Abstract
Pregnane X receptor (PXR) is a ligand-activated nuclear receptor that was originally identified as a master regulator of xenobiotic detoxification. It regulates the expression of drug-metabolizing enzymes and transporters to control the degradation and excretion of endobiotics and xenobiotics, including therapeutic agents. The metabolism and disposition of drugs might compromise their efficacy and possibly cause drug toxicity and/or drug resistance. Because many drugs can promiscuously bind and activate PXR, PXR antagonists might have therapeutic value in preventing and overcoming drug-induced PXR-mediated drug toxicity and drug resistance. Furthermore, PXR is now known to have broader cellular functions, including the regulation of cell proliferation and glucose and lipid metabolism. Thus, PXR might be involved in human diseases such as cancer and metabolic diseases. The importance of PXR antagonists is discussed in the context of the role of PXR in xenobiotic sensing and other disease-related pathways. This review focuses on the development of PXR antagonists, which has been hampered by the promiscuity of PXR ligand binding. However, substantial progress has been made in recent years, suggesting that it is feasible to develop selective PXR antagonists. We discuss the current status, challenges, and the strategies in developing selective PXR antagonists. The strategies are based on the molecular mechanisms of antagonism in related nuclear receptors that can be applied to the design of PXR antagonists, primarily driven by structural information.
Keywords: PXR, antagonist, small molecule, xenobiotics, detoxification
1. Introduction
The nuclear receptor (NR) pregnane X receptor (PXR, NR1I2) plays a prominent role in the detoxification system that humans and other organisms utilize to eliminate endobiotics such as steroid hormones, bile acids, and glucose and xenobiotics such as therapeutic agents. Upon the binding of ligand, PXR transcriptionally upregulates the expression of drug-metabolizing enzymes and transporters, leading to the metabolism and, ultimately, clearance of endobiotics and xenobiotics. These chemicals undergo biotransformation and disposition through a series of processes comprising oxidation and conjugation reactions [involving cytochrome P450 enzymes (CYPs), such as CYP3A4, UDP-glucuronosyltransferases, and glutathione-S-transferases, sulfotransferases] and active efflux [involving the transporter proteins multidrug resistance proteins (MDRs) and multidrug resistance–associated proteins (MRPs)].1–4
PXR and the detoxification system represent a double-edged sword: Although detoxification serves as a beneficial protection mechanism against toxic compounds, it also compromises therapeutic outcomes by decreasing drug efficacy and possibly inducing drug toxicity and resistance. Therefore, a balance between PXR transcriptional activation and repression is needed, not only to maintain appropriate physiologic levels of endogenous chemicals but also to achieve optimal therapeutic efficacy with fewer drug-induced toxicities. Because of its important role in regulating drug efficacy and drug toxicity, as well as its emerging roles in other physiologic and pathologic processes such as energy metabolism and metabolic diseases,5–7 infectious diseases,8 cancer,9 and inflammatory bowel disease (IBD),10 as briefly discussed in sections 1.1–1.4, PXR has become an attractive therapeutic target.1,11 However, PXR is notorious for promiscuously binding structurally diverse chemicals, including clinical drugs, environmental toxins, and endogenous metabolites, making it a challenging therapeutic target partly because of the perceived difficulty of studying its structure–activity relationship.2 These structurally diverse chemicals activate the transcriptional apparatus of PXR by directly engaging in the ligand binding pocket within the ligand binding domain (LBD), which is the most recognizable feature of NRs.12 The LBD of PXR is uniquely adapted to recognize an array of molecules of various sizes and structures because it is larger and more flexible than the LBDs of other NRs, enabling it to conform its shape to accommodate ligands in its mostly hydrophobic pocket. The LBD is part of a major protein architecture typical of NRs, being connected via a flexible hinge to an N-terminal sequence-specific DNA-binding domain (DBD).13 PXR controls gene expression by engaging the PXR-responsive element module (PXRRE) in the proximal promoter and distal enhancer regions of target genes through the zinc fingers located in the DBD.14,15 The multi-functional LBD also contains, at the C-terminus, the activation function 2 (AF-2) domain, which engages coactivator or corepressor proteins, and the heterodimerization interface for association with retinoid X receptor α (RXRα).16–19
As described in recent reviews,20,21 there is growing evidence that PXR activation mediates adverse metabolic phenotypes of xenobiotics and endobiotics and that it induces resistance to chemotherapeutic agents used to treat cancer. Therefore, PXR antagonists might have considerable value for clinical applications. However, the ligand promiscuity of PXR renders the development of specific PXR antagonists challenging. As no recent review has focused on the strategies for developing PXR antagonists, we will present perspectives on the design of antagonists based on work conducted with PXR and other related NRs, which can be translated to the development and optimization of PXR antagonists. The review is heavily oriented toward molecular mechanisms derived from reported structural information. The justification for the discovery of such PXR modulators is presented in context with the important function of PXR in drug metabolism and human disease, complementing recent perspectives on the field.6,7,20,21
1.1. The role of PXR in cancer
PXR has a significant impact on chemotherapy resistance by upregulating target genes involved in drug metabolism and efflux transport that negatively alter drug effectiveness. For instance, PXR overexpression causes resistance to the antineoplastic drug irinotecan in metastatic colorectal cancer by enhancing the degradation of the active drug metabolite SN-38,22,23 which is itself a PXR agonist.24 More recently, the PXR gene, together with its transcriptional target gene CYP3A5, were reported to be upregulated and to cause resistance to therapy with paclitaxel, which is an agonist of PXR and a substrate of CYP3A5, in pancreatic ductal adenocarcinoma. Importantly, such therapy resistance could be reversed by genetically downregulating CYP3A5 or PXR.25
PXR has been implicated in cell proliferation and apoptosis, as reviewed recently.9,26 The activation of PXR suppresses apoptosis by upregulating the antiapoptotic proteins B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia sequence 1 (MCL-1) while downregulating proapoptotic proteins such as p53 and Bcl-2 antagonist/killer 1 (BAK1).27 For example, PXR activation was shown to increase the neoplastic features of LS 174T colorectal adenocarcinoma cells and human primary colon tumor cells through PXR-mediated induction of fibroblast growth factor 19.28 In contrast, PXR was reported to have tumor-suppressive activity in HT-29 colorectal cancer cells (which have undetectable endogenous PXR), in which the restoration of PXR (by ectopic expression) induces cell-cycle arrest to suppress cell proliferation.29 The anticancer role of PXR has also been observed in other studies, including one in which the human PXR (hPXR) agonist rifaximin significantly reduced the effects of colon cancer induction by azoxymethane/dextran sulfate sodium in a PXR-humanized mouse model, probably by inhibiting the inflammatory signal in connection with the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB).30 The discrepancies in the correlation between PXR activation and cancer cell growth are probably caused by differences in methodology, including the induction and quantitation of PXR expression and activity and the experimental models used.28 Therefore, although it is clear that activation of PXR in cancer cells enhances the drug-metabolizing pathway to reduce the sensitivity of the cells to chemotherapeutic agents, the functional relation between PXR expression level and activation and cancer cell growth in different cancer models requires further investigation.
1.2. The role of PXR in glucose metabolism
The role of PXR in glucose metabolism has been reviewed recently.5–7 It has become evident that PXR is interconnected to impaired glucose tolerance, based on findings from human clinical studies involving drugs that activate hPXR, such as rifampicin (also known as rifampin), and from animal studies using rat models and pregnenolone 16α-carbonitrile (PCN), which is an agonist of both rat PXR and mouse PXR (mPXR).31,32 It has been established that PXR is involved in glucose metabolism by repressing gluconeogenic gene expression of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK)33–35 and by repressing the transcription mediated by forkhead box protein O1 (FOXO1), which is among the main transcription factors regulating gluconeogenesis under fasting conditions.34 It has also been reported that PXR associates with the cAMP response element–binding protein (CREB) and suppresses cAMP-mediated transcription of G6Pase.35 PXR interacts with the common coactivator peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1α (PGC-1α), reducing PEPCK expression by interfering with the interaction of PGC-1α with hepatocyte nuclear factor 4 (HNF4), leading to an interrupted glucagon response.33 Expression of PEPCK and G6Pase was impaired in animals (both rats and mice) after treatment with the rat PXR and mPXR agonist PCN,32,35,36 and reduced expression of PEPCK and G6Pase has been detected in transgenic mice with activated human PXR (hPXR).37
It appears that PXR activation would be beneficial for reducing hepatic glucose levels and controlling the glucose response in type 2 diabetes by downregulating PEPCK and G6Pase. This is supported by animal studies, in which activated PXR decreased fasting blood glucose levels38 and impeded the negative effects of a high-fat diet (e.g., obesity).36 However, contradictory results have been reported, whereby the ablation of PXR (in PXR−/− mice) inhibited high-fat diet-induced obesity, hepatic steatosis, and insulin resistance.39 Treatment with PCN and atorvastatin also led to impaired glucose use in rats.40
The differential response of PXR activation in humans and mice adds another layer of complexity to the role of PXR in gluconeogenesis. For example, it was recently reported that the statin simvastatin induces PEPCK1 and G6Pase in human primary hepatocytes and human hepatoma cells in a manner that depends on hPXR, serum/glucocorticoid regulated kinase 2 (SGK2), and protein phosphatase 2C (PP2C).41 Interestingly, the gluconeogenic statin–PXR–SGK2 signaling pathway is not present in mice, which partly explains the contradictory observations of the effect of PXR activation on glucose metabolism in mouse and rat models.34,35,41 Further investigation is needed to establish the role of PXR in glucose metabolism, with particular attention being paid to differences among the cellular models and in the context, the species, and how PXR is modulated (i.e., either genetically by altering the PXR expression levels or pharmacologically by using small molecule modulators).
1.3. The role of PXR in lipid metabolism
The role of PXR in lipid metabolism has also been reviewed recently.5–7 PXR has a profound effect on the homeostasis of lipids, and its agonist-induced activation causes hepatic steatosis, which is characterized by the repression of β-oxidation and the induction of lipogenesis.42 In mice, PXR activation by PCN has a negative impact on carnitine palmitoyltransferase 1A (CPT1A) and 3-hydroxy-3-methylglutarate-CoA synthase 2 (HMGCS2) by repressing the activity of forkhead box protein A2 (FOXA2), a regulator of β-oxidation and ketogenesis. CPT1A aids in the transport of long-chain fatty acids into mitochondria for β-oxidation, whereas HMGCS2 is involved in ketogenesis.38,43 hPXR activation also downregulates the expression of 3-ketoacyl-CoA thiolase, which catalyzes β-oxidation of long-chain fatty acids.37
PXR enhances lipogenesis through the induction of thyroid hormone–responsive spot 14 protein (THRSP),44 long-chain free fatty acid elongase,37 and stearoyl-CoA desaturase 1 (SCD1),38 with the last two proteins being important in fatty acid synthesis. PXR is also involved in the transcriptional regulation of lipin-1, which participates in triglyceride synthesis.39
The effect of PXR activation in reducing β-oxidation and increasing lipogenesis is observed in both mice and humans, but with some differences between the two species. Notably, PPARγ and its target gene CD36 are induced in mouse primary hepatocytes but not in human primary hepatocytes upon PXR activation, and there is no evidence that ketogenesis is downregulated in humans.37,44 Interestingly, the PXR antagonist sulforaphane prevented the upregulation of several lipogenic genes that could be induced by rifampicin.42 The use of small molecules that antagonize rifampicin-induced PXR activity raises the possibility of small molecule modulators of PXR being used as therapeutics. However, the development of such PXR-modulating drugs could be complex, as exemplified by the fact that both pharmacologically induced PXR activation and genetic knockdown of PXR appear to lead to steatosis and lipid accumulation in a case-dependent manner, using different mechanisms and displaying contrasting morphologies.38,42,45
1.4. The role of PXR in inflammatory bowel disease
The results of recent clinical and preclinical studies suggest that PXR agonists can be used to treat IBD in a PXR-dependent way.10,46 IBD is characterized by an immune response–driven inflammation of the gastrointestinal tract. The pathogenesis of IBD was inversely correlated with the expression and activity of PXR and its target genes in the intestines of patients with IBD,47 and treatment with rifaximin improved the symptoms.48 The mPXR agonist PCN was demonstrated to protect mice from dextran sulfate sodium (DSS)-induced colitis in the same manner as the hPXR agonist rifampicin was able to ameliorate the effects of DSS in humanized PXR mouse models.49 The mechanism underlying the positive effects of PXR agonists on IBD is believed to involve crosstalk between PXR and NF-κB, wherein activation of PXR results in the inhibition of the NF-κB signaling cascade and its proinflammatory response.50
Several PXR agonists have been investigated as potential IBD therapeutics, including solomonsterol A,51 artemisinin,52 chrysin,53 isorhamnetin,54 and hydroxyflavone and its structurally related analogs.55 The tissue distribution of PXR agonists appears to be important: rifampicin failed to provide protection against IBD,49 possibly because it concentrates significantly in the liver, where it can lower the plasma level of anti-inflammatory unsaturated fatty acids.49 In contrast, the gut-selective hPXR agonist rifaximin substantially ameliorated IBD.56
2. CURRENT STATUS OF PXR ANTAGONIST DEVELOPMENT, CHALLENGES, AND STRATEGIES
As discussed in section 1, PXR was originally identified as a xenobiotic-sensing receptor that regulates the expression of drug-metabolizing enzymes and transporters to control the disposition of xenobiotics, including therapeutic agents, which could contribute to drug resistance and drug toxicity. Because of its ligand promiscuity, many drugs, such as rifampicin and paclitaxel, and drug candidates can bind and activate PXR. Hence, PXR antagonists might be useful as therapeutics that can be co-administered with other drugs that activate PXR, thereby reducing their PXR-mediated drug resistance and toxicity. PXR is now known to affect other physiologic and pathologic processes, including IBD, for which a PXR agonist might have therapeutic value. In this review, we focus on developing PXR antagonists as co-drugs to prevent or overcome cancer drug resistance and drug-induced toxicity or as therapeutics for other metabolic diseases in which PXR activation is undesirable.
It is important to underscore the necessity of developing selective and potent PXR antagonists that can be used as chemical probes to interrogate PXR-mediated biological pathways. The use of chemical probes provides supporting information, and in many cases, offers advantages over genetic manipulation. This is because deleting the PXR gene not only abrogates its ligand-dependent transcriptional activity but also abolishes the non–ligand-mediated roles of PXR, such as those relating to protein–protein interactions. Such modulators should possess great selectivity in order to avoid off-target effects; otherwise, their use may confound experimental results, leading to contradictory or puzzling conclusions, especially when PXR shares common target genes with the constitutive androstane receptor (CAR).57
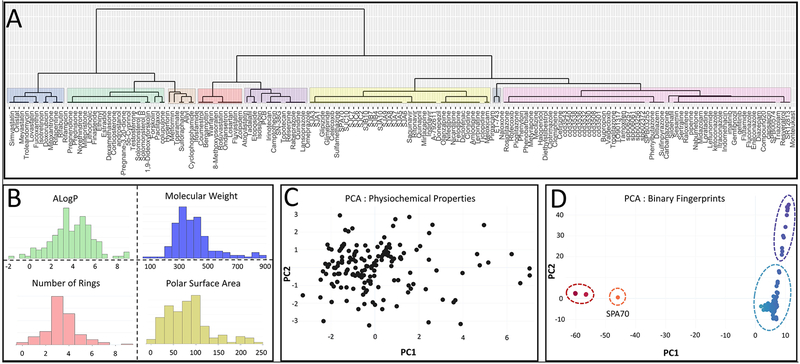
The LBD is characterized by the presence of a hydrophobic pocket that entraps ligands. Unlike the ligand cavity of most NRs, the binding pocket of PXR is prominent for being large and highly flexible, which enables it to accommodate ligands of great diversity in terms of their chemical scaffold, size, lipophilicity, and surface area.12,58,59 Figure 1 shows reported modulators of PXR60–65 in a manner that illustrates the large chemical space that they encompass, thus providing an impression of the extensive range of their physiochemical properties. Most of the chemicals recognized by the ligand binding pocket result in PXR activation, and this agonistic promiscuity is a major impediment to the development of PXR antagonists.
FIGURE 1.
Diversity of reported PXR ligands (agonists and inhibitors). A) Hierarchical clustering of reported PXR ligands. Binary linear fingerprints were generated using Canvas (Schrodinger), and subsequently clustered using the Tanimoto similarity metric. Compounds and their corresponding cluster nodes were then loaded into the R software environment (www.http://.r-project.org). Hierarchical clustering was performed using an average linkage. The dendrogram was plotted using the ggdendrogram package. B) Physiochemical distribution of reported PXR ligands showing the wide range. C) Principal Component Analysis (PCA) of physiochemical properties, illustrating the diverse chemical space of PXR ligands. By using Canvas (Schrodinger), the following physiochemical properties were calculated for each compound: the partition coefficient (AlogP; shown in B), the hydrogen bond acceptors (HBA), the hydrogen bond donors (HBD), the molecular weight (MW; shown in B), the polar surface area (PSA; shown in B), and the polar residues (Polar). These properties were used as input for calculating a two-component PCA. D) Principal Component Analysis of binary fingerprints as an alternative PCA analysis, grouping the ligands into four clusters, represented in red (SJA1, SJA3, SJA5, SJA6, SJA7, SJA8, SJA9, SJA10, SJB4, SJB7, SJB10, SJC6, SJC7, SJC10), orange (SPA70), purple (most of the steroid compounds), and blue (the remaining ligands). Binary and linear fingerprints were generated for all compounds by using Canvas (Schrodinger). These fingerprints were then used as input to calculate a two-component PCA. Clusters were gated into four main groups based on the components. For all graphs, the 164 ligands were prepared using LigPrep (Schrodinger). Each compound was desalted, specified chiralities were retained, and ionization states were not changed. Prepared versions of the compounds were used for all analyses.
Although both hPXR and mPXR bind promiscuously to many structurally diverse chemicals, it is well established that PXR agonists display species selectivity, probably because of the marked variability in the amino acid sequence of the LBD, as exemplified by the human and mouse LBDs.66 It is plausible that antagonists also display such inter-species differences; therefore, caution is needed when interpreting biological data obtained using non-human species. For instance, rifampicin and SR12813 are potent hPXR agonists but poor ligands of mPXR, whereas PCN is a weak agonist of hPXR but exerts strong agonistic behavior for mPXR.67 Because this species selectivity can complicate in vivo studies, humanized mouse models of PXR, in which the mPXR has been replaced with the hPXR, have been developed for evaluating hPXR ligands. In the earlier versions of the humanized mouse models, the expression of hPXR was under the control of the liver-specific albumin promoter68 or the rat fatty acid–binding protein promoter.69 Subsequently, a double-transgenic mouse model expressing both hPXR and human cytochrome P450 3A4 (CYP3A4) was generated that displayed robust CYP3A4 induction by rifampicin.70 The most recent humanized PXR mouse model was generated by expressing a chimeric PXR (mDBD-hLBD)71, which was envisioned as minimizing differences in the DNA binding affinities of different species. Humanized PXR models provide valuable in vivo information and have widespread applications in the development of PXR modulators.
2.1. The current status of PXR antagonist development
Because the LBD of PXR can expand its pocket substantially, it can trap ligands of considerable diversity in terms of their size and shape. Most of the interacting ligands have been shown to be agonists, but a growing number of compounds have been reported to elicit PXR-inhibitory properties (PXR inhibitors have been reviewed elsewhere20,21,60,72). However, most of the compounds described in this section have been insufficiently characterized for them to be considered antagonists from a technical standpoint.
Some notable compounds that inhibit PXR activity have been obtained from natural sources. Ecteinascidin 743 (ET 743) is a natural product with a tetrahydroisoquinoline scaffold that was derived from the Caribbean marine tunicate (sea squirt) Ecteinascidia turbinata and was the first compound reported to be able to repress agonist-induced PXR activity.73 However, ET 743 might be too toxic for broad clinical application because of its ability to bind to the minor groove of DNA and the possibility of its interfering with DNA repair pathways.74,75 It is assumed that phytochemicals already present in the diet are relatively safe for clinical use. Among those phytochemicals reported to suppress agonist-induced PXR activity are sulforaphane (from cruciferous vegetables),76 the phytoestrogen coumestrol (present in legumes and soy beans),77 sesamin (lignan in sesame seed),78 and the alkaloid camptothecin (extracted from the bark of the tree Camptotheca acuminate).79 However, many of these chemicals, such as coumestrol and sulforaphane, display weak potency in repressing PXR function.77 In fact, sulforaphane failed clinical trials in humans because of its ineffectiveness at suppressing hPXR activity.80
Regulatory-approved drugs are attractive candidates for PXR inhibitors because of the wealth of available pharmacokinetic and pharmacodynamic data. Leflunomide (an antirheumatic agent with immunosuppressive properties that is used to treat rheumatoid arthritis and psoriatic arthritis) is recognized as the first approved drug to be repurposed for PXR inhibition.81 Other examples include metformin (an antihyperglycemic agent used in the treatment of diabetes),82 the HIV protease inhibitor A-792611,83 the antifungal ketoconazole and its derivatives,72 the immunomodulator pimecrolimus and the anticancer drug pazopanib.84
As a xenobiotic sensor, PXR is expected to interact with environmental toxins. Even though most of these toxins turn out to be PXR agonists, some, such as the polychlorinated biphenyls (PCBs), have displayed antagonistic properties with potencies in the low micromolar range.85 Interestingly, the highly chlorinated PCBs display species selectivity, antagonizing mPXR but not the human ortholog.
It is noteworthy that even though these compounds were originally reported to be PXR modulators that repressed transcriptional activity, many have not yet been subjected to stringent biochemical and biophysical scrutiny to elucidate the molecular mechanism of inhibition. For many of the reported PXR inhibitors, there is insufficient evidence to determine whether they displace agonists from the ligand binding pocket by competition or by engaging an allosteric site or whether they even interact directly with PXR at all. Because some of these compounds were tested in cell-based assays alone, it is plausible that some of them affect the activity of PXR indirectly through changes in its post-translational modification status or intracellular localization or through another signaling mechanism. Among the compounds known to inhibit PXR, sulforaphane and ketoconazole are the most comprehensively studied, although they are not specific for PXR and are not effective in vivo.80,86 A potent and selective PXR antagonist with activity in animal models, SPA70, has recently been developed, demonstrating that it is feasible to develop selective PXR antagonists.65 SPA70 will be discussed in section 2.7.
2.2. Coactivator/corepressor interaction with the PXR-LBD
Ligand binding to the PXR-LBD induces an active conformation that promotes the recruitment of a coactivator protein, which engages the AF-2 region in NRs. Coactivators are regulatory proteins that enhance the transcriptional activity of agonist-activated nuclear receptors; examples include the steroid receptor coactivator family 1 (SRC-1) and the transcriptional mediator/intermediary factor 2 (TIF2).87 The NR box motif Leu-Xxx-Xxx-Leu-Leu (where Xxx is any other amino acid residue) within a coactivator has been found to be essential for the coupling of the coactivator to the NR. Crystallographic data of PXR in complex with a short SRC-1 peptide show the Leu residue of the Leu-Xxx-Xxx-Leu-Leu motif buried in a hydrophobic groove on the PXR-LBD surface adjacent to the AF-2 helix. This groove is formed by helices α3 and α4 and the AF-2 helix: interactions between Lys259 of α3 and Glu427 of the AF-2 helix fix the peptide by a charge clamp.
Corepressors are the counterparts of coactivators and are involved in negatively regulating the transcriptional activity of NRs. Corepressors, such as nuclear receptor corepressor (NCoR) and silencing mediator for retinoid or thyroid hormone receptors (SMRT), bind to NRs, facilitated by CoRNR boxes with the motif Ile/Leu-Xxx-Xxx-Ile/Val-Ile.88 NRs have been shown to display a preference for a particular CoRNR box or corepressor.89 Even though there are currently no co-crystal structures of PXR with a corepressor peptide, the NR–corepressor interface has been mapped for other NRs by crystallography. The structure of PPAR-α bound to an antagonist showed that the SMRT corepressor peptide is engaged in the same groove that would otherwise be occupied simultaneously by the SRC-1 coactivator peptide and the AF-2 helix. Therefore, the corepressor peptide blocks the AF-2 helix from being oriented in the active conformation.90
Most efforts to develop NR antagonists have relied on the antagonist inducing conformational changes in the NR in order to disrupt coactivator recruitment to the NR LBD or enhance corepressor interaction with the LBD. However, alternative strategies could take advantage of the differences in the tissue- or cell-specific distribution of these coregulatory proteins. For instance, the compounds LAC67b, LAC82a, and LAC82b were shown to be vitamin D receptor (VDR) antagonists in COS-7 cells but potent agonists in HEK-293 cells.91 The authors who reported that finding concluded that the ligand acts as an agonist if there are appropriate levels of intracellular coactivators to bind to the looser VDR–ligand complex but that it acts as an antagonist if the coactivator levels are low.
2.3. AF-2 helix orientation is a determinant of NR activation and repression
The orientation of the AF-2 helix in NRs appears to be the dominant determinant directing the association of the PXR-LBD with coactivators or corepressors. This discriminatory function acts as a switch to turn transcriptional activity on or off. It is not surprising, therefore, that most efforts to develop NR antagonists exploit the modulation of the AF-2 helix positioning.
In the active conformation, the AF-2 helix is folded against the body of the LBD, stabilized by interactions with the ligand binding pocket (Figure 2A). In many cases, further stabilization is accomplished by direct interactions between residues within the AF-2 helix and the agonist. The precise placement of the AF-2 helix in this pose serves as a “lid” to entrap the ligand within the ligand binding pocket, shielding it from the aqueous environment. As a result, the ligand-induced local LBD rearrangement creates a favorable surface (i.e., the AF-2 region) for coactivator binding. This model of ligand-dependent activation has been branded the “mouse trap” model. More recently, a modified model, called the “folding door” model, has been proposed based on structural studies of VDR. In this model, a kink in helix α11 acts as a hinge so that α11 closes the ligand pocket cooperatively with the AF-2 helix.92 This model advocates that ligands have easier access to the ligand binding site than in the “mouse trap” model, and it emphasizes the flexibility in the loop connecting α11 to the AF-2 helix as an important component of NR repression by antagonists.
FIGURE 2.
Position of the AF-2 helix in the agonist-induced and apo forms. A) When bound to the agonist (green), the AF-2 helix (raspberry red) is positioned in the active configuration favorable for coactivator recruitment, as exemplified by the PXR–SJB7 structure (PDB code 5X0R). B) Surprisingly, most apo-NR structures, including all the reported apo-PXR structures, showed the AF-2 helix being held in the agonist-induced form (PDB code 1ILG). C) Apo-RXRα showing the AF-2 helix extending away from the LBD core, which is unfavorable for coactivator recruitment (PDB code 6HN6). D) Apo-form of TR4 showing the AF-2 helix in an autorepressive position that prevents coactivator or corepressor binding (PDB code 3P0U).
It is well established that the position of the AF-2 helix differs in the active and inactive states. Surprisingly, however, most crystallized ligand-free NRs, including all the unliganded PXR-LBD structures reported to date, display the AF-2 helix in the active position (Figures 2A and 2B). It is hypothesized that the nonphysiologic conditions under which diffraction-quality crystals are obtained, such as extreme buffer pH and salt and precipitant concentrations, are major contributors to this phenomenon.93 The presence of a coactivator peptide during protein purification and crystallization, which is essential for achieving a high concentration of PXR protein, can force the AF-2 helix to adopt the agonist-induced form in the absence of ligand. Consequently, multiple agonistic/antagonistic populations present in a solution cannot be revealed in crystal structures because the AF-2 helix is preferentially pre-locked in the agonistic mode during crystal formation.94
Only a few NR crystal structures determined by X-ray crystallography show the AF-2 helix in an orientation distinct from that of the active state. The crystal structure of RXRα shows strikingly that the AF-2 helix extends away from the core of the LBD (Figure 2C).95 The apo testicular receptor 4 (TR4) structure reveals a peculiar arrangement in which the AF-2 helix occupies the coactivator binding site, thereby blocking the association of a coactivator or corepressor with the LBD (Figure 2D).96 This type of autoinhibition is analogous to the inactive autorepressed conformation observed in the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) LBD.97
Although crystallography provides valuable contrast between the active and inactive states of NRs, centered on the arrangement of the AF-2 helix, it is limited in that it provides only snapshots of static situations, whereas the ligand-induced NR conformational change is a dynamic process. In addition, biologically harsh crystallographic conditions and crystal packing constraints can produce inexplicable results.98 Molecular dynamics simulations have been employed in order to understand the allosteric process of the AF-2 helix swing triggered by ligand binding, thereby providing insights into the transition from the inactive to active configurations.99,100 There is a growing consensus that the docking of the ligand in the binding pocket results in a tighter and more stable LBD, as was observed with thyroid hormone receptor (TR) conformation by hydrogen/deuterium exchange coupled with mass spectrometry (HDX-MS).101 NMR studies of VDR in the presence of modulators that elicit diverse transcriptional responses have also demonstrated that the flexibility of the apo-form AF-2 helix is stabilized by agonists.98 Fluorescence anisotropy methods employed to investigate the motion of the AF-2 helix in PPAR revealed that the helix is highly mobile, as compared to the core of the LBD, and that ligand binding reduces its mobility.102
Mounting evidence suggests that the AF-2 helix does not exist only in one or other of the two discrete states of active and inactive conformation but that there are several intermediate states. Backed by observations from X-ray data,103,104 NMR studies revealed that in the unliganded form, the PPARγ structure is present in solution in various conformations because of its fluid nature and that ligand binding affixes a subset of these conformations.105 The dynamic regions were mapped to the coregulatory protein interface and the ligand binding site. By using HDX-MS, differential stabilization of the AF-2 helix by full and partial agonists of VDR has also been observed.106 In these HDX-MS experiments, the graded AF-2 helix stabilization was consistent with the strength of the association of VDR with the coactivator peptide.
2.4. Strategies for developing NR antagonists
An extensive array of NR antagonists has already been reported, and considerable effort has been invested in characterizing their mode of inhibition, in the hope that this will lead to improved antagonists. Two major types of antagonistic mechanisms have been proposed.107 The first type takes advantage of a bulky substituent in the compound to physically push the AF-2 helix away from the active state, creating an unfavorable position for coactivator recruitment.93 Antagonists of the second type reside in the ligand binding site, but they fail to provide sufficient stabilization of the AF-2 helix in the active form, in part because of poor contact between the ligand and key residues.52 This passive mode of action precludes the agonist from residing in the ligand pocket, and it may also increase the compactness of the apo NR by providing a network of interactions, thus acting as a “glue” to hold the different sections of the LBD in a condensed structure for corepressor recruitment. However, in some cases, the antagonist cannot position the AF-2 helix in an appropriate configuration for corepressor binding; instead, the AF-2 helix is left flapping in a disordered fashion, unable to be positioned in the active form. The above-mentioned classification of active vs. passive antagonism is centered on the ability of the antagonist to physically obstruct the AF-2 helix, but a different distinction between the two antagonist types has been employed based on the ability of the antagonist to recruit a corepressor: an active antagonist enhances corepressor interaction with the NR while the passive antagonist prevents recruitment of either coactivator or corepressor.108
2.4.1. VDR antagonists
Although the vast majority of vitamin D analogues function as VDR agonists, the few reported to be VDR antagonists have provided invaluable information about the molecular basis of NR antagonism. Among these antagonists, ZK168281 and related molecules have a bulky ester group that sterically perturbs the AF-2 helix, preventing it from adopting a favorable configuration for coactivator recruitment.109 In contrast, compounds that antagonize VDR passively include the lactone derivatives TEI9647, TET9648, and LAC67a, which are incapable of having contacts with the AF-2 helix.91,110 Although the vast majority of VDR antagonists are chemically related to the secosteroid scaffold of the potent endogenous agonist 1,25(OH)2D3, a few antagonists have been developed with an alternate scaffold, such as those based on GW0742, which is a PPARγ agonist.111
A series of 22-alkyl substituents of the hormone 1α,25-dihydroxyvitamin D3 have been discovered to encompass various types of NR modulation, such as agonism, partial agonism, and antagonism.112 The X-ray structure of VDR in complex with 22S-butyl-25,26,27-trinor-1α,24-dihydroxyvitamin D3 showed that the compound forms poor hydrophobic contacts with the AF-2 helix and, surprisingly, induces the formation of a small cavity within the ligand binding pocket to accommodate the butyl moiety; this ligand-induced structural feature in the canonical VDR LBD had not been observed previously.113 The cavity, known as the “butyl pocket,” is believed to be strongly linked to the agonistic or antagonistic behavior of the ligand, and it serves to demonstrate that flexible regions in the LBD can be exploited for fine-tuning NR activity.114 Based on structure–activity relation studies, the 22S-hexyl analog was originally designed to be a more potent antagonist than its progenitors. Unexpectedly, however, it turned out to be a partial agonist.115 Crystallographic analysis revealed that this compound resides in the binding site in multiple orientations, creating a mixed population of agonists and antagonists. It is not surprising that NRs with a large and flexible ligand pocket would accommodate ligands in several poses, as is the case with the agonist SR12813 in the PXR LBD.12 However, the structure of VDR with the 22S-hexyl analog is an indication that the different orientations of the ligand can have divergent effects on NR activity.
Curiously, co-crystal structures of VDR in complex with the antagonists ADTT,116 ADMI4,116 JB,113 and TEI9647110 display the AF-2 helix in an active orientation similar to that seen in structures of VDR in complex with agonists. Therefore, solution-based structural analyses were conducted, and in contrast to the results of X-ray studies, NMR experiments revealed differences in the conformation of VDR when it was bound to the natural hormone 1,25(OH)2D3, the potent agonist analog 2MD, or the antagonist OU-72.98 The differential NMR chemical shifts exhibited disorder in the region encompassing the α11 and AF-2 helices as a consequence of antagonist binding to the LBD that differed from that seen in the presence of an agonist. Small-angle X-ray scattering (SAXS) was applied to VDR in the apo state and in complex with an antagonist, revealing that conformational changes occurred upon ligand binding in solution.92 This study is noteworthy because there is, at present, no crystal structure of apo VDR; therefore, the behavior of the AF-2 helix of VDR in the unliganded form cannot be known. However, these observations were inconsistent with theoretical profiles calculated from crystallography, and molecular dynamics was used in combination with SAXS to generate the “folding door” model described above.
2.4.2. PPAR antagonists
A recent report described the structural characterization of PPARγ in the presence of the partial inverse agonist SR10171 and the antagonist SR11023,94 which are modified versions of the indole-containing SR1664.117,118 These ligands behave like passive antagonists because they do not appear to clash physically with the AF-2 helix. However, the AF-2 helix adopts an orientation different from that seen in the agonist-bound PPARγ structures that favors corepressor peptide recruitment. Even though SR11023 and SR10171 occupy a space between α3 and the β sheet distal from the AF-2 region, the AF-2 helix is pulled into the antagonist mode. This interpretation has been termed the “tumble and trap” mechanism of inhibition, because the AF-2 helix tumbles in the apo form as a consequence of its elasticity and gets trapped in the antagonistic arrangement by the ligands.
The “tumble and trap” view of passive PPARγ antagonism contrasts with the “push and tumble” mechanism observed in PPARα in the presence of the antagonist GW6471.90,94 Unlike the passive PPARγ inhibitors, GW6471 pushes the AF-2 helix by protruding between α3 and α11, resulting in a tumbling of the mobile AF-2 helix that fails to favor coactivator association. The steric clash with the AF-2 helix was also observed with the antagonist SR1664, the nitro moiety of which collides with Phe282.119 Curiously, the R-enantiomer SR1663 behaves like an agonist, and it makes favorable contacts with Phe282 instead of bumping into it. From the results obtained with a combination of HDX-MS and NMR spectroscopy, it was concluded that the stereo-specific clash with the AF-2 helix resulted in its destabilization. Guided by the AF-2 helix-push approach, the bulkier t-butyl substituent was incorporated into the structure of SR2595 to become an inverse agonist that lost its effectiveness in Phe282Ala mutants because of missing interactions with the side chain of Phe282.119
2.4.3. ER antagonists
Raloxifene is another antagonist that obstructs the AF-2 helix and prevents it from attaining a proper alignment conducive to coactivator binding, as seen in crystal structures of estrogen receptor (ER).120,121 The relatively long (11 Å) molecule cannot be engulfed by the ligand pocket, which is in stark contrast to the binding poses of the agonist 17β-estradiol in ERα120 and the phytoestrogen genistein in ERβ.121 Because the antagonist is too long to be confined within the LBD pocket, its protrusion forces the AF-2 helix to move away from the agonist-inducible position. The ERα antagonist 4-hydroxytamoxifen has been shown to force the AF-2 helix to occupy the groove wherein the coactivator glucocorticoid receptor interacting protein 1 (GRIP1) peptide would otherwise be engaged in the agonist-induced form. In this scenario, the AF-2 helix mimics the hydrophobic character of the NR box contained in the coactivator peptide.122 Similarly to raloxifene, 4-hydroxytamoxifen cannot be fully contained within the ligand pocket, because the large size of the bulky substituent compels it to protrude.
2.4.4. GR antagonists
Mifepristone has been observed to induce agonism and also passive and active antagonism.108,123,124 In the case of glucocorticoid receptor (GR) activity modulation by mifepristone, the passive antagonist prevents the recruitment of a coactivator or corepressor (which latter was previously referred to as a competitive antagonist).108 In contrast, the active antagonist promotes interactions of GR with the corepressor while also enabling nuclear translocation of the GR. This classification of active vs. passive antagonism is not widely applied to other NRs, as discussed in the first paragraph of section 2.4.
The crystal structure of GR with mifepristone illustrates the concept of active and passive antagonist conformations remarkably well (Figure 3).108 Mifepristone has a bulky dimethylamino phenyl group that clashes with the AF-2 helix, preventing it from being positioned in the agonist-induced state (Figures 3A and 3B). GR LBD was crystallized in the presence of mifepristone and the corepressor peptide NCoR to obtain the ternary complex. However, within the same crystal, three different conformations of the AF-2 helix in the antagonistic mode were observed108 (Figures 3D–3F) that were distinct from that seen in the agonistic form in the GR–dexamethasone complex125 (Figure 3C). Two of these conformations positioned a bound corepressor at an orientation almost orthogonal to the AF-2 helix, which is indicative of active antagonism (Figures 3D and 3E). The third conformation showed the AF-2 helix occupying the corepressor site, thereby preventing the NCoR peptide from binding, which is representative of passive antagonism (Figure 3F). One of the active antagonistic states (Figure 3E) displayed a conformation intermediate between the agonistic state (Figure 3C) and the fully pronounced active antagonist form (Figure 3D), which was hypothesized to represent partial agonism.108
FIGURE 3.
Comparisons of the AF-2 helix position of GR in the agonist- and antagonist-induced states. A) The agonist dexamethasone (green) enhances the positioning of the AF-2 helix (raspberry red) in the active conformation for binding the coactivator TIF2 peptide (yellow). B) Mifepristone (green) was taken from the mifepristone–GR structure (PDB code 3H52) and superimposed on the GR LBD of the dexamethasone–GR structure (PDB code 1M2Z) to demonstrate the clash between mifepristone and the agonist-induced AF-2 helix due to the large moiety. C) The GR LBD, showing the AF-2 helix in the active configuration conducive for coactivator TIF2 binding in the presence of dexamethasone (green). D) The fully pronounced active antagonism state, showing the AF-2 helix in a position favoring binding of the corepressor NCoR peptide (blue) in the presence of mifepristone (green). E) The less pronounced active antagonism state (compare to D). Green: mifepristone. F) The passive antagonism state, showing the AF-2 helix in place of the corepressor NCoR peptide. Green: mifepristone.
2.5. RXRα-LBD interaction with the PXR-LBD
As with other NRs, PXR requires interactions with regulatory proteins in order to exert transcriptional activity. The co-crystal structure of the PXR-LBD with the RXRα-LBD shows a large dimer interface region consisting of hPXR helices α5, α9, and α10 and human RXRα (hRXRα) helices α7, α9, and α10 that interact with each other through several electrostatic and polar residues.126 The structure displays many similarities with the structures of RXRα in complex with other NRs, including being positioned in a similar orientation and having similar types of interacting residues. Nevertheless, the superposition of the RXRα-LBD in these complexes reveals certain differences in translation and rotation that can be exploited in developing PXR-selective modulators. More intriguing is the fact that the PXR homodimer, with each PXR-LBD monomer interacting with the RXRα-LBD, forms a heterotetramer complex not previously seen in other NRs.
The behavior of the PXR-LBD differs in the presence or absence of RXRα.126 Agonist binding to PXR condenses the complex, leading to ligand-induced structural stabilization. The formation of the PXR–RXRα complex enhances the affinity of SRC-1 coactivator peptide for each NR LBD, as compared to the PXR-LBD or RXRα-LBD alone, and it is believed that the improved coactivator binding of the heterodimer partnership is important in the regulation of target gene expression.
Ligand binding stabilizes the heterodimerization of NRs with RXRα, as demonstrated by HDX-MS studies of VDR.106 In those experiments, the full-length VDR remained unstable in the absence of full-length RXRα and the presence of agonist enhanced the interaction of the hetero complex. Remote regions far from the ligand binding pocket, including the heterodimerization interface, were perturbed. Therefore, certain compounds might be able to modulate NR activity negatively by allosterically affecting interactions with RXRα. Conversely, native mass-spectrometry studies found that the partial inverse agonist SR10171 and the antagonist SR11023 could bind to the PPARγ-RXRα complex without disrupting heterodimerization.94 These findings emphasize the difficulty of suppressing NR activity by disturbing dimerization with RXRα.
2.6. Exploiting allosteric sites for NR antagonism
The most prominent site devoted to the modulation (up- or downregulation) of NR activity has been the ligand binding pocket in the LBD. However, several additional sites by which chemicals affect NR function have been uncovered, and molecules that bind to these alternative locations are known as NR alternate-site modulators.127 Some of the earliest observations of compounds targeting alternate regions came from the development of anti-ER inhibitors, such as pyrimidines that block the association of ERα with an SRC-1 peptide.128,129 These types of modulators are known as coactivator-binding inhibitors, and they represent the initial efforts to design drugs by employing alternative approaches. Unexpectedly, hydroxytamoxifen was shown to bind in the coactivator-binding groove of ERβ, in addition to the ligand binding pocket, as elucidated by crystallography.130 Even though it is suspected that high concentrations of antagonist led to the saturation of the second unexpected site, this alternative surface should be explored in relation to drug design. The potential value of exploiting the AF-2 surface was also demonstrated by the crystal structure of the thyroid hormone receptor β (TRβ), in which the compound 1-(4hexylphenyl)prop-2-en-1-one (HPPE) was seen located in the concave pocket of the AF-2 surface that would otherwise be occupied by a coactivator peptide (Figure 4A and Figure 4B).131
FIGURE 4.
Allosteric binding sites of NR antagonists. A) The crystal structure of TRβ in complex with the antagonist HPPE (blue) shows the antagonist residing at the surface area where a coactivator peptide would otherwise engage (PDB code 2PIN). B) For comparison, the coactivator peptide GRIP1 (yellow) is shown positioned at the AF-2 interface of TRβ (PDB code 1BSX). C) Binding of the antagonist 3,3’,5-triiodothyroacetic acid (blue) to the alternative site termed BF-3 (green) on the surface of AR weakens coactivator interaction with AR (PDB code 2PIV). The AF-2 helix is colored raspberry red.
The azole class of chemicals are among the most extensively investigated inhibitors of PXR, and they include ketoconazole and its derivatives.72 The antifungal ketoconazole was shown to disrupt coactivator association with PXR and other NRs,132 but interactions of PXR with ligand, DNA elements, or RXR were not disturbed.86 Based on data obtained through biochemical techniques, cell-based assays, and molecular docking analysis, it was concluded that ketoconazole binds at the AF-2 surface of PXR instead of residing in the ligand binding pocket.63,86,133 Pharmacophore studies targeting the AF-2 surface of PXR uncovered a small pocket in which hydrophobic and hydrogen bond acceptor interactions can be used to dock relatively small compounds, and this led to the discovery of novel PXR inhibitors such as SPB03255 and SPB00574.63
An additional site of interest on the surface of NRs has been discovered. Termed binding function 3 (BF-3), this site is a hydrophobic section of similar size to the AF-2 region and was initially identified in androgen receptor (AR).134 Compounds were crystallized in complex with AR at the BF-3 site and were shown to affect coactivator binding allosterically (Figure 4C).
2.7. SPA70 as a selective PXR antagonist and a template for future PXR antagonist development
A high-throughput screening campaign identified SPA70 as representing a novel class of compound that antagonized hPXR,65 which was shown to have therapeutic potential by mitigating the effects of PXR-mediated hemorrhagic shock-induced liver injury in mouse models.135 SPA70 displayed marked selectivity across multiple NRs while retaining potency for PXR, and it could suppress PXR activity in human hepatocytes and humanized mouse models. A series of SPA70 analogs have been synthesized, and these have revealed that small structural changes in the chemical scaffold can turn an antagonist into an agonist, supporting the notion that the development of PXR antagonists is challenging. Efforts to obtain crystals of the PXR–SPA70 complex failed when the crystal conditions were optimized for agonists (e.g., with SRC-1 peptide being present). Such setbacks are characteristic of attempts to obtain co-crystals of NRs with antagonists. However, crystals could be obtained with the structurally related agonist SJB7. In conjunction with HDX-MS and computational docking studies, it was rationalized that SPA70 has poorer interactions with residues in the AF-2 helix when compared to SJB7. The lack of a sizeable chemical group that clashes sterically with the AF-2 helix would indicate a passive mode of AF-2 helix destabilization comparable to that observed in other NRs.
There is no evidence that SPA70 resides in the ligand binding pocket of PXR in mixed agonistic and antagonistic conformations, as seen with the VDR antagonist 22S-hexyl derivative.115 Because of the large and expansible LBD of PXR, future analogs could adopt multiple poses with opposing cellular transcriptional activity. The incorporation of an extended chemical functional group to create a clash with the AF-2 helix should be considered, as this strategy has proved effective with other NRs. However, it would not be surprising if some of these molecules with a bulky substituent are ineffective antagonists because of the potential for ligand rearrangement within the fluid PXR-LBD.
3. Concluding remarks and perspectives
There is a great need to develop PXR antagonists that can be used as chemical probes to obtain a full understanding of PXR antagonism and as drug leads to develop therapy to prevent or overcome adverse PXR-mediated effects, such as cancer drug resistance and drug-induced toxicity. It is rationalized that preventing the drug-induced expression of PXR target genes at the transcriptional level may be less toxic than directly inhibiting the activity of PXR downstream targets, because they can be constitutively present in normal tissues performing house-keeping functions: such could be the case for multidrug resistance 1 (MDR1) inhibitors that failed clinical trials due to toxic side effects.13 On the other hand, there can be circumstances where selective inhibition of the activity of PXR downstream targets is more desirable. For instance, it was reported that overexpression of CYP3A5 in pancreatic ductal adenocarcinoma mediates its chemoresistance,25 and CYP3A5-selective inhibitors would overcome CYP3A5-mediated chemoresistance with reduced toxicity by not affecting CYP3A4 activity in the liver. Even though PXR has become an attractive target in early drug discovery, it is noteworthy to recognize that PXR is not necessarily the only or major factor that ultimately impacts the fate of drugs in vivo, because there are other variables that affect absorption, distribution, metabolism and excretion (ADME) profiles.64,136
Efforts to developed selective and potent PXR antagonists have been hampered by the promiscuous ligand-binding nature of the protein. However, considerable advances have been made in recent years, including the repurposing and characterization as potential PXR antagonists of bioactive compounds that affect other biological pathways, such as sulforaphane and ketoconazole and its derivatives. The recent development of SPA70 and analogs thereof as novel, potent, and selective PXR antagonists has demonstrated the feasibility of developing selective PXR antagonists, and it provides a template for designing improved PXR antagonists, guided by information gained from the growing number of antagonists that target other NRs with proven molecular mechanisms. The cumulative knowledge gathered from the many atomic-level structures of related NRs indicates that the two main approaches for designing PXR antagonists are based on the ability of the ligand to disrupt the AF-2 helix from the active conformation by directly clashing with it via bulky substituents or to destabilize the AF-2 helix by removing favorable interactions between its side chains and the ligand. PXR antagonists that engage alternative sites that are more rigid than the dynamic ligand binding pocket should also be considered, even though potency could be an issue due to the relatively shallow grooves.
To tackle such a challenging target as PXR effectively, out-of-the-box thinking is needed (Figure 5). At the target level, the ligand promiscuity of PXR, although expanding the ligand profile and enabling PXR to act as a master xenobiotic sensor, appears to hamper the development of selective antagonists. However, the species selectivity of ligands in mice and humans, together with the unexpected observation that subtle chemical modification converts an antagonist (SPA70) to an agonist (SJB7), provides hints that it may be possible to identify PXR structural motifs that will facilitate an understanding of PXR ligand promiscuity and the development of selective PXR antagonists. At the compound level, hybrid compounds based on the existing selective PXR antagonists may be developed to engage an intramolecular site, such as an allosteric site, to improve potency and selectivity or an intermolecular partner, such as the E3 ubiquitin ligase (i.e., a proteolysis targeting chimera [PROTAC]), to inhibit PXR. At the methodology level, although X-ray crystallography can provide details of compound–PXR interactions, the dynamic nature of ligand-induced PXR conformational changes (including the movement of the AF-2 helix) means that solution-based approaches, such as HDX-MS and SAXS, newer technology, such as cryo-electron microscopy (cryoEM), and in silico approaches, such as molecular dynamics simulations, will provide additional information.
FIGURE 5.
Strategies and considerations in the development of PXR antagonists. A) Inter-species differences should be considered when using non-human models. B) Tissue-dependent levels of coregulatory proteins and ligand distribution can have an effect on NR antagonism. C) Disruption of the AF-2 helix (red) from the agonistic configuration by an antagonist (blue) has been the most studied approach for NR antagonist development. D) Coupling of PXR ligands (blue) to a bait (green) in order to recruit machinery (orange) for degradation or inhibition of PXR (grey), such as the recruitment of E3 ubiquitin ligase by PROTACs. E) Molecules that bind in alternate sites (pink) can be linked to PXR ligands (blue) for enhanced potency and selectivity. All protein and ligand models are hypothetical representations.
ACKNOWLEDGMENTS
We thank other members of the Chen research laboratory for valuable discussions and Dr. Keith A. Laycock (St. Jude Department of Scientific Editing) for editing the manuscript. This work was supported, in part, by ALSAC, St. Jude Children’s Research Hospital, and the National Institutes of Health [grants R35-GM118041 (to TC) and P30-CA21765 (to the St. Jude Cancer Center)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR BIOGRAPHIES
Taosheng Chen is a Full Member of the Department of Chemical Biology & Therapeutics and of the St. Jude Graduate School of Biomedical Sciences, St. Jude Children’s Research Hospital (St. Jude). He is also a Full Professor at the University of Tennessee Health Science Center and the Director of the High Throughput Bioscience Center at St. Jude. He received his BSc and MS degrees from Fudan University, China, and he completed his PhD studies at the University of Vermont and his postdoctoral studies at the University of Virginia. Before joining St. Jude, he was a Senior Research Investigator at Bristol-Myers Squibb and a Research Scientist at SAIC-Frederick/National Cancer Institute. He serves on the editorial boards of several journals and on NIH grant review panels and study sections. He has authored more than 130 publications (including two books) and multiple patents. His research laboratory studies the regulation of the nuclear receptors PXR and CAR, and it uses a multidisciplinary approach to develop novel PXR and CAR modulators for use as chemical tools for investigating the broad cellular function of PXR and CAR and as lead compounds for therapeutic development.
Sergio C. Chai is a high-throughput screening (HTS) specialist at St. Jude Children’s Research Hospital, where he is involved in the development and implementation of HTS assays, data analysis and modeling, and automation. His multidisciplinary expertise lies at the interface of biochemistry, chemical biology, and analytical and inorganic chemistry. He received his PhD degree from the Department of Chemistry at the University of Massachusetts Amherst and pursued his postdoctoral fellowship at the Indiana University School of Medicine.
William C. Wright is a PhD student under the guidance of Dr. Taosheng Chen in the Department of Chemical Biology and Therapeutics at St. Jude Children’s Research Hospital. His current research broadly involves the intersection of drug metabolism pathways and cancer. He uses and develops computational and experimental techniques for target identification and drug discovery/development.
Footnotes
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Wang YM, Ong SS, Chai SC, Chen TS. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Met. 2012;8(7):803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1(4):259–266. [DOI] [PubMed] [Google Scholar]
- 3.Tolson AH, Wang HB. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliver Rev. 2010;62(13):1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YM, Lin WW, Chai SC, et al. Piperine activates human pregnane X receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol Appl Pharm. 2013;272(1):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Zhong JW, Hu L, et al. The Role of Xenobiotic Receptors on Hepatic Glycolipid Metabolism. Curr Drug Metab. 2019;20(1):29–35. [DOI] [PubMed] [Google Scholar]
- 6.Mackowiak B, Hodge J, Stern S, Wang HB. The Roles of Xenobiotic Receptors: Beyond Chemical Disposition. Drug Metab Dispos. 2018;46(9):1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakkola J, Rysa J, Hukkanen J. Regulation of hepatic energy metabolism by the nuclear receptor PXR. Bba-Gene Regul Mech. 2016;1859(9):1072–1082. [DOI] [PubMed] [Google Scholar]
- 8.Shehu AI, Li GM, Xie W, Ma XC. The pregnane X receptor in tuberculosis therapeutics. Expert Opin Drug Met. 2016;12(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Mattia E, Cecchin E, Roncato R, Toffoli G. Pregnane X receptor, constitutive androstane receptor and hepatocyte nuclear factors as emerging players in cancer precision medicine. Pharmacogenomics. 2016;17(14):1547–1571. [DOI] [PubMed] [Google Scholar]
- 10.Klepsch V, Moschen AR, Tilg H, Baier G, Hermann-Kleiter N. Nuclear Receptors Regulate Intestinal Inflammation in the Context of IBD. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic Metabolomics: Major Impact on the Metabolome. Annu Rev Pharmacol. 2012;52:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins RE, Wisely GB, Moore LB, et al. The human nuclear xenobiotic receptor PXR: Structural determinants of directed promiscuity. Science. 2001;292(5525):2329–2333. [DOI] [PubMed] [Google Scholar]
- 13.Chen TS. Overcoming drug resistance by regulating nuclear receptors. Adv Drug Deliver Rev. 2010;62(13):1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HB, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42(15):1331–1357. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg B, Sabbagh W, Juguilon H, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Gene Dev. 1998;12(20):3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dussault I, Lin M, Hollister K, et al. A structural model of the constitutive androstane receptor defines novel interactions that mediate ligand-independent activity. Mol Cell Biol. 2002;22(15):5270–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins RE, Davis-Searles PR, Lambert MH, Redinbo MR. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331(4):815–828. [DOI] [PubMed] [Google Scholar]
- 18.Shan L, Vincent J, Brunzelle JS, et al. Structure of the murine constitutive androstane receptor complexed to androstenol: A molecular basis for inverse agonism. Mol Cell. 2004;16(6):907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YM, Chai SC, Lin W, et al. Serine 350 of human pregnane X receptor is crucial for its heterodimerization with retinoid X receptor alpha and transactivation of target genes in vitro and in vivo. Biochem Pharmacol. 2015;96(4):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staudinger JL. Clinical applications of small molecule inhibitors of Pregnane X receptor. Mol Cell Endocrinol. 2019;485:61–71. [DOI] [PubMed] [Google Scholar]
- 21.Chai SC, Cherian MT, Wang YM, Chen T. Small-molecule modulators of PXR and CAR. Biochim Biophys Acta. 2016;1859(9):1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita K, Kubota Y, Ishida H, Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroentero. 2015;21(43):12234–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raynal C, Pascussi JM, Legueline G, et al. Pregnane x Receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation. Mol Cancer. 2010;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basseville A, Preisser L, Trecesson SD, et al. Irinotecan induces steroid and xenobiotic receptor (SXR) signaling to detoxification pathway in colon cancer cells. Mol Cancer. 2011;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noll EM, Eisen C, Stenzinger A, et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat Med. 2016;22(3):278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oladimeji PO, Chen T. PXR: more than just a master xenobiotic receptor. Mol Pharmacol. 2018;93(2):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Liu MJ, Zhai Y, Xie W. The antiapoptotic role of pregnane x receptor in human colon cancer cells. Mol Endocrinol. 2008;22(4):868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang HW, Venkatesh M, Li H, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121(8):3220–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang N, Ke S, Eagleton N, et al. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Brit J Cancer. 2010;102(12):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng J, Fang ZZ, Nagaoka K, et al. Activation of Intestinal Human Pregnane X Receptor Protects against Azoxymethane/Dextran Sulfate Sodium-Induced Colon Cancer. J Pharmacol Exp Ther. 2014;351(3):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hukkanen J Induction of cytochrome P450 enzymes: a view on human in vivo findings. Expert Rev Clin Phar. 2012;5(5):569–585. [DOI] [PubMed] [Google Scholar]
- 32.Rysa J, Bulerl M, Savolainen MJ, Ruskoaho H, Hakkola J, Hukkanen J. Pregnane X Receptor Agonists Impair Postprandial Glucose Tolerance. Clin Pharmacol Ther. 2013;93(6):556–563. [DOI] [PubMed] [Google Scholar]
- 33.Bhalla S, Ozalp C, Fang SS, Xiang LJ, Kemper K. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1 alpha - Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279(43):45139–45147. [DOI] [PubMed] [Google Scholar]
- 34.Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24(18):7931–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodama S, Moore R, Yamamoto Y, Negishi M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J. 2007;407:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma YJ, Liu DX. Activation of Pregnane X Receptor by Pregnenolone 16 alpha-carbonitrile Prevents High-Fat Diet-Induced Obesity in AKR/J Mice. Plos One. 2012;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Zhai YG, Mu Y, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281(21):15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282(13):9768–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He JH, Gao J, Xu MS, et al. PXR Ablation Alleviates Diet-Induced and Genetic Obesity and Insulin Resistance in Mice. Diabetes. 2013;62(6):1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling ZL, Shu N, Xu P, et al. Involvement of pregnane X receptor in the impaired glucose utilization induced by atorvastatin in hepatocytes. Biochem Pharmacol. 2016;100:98–111. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh S, Negishi M. Statin-activated nuclear receptor PXR promotes SGK2 dephosphorylation by scaffolding PP2C to induce hepatic gluconeogenesis. Sci Rep-Uk. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitter A, Rummele P, Klein K, et al. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch Toxicol. 2015;89(11):2089–2103. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–144. [DOI] [PubMed] [Google Scholar]
- 44.Moreau A, Teruel C, Beylot M, et al. A Novel Pregnane X Receptor and S14-Mediated Lipogenic Pathway in Human Hepatocyte. Hepatology. 2009;49(6):2068–2079. [DOI] [PubMed] [Google Scholar]
- 45.Spruiell K, Richardson RM, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA. Role of Pregnane X Receptor in Obesity and Glucose Homeostasis in Male Mice. J Biol Chem. 2014;289(6):3244–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng J, Shah YM, Gonzalez FJ. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci. 2012;33(6):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langmann T, Moehle C, Mauerer R, et al. Loss of detoxification in inflammatory bowel disease: Dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127(1):26–40. [DOI] [PubMed] [Google Scholar]
- 48.Khan KJ, Ullman TA, Ford AC, et al. Antibiotic Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2011;106(4):661–673. [DOI] [PubMed] [Google Scholar]
- 49.Cheng J, Shah YM, Ma XC, et al. Therapeutic Role of Rifaximin in Inflammatory Bowel Disease: Clinical Implication of Human Pregnane X Receptor Activation. J Pharmacol Exp Ther. 2010;335(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou C, Tabb MM, Nelson EL, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappa B signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116(8):2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepe V, Ummarino R, D’Auria MV, et al. Total Synthesis and Pharmacological Characterization of Solomonsterol A, a Potent Marine Pregnane-X-Receptor Agonist Endowed with Anti-Inflammatory Activity. J Med Chem. 2011;54(13):4590–4599. [DOI] [PubMed] [Google Scholar]
- 52.Hu DH, Wang YG, Chen ZW, et al. Artemisinin protects against dextran sulfate-sodium-induced inflammatory bowel disease, which is associated with activation of the pregnane X receptor. Eur J Pharmacol. 2014;738:273–284. [DOI] [PubMed] [Google Scholar]
- 53.Dou W, Zhang JJ, Zhang EY, et al. Chrysin Ameliorates Chemically Induced Colitis in the Mouse through Modulation of a PXR/NF-kappa B Signaling Pathway. J Pharmacol Exp Ther. 2013;345(3):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dou W, Zhang JJ, Li H, et al. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem. 2014;25(9):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau AJ, Chang TKH. 3-Hydroxyflavone and structural analogues differentially activate pregnane X receptor: Implication for inflammatory bowel disease. Pharmacol Res. 2015;100:64–72. [DOI] [PubMed] [Google Scholar]
- 56.Ma XC, Shah YM, Guo GL, et al. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther. 2007;322(1):391–398. [DOI] [PubMed] [Google Scholar]
- 57.Cherian MT, Chai SC, Wright WC, et al. CINPA1 binds directly to constitutive androstane receptor and inhibits its activity. Biochem Pharmacol. 2018;152:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchman CD, Chai SC, Chen TS. A current structural perspective on PXR and CAR in drug metabolism. Expert Opin Drug Met. 2018;14(6):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ingraham HA, Redinbo MR. Orphan nuclear receptors adopted by crystallography. Curr Opin Struc Biol. 2005;15(6):708–715. [DOI] [PubMed] [Google Scholar]
- 60.Wang YM, Chai SC, Brewer CT, Chen TS. Pregnane X receptor and drug-induced liver injury. Expert Opin Drug Met. 2014;10(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92(1):73–82. [DOI] [PubMed] [Google Scholar]
- 62.Hodnik Z, Masic LP, Tomasic T, et al. Bazedoxifene-Scaffold-Based Mirnetics of Solomonsterols A and B as Novel Pregnane X Receptor Antagonists. J Med Chem. 2014;57(11):4819–4833. [DOI] [PubMed] [Google Scholar]
- 63.Ekins S, Chang C, Mani S, et al. Human pregnane X receptor antagonists and Agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72(3):592–603. [DOI] [PubMed] [Google Scholar]
- 64.Sinz M, Kim S, Zhu Z, et al. Evaluation of 170 xenobiotics as transactivators of human pregnane X receptor (hPXR) and correlation to known CYP3A4 drug interactions. Curr Drug Metab. 2006;7(4):375–388. [DOI] [PubMed] [Google Scholar]
- 65.Lin W, Wang YM, Chai SC, et al. SPA70 is a potent antagonist of human pregnane X receptor. Nat Commun. 2017;8(1):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekins S, Mirny L, Schuetz EG. A ligand-based approach to understanding selectivity of nuclear hormone receptors PXR, CAR, FXR, LXR alpha, and LXR beta. Pharm Res-Dordr. 2002;19(12):1788–1800. [DOI] [PubMed] [Google Scholar]
- 67.Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275(20):15122–15127. [DOI] [PubMed] [Google Scholar]
- 68.Xie W, Barwick JL, Downes M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406(6794):435–439. [DOI] [PubMed] [Google Scholar]
- 69.Gong HB, Singh SV, Singh SP, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20(2):279–290. [DOI] [PubMed] [Google Scholar]
- 70.Ma X, Cheung C, Krausz KW, et al. A Double Transgenic Mouse Model Expressing Human Pregnane X Receptor and Cytochrome P450 3A4. Drug Metab Dispos. 2008;36(12):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Igarashi K, Kitajima S, Aisaki K, et al. Development of humanized steroid and xenobiotic receptor mouse by homologous knock-in of the human steroid and xenobiotic receptor ligand binding domain sequence. J Toxicol Sci. 2012;37(2):373–380. [DOI] [PubMed] [Google Scholar]
- 72.Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45(1):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7(5):584–590. [DOI] [PubMed] [Google Scholar]
- 74.Aune GJ, Furuta T, Pommier Y. Ecteinascidin 743: a novel anticancer drug with a unique mechanism of action. Anti-Cancer Drug. 2002;13(6):545–555. [DOI] [PubMed] [Google Scholar]
- 75.van Kesteren C, de Vooght MMM, Lopez-Lazaro L, et al. Yondelis (R) (trabectedin, ET-743): the development of an anticancer agent of marine origin. Anti-Cancer Drug. 2003;14(7):487–502. [DOI] [PubMed] [Google Scholar]
- 76.Zhou CC, Poulton EJ, Grun F, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71(1):220–229. [DOI] [PubMed] [Google Scholar]
- 77.Wang HW, Li H, Moore LB, et al. The phytoestrogen Coumestrol is a naturally occurring antagonist of the human pregnane x receptor. Mol Endocrinol. 2008;22(4):838–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim YP, Ma CY, Liu CL, et al. Sesamin: A Naturally Occurring Lignan Inhibits CYP3A4 by Antagonizing the Pregnane X Receptor Activation. Evid-Based Compl Alt. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wall ME, Wani M, Cook C, Palmer KH, McPhail Aa, Sim G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J Am Chem Soc. 1966;88(16):3888–3890. [Google Scholar]
- 80.Poulton EJ, Levy L, Lampe JW, et al. Sulforaphane is not an effective antagonist of the human pregnane X-receptor in vivo. Toxicol Appl Pharm. 2013;266(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekins S, Kholodovych V, Ai N, et al. Computational discovery of novel low micromolar human pregnane X receptor antagonists. Mol Pharmacol. 2008;74(3):662–672. [DOI] [PubMed] [Google Scholar]
- 82.Krausova L, Stejskaova L, Wang HW, et al. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem Pharmacol. 2011;82(11):1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Healan-Greenberg C, Waring JF, Kempf DJ, Blomme EAG, Tirona RG, Kim RB. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane x receptor. Drug Metab Dispos. 2008;36(3):500–507. [DOI] [PubMed] [Google Scholar]
- 84.Burk O, Kuzikov M, Kronenberger T, et al. Identification of approved drugs as potent inhibitors of pregnane X receptor activation with differential receptor interaction profiles. Arch Toxicol. 2018;92(4):1435–1451. [DOI] [PubMed] [Google Scholar]
- 85.Tabb MM, Kholodovych V, Grun F, Zhou CC, Welsh WJ, Blumberg B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR). Environ Health Persp. 2004;112(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang H, Wang H, Sinz M, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26(2):258–268. [DOI] [PubMed] [Google Scholar]
- 87.Smith CL, O’Malley BW. Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25(1):45–71. [DOI] [PubMed] [Google Scholar]
- 88.di Masi A, De Marinis E, Ascenzi P, Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol Aspects Med. 2009;30(5):297–343. [DOI] [PubMed] [Google Scholar]
- 89.Hu X, Li Y, Lazar MA. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21(5):1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu HE, Stanley TB, Montana VG, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPAR alpha. Nature. 2002;415(6873):813–817. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimoto N, Inaba Y, Yamada S, Makishima M, Shimizu M, Yamamoto K. 2-Methylene 19-nor-25-dehydro-1 alpha-hydroxyvitamin D-3 26,23-lactones: Synthesis, biological activities and molecular basis of passive antagonism. Bioorgan Med Chem. 2008;16(1):457–473. [DOI] [PubMed] [Google Scholar]
- 92.Anami Y, Shimizu N, Eldmoto T, et al. Apo- and Antagonist-Binding Structures of Vitamin D Receptor Ligand-Binding Domain Revealed by Hybrid Approach Combining Small-Angle X-ray Scattering and Molecular Dynamics. J Med Chem. 2016;59(17):7888–7900. [DOI] [PubMed] [Google Scholar]
- 93.Kojetin DJ, Burris TP. Small Molecule Modulation of Nuclear Receptor Conformational Dynamics: Implications for Function and Drug Discovery. Mol Pharmacol. 2013;83(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frkic RL, Marshall AC, Blayo AL, et al. PPARgamma in Complex with an Antagonist and Inverse Agonist: a Tumble and Trap Mechanism of the Activation Helix. iScience. 2018;5:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal-Structure of the Ligand-Binding Domain of the Human Nuclear Receptor Rxr-Alpha. Nature. 1995;375(6530):377–382. [DOI] [PubMed] [Google Scholar]
- 96.Zhou XE, Suino-Powell KM, Xu Y, et al. The Orphan Nuclear Receptor TR4 Is a Vitamin A-activated Nuclear Receptor. J Biol Chem. 2011;286(4):2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kruse SW, Suino-Powell K, Zhou XE, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. Plos Biol. 2008;6(9):2002–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singarapu KK, Zhu JG, Tonelli M, et al. Ligand-Specific Structural Changes in the Vitamin D Receptor in Solution. Biochemistry. 2011;50(51):11025–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watanabe C, Watanabe H, Tanaka S. An interpretation of positional displacement of the helix12 in nuclear receptors: Preexistent swing-up motion triggered by ligand binding. Bba-Proteins Proteom. 2010;1804(9):1832–1840. [DOI] [PubMed] [Google Scholar]
- 100.Celik L, Lund JDD, Schiott B. Conformational dynamics of the estrogen receptor alpha: Molecular dynamics simulations of the influence of binding site structure on protein dynamics. Biochemistry. 2007;46(7):1743–1758. [DOI] [PubMed] [Google Scholar]
- 101.Figueira ACM, Saidemberg DM, Souza PCT, et al. Analysis of Agonist and Antagonist Effects on Thyroid Hormone Receptor Conformation by Hydrogen/Deuterium Exchange. Mol Endocrinol. 2011;25(1):15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kallenberger BC, Love JD, Chatterjee VKK, Schwabe JWR. A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat Struct Biol. 2003;10(2):136–140. [DOI] [PubMed] [Google Scholar]
- 103.Uppenberg J, Svensson C, Jaki M, Bertilsson G, Jendeberg L, Berkenstam A. Crystal structure of the ligand binding domain of the human nuclear receptor PPAR gamma. J Biol Chem. 1998;273(47):31108–31112. [DOI] [PubMed] [Google Scholar]
- 104.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395(6698):137–143. [DOI] [PubMed] [Google Scholar]
- 105.Johnson BA, Wilson EM, Li Y, Moller DE, Smith RG, Zhou GC. Ligand-induced stabilization of PPAR gamma monitored by NMR spectroscopy: Implications for nuclear receptor activation. J Mol Biol. 2000;298(2):187–194. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Chalmers MJ, Stayrook KR, et al. Hydrogen/Deuterium Exchange Reveals Distinct Agonist/Partial Agonist Receptor Dynamics within Vitamin D Receptor/Retinoid X Receptor Heterodimer. Structure. 2010;18(10):1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shiau AK, Barstad D, Radek JT, et al. Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat Struct Biol. 2002;9(5):359–364. [DOI] [PubMed] [Google Scholar]
- 108.Schoch GA, D’Arcy B, Stihle M, et al. Molecular Switch in the Glucocorticoid Receptor: Active and Passive Antagonist Conformations. J Mol Biol. 2010;395(3):568–577. [DOI] [PubMed] [Google Scholar]
- 109.Bury Y, Steinmeyer A, Carlberg C. Structure activity relationship of carboxylic ester antagonists of the vitamin D-3 receptor. Mol Pharmacol. 2000;58(5):1067–1074. [DOI] [PubMed] [Google Scholar]
- 110.Miura D, Manabe K, Ozono K, et al. Antagonistic action of novel 1 alpha,25-dihydroxyvitamin D-3–26,23-lactone analogs on differentiation of human leukemia cells (HL-60) induced by 1 alpha,25-dihydroxyvitamin D-3. J Biol Chem. 1999;274(23):16392–16399. [DOI] [PubMed] [Google Scholar]
- 111.Teske KA, Bogart JW, Arnold LA. Novel VDR antagonists based on the GW0742 scaffold. Bioorg Med Chem Lett. 2018;28(3):351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakamaki Y, Inaba Y, Yoshimoto N, Yamamoto K. Potent Antagonist for the Vitamin D Receptor: Vitamin D Analogues with Simple Side Chain Structure. J Med Chem. 2010;53(15):5813–5826. [DOI] [PubMed] [Google Scholar]
- 113.Inaba Y, Yoshimoto N, Sakamaki Y, et al. A New Class of Vitamin D Analogues that Induce Structural Rearrangement of the Ligand-Binding Pocket of the Receptor. J Med Chem. 2009;52(5):1438–1449. [DOI] [PubMed] [Google Scholar]
- 114.Yoshimoto N, Sakamaki Y, Haeta M, et al. Butyl Pocket Formation in the Vitamin D Receptor Strongly Affects the Agonistic or Antagonistic Behavior of Ligands. J Med Chem. 2012;55(9):4373–4381. [DOI] [PubMed] [Google Scholar]
- 115.Anami Y, Sakamaki Y, Itoh T, et al. Fine tuning of agonistic/antagonistic activity for vitamin D receptor by 22-alkyl chain length of ligands: 22S-Hexyl compound unexpectedly restored agonistic activity. Bioorgan Med Chem. 2015;23(22):7274–7281. [DOI] [PubMed] [Google Scholar]
- 116.Nakabayashi M, Yamada S, Yoshimoto N, et al. Crystal structures of rat vitamin D receptor bound to adamantyl vitamin D analogs: Structural basis for vitamin D receptor antagonism and partial agonism. J Med Chem. 2008;51(17):5320–5329. [DOI] [PubMed] [Google Scholar]
- 117.Zheng J, Corzo C, Chang MR, et al. Chemical Crosslinking Mass Spectrometry Reveals the Conformational Landscape of the Activation Helix of PPAR gamma; a Model for Ligand-Dependent Antagonism. Structure. 2018;26(11):1431-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stechschulte LA, Czernik PJ, Rotter ZC, et al. PPARG Post-translational Modifications Regulate Bone Formation and Bone Resorption. Ebiomedicine. 2016;10:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marciano DP, Kuruvilla DS, Boregowda SV, et al. Pharmacological repression of PPAR gamma promotes osteogenesis. Nat Commun. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brzozowski AM, Pike ACW, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758. [DOI] [PubMed] [Google Scholar]
- 121.Pike ACW, Brzozowski AM, Hubbard RE, et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J. 1999;18(17):4608–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–937. [DOI] [PubMed] [Google Scholar]
- 123.Wagner BL, Pollio G, Giangrande P, et al. The novel progesterone receptor antagonists RTI 3021-012 and RTI 3021-022 exhibit complex glucocorticoid receptor antagonist activities: Implications for the development of dissociated antiprogestins. Endocrinology. 1999;140(3):1449–1458. [DOI] [PubMed] [Google Scholar]
- 124.Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R. RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding. J Biol Chem. 2002;277(29):26238–26243. [DOI] [PubMed] [Google Scholar]
- 125.Bledsoe RK, Montana VG, Stanley TB, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110(1):93–105. [DOI] [PubMed] [Google Scholar]
- 126.Wallace BD, Betts L, Talmage G, Pollet RM, Holman NS, Redinbo MR. Structural and Functional Analysis of the Human Nuclear Xenobiotic Receptor PXR in Complex with RXR alpha. J Mol Biol. 2013;425(14):2561–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moore TW, Mayne CG, Katzenellenbogen JA. Minireview: Not Picking Pockets: Nuclear Receptor Alternate-Site Modulators (NRAMs). Mol Endocrinol. 2010;24(4):683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodriguez AL, Tamrazi A, Collins ML, Katzenellenbogen JA. Design, synthesis, and in vitro biological evaluation of small molecule inhibitors of estrogen receptor a coactivator binding. J Med Chem. 2004;47(3):600–611. [DOI] [PubMed] [Google Scholar]
- 129.Parent AA, Gunther JR, Katzenellenbogen JA. Blocking Estrogen Signaling After the Hormone: Pyrimidine-Core Inhibitors of Estrogen Receptor-Coactivator Binding. J Med Chem. 2008;51(20):6512–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP. A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor beta. P Natl Acad Sci USA. 2006;103(26):9908–9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Estebanez-Perpina E, Arnold LA, Jouravel N, et al. Structural insight into the mode of action of a direct inhibitor of coregulator binding to the thyroid hormone receptor. Mol Endocrinol. 2007;21(12):2919–2928. [DOI] [PubMed] [Google Scholar]
- 132.Takeshita A, Taguchi M, Koibuchi N, Ozawa Y. Putative role of the orphan nuclear receptor SXR (steroid and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by xenobiotics. J Biol Chem. 2002;277(36):32453–32458. [DOI] [PubMed] [Google Scholar]
- 133.Wang HW, Huang HY, Li H, et al. Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin Cancer Res. 2007;13(8):2488–2495. [DOI] [PubMed] [Google Scholar]
- 134.Estebanez-Perpina E, Arnold AA, Nguyen P, et al. A surface on the androgen receptor that allosterically regulates coactivator binding (vol 104, pg 16074, 2007). P Natl Acad Sci USA. 2007;104(47):18874–18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie Y, Xu MS, Deng MH, et al. Activation of Pregnane X Receptor Sensitizes Mice to Hemorrhagic Shock-Induced Liver Injury. Hepatology. 2019;70(3):995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hogle BC, Guan XD, Folan MM, Xie W. PXR as a mediator of herb-drug interaction. J Food Drug Anal. 2018;26:S26–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]