Introduction:
Migraine is an idiopathic, episodic, recurrent neurological disorder characterized as a unilateral, throbbing headache that affects 27 million women and 10 million men in the US [45]. A large body of evidence supports the idea that the headache phase of migraine results from the activation of a nociceptive sensory pathway that innervates the intracranial meninges [7; 46]. The events that cause the nociceptive meningeal nerve endings to become activated during a migraine attack are largely unknown – likely due to the difficulty of studying them in humans. For lack of reliable ways to detect and measure molecular and cellular events that occur in the cortex, pia, arachnoid and dura of migraine patients between the time an attack is triggered and a headache is initiated, attempts to determine how meningeal nociceptors become activated are limited to pre-clinical studies in acceptable animal models.
To date, one of the most commonly used and widely accepted animal models of migraine is cortical spreading depression (CSD), a slowly propagating wave of altered neural activity, consisting of a brief excitation followed by a prolonged inhibition, which can be evoked experimentally by brief focal stimulation of the cerebral cortex [22; 24; 36]. Inside the brain, CSD is commonly associated with activation of glia cells, astrocytes and other neuroinflammatory events [17]. Outside the cortex, CSD triggers delayed activation of meningeal nociceptors [31; 55; 56], and that this delayed activation is preceded by arterial dilatation [3; 5; 38], plasma protein extravasation [39], activation of pial and dural macrophages and dendritic cells [37], transient closure of the glymphatic space [38], Caspase-1 activation and release of High-mobility group protein B1 (HMGB1) and interleukin 1 beta (IL-1β) into the cerebrospinal fluid [11; 19] – all events that can associate inflammatory responses with the process by which CSD or aura activates nociceptors in the dura.
The rationale for continuing to investigate the association between inflammation and the headache phase of migraine is that prostaglandins are found in smooth muscles of cranial arteries [2], that non-steroidal anti-inflammatory drugs (NSAID) are effective in treating migraine of mild to moderate severity [35; 44; 48], that they are most commonly used as migraine abortive drugs [27; 28], that they slow down the progression from episodic to chronic migraine [4; 40], and that the American Academy of Family Physicians, American College of Physicians, and American Society of Internal Medicine guideline recommend using NSAIDs as a first-line treatment choice for all migraine attacks [41].
Accordingly, the main goal of the current study was to determine the extent to which inhibition of prostaglandin synthesis by the selective cyclooxygenase-2 inhibitor celecoxib, can reduce CSD-induced blood vessel responses, plasma protein extravasation, and immune cell activation in the dura and pia.
Methods:
Animals.
All procedures involving animals were in compliance with the experimental protocol approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Harvard Medical School and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. Female Sprague Dawley Rats (Taconic) weighing 230–310 grams were used for vascular and plasma protein extravasation experiments. Only female rats were used in order to best compare to previous data for dural dilatation and plasma protein extravasation [39]. These experiments required rats because mice did not have such a dural response in our hands (data not shown). Male and female CX3CR1-GFP heterozygote mice (Jackson labs strain 5582) on C57BL/6J background aged 5–11 months old were used for macrophage experiments. Animals were kept in 12-hour light/dark cycles, and were housed 2 rats or 5 mice per cage.
Experimental Design:
Rats were anesthetized, intubated, and their femoral vein was cannulated. The skull was thinned over the parietal cortex and a craniotomy was performed over the frontal cortex. Celecoxib (20 mg/kg) or vehicle (total volume = 0.7 ml) was then injected via intraperitoneal (IP) injection. One hour later, rats were placed into the microscope and an image stack was taken at 800nm excitation for the identification of, and differentiation between arteries and veins. We then injected 2MDalton FITC Dextran into the femoral cannula in order to label the blood, and began imaging at 890nm excitation. After at least 5 minutes of baseline, we induced a single wave of CSD by pinprick in the frontal cortex, and then continued to image for 45 minutes, with image stacks taken about every 10 seconds. We could then determine the diameter of pial and dural blood vessels over the imaging time as well as quantify dural plasma protein extravasation events.
Mice were anesthetized, and then the skull was thinned and craniotomy placed. Mice were then given celecoxib or vehicle via IP injection. One hour later, macrophages in the dura and pia were imaged at baseline for at least 15 minutes before and 40 minutes after induction of CSD via pinprick through the frontal craniotomy, with image stacks taken every three minutes.
Anesthesia.
Rats were briefly anesthetized using isoflurane and injected with urethane (IP, 1.5 g/kg) and atropine (subcutaneous, 0.3 ml). Mice were anesthetized using urethane (IP, 1.5 g/kg) and atropine (IP, 0.15 mg/kg). Rats were intubated with an endotracheal tube and provided oxygen through a nose cone. Mice were provided oxygen through a nose cone. Core temperature was maintained at 37°C and blood oxygen levels were monitored with a PhysioSuite (Kent Scientific Corp., Torrington, CT) throughout the length of the experiment for both mice and rats.
Injections.
For rats, the femoral vein was cannulated for dye infusion. FITC-conjugated 2 mega-Dalton dextran (Thermo Fisher Scientific) was used to label the blood (150 μl of 5 mg/ml in saline). Injections into the femoral vein were followed by at least 1 ml of saline to flush the dead volume within the cannula and attached tube. Celecoxib was obtained from Dr. Reddy’s Laboratories Ltd.. (Princeton, NJ). For rats and mice, celecoxib was injected at 20 mg/kg via intraperitoneal injection. This dose is consistent with previous reports in rodents and was recommended by Dr. Reddy.
Skull thinning.
To create an imaging window, rats were placed on a modified stereotaxic frame with a movable, rotatable ball joint in order to tilt the head. The scalp was shaved and sterilized, and the skin above the superficial skull was removed. Mice scalps were shaved and sterilized, a midline incision was made, and a metal plate was affixed to the skull with cyanoacrylate. For both, the periosteum was removed over the right parietal and frontal skull plates. For rats, a layer of cyanoacrylate was placed around the perimeter of the plates for its hydrophobicity, and an area of 3 mm in diameter was thinned on the right parietal skull plate above a branch of the middle meningeal artery to a thickness of 70–100 μm. For mice, an area of about 1 mm diameter was thinned to a thickness of about 40–50 μm. A high speed drill was used to thin the skulls. For placement of CSD-inducing electrode, a similar area of about 1 mm was thinned in the frontal plate, and a small craniotomy was performed using an 18 gauge needle (both animals). To allow the dura to recover from any stress of thinning, imaging started at least one hour after all surgical procedures were completed (both animals).
In vivo imaging.
Fully anesthetized animals were imaged in an Olympus FV1000MPE-E multiphoton Imaging System using a Spectra-Physics Maitai Deepsee laser (~70 fs pulse width) and a PLAN 25X 1.05 NA objective. Three color channels were collected simultaneously (420–460 nm blue in a PMT; 495–540 nm green and 575–630 nm red in GaAsP detectors). A wavelength of 890 nm was used to excite all fluorophores, and produce second harmonic generation at 445 nm (blue). An excitation of 800 nm was used for the detection of intrinsic fluorescence (green) emitted by the outermost tissue layer of arteries, which allowed the distinguishing of arteries from veins. Time-lapse three-dimensional image stacks were taken covering a space from the skull through the meninges to the brain. Image resolution was 512 × 512 pixels for rat vasculature and 1024 × 1024 for mice macrophages, covering an area of 509 × 509 μm. For rat vascular imaging, custom software was written to allow special imaging parameters that ensured fast imaging while still correcting for any up-down motion of the rat brain: Two sets of three image slices were taken 10 μm apart, one set in the dura, and one in the pia, for each time point. This allowed us to skip slices in the 50–100 μm of subarachnoid space that we were not interested in for the sake of time, yielding time points about 10 s apart.
CSD initiation.
For CSD initiation, a metal electrode was inserted into the cortex through the dura to about 3 mm in rats, 1mm in mice, for 20 seconds. The CSD was initiated from a site in the frontal cortex that was sufficiently distant from the imaging site to minimize the possibility that the craniotomy or electrode insertion would affect the imaged area.
Image analysis.
Images were analyzed using Fiji (http://www.fiji.sc), a version of ImageJ (http://imagej.net). Blood vessel diameter was determined programmatically with a custom-written plugin that took a line ROI across the blood vessel as an input, averaged the brightness over the adjacent ~50 lines, then, using a threshold at the mean brightness to count pixels, determined the width of the blood vessel for each time point. Dural arteries were determined to start dilatation when their diameter rose 5% above baseline, and the dilatation ended when it returned to within 5% of baseline. Quantification of plasma protein extravasation events were inspected manually and visually, where one event was counted at the first detection of an increase of brightness next to a blood vessel. Frequency of these events were counted per 250 s bins. Macrophages shape was quantified as described previously. Briefly, a maximum intensity projection over three or four slices of an image stack containing dural or pial macrophages was taken, and the brightness was normalized over time. We then used a custom written Fiji macro to threshold the images and select individual macrophages using the wand tool, and measured the shape characteristics. Circularity is defined as ((4*pi*area)/perimeter2), in which a perfect circle would have a value of 1.0, and shapes with many projections would have lower values.
Determination of Arrival of CSD Wave:
The time of the arrival of the CSD wave under the imaging window was determined by changes in the diameter of pial arteries; typically occurring 2–3 minutes after CSD initiation. Time point 0 for “Time post CSD” was chosen as 30 seconds previous to the time point with the largest pial artery dilatation.
Statistics.
Multiple two-tailed paired student’s t-tests with Holm-Bonferroni correction were used to compare individual time points after CSD to baseline for blood vessel diameter. Two-way ANOVA was performed to compare blood vessel diameters and plasma protein extravasation event frequencies, with time post CSD as one variable, and treatment group as the other. Two-way ANOVA was performed to compare macrophage circularity, with time post CSD/injection as one variable, and treatment group as the other. Values given are averages ± standard error unless otherwise noted, and error bars in figures all represent standard error.
Results:
Dural but not pial arterial responses to CSD are reduced by IP Celecoxib
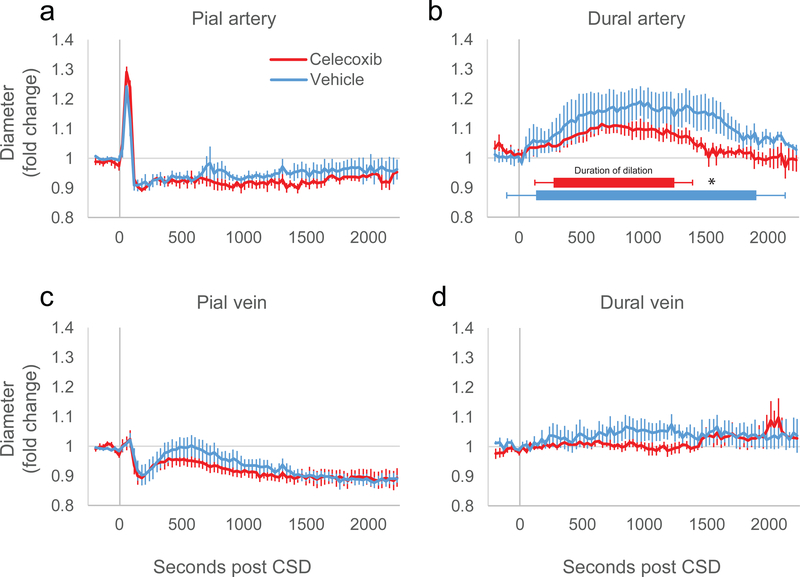
Treatment with celecoxib had no effect on the responses of pial arteries to CSD (Fig. 1a). Pial arteries dilated to a maximum of 36% ± 3.9 in the vehicle group and 43% ± 0.8 in the celecoxib group (not significantly different, p = 0.10), and compared to their baseline diameter, constricted about 4.2% ± 0.52 in the vehicle group and 8.2% ± 1.7 in the celecoxib group (p = 0.08). There was no significant interaction between treatment group and time post CSD (two-way ANOVA, F(71,1) = 1.1, p = 0.25 comparing 72 time points, F(1,1) = 3.3, p = 0.11 comparing just 2 time points, baseline to dilatation, and F(1,1) = 1.4, p = 0.28 comparing baseline to delayed constriction averaged over 320 s to 1310 s post CSD).
Figure 1.
Rat vascular responses to CSD in the presence of celecoxib or vehicle. Vascular diameters as measured from in vivo multiphoton imaging are plotted as fold change from average baseline diameter pre- and post-CSD for (a) pial arteries, (b) dural arteries, (c) pial veins, (d) dural veins. For (b), duration of dilatation is plotted based on average time of dilatation start and stop for each group. (* indicates p<0.05 student’s t-test, between dilatation durations).
Celecoxib did reduce the dilatation of dural arteries post CSD compared to vehicle (Fig. 1b, 2-way ANOVA interaction between time and treatment, F(71,1) = 1.7, p = 5.6*10−4, comparing 72 time points). This reduction was manifested mostly as a shortened time of dilatation. While there was a trend for the maximum amount of dilatation of the dural artery to be reduced in rats treated with celecoxib, it did not reach significance (dilatation of 25% ± 5.9 for vehicle, 13% ± 3.2 for celecoxib, p = 0.097, t-test), however the time at which the dura returned to baseline was significantly shortened (1890 ± 150 s vehicle vs. 1330 ± 73 celecoxib, p = 8.3*10−3, t-test), as well as the total duration of the dilatation (1750 ± 230 s vehicle vs. 955 ± 150 s celecoxib, p = 0.02, t-test). The time of the start of the dilatation was later on average but was not significantly different (150 ± 82 s vehicle vs 280 ± 110 s celecoxib, p = 0.39, t-test).
Pial and dural veins are unaffected by celecoxib
Previously, we have reported that pial veins have characteristic changes in diameter post CSD which can be affected by drug treatment [cite]. However, we did not find that celecoxib changed the pial vein response as compared to vehicle (Fig 1c, 2-way ANOVA interaction, F(71,1) = 0.82, p = 0.85). We observed an initial dilatation (5.8% ± 2.2 vehicle, 5.1% ± 2.3 celecoxib, p = 0.83) and constriction (9.2% ± 3.6 vehicle, 12% ± 3.0 celecoxib, p = 0.53), that seemed to closely match the behavior of the pial artery. This was followed by a recovery to baseline that lasted an average 817 ± 130 s for vehicle and 776 ± 170 s for celecoxib (p = 0.86 between treatment groups), ending in a moderately constricted state (9.2% ± 3.6 vehicle, 12.3% ± 3.1 celecoxib, p = 0.53 between treatment groups).
Dural veins did not respond to CSD (Fig. 1d), in both vehicle and celecoxib treated rats (two-way ANOVA interaction, F(71,1) = 0.86, p = 0.78).
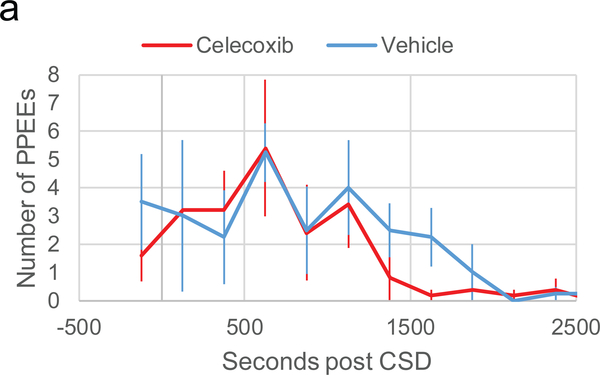
CSD-induced plasma protein extravasation events are unaffected by celecoxib
Plasma protein extravasation (PPE) is a leakage of proteins from blood vessels into the surrounding tissue. Previously, we have shown plasma protein can leak into the dura as discreet events at particular locations along a blood vessel, which we called plasma protein extravasation events (PPEEs). Each event was marked by a cloud of fluorescent expulsion that spreads from a small point along the endothelium, covering a small area nearby, and then partially diffusing away [citation]. We did not find any difference between vehicle and celecoxib treated animals in the amount of PPEEs post CSD (Fig 2, two-way ANOVA interaction, F(9,1) = 0.35 p = 0.95). In the first 750 s post CSD (the time of highest PPEE output), the average amount of PPEEs per 250 s bin was 4.2 ± 1.3 for vehicle and 4.3 ± 0.96 for celecoxib (p = 0.98 between treatment groups).
Figure 2.
Plasma protein extravasation events post-CSD in the presence of celecoxib or vehicle. Plot of plasma protein extravasation events counted per 250 s bin and averaged per group.
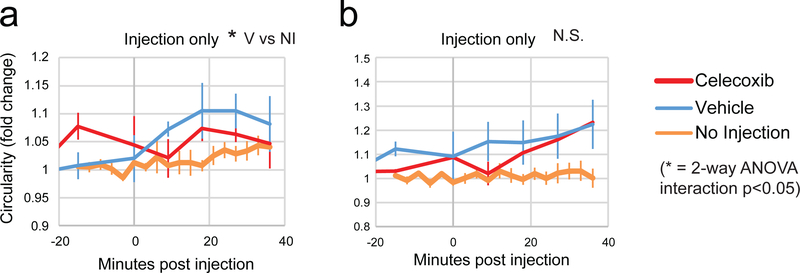
In the absence of CSD, celecoxib reduces vehicle-induced dural macrophage activation.
We have previously reported that CX3CR1-driven GFP-expressing macrophages in the pia and dura of transgenic mice become more round post CSD, and that this roundness is likely an indicator of pro-inflammatory macrophage activation. We therefore sought to test whether celecoxib was able to reduce the activation of macrophages post CSD. In order to do this, we first needed to test whether the injection of celecoxib or its vehicle itself had an effect on macrophage roundness. Therefore we imaged macrophages in the dura and pia of CX3CR1-GFP mice before and after injection of celecoxib or vehicle, and compared it to our previous data where we imaged for a similar total length of time with no injection. In the dura, injection of vehicle did cause macrophages to become significantly more round according to a 2-way ANOVA interaction compared to no injection (Fig. 3a, F(5,1) = 3.6, p = 0.017). Rounding of macrophages following injection of celecoxib, however, was not significantly different than mice that were not injected (Fig. 3a, F(5,1) = 1.5, p = 0.25 2-way ANOVA interaction), or mice injected with the vehicle (F(5,1) = 0.76, p = 0.59). Compared to no injection, in the pia, neither vehicle nor celecoxib induced significant rounding (Fig. 3b, F(5,1) = 2.5, p = 0.062 for vehicle, F(5,1) = 1.8, p = 0.16 for celecoxib). Comparison of celecoxib and vehicle showed no significant difference in rounding of macrophages in the pia (Fig. 3b, F(5,1) = 0.65, p = 0.66).
Figure 3.
Effect of celecoxib or vehicle on activation of dural and pial macrophages. (a-b) Plot of macrophage circularity normalized to a 40 m baseline (only 20 m of baseline is shown) before and after injection of celecoxib or vehicle in the (a) dura or (b) pia. Also shown is previously published data of control macrophages imaged for a similar amount of time with no injection. For (a), a 2-way ANOVA interaction was significant for vehicle vs no injection, but not celecoxib vs no injection. For all plots, red is celecoxib, blue is vehicle, and orange is no injection.
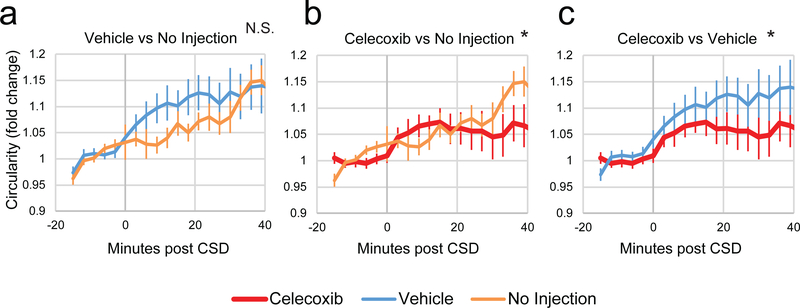
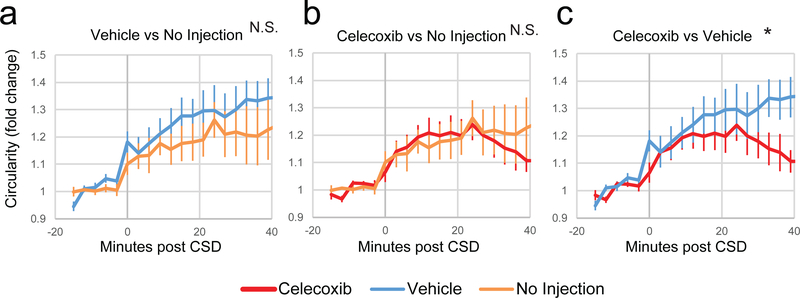
Celecoxib reduces CSD-induced dural macrophage activation.
CX3CR1-GFP mice were injected with vehicle or celecoxib and then macrophages in the dura and pia were imaged before and after CSD, and compared to previous data where mice were imaged before and after CSD with no injection. While vehicle injection in mice did not cause significantly more rounding on top of the rounding due to CSD in the dura (Fig. 4a, F(15,1)=1.0, p = 0.45, 2-way ANOVA interaction), injection of celecoxib significantly reduced rounding in the dura post CSD compared to no injection (Fig. 4b, F(15,1) = 3.5, p = 3.69*10−5, 2-way ANOVA interaction), as well as compared to vehicle (Fig. 4c, F(15,1) = 1.9, p = 0.03).
Figure 4.
Effect of celecoxib on CSD-induced activation of dural macrophages. All plots show normalized macrophage circularity before and after induction of CSD. Shown are comparisons between vehicle and previously published control data of macrophage circularity post-CSD with no injection (a), between celecoxib and no injection (b), and celecoxib and vehicle (c). At the top of the plots, significance of a 2-way ANOVA interaction is indicated. For all plots, red is celecoxib, blue is vehicle, and orange is no injection.
Celecoxib reduces CSD/vehicle combined effect on pial macrophage activation
In the pia, vehicle did not cause any significant changes in rounding post CSD when compared to no injection (Fig. 5a, F(15,1) = 0.67, p = 0.81, 2-way ANOVA interaction). Injection of celecoxib was also not different compared to no injection (Fig. 5b, F(15,1) = 1.2, p = 0.30). However, celecoxib was significantly different than vehicle (F(15,1) = 3.2, p = 1.2*10−4).
Figure 5.
Effect of celecoxib on CSD-induced activation of pial macrophages. All plots show normalized macrophage circularity before and after induction of CSD. Shown are comparisons between vehicle and previously published control data of macrophage circularity post-CSD with no injection (a), between celecoxib and no injection (b), and celecoxib and vehicle (c). At the top of the plots, significance of a 2-way ANOVA interaction is indicated. For all plots, red is celecoxib, blue is vehicle, and orange is no injection.
Discussion:
Using in vivo multiphoton imaging, we show here that celecoxib is capable of reducing CSD-induced dilatation of dural arteries, and activation of dural and pial macrophages but not dilatation or constriction of pial arteries and veins, or the occurrence of PPE. Given the role of macrophages in inflammation[49], the anti-inflammatory effects of celecoxib through inhibition of COX-2, and celecoxib’s changes in protein expression in macrophages[21], the findings that celecoxib is capable of attenuating macrophage activation is somewhat expected. Less expected, however, is the finding that celecoxib shortened the dilatation of dural arteries; a vascular event that is closely linked to migraine pathophysiology [1; 3; 5; 20]. While the debate over the role of the vasculature in migraine is far from being resolved [13; 18], it is worthwhile noting that effective anti-migraine drugs such as triptans and CGRP monoclonal antibodies, also reduce dilatation of dural arteries. Collectively, these findings suggest that celecoxib, and potentially other non-steroidal anti-inflammatory drugs ease the intensity of migraine headache and potentially terminate an attack by attenuating dural macrophages activation and arterial dilatation outside the blood brain barrier (BBB), and pial macrophages inside the BBB.
In principle, dilatation and constriction of dural arteries is mediated by smooth muscle cells whose contraction and relaxation tones are regulated by sensory, sympathetic and parasympathetic nerves that upon their stimulation release CGRP, substance P, neurokinins, PACAP, neuropeptide Y, norepinephrine, VIP, nitric oxide and other chemicals that control vascular tone in the meninges [6; 15; 16]. Theoretically, the attenuation of the duration of dural arterial dilatation by celecoxib following the occurrence of CSD could be mediated by a reduction in the release of one or more of these vasodilators from trigeminal sensory afferents (e.g., CGRP, SP, PACAP, neurokinin A) or parasympathetic efferents (e.g., VIP, Acetylcholine, NOS). Expression of constitutive COX-2 in dural axons containing CGRP [57] raise the possibility that COX-2 inhibition can directly reduce axonal firing and consequently synaptic release of vesicles containing these chemicals. Presence of CGRP in trigeminal [30; 34; 47] and parasympathetic [26; 43] axons calls attention to the possibility that COX-2 inhibition can potentially reduce synaptic release in both. Growing evidence for modulation of voltage-dependent calcium channels in the brain by prostaglandins [54], and suppression of voltage-dependent potassium and sodium currents in retinal ganglion cells by celecoxib [14] give some support to the view that COX-1/COX-2 inhibitors may achieve their analgesic effects not only by reducing inflammation but also by reducing neuronal firing and the consequential release of vasodilators and/or pro-inflammatory neuropeptides from their nerve endings. Another possibility is that celecoxib decreases the synthesis of vasodilating prostaglandins (such as PGE2, PGI2 and PGD2) whose receptors are found in smooth muscles of dural arteries and their infusion trigger headache[2].
At least 2 scenarios may account for celecoxib lack of effects on the responses of pial blood vessels to CSD. The first is that their responses to CSD are influenced mainly by their proximity to the cortex (i.e., by their anatomical and physiological coupling with events that alter the functions of cortical neurons and astrocytes) and to a lesser degree by sensory and autonomic innervation[8], and the second is that celecoxib does not reach the pia at a sufficient concentration. Given celecoxib effects on pial macrophages, however (see below), this explanation appears less likely.
Plasma protein extravasation has long been linked to migraine pathophysiology and the process by which abnormal brain functioning can give rise to the headache phase of a migraine attack[29; 32; 33]. Although attractive and supported by data, this hypothesis had been a subject of long and bitter debate which was fueled by opposing sets of data demonstrating prevention of PPE by drugs that terminate migraine[9; 25] as well as prevention of PPE by drugs that do not terminate migraine[12]. In agreement with the latter, the current study shows that celecoxib, a drug with proven efficacy in acute migraine treatment, does not prevent a CSD-induced PPE. While it is tempting to suggest that our findings support the view that PPE may not be critically relevant to the activation of the nociceptors by CSD or the initiation of headache by aura, we cannot rule out the possibility that celecoxib site of action is downstream from the occurrence of PPE. Further complicating this discussion are studies showing that both COX-1/COX-2 inhibitors[10] as well as COX-2 only inhibitors[42] reduce occurrence of PPE in animals in which PPEEs were induced by stimulation of trigeminal afferent rather than CSD. As discussed in our recent study[39], the occurrence of PPE may be correlated with the dilatation of dural arteries if such dilatation was triggered by CSD, but, as shown in that study, the PPE can be dissociated from the arterial dilatation, for example if such dilatation was triggered by CGRP infusion alone. Regardless of the complexity of factors that determine occurrence of CSD, our findings suggest that while CSD-induced dilatation of dural arteries is partially dependent on COX-2 synthesis of prostaglandins, the resultant PPE appears independent of trigeminal afferents activation (since PPE induction by trigeminal nerve stimulation was blocked by COX-2 inhibitor) or alternatively, is mediated by yet unknown events.
Macrophages are white blood cells that phagocyte cellular debris, microbes or anything else that may appear during disease [23]. Beside phagocytosis, they play important role in both innate and adaptive immunity by facilitating the recruitment of other immune cells to sites of inflammation [53]. Secondary to their pivotal role in immunity and the inflammation, macrophages are capable of releasing molecules (e.g., cytokines) that activate and sensitize nociceptors through multiple signaling cascades that eventually lead to increased neuronal firing and pain [52]. Relevance to the current study, we showed recently that CSD causes pial and dural macrophages, which are present mainly around blood vessels, to retract their podia (cytoplasmic processes), and interpreted this behavior as an indication of activation [37; 50; 51]. Time-wise, we noted that while pial macrophages became activated minutes after occurrence of CSD, dural macrophages became activated 20 min later; suggesting that different mechanisms may be responsible for the activation of these immune cells in the pia and dura. The delayed activation of dural macrophages provides a logical explanation for our current observation regarding the delayed effect of celecoxib on the activation of dural macrophages by CSD (i.e., it seems to prevent their activation only when they eventually become active). What is surprising, however, is that in pia, where macrophages activation begins immediately after occurrence of CSD, celecoxib ability to prevent their activation is also delayed by about 20 minutes. A reasonable way to interpret these findings is to suggest that celecoxib can prevent the activation of dural macrophages and inhibit (deactivate) the already-activated pial macrophages. Mechanistically, the prevention of macrophages activation by celecoxib suggest that COX-2 may play a role in their activation and that inhibition of prostaglandins synthesis in dural and pial immune cells or neurons can mediate the celecoxib effects. Presence of COX-2 in meningeal macrophages and sensory or parasympathetic nerve terminals lends further support to this notion.
Technical note:
Because celecoxib cannot be diluted in saline alone, it was diluted in a vehicle containing PEG-400. As shown in the results, PEG-400 may have caused some activation of meningeal macrophages that was independent of CSD. While this effect of the vehicle was undesirable, it serves to strengthen the proof that celecoxib inhibits the activation of meningeal macrophages as it had to counter their activation by CSD as well as by the vehicle.
Acknowledgement:
This study was supported by a grant from Dr. Reddy’s Laboratories Inc., and NIH grants R37-NS079678, RO1 NS069847, RO1 NS094198 (RB). Conflict of interest disclosures: Dr. Reddy’s Laboratories Inc. holds the patent for DFN-15. DFN15 is being developed for migraine indication. Celecoxib is the active ingredient of DFN15. Dr. Reddy’s Laboratories Inc. funded parts of the study. Dr. Burstein is a consultant to Dr. Reddy.
References
- [1].Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, de Koning PJ, Andersen MR, Larsson HB, Fahrenkrug J, Olesen J, Ashina M. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014;137(Pt 3):779–794. [DOI] [PubMed] [Google Scholar]
- [2].Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandins in migraine: update. Curr Opin Neurol 2013;26(3):269–275. [DOI] [PubMed] [Google Scholar]
- [3].Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol 2011;69(4):635–645. [DOI] [PubMed] [Google Scholar]
- [4].Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008;48(8):1157–1168. [DOI] [PubMed] [Google Scholar]
- [5].Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002;8(2):136–142. [DOI] [PubMed] [Google Scholar]
- [6].Brennan KC, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol 2010;23(3):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 2015;35(17):6619–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol 2008;86(4):379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buzzi MG, Moskowitz MA, Peroutka SJ, Byun B. Further characterization of the putative 5-HT receptor which mediates blockade of neurogenic plasma extravasation in rat dura mater. Br J Pharmacol 1991;103(2):1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Buzzi MG, Sakas DE, Moskowitz MA. Indomethacin and acetylsalicylic acid block neurogenic plasma protein extravasation in rat dura mater. Eur J Pharmacol 1989;165(2–3):251–258. [DOI] [PubMed] [Google Scholar]
- [11].Costa C, Tozzi A, Rainero I, Cupini LM, Calabresi P, Ayata C, Sarchielli P. Cortical spreading depression as a target for anti-migraine agents. J Headache Pain 2013;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Diener HC, Group RPRS. RPR100893, a substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia 2003;23(3):183–185. [DOI] [PubMed] [Google Scholar]
- [13].Dodick DW. Examining the essence of migraine--is it the blood vessel or the brain? A debate. Headache 2008;48(4):661–667. [DOI] [PubMed] [Google Scholar]
- [14].Frolov RV, Slaughter MM, Singh S. Effects of celecoxib on ionic currents and spontaneous firing in rat retinal neurons. Neuroscience 2008;154(4):1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 1988;23(2):193–196. [DOI] [PubMed] [Google Scholar]
- [16].Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28(2):183–187. [DOI] [PubMed] [Google Scholar]
- [17].Hadjikhani N, Vincent M. Neuroimaging clues of migraine aura. J Headache Pain 2019;20(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jacobs B, Dussor G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016;338:130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013;339(6123):1092–1095. [DOI] [PubMed] [Google Scholar]
- [20].Khan S, Amin FM, Christensen CE, Ghanizada H, Younis S, Olinger ACR, de Koning PJH, Larsson HBW, Ashina M. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain 2019;142(1):93–102. [DOI] [PubMed] [Google Scholar]
- [21].Kondreddy VK, Kamatham AN. Celecoxib, a COX-2 inhibitor, synergistically potentiates the anti-inflammatory activity of docosahexaenoic acid in macrophage cell line. Immunopharmacol Immunotoxicol 2016;38(2):153–161. [DOI] [PubMed] [Google Scholar]
- [22].Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994;117(Pt 1):199–210. [DOI] [PubMed] [Google Scholar]
- [23].Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015;15(12):731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leao A. Spreading depression of activity in cerebral cortex. J Neurophysiol 1944;7:359–390. [DOI] [PubMed] [Google Scholar]
- [25].Lee WS, Limmroth V, Ayata C, Cutrer FM, Waeber C, Yu X, Moskowitz MA. Peripheral GABAA receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance P and trigeminal stimulation. Br J Pharmacol 1995;116(1):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience 1985;15(4):1227–1237. [DOI] [PubMed] [Google Scholar]
- [27].Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001;41(7):638–645. [DOI] [PubMed] [Google Scholar]
- [28].Lipton RB, Scher AI, Steiner TJ, Bigal ME, Kolodner K, Liberman JN, Stewart WF. Patterns of health care utilization for migraine in England and in the United States. Neurology 2003;60(3):441–448. [DOI] [PubMed] [Google Scholar]
- [29].Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci 1987;7(12):4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A 1986;83(15):5731–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM, Burstein R. Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody. J Neurosci 2017;37(30):7149–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moskowitz MA. Basic mechanisms in vascular headache. Neurol Clin 1990;8(4):801–815. [PubMed] [Google Scholar]
- [33].Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev 1993;5(3):159–177. [PubMed] [Google Scholar]
- [34].O’Connor TP, van der Kooy D. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J Neurosci 1986;6(8):2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pardutz A, Schoenen J. NSAIDs in the Acute Treatment of Migraine: A Review of Clinical and Experimental Data. Pharmaceuticals (Basel) 2010;3(6):1966–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pietrobon D, Brennan KC. Genetic mouse models of migraine. J Headache Pain 2019;20(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schain AJ, Melo-Carrillo A, Borsook D, Grutzendler J, Strassman AM, Burstein R. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol 2018;83(3):508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci 2017;37(11):2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R. CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP’s Role in Migraine with Aura. J Neurosci 2019;39(30):6001–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Scher AI, Lipton RB, Stewart WF, Bigal M. Patterns of medication use by chronic and episodic headache sufferers in the general population: results from the frequent headache epidemiology study. Cephalalgia 2010;30(3):321–328. [DOI] [PubMed] [Google Scholar]
- [41].Schroeder BM. AAFP/ACP-ASIM release guidelines on the management and prevention of migraines. Am Fam Physician 2003;67(6):1392, 1395–1397. [PubMed] [Google Scholar]
- [42].Schuh-Hofer S, Tayefeh M, Reuter U, Dirnagl U, Arnold G. Effects of parecoxib on plasma protein extravasation and c-fos expression in the rat. Headache 2006;46(2):276–285. [DOI] [PubMed] [Google Scholar]
- [43].Silverman JD, Kruger L. Calcitonin-gene-related-peptide-immunoreactive innervation of the rat head with emphasis on specialized sensory structures. Journal of Comparative Neurology 1989;280(2):303–330. [DOI] [PubMed] [Google Scholar]
- [44].Snow V, Weiss K, Wall EM, Mottur-Pilson C. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med 2002;137(10):840–849. [DOI] [PubMed] [Google Scholar]
- [45].Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992;267(1):64–69. [PubMed] [Google Scholar]
- [46].Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996;384(6609):560–564. [DOI] [PubMed] [Google Scholar]
- [47].Tajti J, Uddman R, Moller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 1999;76(2–3):176–183. [DOI] [PubMed] [Google Scholar]
- [48].Tfelt-Hansen P. Triptans vs other drugs for acute migraine. Are there differences in efficacy? A comment. Headache 2008;48(4):601–605. [DOI] [PubMed] [Google Scholar]
- [49].Valledor AF, Comalada M, Santamaria-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol 2010;108:1–20. [DOI] [PubMed] [Google Scholar]
- [50].Vereyken EJ, Heijnen PD, Baron W, de Vries EH, Dijkstra CD, Teunissen CE. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation 2011;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vogel DY, Heijnen PD, Breur M, de Vries HE, Tool AT, Amor S, Dijkstra CD. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation 2014;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol 2004;61(1):55–71. [DOI] [PubMed] [Google Scholar]
- [53].Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yagami T, Koma H, Yamamoto Y. Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System. Mol Neurobiol 2016;53(7):4754–4771. [DOI] [PubMed] [Google Scholar]
- [55].Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 2011;69(5):855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 2010;30(26):8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang XC, Kainz V, Jakubowski M, Burstein R, Strassman A, Levy D. Localization of COX-1 and COX-2 in the intracranial dura mater of the rat. Neurosci Lett 2009;452(1):33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]