N, et al. Predominance of the contralateral movement-related activity in the subthalamo-cortical loop. Clin Neurophysiol. 2006;117:2315–2327. doi: 10.1016/j.clinph.2006.06.719. [DOI] [PubMed] [Google Scholar]
- 57.Androulidakis AG, Kühn AA, Chen CC, et al. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson’s disease. Brain. 2007;130:457–468. doi: 10.1093/brain/awl358. [DOI] [PubMed] [Google Scholar]
- 58.Anidi C, O’Day JJ, Anderson RW, et al. Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease. Neurobiol Dis. 2018;120:107–117. doi: 10.1016/j.nbd.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan H, Zavala B, Pogosyan A, et al. Human subthalamic nucleus in movement error detection and its evaluation during visuomotor adaptation. J Neurosci. 2014;34:16744–16754. doi: 10.1523/JNEUROSCI.3414-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eusebio A, Thevathasan W, Doyle Gaynor L, et al. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82:569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]