Abstract
Damage to osteochondral (OC) tissues can lead to pain, loss of motility, and progress to osteoarthritis. Tissue engineering approaches offer the possibility of replacing damaged tissues and restoring joint function; however, replicating the spatial and functional heterogeneity of native OC tissue remains a pressing challenge. Chondrocytes in healthy cartilage exist in relatively low-oxygen conditions, while osteoblasts in the underlying bone experience higher oxygen pressures. Such oxygen gradients also exist in the limb bud, where they influence OC tissue development. The cellular response to these spatial variations in oxygen pressure, which is mediated by the hypoxia inducible factor (HIF) pathway, plays a central role in regulating osteo- and chondrogenesis by directing progenitor cell differentiation and promoting and maintaining appropriate extracellular matrix production. Understanding the role of the HIF pathway in OC tissue development may enable new approaches to engineer OC tissue. In this review, we discuss strategies to spatially and temporarily regulate the HIF pathway in progenitor cells to create functional OC tissue for regenerative therapies.
Impact statement
Strategies to engineer osteochondral (OC) tissue are limited by the complex and varying microenvironmental conditions in native bone and cartilage. Indeed, native cartilage experiences low-oxygen conditions, while the underlying bone is relatively normoxic. The cellular response to these low-oxygen conditions, which is mediated through the hypoxia inducible factor (HIF) pathway, is known to promote and maintain the chondrocyte phenotype. By using tissue engineering scaffolds to spatially and temporally harness the HIF pathway, it may be possible to improve OC tissue engineering strategies for the regeneration of damaged cartilage and its underlying subchondral bone.
Keywords: HIF-1α, hypoxia, cartilage, osteochondral tissue engineering
Introduction
Cartilage has a poor capacity for self-repair after injury, which can lead to joint pain, immobility, and eventually osteoarthritis (OA). By repairing cartilage, it may be possible to restore joint function and prevent the development of OA. Tissue engineering (TE), a field whose primary aim is to form new tissue,1 has the potential to revolutionize treatments for cartilage damage. Native cartilage's primary function is to cushion bones and support the smooth movement of articular joints. Cartilage achieves this by seamlessly integrating with its underlying bone. Therefore, cartilage TE strategies often aim to engineer bone and cartilage together to create osteochondral (OC) constructs that can integrate with the supportive subchondral bone. However, engineering such disparate tissues in a single TE construct is challenging as cartilage is dominated by a collagen type II/proteoglycan-rich matrix with embedded chondrocytes, while the underlying bone comprises a mineralized collagen type I structure that is maintained by osteocytes. However, fundamental to both the development and maintenance of native OC tissue is oxygen pressure. Oxygen pressure is low in native cartilage, but higher in subchondral bone. The cellular response to oxygen pressure, which is mediated by the hypoxia inducible factor (HIF) pathway, is central in controlling the differentiation of progenitor cells during development, their production of appropriate extracellular matrix (ECM), and maintenance of their correct phenotype. Therefore, by creating TE scaffolds that can spatially harness the cellular response to oxygen pressure, it may be possible to effectively engineer functional OC tissue. In this study, we review the role of the HIF pathway in OC development and maintenance and discuss its potential for use in OC TE.
The Effect of Oxygen Pressure and the HIF Pathway in OC Tissue Development and Maintenance
The cellular response to oxygen plays an important role in both the development and maintenance of OC tissue2–7 and is primarily mediated through the HIF pathway (Fig. 1). Under normoxic conditions, HIF-1α, the oxygen-responsive subunit of the HIF complex, is continually degraded. However, under hypoxic conditions, HIF-1α accumulates within the cytoplasm and translocates to the nucleus where it regulates expression of target (hypoxia-responsive element, HRE) genes. Moreover, hypoxia enhances the binding between HIF-1α and its transcriptional cofactors, which further augments HIF complex-mediated regulation of gene expression.8 During development, the HIF pathway plays fundamental roles in directing the differentiation of OC progenitors. In general, increased HIF-1α stabilization (under low-oxygen conditions) stimulates a prochondrogenic, antiosteogenic, and antihypertrophic transcriptome.9 This effect is reversed in the presence of higher oxygen concentrations as HIF-1α is degraded, which promotes a more hypertrophic/osteoblastic fate.
FIG. 1.
HIF pathway-mediated regulation of OC progenitor cells. Under hypoxic conditions, the HIF complex binds to its response elements (HREs) on target genes (A) where it acts to enhance SOX9 expression and activity (B), resulting in increased production of cartilage ECM (C), dampened activity of RUNX2 (D), and reduced expression of genes involved in osteogenesis (E) and chondrocyte hypertrophy (F). HIF has also been shown to increase expression of glycolytic enzymes (G) as well as Wnt antagonists involved in delaying chondrocyte hypertrophy (H). Yellow arrows indicate pathways/genes that are activated, while red arrows show pathways/genes that are inhibited under hypoxic conditions. ECM, extracellular matrix; HIF, hypoxia inducible factor; HRE, hypoxia-responsive element; OC, osteochondral. Color images are available online.
The Role of HIF-1α in Differentiation of OC Progenitor Cells, Their Survival, and Phenotypic Maintenance
During development, articular cartilage forms during endochondral ossification,10 the process by which the axial skeleton is created. To achieve this, a condensed population of mesenchymal precursors form the initial cartilaginous anlage, which is subsequently infiltrated by the vasculature and ossified.11 Concomitant with this process, cartilage is maintained at the ends of long bones and within it, a population of chondrocytes, which will go on to form the articular cartilage. HIF-1α is essential in this process as under the low-oxygen conditions of the developing growth plate, knockdown of HIF-1α results in chondrocyte cell death.2 Moreover, knockdown of the enzyme required for HIF-1α degradation in the growth plate deregulates mesenchymal precursor and chondrocyte proliferation12,13 and its conditional inactivation in the developing mouse limb bud mesenchyme negatively impacts both the formation of cartilage and joint development.7 When HIF-1α is conditionally inactivated, expression of SOX9, the master transcriptional regulator of chondrogenesis, and its downstream targets, the genes that encode collagen type II and aggrecan, the main constituents of cartilage tissue, are all reduced.7,9 In vitro activation of the HIF pathway has similarly been shown to upregulate SOX9 expression and that of its downstream targets in murine14 and rat15 mesenchymal stromal cells (MSCs) as well as in human articular chondrocytes (hACs).16 Furthermore, engineering murine MSCs to stably express HIF-1α under normoxic conditions has been shown to potentiate their BMP2-induced chondrogenic differentiation.17
In addition to driving the differentiation of progenitors, hypoxia and HIF-1α also play a role in maintaining cells' chondrogenic phenotypes by preventing their hypertrophic or osteogenic differentiation. During endochondral ossification, signaling gradients, including those triggered by oxygen pressure, are responsible for retaining populations of chondrocytes in their nonhypertrophic state, priming them for a permanent, articular chondrocyte fate.18 Hypoxia and HIF-1α achieve this in hACs by downregulating the expression of hypertrophic fibroblast-like markers such as COL1A1 and COL3A1.19 Moreover, hypoxia suppresses the expression of matrix metalloproteinases (MMPs) and aggrecanases in hACs, both of which degrade the cartilage matrix.20 Similarly, human and other mammalian cartilage explants cultured under hypoxic conditions show HIF-1α-mediated suppression of cartilage catabolism by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-5) and MMP-13.21 The ability of HIF signaling to promote a stable articular phenotype is also supported by observations that hypoxia enhances the expression of antihypertrophic Wnt antagonists,22 and HIF-1α conditional knockout in developing cartilage results in reduced expression of Wnt9a and GDF5.9
The Role of HIF-1α in Cartilage ECM Formation
Not only do physiological hypoxia and the HIF pathway play important roles in regulating the differentiation of OC progenitor cells but they also appear to drive the formation of appropriate ECM. The ECM of native cartilage is dominated by a combination of collagen type II and proteoglycans, and hypoxia and the HIF pathway have been shown to regulate the formation of this matrix (Fig. 2). For example, physiological hypoxia enhances the production of cartilage-specific ECM in cultured hACs when compared with that formed under normoxic conditions.23 Similarly, hACs in pellet cultures synthesize collagen fibrils with more ordered morphologies when cultured in 5% oxygen compared with under normoxic conditions.16,21,23 Similar observations have been made in chondrocytes embedded in alginate hydrogels24 and seeded on 3D PLGA scaffolds.25 Moreover, human bone marrow-derived MSCs and hACs pretreated with hypoxia before encapsulation in alginate and implantation in a nude mouse model showed enhanced cartilage ECM formation compared with that observed when cells were precultured under normoxic conditions.26
FIG. 2.
Oxygen, HIF-1α, transcription factor, and ECM gradients in OC tissue. Oxygen pressure is low in native cartilage, but high in the subchondral bone. The cellular response to these varying oxygen pressures, which are mediated by HIF-1α, plays a role in regulating transcription factors important in progenitor cell differentiation toward chondrogenic and osteogenic phenotypes and in promoting the production of proteins that are abundant in cartilage compared with bone. (a) A histological section of the OC tissue from a rabbit metacarpophalangeal joint stained with hematoxylin, Safranin O, and fast green. Cartilage appears red, calcified cartilage appears dark green, and bone appears blue. Image is adapted from106 (CC-BY 2.0). (b) A schematic of OC tissue showing expression gradients of HIF-1α, transcription factors, proteins, and enzymes. Factors in blue are upregulated or promoted in response to low oxygen pressures, while those in red are downregulated.2,107–112 col II, collagen type II; acan, aggrecan; col I, collagen type I; col X, collagen type X. Color images are available online.
Regulation of ECM synthesis is dependent on the activity of HIF-1α as its conditional knockdown in developing murine limbs results in abnormal ECM morphology as well as reduced production of proteoglycans and collagen type II in the growth plate.7,9,27 Similarly, stabilizing HIF-1α chemically by inhibiting its ubiquitination and degradation causes hACs to increase their secretion of collagen type II and aggrecan.28 Hypoxia has also been shown to increase cartilage-like ECM production in MSCs.29 Indeed, delivery of a HIF-1α expression vector enhances the expression of genes for both collagen type II and the proteoglycan aggrecan, as well as a panel of chondrogenic transcription factors.26 Moreover, stabilization of HIF-1α promotes the formation of cartilage-specific ECM by both upregulating the expression of SOX9 and through post-translational modifications to collagen type II.30 HIF-1α's role in regulating ECM production is thought to be mediated by its regulation of collagen prolyl 4-hydroxylase, which is required for the addition of 4-hydroxyproline residues to collagen fibrils, allowing them to form triple helices.30 In addition, the HIF pathway is also thought to control expression of lysyl oxidase, an enzyme required for the cross-linking of collagen triple helices.31,32
Strategies for OC TE
A common approach to engineer cartilage tissue is to mimic aspects of the native, in vivo cellular microenvironment in 3D scaffolds seeded with appropriate progenitor cells.33–35 A clinical example of this is matrix-assisted, autologous chondrocyte transplantation/implantation (MACT/MACI), which involves seeding autologous chondrocytes in a 3D scaffold before surgical implantation. However, despite providing chondrocytes with a 3D environment,36 the MACT matrix often does not adequately mimic many microenvironmental conditions within native cartilage, including local oxygen pressure. Moreover, integration with the underlying subchondral bone remains an issue.
Because of these drawbacks, researchers have attempted to engineer OC tissue that can seamlessly integrate with the underlying subchondral bone. This can be accomplished either by engineering a monophasic cartilage construct and then relying on the native local environment to drive ossification at the bone interface or by engineering a bone–cartilage construct that contains both tissues before implantation. Monophasic cartilage TE approaches have been applied widely. For example, Koga et al. created cartilage using synovium-derived MSCs. When implanted in a rabbit model, MSCs produced extensive cartilage matrix, while cells adjacent to the subchondral bone differentiated into osteoblasts.37 However, such endogenous processes are uncontrolled, and movement of the tidemark, the interface between the bone and the cartilage, has been observed. Therefore, others have attempted to create OC constructs that contain either bone and cartilage or bone, cartilage, and an interfacial region. Engineering a single construct that contains bone and cartilage, however, remains challenging because of the tissues' distinct compositions, architectures, and cellular microenvironments. One strategy to address this is to create biphasic scaffolds formed from separate osteogenic and chondrogenic constructs that are later combined. Such strategies have been tested in vitro38 and in large animal models.39 However, unlike in native OC tissue in which a calcified hypertrophic zone exists between the bone and cartilage, in many biphasic scaffolds, an abrupt artificial interface may form, which may impact the construct's mechanical integrity. For example, when Grayson et al. synthesized a biphasic construct by placing agarose within a decellularized bone scaffold, they reported the absence of a hypertrophic transition zone.40
Researchers have also attempted to form triphasic scaffolds, which contain an interfacial region between the cartilage and bone scaffolds, to more faithfully recapitulate the native OC interface. For example, Da et al. formed a compact interfacial layer by placing poly(lactic-co-glycolic acid)-β-tricalcium phosphate between the chondrogenic and bony components of a biphasic scaffold. They observed enhanced mechanical properties in the interface-containing scaffolds compared with those that lacked the interfacial region, as well as increased OC tissue regeneration in a rabbit model.41 Similarly, Kon et al. formed triphasic scaffolds by varying the ratios of type I collagen and hydroxyapatite in their constructs. When tested in 15 patients with cartilage lesions, they were able to demonstrate safety and short-term follow-up appeared promising.42 Nevertheless, like biphasic scaffolds, triphasic scaffolds may still not fully recapitulate the native tissue's seamless interface and thus may separate in vivo. This has been observed in polycaprolactone/alginate scaffolds upon subcutaneous implantation in a rat model, where the osteogenic and chondrogenic portions often became separated.43 Moreover, biphasic and triphasic scaffolds may require separate chondrogenic and osteogenic culture conditions, which may create logistical challenges for their scale-up and clinical use.
Alternatives to multiphasic scaffolds are continuous OC scaffolds designed to enable synchronous formation of both cartilage and bone with a seamless transition, mimicking the calcified hypertrophic interface in native OC tissue. Continuous scaffolds may also preclude the need for separate culture conditions as they can be designed to differentiate a single progenitor population down different lineages depending on location within the biomaterial construct. For example, Harley et al. created continuous OC scaffolds by lyophilizing mineralized and unmineralized type I collagen–glycosaminoglycan suspensions to form a natural interface.44 Researchers have also achieved continuous scaffolds by creating morphogen gradients. For example, Wang et al. utilized BMP2 and IGF-1-containing microspheres to create inverse gradients in alginate hydrogels and observed corresponding differentiation of encapsulated human MSCs (hMSCs) down chondrogenic and osteogenic lineages.45 Similarly, Mohan et al. utilized inverse gradients of microspheres containing BMP2 and TGF-β1. When implanted in a rat femoral defect model, they showed region-specific regeneration of cartilage and bone and formation of a stable interface.46
Exploiting the HIF-1α Regulatory Network for OC TE
As oxygen gradients form during OC tissue development and aid in the maintenance of OC tissue in the adult, controlling oxygen pressure may be an effective strategy to engineer OC tissue. Researchers have described strategies to locally regulate oxygen pressure in situ within biomaterials. For example, oxygen-releasing molecules such as perfluorocarbons47 and hemoglobin48 or myglobin49 can be incorporated into biomaterials to increase local oxygen levels, using strategies amenable for OC TE. Similarly, manganese dioxide nanoparticles50 and calcium peroxide51 can be used to generate oxygen within a TE construct. Alternatively, oxygen scavengers can mediate the opposite effect and lower local levels of oxygen. Indeed, it is possible to locally decrease the oxygen pressure within a biomaterial either by incorporating various molecules52 or simply by limiting oxygen diffusion, which has been shown to stimulate the chondrogenesis of progenitor cells.53
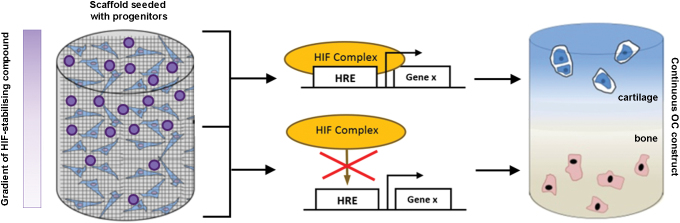
However, regulating oxygen itself may not be ideal as hypoxia is also known to cause oxidative stress, prompt potentially undesirable effects on cell metabolism, and negatively impact cell growth and viability,54 all of which may be detrimental to forming tissue. An alternative approach is to stabilize HIF-1α under normoxic conditions as this has the potential to provide the beneficial prochondrogenic effects of hypoxia, but in a more controlled and potentially less deleterious manner. Indeed, as stabilization of HIF-1α enhances the chondrogenic differentiation of progenitor cells,55 minimizes chondrocyte hypertrophy, and stimulates the production of cartilage-like ECM, manipulating its regulatory network within TE scaffolds may be an effective strategy to engineer OC tissue. A number of compounds have been reported to ectopically stabilize HIF-1α at normoxia and thus stimulate cellular responses that mimic those elicited by low oxygen pressure. Therefore, by incorporating these HIF mimetics into the chondrogenic region of a TE scaffold, it may be possible to stimulate progenitor cells to undergo region-specific formation of articular cartilage (Fig. 3). To accomplish this, appropriate components of the HIF complex that can be targeted pharmacologically need to be recognized and compounds that act against them identified and incorporated into scaffolds.
FIG. 3.
Exploiting the HIF pathway in OC TE. An example of a progenitor-seeded biomaterial scaffold containing a gradient of an HIF-1α-stabilizing compound. During differentiation and tissue formation, the differential levels of the HIF-1α-stabilizing agent promote a continuous interface that mimics that in native OC tissue. The resulting continuous OC construct would then contain spatially restricted regions of articular cartilage and subchondral bone. TE, tissue engineering. Color images are available online.
Regulation of the HIF Transcriptional Complex
The α subunit of HIF exists in two forms—HIF-1α and HIF-2α—both of which play roles in the regulation of cartilage formation. Knockout of HIF-1α results in cell death and cartilage catabolism in the developing limb bud mesenchyme,2,56 and suppression of HIF-1α negatively impacts the production of cartilage-associated matrix proteins in cultured chondrocytes. HIF-2α, on the other hand, regulates endochondral ossification by mediating angiogenesis and ossification of the cartilage template56 and plays important roles in cartilage degradation during OA.57 Therefore, because of HIF-1α's central role in maintaining the chondrocyte phenotype and cartilage ECM,30 it is the more obvious target for OC TE strategies.
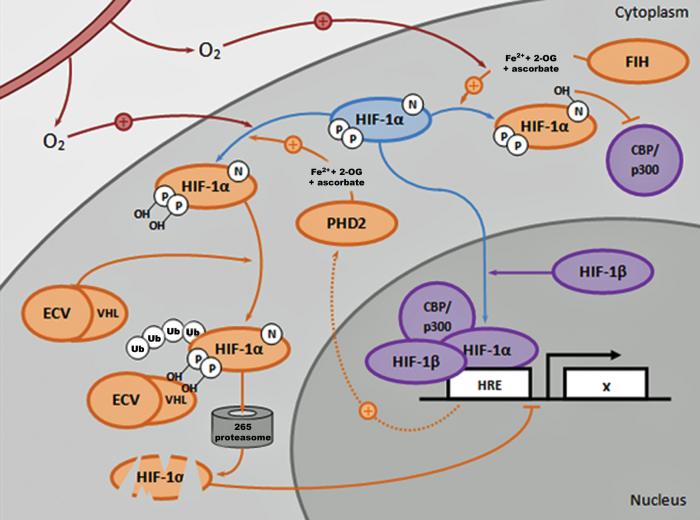
Central to regulation of HIF-1α is the prolyl hydroxylase 2–von Hippel-Lindau (PHD2-VHL) signaling cascade58 (Fig. 4). At normoxia, PHD2 utilizes molecular oxygen and other cofactors to hydroxylate residues on the oxygen-dependent degradation domain (ODDD) of HIF-1α.59 The hydroxylated residues then serve as recognition motifs by the VHL tumor suppressor protein. As part of the E3 ubiquitin ligase complex, VHL binds and ubiquitinates the hydroxylated residues of HIF-1α, targeting the molecule for degradation by the proteasome.60–62 However, under hypoxic conditions, PHD2's ability to hydroxylate HIF-1α is diminished, enabling its cytosolic accumulation and nuclear translocation, where together with transcriptional cofactors, it activates expression of its target genes in the HIF complex.63 Other pathways central to regulating HIF-1α degradation are RACK1 and HSP90. HSP90 normally binds to HIF-1α, thus preventing its degradation. However, RACK1 can compete with HSP90 in its binding to HIF-1α and, in doing so, recruits the same ubiquitinating complex utilized by VHL, thereby resulting in PHD2/VHL-independent HIF-1α degradation.64–66
FIG. 4.
The PHD2-VHL signaling cascade: an opportunity to regulate the HIF pathway. In response to molecular oxygen (O2), FIH and PHD2 (in the presence of Fe2+, 2-OG, and ascorbate) hydroxylate specific amino acid residues on HIF-1α. FIH-mediated hydroxylation blocks cofactors CBP/p300 from binding to HIF-1α, thereby reducing HIF transcriptional activity. PHD2-mediated hydroxylation results in ubiquitination of the ODDD domain of HIF-1α by the VHL component of the ECV (elongin/culin/VHL) ubiquitin ligase complex, thus promoting degradation of HIF-1α in the 265 proteasome and reducing HIF transcriptional activity. Under hypoxic conditions, PHD2 and FIH activity are reduced, thus enabling HIF-1α to translocate and accumulate in the nucleus, where it activates expression of HIF target genes in the HRE as part of a transcriptional complex with HIF-1β, CBP/p300, and other cofactors. A negative feedback mechanism exists in which PHD2 expression is also enhanced by HIF activity. CBP, CREB-binding protein; FIH, factor inhibiting HIF; 2-OG, 2-oxoglutarate; ODDD, oxygen-dependent degradation domain; PHD2-VHL, prolyl hydroxylase 2–von Hippel-Lindau. Color images are available online.
In addition to the PHD2-VHL pathway, HIF-1α also requires cofactors to be recruited to the HIF transcriptional complex to activate gene expression when bound to the HRE in target gene promoters. Two important factors in this complex are p300 and the CREB-binding protein (CBP).67 One key residue on HIF-1α involved in its binding with p300/CBP is asparagine-803 (Asn-803).8 Indeed, Asn-803 is also the target of another 2-oxoglutarate (2-OG)-utilizing hydroxylase, factor inhibiting HIF (FIH), which similarly regulates HIF transcriptional activity.8 FIH hydroxylates Asn-803 on HIF-1α, preventing the binding of p300/CBP to HIF-1α, and therefore disrupts the formation of a functional HIF transcriptional complex.8
Harnessing the HIF Pathway for OC TE by Stabilizing HIF-1α
Over the past two decades, there has been tremendous interest in identifying compounds that are able to stabilize HIF-1α and enhance its binding by transcriptional cofactors at normoxia (Fig. 5) for potential use as therapeutic agents to treat a range of conditions. The most common HIF mimetics include dimethyloxalylglycine (DMOG), desferrioxamine (DFX), and cobalt chloride (CoCl2), all of which target PHD2 and/or FIH.68,69 By targeting PHD2 and FIH, HIF mimetics reduce HIF-1α's prolyl and asparagine hydroxylation, reducing its subsequent degradation, and enhance its binding by transcriptional cofactors. DMOG acts through competition with 2-OG by engaging the binding pocket of the prolyl hydroxylase active site on both FIH and PHD2.70 DFX is an iron chelator and sequesters available Fe2+, which is required by both FIH and PHD2, thereby reducing their activity.71 CoCl2, on the other hand, may directly compete with Fe2+ binding to the PHD2 active site.72
FIG. 5.
Pharmacological strategies to manipulate the HIF pathway. (A) High-density lipoproteins regulate HIF-1α levels through activation of Siah2, an E3 ubiquitin ligase, which targets PHD2 for proteasomal degradation. (B) Blocking binding of the VHL component of the E3 ubiquitin ligase to HIF-1α, following its proline hydroxylation, reduces ubiquitination and degradation. This can be achieved by molecules that bind and occupy the HIF-1α binding site of VHL or through the use of nitric oxide donors, which chemically modify VHL or the ODDD of HIF-1α. (C) HIF-1α hydroxylation reactions can be inhibited to reduce the subsequent ubiquitination and degradation of HIF-1α. For example, hydroxylation can be inhibited by specific hydroxylase inhibitors such as cofactor analogs, agents that sequester the Fe2+ that is required at the hydroxylase active sites, or ROS, which downregulate expression of PHD2. (D) HIF hydroxylases are susceptible to downregulation at the transcriptional level by interfering RNA molecules, which reduce their translation and thus HIF-1α hydroxylation. Asn803, asparagine-803; pro564/pro402, proline564/proline402; ROS, reactive oxygen species. Color images are available online.
Recent work to compare how DFX, DMOG, and CoCl2 impact hMSC chondrogenesis in vitro showed that DMOG upregulated expression of HIF target genes and induced a more chondrogenic transcriptional profile compared with either DFX or CoCl2.55 These observations suggest that hMSC chondrogenesis may be regulated by mechanisms with a greater dependence on 2-OG than Fe2+ availability and suggest that compounds that target 2-OG may be more effective for OC TE. These findings are supported by observations that cobalt, when released from a bioactive glass, reduces hMSC chondrogenic differentiation despite stabilizing HIF-1α.73 Sathy et al. have since exploited the hypoxia-mimicking properties of DMOG for cartilage TE by placing it within porcine-MSC-laden alginate hydrogels. They showed that DMOG-containing constructs enhanced MSC chondrogenesis in vitro and cartilage-like tissue formation in vivo.74 However, the HIF mimetic type, specificity, concentration, and duration of exposure may also play roles in their efficacy in promoting chondrogenesis, as highlighted by conflicting results in the literature. Indeed, while cobalt has been shown to promote chondrogenesis,75 others have demonstrated that it inhibits chondrogenesis73 and that this may be dependent on cell source.76
Nevertheless, although promising, the three most widely tested HIF mimetics lack a high degree of specificity for PHD2 FIH. Indeed, DMOG may also target similarly structured enzymes that are essential for formation of the collagen triple helix.77 Similarly, chelating Fe2+ ions or displacing them in enzymatic reactions lends a poor degree of control as iron is central in a range of other vital biological processes, including the mitochondrial respiratory chain or PHDs involved in collagen synthesis.78 More recently, screens have identified additional 2-OG inhibitors,79 including Kreb's cycle metabolites80,81 and metal chelators,82 some of which have been tested in clinical trials.83 GSK360A, for example, has been shown to improve ventricular remodeling following myocardial infarction,84 and FibroGen's FG-2216 alleviates erythropoietin deficiency in various anemic conditions.85 It will be particularly interesting to learn how these compounds influence cell behavior in OC TE applications.
Alternative approaches can also be used to ectopically stabilize HIF-1α by targeting the PHD2-VHL pathway. For example, high-density lipoproteins (HDLs) enhance HIF-dependent VEGF signaling through regulation of HIF-1α post-translational modification.86,87 HDLs act through activation of the ubiquitin ligase Siah2,88 which (when active) inhibits PHD2/PHD3, leading to HIF-1α accumulation.87,89 PHD2/FIH inhibitors designed to mimic cofactors that act with hydroxylases or interfering RNA molecules are promising tools in this regard as they can specifically target PHD2 and FIH. Indeed, RNAi against PHD228,90 and native hypoxia-driven microRNA91,92 have been shown to enhance HIF-1α stabilization.
Nitric oxide (NO) has also been implicated as a regulator of HIF-1α,93 an effect that can also be induced by NO donors such as GSNO, SNAP, NAC, and DetaNONOATE, which similarly increase intracellular HIF-1α levels.94 These compounds modify the HIF-1α ODDD through N-nitrosylation and, in doing so, block VHL binding and subsequent HIF-1α ubiquitination.95,96 GSNO has also been shown to inhibit PHD2 and FIH activity, at least in part, by blocking the binding of Fe2+ to the active site, leading to similar levels of HIF-1α stabilization as those observed in CoCl2-treated cell cultures.97,98 SNAP similarly promotes HIF-dependent gene expression by inhibiting VHL-HIF-1α binding and FIH activity,99 and NAC has been shown to mimic the physiological effect of chronic hypoxia in murine, vascular, pulmonary endothelial cells through nitrosylation of proteins in the PHD2/VHL pathway.100 Nevertheless, like the HIF mimetics, targeting NO for OC TE is not specific as NO has a variety of biological roles.
Researchers have also attempted to target the HIF-1α-regulating effects of VHL.101 Peptides that mimic the hydroxylated ODDD of HIF-1α compete with native HIF-1α to bind to VHL, reducing HIF-1α ubiquitination.102,103 This is a particularly promising approach for OC TE as the peptide-based inhibitors can be highly specific. Calcium signaling is also a potential target. A calcium ionophore, which facilitates Ca2+ entry into the cell, has been shown to inhibit dimerization and activation of RACK1, thereby inhibiting its role in HIF-1α degradation.104 An alternative to reducing HIF-1α degradation is augmenting HIF-1α translation. Calcium ionophores or a calcium compound may tap into calcium's role in HIF-1α translation. However, the use of factors that enhance HIF-1α translation may be most effective when used in combination with those that inhibit HIF-1α degradation to increase overall levels of HIF-1α and increase biological function of the HIF transcriptional complex.
Outlook
As hypoxia plays fundamental roles in development and maintenance of OC tissue, attempting to mimic its effects on progenitor cells may be an effective means to engineer OC tissue. Indeed, a biomaterial that is able to spatially control the intracellular stabilization and cofactor binding of HIF-1α may stimulate region-specific formation of articular cartilage where HIF-1α is active, while promoting the formation of a subchondral bone region where HIF-1α activity is depleted, all within a single construct cultured under normoxic conditions. Stabilization of HIF-1α at normoxia can be achieved by using HIF mimetics such as DMOG, DFX, and CoCl2. However, targeting the native regulatory signaling pathways that control intracellular levels of HIF-1α, such as the ODDD domain of HIF-1α, using peptides or RNAi may be an even more effective means to control intracellular levels of HIF-1α.
Utilizing HIF mimetics for OC TE will likely require them to be stably incorporated into scaffolds in a regional or gradient manner and for their controlled release. This could be achieved by tethering HIF mimetics directly to the scaffold or by incorporating soluble factors within degradable microspheres, whose locations within the scaffold are spatially controlled.45 Light-based chemistries could also be used to locally attach a HIF mimetic to a scaffold by applying differential levels of UV light along the length of a presynthesized biomaterial.105 Indeed, strategies to achieve localized delivery of HIF mimetics are already within reach and thus can be quickly incorporated into OC TE designs with the potential to deliver on the promise of OC TE to repair cartilage lesions and prevent OA.
Disclosure Statement
No competing financial interests exist.
Funding Information
D.K.T. acknowledges a PhD studentship from Orthopaedic Research United Kingdom and support from the Rosetrees Trust. E.G. was supported by a Research Career Development Fellowship from the Wellcome Trust (WT093687) and a Philip Leverhulme Prize from the Leverhulme Trust.
References
- 1. Foyt D.A., Norman M.D.A., Yu T.T.L., and Gentleman E.. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv Healthc Mater 7, e1700939, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schipani E., Ryan H.E., Didrickson S., et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15, 2865, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hatta T., Kishimoto K.N., Okuno H., and Itoi E.. Oxygen tension affects lubricin expression in chondrocytes. Tissue Eng Part A 20, 2720, 2014 [DOI] [PubMed] [Google Scholar]
- 4. Gelse K., Pfander D., Obier S., et al. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res Ther 10, R111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leijten J., Georgi N., Moreira Teixeira L., et al. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A 111, 13954, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maes C., Carmeliet G., and Schipani E.. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 8, 358, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Provot S., Zinyk D., Gunes Y., et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol 177, 451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freedman S.J., Sun Z.Y., Poy F., et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A 99, 5367, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amarilio R., Viukov S.V., Sharir A., et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 134, 3917, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Mackie E.J., Tatarczuch L., and Mirams M.. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol 211, 109, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Long F., and Linsenmayer T.F.. Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development 125, 1067, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Pfander D., Kobayashi T., Knight M.C., et al. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 131, 2497, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Mangiavini L., Merceron C., Araldi E., et al. Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev Biol 393, 124, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robins J.C., Akeno N., Mukherjee A., et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 37, 313, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kanichai M., Ferguson D., Prendergast P.J., and Campbell V.A.. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol 216, 708, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Lafont J.E., Talma S., and Murphy C.L.. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum 56, 3297, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Zhou N., Hu N., Liao J.Y., et al. HIF-1alpha as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell Physiol Biochem 36, 44, 2015 [DOI] [PubMed] [Google Scholar]
- 18. Nilsson O., Parker E.A., Hegde A., et al. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol 193, 75, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Duval E., Leclercq S., Elissalde J.M., et al. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum 60, 3038, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Markway B.D., Cho H., and Johnstone B.. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res Ther 15, R92, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thoms B.L., Dudek K.A., Lafont J.E., and Murphy C.L.. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum 65, 1302, 2013 [DOI] [PubMed] [Google Scholar]
- 22. Leijten J.C., Emons J., Sticht C., et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum 64, 3302, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Strobel S., Loparic M., Wendt D., et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res Ther 12, R34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coyle C.H., Izzo N.J., and Chu C.R.. Sustained hypoxia enhances chondrocyte matrix synthesis. J Orthop Res 27, 793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan G.K., Dinnes D.L., Myers P.T., and Cooper-White J.J.. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials 32, 5600, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Duval E., Bauge C., Andriamanalijaona R., et al. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 33, 6042, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Pfander D., Cramer T., Schipani E., and Johnson R.S.. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci 116, 1819, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Thoms B.L., and Murphy C.L.. Inhibition of hypoxia-inducible factor-targeting prolyl hydroxylase domain-containing protein 2 (PHD2) enhances matrix synthesis by human chondrocytes. J Biol Chem 285, 20472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foyt D.A., Taheem D.K., Ferreira S.A., et al. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater 89, 73, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aro E., Khatri R., Gerard-O'Riley R., et al. Hypoxia-inducible factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction of collagen prolyl 4-hydroxylases in primary newborn mouse epiphyseal growth plate chondrocytes. J Biol Chem 287, 37134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makris E.A., Hu J.C., and Athanasiou K.A.. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21, 634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makris E.A., Responte D.J., and Paschos N.K.. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A 111, E4832, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCullen S.D., Autefage H., Callanan A., Gentleman E., and Stevens M.M.. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng Part A 18, 2073, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamaddon M., Burrows M., Ferreira S.A., et al. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci Rep 7, 43519, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Undt G., Jahl M., Pohl S., et al. Matrix-associated chondrocyte transplantation for reconstruction of articulating surfaces in the temporomandibular joint: a pilot study covering medium- and long-term outcomes of 6 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 126, 117, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kon E., Filardo G., Di Matteo B., Perdisa F., and Marcacci M.. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res 2, 18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koga H., Muneta T., Ju Y.J., et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 25, 689, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Lam J., Lu S., Meretoja V.V., et al. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater 10, 1112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swieszkowski W., Tuan B.H., Kurzydlowski K.J., and Hutmacher D.W.. Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng 24, 489, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Grayson W.L., Bhumiratana S., Grace Chao P.H., Hung C.T., and Vunjak-Novakovic G.. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage 18, 714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Da H., Jia S.J., Meng G.L., et al. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS One 8, e54838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kon E., Delcogliano M., Filardo G., et al. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury 41, 693, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Jeon J.E., Vaquette C., Theodoropoulos C., Klein T.J., and Hutmacher D.W.. Multiphasic construct studied in an ectopic osteochondral defect model. J R Soc Interface 11, 20140184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harley B.A., Lynn A.K., Wissner-Gross Z., et al. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res Part A 92, 1078, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Wang X., Wenk E., Zhang X., et al. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release 134, 81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohan N., Dormer N.H., Caldwell K.L., et al. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A 17, 2845, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Chin K., Khattak S.F., Bhatia S.R., and Roberts S.C.. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol Prog 24, 358, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Paciello A., Amalfitano G., Garziano A., Urciuolo F., and Netti P.A.. Hemoglobin-conjugated gelatin microsphere as a smart oxygen releasing biomaterial. Adv Healthc Mater 5, 2655, 2016 [DOI] [PubMed] [Google Scholar]
- 49. Armstrong J.P.K., Shakur R., Horne J.P., et al. Artificial membrane-binding proteins stimulate oxygenation of stem cells during engineering of large cartilage tissue. Nat Commun 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim J., Kim H.Y., Song S.Y., et al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13, 3206, 2019 [DOI] [PubMed] [Google Scholar]
- 51. Alemdar N., Leijten J., Camci-Unal G., et al. Oxygen-generating photo-cross-linkable hydrogels support cardiac progenitor cell survival by reducing hypoxia-induced necrosis. ACS Biomater Sci Eng 3, 1964, 2017 [DOI] [PubMed] [Google Scholar]
- 52. Sthijns M.M.J.P.E., van Blitterswijk C.A., and LaPointe V.L.S.. Redox regulation in regenerative medicine and tissue engineering: the paradox of oxygen. J Tissue Eng Regen Med 12, 2013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo L., O'Reilly A.R., Thorpe S.D., Buckley C.T., and Kelly D.J.. Engineering zonal cartilaginous tissue by modulating oxygen levels and mechanical cues through the depth of infrapatellar fat pad stem cell laden hydrogels. J Tissue Eng Regen Med 11, 2613, 2017 [DOI] [PubMed] [Google Scholar]
- 54. Majmundar A.J., Wong W.J., and Simon M.C.. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40, 294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taheem D.K., Foyt D.A., Loaiza S., et al. Differential regulation of human bone marrow mesenchymal stromal cell chondrogenesis by hypoxia inducible factor-1alpha hydroxylase inhibitors. Stem Cells 36, 1380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Araldi E., Khatri R., Giaccia A.J., Simon M.C., and Schipani E.. Lack of HIF-2alpha in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat Med 17, 25, 2011; author reply 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang S., Kim J., Ryu J.H., et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med 16, 687, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Dery M.A., Michaud M.D., and Richard D.E.. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37, 535, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Berra E., Benizri E., Ginouves A., et al. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22, 4082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanimoto K., Makino Y., Pereira T., and Poellinger L.. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J 19, 4298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cockman M.E., Masson N., Mole D.R., et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 275, 25733, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Maxwell P.H., Wiesener M.S., Chang G.W., et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271, 1999 [DOI] [PubMed] [Google Scholar]
- 63. Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Science's STKE 2007, cm8, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Isaacs J.S., Jung Y.J., and Neckers L.. Aryl hydrocarbon nuclear translocator (ARNT) promotes oxygen-independent stabilization of hypoxia-inducible factor-1alpha by modulating an Hsp90-dependent regulatory pathway. J Biol Chem 279, 16128, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Mabjeesh N.J., Post D.E., Willard M.T., et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res 62, 2478, 2002 [PubMed] [Google Scholar]
- 66. Liu Y.V., Baek J.H., Zhang H., et al. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell 25, 207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arany Z., Huang L.E., Eckner R., et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A 93, 12969, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nagel S., Talbot N.P., Mecinovic J., et al. Therapeutic manipulation of the HIF hydroxylases. Antioxid Redox Signal 12, 481, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Holden P., and Nair L.S.. Deferoxamine: an angiogenic and antioxidant molecule for tissue regeneration. Tissue Eng Part B Rev, 2019 [DOI] [PubMed] [Google Scholar]
- 70. Nguyen L.K., Cavadas M.A., Scholz C.C., et al. A dynamic model of the hypoxia-inducible factor 1alpha (HIF-1alpha) network. J Cell Sci 126, 1454, 2013 [DOI] [PubMed] [Google Scholar]
- 71. Wang G.L., and Semenza G.L.. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82, 3610, 1993 [PubMed] [Google Scholar]
- 72. Tian Y.M., Yeoh K.K., Lee M.K., et al. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J Biol Chem 286, 13041, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Littmann E., Autefage H., Solanki A.K., et al. Cobalt-containing bioactive glasses reduce human mesenchymal stem cell chondrogenic differentiation despite HIF-1alpha stabilisation. J Eur Ceram Soc 38, 877, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sathy B.N., Daly A., Gonzalez-Fernandez T., et al. Hypoxia mimicking hydrogels to regulate the fate of transplanted stem cells. Acta Biomater 88, 314, 2019 [DOI] [PubMed] [Google Scholar]
- 75. Li H., Li X.J., Jing X.Z., et al. Hypoxia promotes maintenance of the chondrogenic phenotype in rat growth plate chondrocytes through the HIF-1 alpha/YAP signaling pathway. Int J Mol Med 42, 3181, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Teti G., Focaroli S., Salvatore V., et al. The hypoxia-mimetic agent cobalt chloride differently affects human mesenchymal stem cells in their chondrogenic potential. Stem Cells Int 2018, 3237253, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol 22, 15, 2003 [DOI] [PubMed] [Google Scholar]
- 78. Gorres K.L., and Raines R.T.. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol 45, 106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chowdhury R., Candela-Lena J.I., Chan M.C., et al. Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. ACS Chem Biol 8, 1488, 2013 [DOI] [PubMed] [Google Scholar]
- 80. Serra-Perez A., Planas A.M., Nunez-O'Mara A., et al. Extended ischemia prevents HIF1alpha degradation at reoxygenation by impairing prolyl-hydroxylation: role of Krebs cycle metabolites. J Biol Chem 285, 18217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koivunen P., Hirsila M., Remes A.M., et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 282, 4524, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Flagg S.C., Martin C.B., Taabazuing C.Y., Holmes B.E., and Knapp M.J.. Screening chelating inhibitors of HIF-prolyl hydroxylase domain 2 (PHD2) and factor inhibiting HIF (FIH). J Inorg Biochem 113, 25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beuck S., Schanzer W., and Thevis M.. Hypoxia-inducible factor stabilizers and other small-molecule erythropoiesis-stimulating agents in current and preventive doping analysis. Drug Test Anal 4, 830, 2012 [DOI] [PubMed] [Google Scholar]
- 84. Bao W., Qin P., Needle S., et al. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol 56, 147, 2010 [DOI] [PubMed] [Google Scholar]
- 85. Hong Y.R., Kim H.T., Lee S.C., et al. [(4-Hydroxyl-benzo[4,5]thieno[3,2-c]pyridine-3-carbonyl)-amino]-acetic acid derivatives; HIF prolyl 4-hydroxylase inhibitors as oral erythropoietin secretagogues. Bioorg Med Chem Lett 23, 5953, 2013 [DOI] [PubMed] [Google Scholar]
- 86. Prosser H.C., Tan J.T., Dunn L.L., et al. Multifunctional regulation of angiogenesis by high-density lipoproteins. Cardiovasc Res 101, 145, 2014 [DOI] [PubMed] [Google Scholar]
- 87. Tan J.T., Prosser H.C., Vanags L.Z., et al. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha. FASEB J 28, 206, 2014 [DOI] [PubMed] [Google Scholar]
- 88. Nakayama K., Qi J., and Ronai Z.. The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res 7, 443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang X.M., Wang Y.S., Zhang J., et al. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci 50, 1873, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Wetterau M., George F., Weinstein A., et al. Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen 19, 481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sun G., Zhou Y., Li H., et al. Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J Biomed Sci 20, 100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kelly T.J., Souza A.L., Clish C.B., and Puigserver P.. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol 31, 2696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mateo J., Garcia-Lecea M., Cadenas S., Hernandez C., and Moncada S.. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J 376, 537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brix B., Mesters J.R., Pellerin L., and Johren O.. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1alpha-mediated target gene activation. J Neurosci 32, 9727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li F., Sonveaux P., Rabbani Z.N., et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26, 63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yasinska I.M., and Sumbayev V.V.. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 549, 105, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Metzen E., Zhou J., Jelkmann W., Fandrey J., and Brune B.. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell 14, 3470, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chowdhury R., Flashman E., Mecinovic J., et al. Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1). J Mol Biol 410, 268, 2011 [DOI] [PubMed] [Google Scholar]
- 99. Park Y.K., Ahn D.R., Oh M., et al. Nitric oxide donor, (+/-)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1alpha by inhibiting von Hippel-Lindau recruitment and asparagine hydroxylation. Mol Pharmacol 74, 236, 2008 [DOI] [PubMed] [Google Scholar]
- 100. Palmer L.A., Doctor A., Chhabra P., et al. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Investig 117, 2592, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Buckley D.L., Van Molle I., Gareiss P.C., et al. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J Am Chem Soc 134, 4465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahn D.R., Kim S.Y., Lee M.J., and Yang E.G.. HIF-1alpha peptide derivatives with modifications at the hydroxyproline residue as activators of HIF-1alpha. Bioorg Med Chem Lett 19, 4403, 2009 [DOI] [PubMed] [Google Scholar]
- 103. Mcgrath K. Peptide activators of Vegf. Available at www.lens.org/lens/patent/US_7053046_B2/fulltext (accessed December19, 2019)
- 104. Liu Y.V., Hubbi M.E., Pan F., et al. Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem 282, 37064, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sant S., Hancock M.J., Donnelly J.P., Iyer D., and Khademhosseini A.. Biomimetic gradient hydrogels for tissue engineering. Can J Chem Eng 88, 899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saadat E., Lan H., Majumdar S., Rempel D.M., and King K.B.. Long-term cyclical in vivo loading increases cartilage proteoglycan content in a spatially specific manner: an infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res Ther 8, R147, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brucker P.U., Izzo N.J., and Chu C.R.. Tonic activation of hypoxia-inducible factor 1alpha in avascular articular cartilage and implications for metabolic homeostasis. Arthritis Rheum 52, 3181, 2005 [DOI] [PubMed] [Google Scholar]
- 108. Hattori T., Muller C., Gebhard S., et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137, 901, 2010 [DOI] [PubMed] [Google Scholar]
- 109. Henry S.P., Liang S., Akdemir K.C., and de Crombrugghe B.. The postnatal role of Sox9 in cartilage. J Bone Miner Res 27, 2511, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Grogan S.P., Miyaki S., Asahara H., D'Lima D.D., and Lotz M.K.. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 11, R85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dy P., Wang W., Bhattaram P., et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell 22, 597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Maes C., Araldi E., Haigh K., et al. VEGF-independent cell-autonomous functions of HIF-1alpha regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res 27, 596, 2012 [DOI] [PubMed] [Google Scholar]