Abstract
Background
Stage 1 of the NIA-AA’s proposed Alzheimer’s disease (AD) continuum is defined as β-amyloid (Aβ) positive but cognitively normal. Identifying at-risk individuals before Aβ reaches pathological levels could have great benefits for early intervention. Although Aβ levels become abnormal long before severe cognitive impairments appear, increasing evidence suggests subtle cognitive changes may begin early, potentially before Aβ surpasses the threshold for abnormality. We examined whether baseline cognitive performance would predict progression from normal to abnormal levels of Aβ.
Methods
We examined the association of baseline cognitive composites (Preclinical Alzheimer Cognitive Composite [PACC]; ADNI memory factor score [ADNI_MEM]) with progression to Aβ-positivity in 292 non-demented, Aβ-negative Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants. Additional analyses included continuous CSF biomarker levels to examine the effects of subthreshold pathology.
Results
Forty participants progressed to Aβ-positivity during follow-up. Poorer baseline performance on both cognitive measures was significantly associated with increased odds of progression. More abnormal levels of baseline CSF p-tau and subthreshold Aβ were associated with increased odds of progression to Aβ-positivity. Nevertheless, baseline ADNI_MEM performance predicted progression even after controlling for baseline biomarker levels and APOE genotype (PACC was trend level). Survival analyses were largely consistent: controlling for baseline biomarker levels, baseline PACC still significantly predicted progression time to Aβ-positivity (ADNI_MEM was trend level).
Conclusions
The possibility of intervening before Aβ reaches pathological levels is of obvious benefit. Low cost, non-invasive cognitive measures can be informative for determining who is likely to progress to Aβ-positivity, even after accounting for baseline subthreshold biomarker levels.
Keywords: biomarker trajectories, β-amyloid, cognition, amyloid accumulation, Alzheimer’s disease (AD), mild cognitive impairment (MCI)
INTRODUCTION
Given its long prodromal period, Alzheimer’s disease (AD) treatment should begin as early as possible (1). Early intervention may be possible after identifying Aβ-positive individuals who are still cognitively normal, defined as preclinical/Stage 1 of the AD continuum proposed by the National Institute on Aging-Alzheimer’s Association (NIA-AA) research framework (2). Yet being Aβ-positive means significant pathology is already present. It may be critically important to identify at-risk individuals before they develop substantial amyloid burden (i.e., at Stage 0) to improve treatment efficacy and slow progression to AD dementia.
Examinations of AD biomarkers primarily focus on biomarkers as predictors of cognitive decline, but here our focus was on biomarker positivity as an outcome. Abnormal biomarkers precede clinical symptom onset by years or even decades (3–5). However, there is also evidence that cognition demonstrates subtle change earlier than is typically appreciated. Cognition begins to show accelerated change across individuals with a range of baseline Aβ values, including those who are Aβ-negative (6, 7). Delayed recall has been shown to demonstrate accelerating change prior to other biomarker and clinical measures (8–10). Change in amyloid is also correlated with change in cognition (11, 12). Thus, Aβ accumulation, including subthreshold levels, is related to concurrent or future cognitive outcomes. However, none of these studies addressed whether baseline cognitive performance can predict progression to Aβ-positivity as an outcome. According to the NIA-AA framework staging, Aβ-positivity precedes cognitive impairment, consistent with serial models of AD trajectories. Here, we examined whether baseline cognition among Aβ-negative individuals could predict later progression to Aβ-positivity, even among cognitively unimpaired individuals.
Increasing postmortem evidence indicates that abnormal tau appears in the brainstem during the earliest stages of AD – potentially before cortical Aβ plaque deposition – and tau is associated with poorer memory performance even in the absence of Aβ (13–16). However, individuals classified as A−/T+ are not considered to be on the AD continuum. Although tau deposition in the absence of Aβ might be age-related rather than Alzheimer’s-related, we also examined whether individuals with elevated tau would be more likely to progress to Aβ-positivity, indicating increased risk of AD.
METHODS
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
Participants from the ADNI-1, ADNI-GO, and ADNI-2 cohorts were included if they 1) had valid cognitive and cerebrospinal fluid (CSF) Aβ and phosphorylated tau (p-tau) data at baseline, 2) had at least one follow-up of amyloid data based on CSF or positron emission tomography (PET), 3) were Aβ-negative at baseline, and 4) did not have a diagnosis of Alzheimer’s dementia at baseline (see Table 1 for participant characteristics). In total, baseline and follow-up amyloid status were based on 585 assessments of CSF Aβ, 646 florbetapir PET scans, and 10 11C-Pittsburgh Compound B (PIB) scans. Individuals were classified as Aβ-stable if they had no abnormal amyloid levels at any follow-up, or as Aβ-converter if they showed evidence of abnormal Aβ at a follow-up assessment. Individuals who were Aβ-positive at multiple assessments followed by a subsequent reversion to Aβ-negative status on only a single timepoint were included as Aβ-converters. Individuals who were only Aβ-positive at one assessment followed by reversion to Aβ-negative were excluded (n=9). Individuals diagnosed as having MCI in ADNI (17) were included if they were Aβ-negative at baseline because our focus was to determine whether poorer cognition may precede amyloid positivity, and some of these Aβ-negative individuals with MCI may progress to Aβ-positive. Excluding them would truncate the distribution of cognitive performance, our predictor of primary interest. A total of 292 individuals were included (252 Aβ-stable, 40 Aβ-converters). Despite being Aβ-negative, 138 (47.3%) were diagnosed with MCI at baseline.
Table 1. Baseline sample characteristics of Aβ-stable versus Aβ-converters.
Descriptive statistics of Aβ-stable and Aβ-converter participants at baseline. Mean (SD) presented for continuous variables, count (%) presented for categorical variables. An asterisk indicates a significant (p < 0.05) difference between the two groups.
| Measures (units) | Aβ-stable | Aβ-converter |
|---|---|---|
| n | 252 | 40 |
| Age (years) | 71.62 (7.20) | 71.69 (6.71) |
| Gender (male) | 128 (50.8%) | 25 (62.5%) |
| APOE-ε4 status (ε4+) | 41 (16.3%) | 12 (30.0%) |
| MCI Diagnosis (MCI) | 117 (46.4%) | 21 (52.5%) |
| Education* (years) | 16.21 (2.56) | 17.20 (2.22) |
| Length of follow-up (years)* | 3.22 (1.59) | 4.30 (2.44) |
| ADNI_MEM | 0.89 (0.68) | 0.70 (0.59) |
| PACC | −1.32 (3.31) | −1.97 (3.03) |
Procedures were approved by the Institutional Review Board of participating institutions and informed consent was obtained from all participants.
CSF and amyloid imaging measures
CSF samples were collected and processed as previously described (18). CSF Aβ42 and p-tau were measured with the fully automated Elecsys immunoassay (Roche Diagnostics) by the ADNI biomarker core (University of Pennsylvania). Established cutoffs designed to maximize sensitivity in the ADNI study population were used to classify biomarker positivity [Aβ+: Aβ42<977 pg/mL; p-tau+: p-tau>21.8 pg/mL] (http://adni.loni.usc.edu/methods) (19).
PET Aβ data were processed according to previously published methods (http://adni.loni.usc.edu/methods) (20, 21). Mean standardized uptake value ratios (SUVR) were taken from a set of regions including frontal, temporal, parietal and cingulate cortices using whole cerebellum (florbetapir) or cerebellar gray matter (PIB) as a reference region. Established cutoffs to determine Aβ+ were used for PIB-PET (SUVR>1.44) and florbetapir-PET (SUVR>1.11) (20).
CSF Aβ assessment was more common at earlier study timepoints, whereas PET assessments became more common at later timepoints. We included both modalities to maximize the number of individuals with baseline data and increase the length of follow-up assessment for dichotomized outcomes. However, it was necessary to restrict analyses of continuous baseline values to a single modality so that values were equivalent. CSF was chosen to examine continuous levels of baseline Aβ because it was available for more participants compared with PET.
Cognitive measures
We used two composite measures of baseline cognition. ADNI_MEM is based on a factor model of scores from four episodic memory tests: Rey Auditory Verbal Learning Test, Alzheimer’s Disease Assessment Schedule–Cognition (ADAS-Cog) word list and recognition, Mini-Mental State Examination (MMSE) word recall, and Logical Memory immediate and delayed recall (22). The Preclinical Alzheimer Cognitive Composite (PACC) (23, 24) is designed to detect amyloid-related cognitive decline and is based on delayed recall from the ADAS-Cog and Logical Memory, MMSE total score, and Trails B time. ADNI_MEM and PACC scores were converted to z-scores and coded such that higher scores reflect poorer performance. In a secondary analysis, we examined the ADNI_EF factor score (25) to test whether a composite baseline executive function measure also predicted conversion to Aβ-positivity.
Covariates
Age and APOE genotype (ε4+ vs. ε4−) were included because of their association with increased amyloid (26). Although age and cognitive performance are correlated, the variance inflation factor (VIF) for these variables was ≤1.30 in all models, well below the common threshold indicating excessive multicollinearity. Length of follow-up was included to account for its effect on odds of observing eventual progression to Aβ-positivity. Education was included to account for long-standing differences in cognitive ability or cognitive reserve that might influence the relationship between amyloid and cognition. In other analyses, baseline biomarkers were included to assess the effect of AD-related pathology on progression to Aβ-positivity. P-tau status (p-tau+ vs. p-tau-) was included to account for differences in cognition due to other AD-related pathology. An additional set of models included continuously measured CSF Aβ42 and p-tau as covariates to determine whether subthreshold levels of pathology predict later progression to Aβ-positivity. These measures were converted to z-scores and values of CSF Aβ42 were reverse coded such that higher values of both indicated abnormality.
Statistical analysis
We tested Aβ-stable and Aβ-converter groups for differences in the covariates using χ2 and t-tests. Logistic regression models were used to test whether baseline cognition in Aβ-negative individuals was associated with increased odds of future progression to Aβ-positivity. We chose this approach over a generalized linear mixed-effects (GLMM) logistic regression that includes data from all timepoints because the issue of primary interest was the odds of progressing to Aβ-positivity at any point during follow-up, not the odds of being Aβ-positive at each individual timepoint (see Supplemental Material for further discussion). The first set of models separately tested the ADNI_MEM and PACC, with baseline cognitive performance on these measures as predictors and group (Aβ-stable or Aβ-converter) as the outcome. The second set of models additionally included p-tau status to assess whether lower cognitive performance was driven by abnormal levels of p-tau, the other hallmark AD pathology. Although no subject met criteria for abnormal Aβ at baseline, that does not mean they were completely pathology-free. Therefore, we ran a third set of models to determine whether poorer baseline cognition was driven by sub-threshold levels of amyloid or tau. These models included continuous levels of CSF Aβ42 and p-tau as predictors. All models included age at baseline, APOE genotype (ε4+ vs. ε4−), education, and length of follow-up as covariates. To determine whether effects were driven primarily by the subgroup with MCI at baseline, we conducted follow-up analyses excluding those individuals.
We also examined Cox proportional hazards models to test the association of baseline cognitive performance with time to conversion to Aβ-positive (or censored at last follow-up). Two sets of models were run: the first included baseline cognitive performance as the predictor of interest; the second added continuous levels of baseline CSF Aβ42 and p-tau. These models additionally controlled for age at baseline, APOE genotype, and education. The survival analyses are useful for directly addressing the question of differential follow-up time. However, they consider individuals with differential times to conversion differently, and the use of multiple modalities may further affect time to conversion. Because our primary question of interest was about progression to Aβ-positivity at any point during follow-up rather than its time to progression, we consider these models to be supplemental to the primary logistic regression analyses. Analyses were conducted with R version 3.5 (27).
RESULTS
Descriptive statistics
Descriptive statistics are presented in Table 1 and Table 2. There were no significant differences between groups for age (P=0.94), gender (P=0.18), or proportion of individuals with MCI (P=0.47). Aβ-converters were more likely to be APOE-ε4+ at a trend level (P=0.08). The Aβ-converter group had a higher average education (17.3 vs. 16.2 years; t=2.78, P=0.007). Follow-up interval was significantly longer for the Aβ-converter group (4.22 vs. 3.23 years; t=2.50, P=0.02). The mean time between baseline cognitive testing and the assessment at which Aβ-converters first demonstrated progression to Aβ-positivity was 2.8 years (interquartile range: 1.98–4.01 years). Of the 138 individuals who were Aβ-negative and had MCI at baseline, 21 (15%) progressed to Aβ-positivity. The MCI group did not have significantly different levels of baseline CSF Aβ (P=0.119) or p-tau (P=0.930) compared to cognitively normal participants. However, individuals with MCI who progressed to Aβ-positivity did have lower baseline CSF Aβ (t=3.158, P=0.004) and higher p-tau (t=2.389, P=0.024) compared to those with MCI that did not (see Supplemental Table S1).
Table 2. Baseline sample characteristics of cognitively normal versus mild cognitive impairment.
Descriptive statistics of cognitively normal participants versus those with mild cognitive impairment at baseline. Mean (SD) presented for continuous variables, count (%) presented for categorical variables. An asterisk indicates a significant (p < 0.05) difference between the two groups.
| Measure (units) | Cognitively Normal | MCI |
|---|---|---|
| n | 154 | 138 |
| Age (years) | 72.67 (5.97) | 70.47 (8.09) |
| Gender (male) | 80 (51.9%) | 73 (52.9%) |
| APOE-ε4 status (ε4+) | 25 (16.2%) | 28 (20.3%) |
| Education* (years) | 16.50 (2.50) | 16.18 (2.57) |
| Baseline CSF Aβ* (pg/ml) | 1488.68 (233.40) | 1443.50 (260.50) |
| Baseline CSF P-tau* (pg/ml) | 19.47 (5.75) | 19.54 (7.86) |
| ADNI_MEM* | 0.59 (0.69) | 0.46 (0.57) |
| PACC* | −3.25 (3.15) | −3.40 (2.42) |
Baseline cognition predicts progression to Aβ-positivity during follow-up
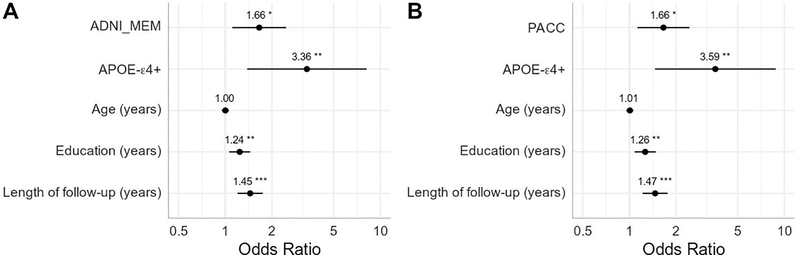
In the first set of models, Aβ-converters were also more likely to be APOE-ε4+, have more education, and longer duration of follow-up. Age was not significantly associated with progression to Aβ-positivity in either model. After accounting for covariates, individuals with poorer performance on either cognitive composite at baseline showed higher odds of progressing to Aβ-positivity at follow-up (ADNI_MEM: OR=1.66, P=0.013; PACC: OR=1.66, P=0.01). Full results of the regression models are presented in Figure 1.
Figure 1. Baseline cognitive performance predicting future conversion to Aβ-positivity.
Results of two logistic regression models using A) the ADNI Memory composite (ADNI_MEM) and B) the Preclinical Alzheimer Cognitive Composite (PACC). Measures are all taken from baseline and predict future progression to Aβ-positivity. Cognitive scores were converted to z-scores and reverse coded such that higher scores indicate poorer performance. Odds ratios are presented with asterisks indicating significant estimates (*p<0.05, **p<0.01, ***p<0.001). Lines represent 95% confidence intervals.
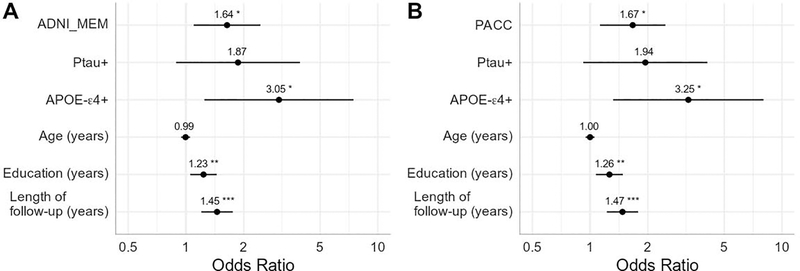
The second set of models included a dichotomous classification for baseline CSF p-tau (Figure 2). Aβ-converters were again more likely to be APOE-ε4+, have more education, and longer duration of follow-up. Age and dichotomous p-tau status were not significantly associated with progression to Aβ-positivity in either model. After controlling for covariates, poorer baseline performance on either cognitive composite remained significantly associated with increased odds of progressing to Aβ-positivity at follow-up (ADNI_MEM: OR=1.64, P=0.016; PACC: OR=1.67, P=0.011).
Figure 2. Baseline cognitive performance and p-tau+ status predicting future conversion to Aβ-positivity.
Results of two logistic regression models using A) the ADNI Memory composite (ADNI_MEM) and B) the Preclinical Alzheimer Cognitive Composite (PACC). Measures are all taken from baseline and predict future progression to Aβ-positivity. Cognitive scores were converted to z-scores and reverse coded such that higher scores indicate poorer performance. P-tau-positivity is entered as a dichotomous variable. Odds ratios are presented with asterisks indicating significant estimates (*p<0.05, **p<0.01, ***p<0.001). Lines represent 95% confidence intervals.
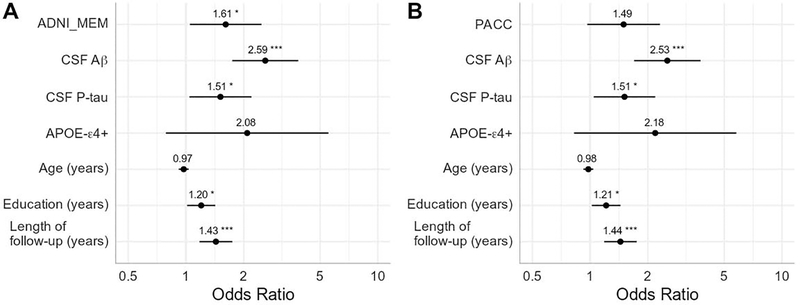
The third set of models addressed the question of whether subthreshold levels of AD pathology could account for the effect of lower cognitive performance on progression by including continuous CSF Aβ and p-tau measures (Figure 3). More abnormal baseline CSF Aβ and p-tau were associated with increased odds of progression to Aβ-positivity (CSF Aβ: OR=2.53 – 2.59, P<0.001; CSF p-tau: OR=1.51, P=0.03). Note that for CSF Aβ, these values were all in the normal range according to standard cut-offs. After controlling for baseline biomarkers, the performance on the ADNI_MEM remained a significant predictor (OR=1.61, P=0.03), but the effect of the PACC was reduced to trend level (OR=1.49, P=0.071). Education and length of follow-up remained significant predictors of progression, whereas the effect of APOE-ε4 status was reduced to trend level.
Figure 3. Baseline cognitive performance and continuous measures of CSF Aβ and p-tau predicting future conversion to Aβ-positivity.
Results of two logistic regression models using A) the ADNI Memory composite (ADNI_MEM) and B) the Preclinical Alzheimer Cognitive Composite (PACC). Measures are all taken from baseline and predict future progression to Aβ-positivity. Cognitive scores were converted to z-scores and reverse coded such that higher scores indicate poorer performance. CSF Aβ and P-tau were entered as continuous variables. Both measures were z-scored and CSF Aβ was reverse coded such that higher values on both indicates abnormality. Odds ratios are presented with asterisks indicating significant estimates (*p<0.05, **p<0.01, ***p<0.001). Lines represent 95% confidence intervals.
To determine whether these results may be driven by the MCI participants, we conducted analyses on cognitively normal and MCI groups separately. The large drop in sample size resulted in non-significant results for most analyses, but effect sizes of cognition predicting progression to Aβ-positivity tended to be larger for the cognitively normal group.
Baseline performance on ADNI_EF also significantly predicted later conversion at Aβ-positivity. This effect remained when including dichotomous p-tau status, but became non-significant when including continuous levels of baseline CSF Aβ and p-tau. (See Supplemental Table S2.)
Baseline cognition predicts progression time to Aβ-positivity
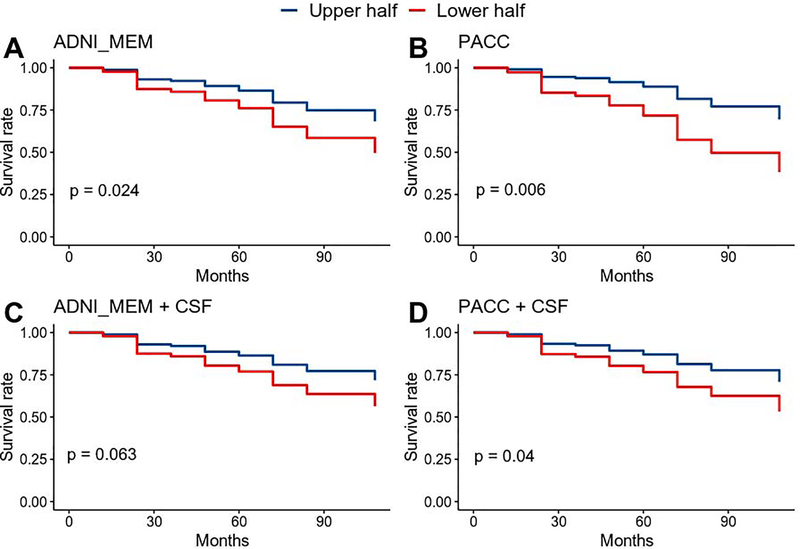
The Cox models were largely consistent with the logistic regression models (Figure 4, Supplemental Figure S1). In models including only baseline cognitive performance and covariates, APOE-ε4 and higher education were associated with significantly higher risk whereas age was not. After accounting for covariates, lower cognitive performance was associated with increased risk of progression to Aβ-positivity (ADNI_MEM: HR=1.48, P=0.024; PACC: HR=1.61, P=0.006).
Figure 4. Survival estimates of progression to Aβ-positivity based on baseline cognitive performance.
Cox proportional hazard models were run using continuous measures of baseline performance. For display purposes, scores were grouped based on a median split and adjusted survival curves are shown for better (upper half) and worse (lower half) performance on baseline cognitive measures. Results from 4 models are presented: A) ADNI Memory composite (ADNI_MEM) + covariates; B) the Preclinical Alzheimer Cognitive Composite (PACC) + covariates; C) ADNI_MEM + covariates + baseline CSF Aβ and p-tau; D) PACC + covariates + baseline CSF Aβ and p-tau. CSF Aβ and P-tau were entered as continuous variables. Covariates include: APOE-ε4+ status, age at baseline, and education. P-values of hazard ratios for cognitive measures are shown for each model.
Additional Cox models were conducted including baseline CSF Aβ and p-tau to assess the impact of subthreshold pathology on risk of progression to Aβ-positivity (Figure 4, Supplemental Figure S2). More abnormal baseline CSF Aβ and p-tau were associated with increased risk of progression to Aβ-positivity (CSF Aβ: HR=2.3, P<0.001; CSF p-tau: OR=1.5, P<0.001). The PACC remained significantly associated with increased risk of progression (HR=1.45, P=0.04) whereas the effect of the ADNI_MEM was reduced to trend level (HR=1.41, P=0.063). Age was not associated with increased effects, and both APOE-ε4 and education were reduced to trend level.
DISCUSSION
Cognitive function predicts Aβ-positivity
Here we found that cognition can be a useful early risk indicator. The ability to identify individuals at risk before substantial Aβ accumulation would enhance prospects for slowing AD progression and may be useful for selection of participants in clinical trials. The NIA-AA research framework represents a move toward defining AD as a biological construct (2). However, as noted by the NIA-AA workgroups on diagnostic guidelines for AD (28), behavioral markers may still hold great promise for early identification. Cognitive measures can predict progression from MCI to AD as well as or better than biomarkers (29–32). It is not surprising that cognitive measures predict future cognition, but we found that cognitive measures can predict progression to Aβ-positivity even after accounting for baseline biomarker levels. Furthermore, composite measures such as those used here may provide substantial boosts in sensitivity compared to individual test scores (33, 34).
Impact of subthreshold Aβ
Why would cognition would predict future accumulation of AD pathology? There may be several potential explanations. Pathological processes may already be underway, and lower cognitive function may represent decline driven by subthreshold pathology. In a smaller (n=35) study of ADNI participants, baseline cognition did not predict later progression to Aβ-positivity (35). However, with the larger sample in our analysis, cognitive function was a significant predictor. Controlling for subthreshold Aβ in our analysis attenuated the effect of cognition, lending support to the idea that even low levels of Aβ are at least partially contributing to lower cognitive performance. This fits with growing evidence that subthreshold levels of Aβ are clinically relevant. Cognitive tests at this early stage seem to be more sensitive than dichotomous classifications of biomarker abnormality at current detection thresholds. As biomarker measures become more sensitive, classification of biomarker abnormality may more consistently appear before cognitive differences.
On the other hand, cognition still predicted future progression to Aβ-positivity even after controlling for subthreshold Aβ. Therefore, cognitive performance contributes independent information, and the effect is not driven solely by individuals closer to the Aβ-positivity threshold. Cognitive testing early on is also more practical, non-invasive, and far less costly than CSF or PET biomarkers.
Although CSF and PET measure different aspects of the amyloid process, both are considered valid indicators of abnormal Aβ and use of both is consistent with the goals of the A/T/(N) framework. On the other hand, it may introduce some inconsistencies such as timing of conversion. Of the 40 Aβ-converters, only 6 (15%) were based on different modalities (baseline CSF-negative; follow-up PET-positive), largely because later follow-ups were with PET. Moreover, these measures show high concordance (36–38) such that it is likely that if an individual is positive on one, it is likely they would be positive on the other at some point in the near future. Most importantly, our primary analyses only assess if—not when—someone converts to Aβ-positivity, which should mitigate differences in these modalities.
The relevance of subthreshold pathology also has implications for the use of dichotomous versus continuous biomarker measures. Some have argued that making Aβ thresholds less conservative may improve sensitivity without a substantial sacrifice of specificity (39). Our findings suggest that current thresholds may not detect meaningful early Aβ accumulation, so the development of thresholds optimized for detecting the earliest stages of Aβ deposition is an important goal. Analysis of continuous measures should also be conducted when possible because continuous and binary A/T/(N) measures may lead to inconsistent inferences. An alternative approach is to examine Aβ accumulation over time. Several studies have examined individuals who do not meet the criteria for abnormal Aβ but do demonstrate evidence of change in Aβ (11, 12, 40–42). These studies find that change in Aβ levels is correlated with concurrent cognitive decline, commonly assumed to result from Aβ accumulation. Here we shifted the focus earlier in time and found that baseline cognition itself can predict later Aβ accumulation.
Non-AD-related processes and ordering of AD-related changes
An alternative explanation for cognition predicting Aβ-positivity is that lower cognitive function at baseline may result from non-AD-related processes. Individuals who progress to MCI while being Aβ-negative exhibit different biomarkers and cognitive profiles and tend to be on a non-AD trajectory (43). As a whole, the Aβ-negative MCI group in our analysis did not differ from the cognitively normal group on baseline Aβ or p-tau, perhaps suggesting a non-AD etiology for cognitive impairment. However, the significant association between baseline cognition and later Aβ-positivity suggests that such processes are still somehow a risk factor for AD. Indeed, 15% of Aβ-negative MCI participants did progress to Aβ-positivity, at which point they would be classified as Stage 3 in the AD continuum. This 15% had more abnormal levels of baseline Aβ (although still subthreshold) and p-tau compared to MCI participants that did not progress, suggesting that AD pathology may at least partially contribute to their cognitive impairment. Some individuals may be more sensitive to the effects of Aβ such that even subthreshold levels result in cognitive impairment.
It is, of course, possible to have mixed etiology driving impairment whether it appears before or after an individual surpasses the threshold for Aβ-positivity. Although the A/T/(N) framework is agnostic to the sequence of AD-related changes (44), these Aβ-negative (A-) MCI cases would not be considered to be on the AD continuum. As such, cognitive impairment prior to Aβ-positivity is assumed to have a non-AD etiology. However, as pointed out in the NIA-AA framework, it is also uncertain that cognitive impairment arising after Aβ-positivity is solely due to AD pathology (2). Indeed, it is well known that there can be significant AD pathology without cognitive impairment (45–47).Although the proposed NIA-AA research framework staging captures the typical progression, it will be beneficial to maintain a degree of flexibility to account for individuals who may progress through these stages in a non-typical trajectory.
Tau-PET studies find that tau is confined to the medial temporal lobe and only spreads to the rest of the isocortex once Aβ is present (48–51). However, some have suggested that tau and Aβ develop independently, which may give rise to variable ordering in their progression (14, 15, 52). These different findings may raise questions about serial models of AD biomarker trajectories, i.e., that Aβ always precedes tau. We found that continuous – but not dichotomous – levels of CSF p-tau were associated with significantly higher odds of progression to Aβ-positivity. Thus, some individuals with elevated tau and subthreshold Aβ do develop more typical AD-like profiles. Being at heightened risk of entering the AD continuum, they would be worth monitoring more closely.
Long-standing individual differences
Another explanation for why cognition predicts Aβ-positivity is that lower baseline cognition might reflect long-standing individual differences. Lower cognitive function may reflect less efficient neural processing, which would in turn require higher activity. It has been proposed that elevated synaptic activity across the lifespan could result in increased release and aggregation of Aβ (53). Individuals with less efficient processing (indexed by lower cognitive function) may therefore be at greater risk of accumulating Aβ.
However, this idea may seem to be contradicted by the unexpected finding that higher education was associated with increased odds of progression to Aβ-positivity. We propose two potential explanations. First, individuals with lower education may be at greater risk of becoming Aβ-positive prior to their baseline visit, and thus would not have been included in our analysis. Those with lower education who remained Aβ-negative until their baseline visit may be more resistant to Aβ deposition, and thus less likely to progress in the future. Second, the seemingly paradoxical education finding might be, in part, a function of ADNI ascertainment. Average education was 16+ years, yet only about 10% of this age cohort in the U.S. attained a 4-year college degree (54). ADNI participants were recruited at AD Research Centers, which are likely to attract people with concerns about memory and AD risk. There might, in turn, be a link between well-educated older adults with memory concerns and increased likelihood of progressing to Aβ-positivity.
Are the results driven by MCI cases?
We considered that the present results might be driven by the 47.3% of the sample diagnosed with MCI at baseline. However, ORs were in the direction of greater magnitude among cognitively normal participants when analyzed separately. It is also worth emphasizing that the results for the majority (52.7%) of the sample are consistent with typical disease progression because these non-MCI individuals did not have cognitive impairment prior to reaching Aβ-positivity. Rather, differences within the range of normal cognitive function were informative about who is more likely to become Aβ-positive.
Implications for study participant selection
Use of Aβ-positivity as inclusion criteria should be context dependent. Defined cut-points are necessary for clinical diagnosis and for clinical trials targeting Aβ pathology. Including only biomarker-confirmed MCI cases will reduce the number of false-positive diagnoses and provide more certainty that cognitive deficits arise from AD pathology. Our results suggest that early cognitive testing may also have utility as a screening tool for identifying who should receive biomarker assessments to more directly assess disease etiology or suitability for clinical trials. However, it would exclude Aβ-negative MCI cases who may later enter the AD continuum upon progression to Aβ-positivity. If the goal is to understand the earliest stages of the AD continuum, it will be important to capture individuals who demonstrate putative atypical disease progression to better detect and identify sources of variability.
Summary
Despite much evidence for the standard model of biomarker and cognitive trajectories, the current results demonstrate the complex nature of disease progression. Differences in cognition that predict future progression to Aβ-positivity may be driven by subthreshold pathology, perhaps suggesting a need to reconsider current biomarker thresholds or to focus more on approaches that measure Aβ accumulation. Additionally, higher levels of tau are associated with increased risk of becoming Aβ-positive. Thus, elevated tau should be considered when identifying those at risk for developing AD. A subset of individuals with MCI but normal Aβ levels may similarly end up on the AD pathway as indicated by later progression to Aβ-positivity. Importantly, the results strongly suggest that cognition should not be considered important only as a late-stage endpoint of AD. Rather, even when cognitive function is still within the normal range, it can provide a sensitive, low-cost, non-invasive predictor of risk, potentially before current thresholds for Aβ-positivity are reached.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute on Aging R01 AG050595 (W.S.K., M.J.L., C.E.F.), R01 AG022381 (W.S.K.), R01 AG059329 (sub PI C.E.F), R01 AG056410 (M.S.P) and K08 AG047903 (M.S.P). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Results of these analyses were reported at the 2019 Alzheimer’s Association International Conference and on the bioRxiv preprint server.
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sperling R, Mormino E, Johnson K (2014): The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 84:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. (2018): NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. (2013): Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12:357–367. [DOI] [PubMed] [Google Scholar]
- 4.Beason-Held LL, Goh JO, An Y, Kraut MA, O’Brien RJ, Ferrucci L, et al. (2013): Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 33:18008–18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. (2012): Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England journal of medicine. 367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel PS, Ossenkoppele R, Gessert D, Jagust W, Landau S, Hansson O, et al. (2017): Time to Amyloid Positivity and Preclinical Changes in Brain Metabolism, Atrophy, and Cognition: Evidence for Emerging Amyloid Pathology in Alzheimer’s Disease. Front Neurosci. 11:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel PS, Mattsson N, Mackin RS, Scholl M, Nosheny RL, Tosun D, et al. (2016): Accelerating rates of cognitive decline and imaging markers associated with beta-amyloid pathology. Neurology. 86:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jedynak BM, Liu B, Lang A, Gel Y, Prince JL, Alzheimer’s Disease Neuroimaging I (2015): A computational method for computing an Alzheimer’s disease progression score; experiments and validation with the ADNI data set. Neurobiol Aging. 36 Suppl 1:S178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT, et al. (2012): A computational neurodegenerative disease progression score: Method and results with the Alzheimer’s disease neuroimaging initiative cohort. NeuroImage. 63:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes L, Albert M, Moghekar A, Soldan A, Pettigrew C, Miller MI (2019): Identifying Changepoints in Biomarkers During the Preclinical Phase of Alzheimer’s Disease. Front Aging Neurosci. 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landau SM, Horng A, Jagust WJ, Alzheimer’s Disease Neuroimaging I (2018): Memory decline accompanies subthreshold amyloid accumulation. Neurology. 90:e1452–e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell ME, Chen X, Rundle MM, Chan MY, Wig GS, Park DC (2018): Regional amyloid accumulation and cognitive decline in initially amyloid-negative adults. Neurology. 91:e1809–e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, et al. (2018): Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J Neurosci. 38:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K (2015): The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 138:2814–2833. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011): Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 70:960–969. [DOI] [PubMed] [Google Scholar]
- 16.Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP, et al. (2017): Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 13:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. (2010): Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. (2009): Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. (2018): CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. (2013): Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 54:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, et al. (2012): Association of lifetime cognitive engagement and low β-amyloid deposition. Archives of Neurology. 69:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, et al. (2012): Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS, et al. (2017): Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA. 317:2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. (2014): The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, et al. (2012): A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 6:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen WJ, Wilson RS, Visser PJ, Nag S, Schneider JA, James BD, et al. (2018): Age and the association of dementia-related pathology with trajectories of cognitive decline. Neurobiol Aging. 61:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team (2017): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. (2011): Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamberger D, Lavrac N, Srivatsa S, Tanzi RE, Doraiswamy PM (2017): Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Scientific reports. 7:6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE, Alzheimer’s Disease Neuroimaging I (2011): Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. 68:961–969. [DOI] [PubMed] [Google Scholar]
- 31.Hinrichs C, Singh V, Xu G, Johnson SC, Alzheimers Disease Neuroimaging I (2011): Predictive markers for AD in a multi-modality framework: an analysis of MCI progression in the ADNI population. Neuroimage. 55:574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korolev IO, Symonds LL, Bozoki AC, Initi AsDN (2016): Predicting Progression from Mild Cognitive Impairment to Alzheimer’s Dementia Using Clinical, MRI, and Plasma Biomarkers via Probabilistic Pattern Classification. Plos One. 11:e0138866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonaitis EM, Koscik RL, Clark LR, Ma Y, Betthauser TJ, Berman SE, et al. (2019): Measuring longitudinal cognition: Individual tests versus composites. Alzheimers Dement (Amst). 11:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustavson DE, Sanderson-Cimino M, Elman JA, Franz CE, Panizzon MS, Jak AJ, et al. (In press): Extensive memory testing improves prediction of progression to MCI in late middle age. Alzheimers Dement (Amst). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattsson N, Insel PS, Donohue M, Jagust W, Sperling R, Aisen P, et al. (2015): Predicting Reduction of Cerebrospinal Fluid beta-Amyloid 42 in Cognitively Healthy Controls. JAMA Neurol. 72:554–560. [DOI] [PubMed] [Google Scholar]
- 36.Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. (2013): Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol. 74:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H (2015): Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 36:297–309. [DOI] [PubMed] [Google Scholar]
- 38.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Alzheimer’s Disease Neuroimaging I, Minthon L, et al. (2015): Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 85:1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. (2015): Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 138:2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villain N, Chételat G, Grassiot B, Bourgeat P, Jones G, Ellis KA, et al. (2012): Regional dynamics of amyloid-β deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB–PET longitudinal study. Brain. 135:2126–2139. [DOI] [PubMed] [Google Scholar]
- 41.Insel PS, Mattsson N, Donohue MC, Mackin RS, Aisen PS, Jack CR Jr., et al. (2015): The transitional association between beta-amyloid pathology and regional brain atrophy. Alzheimers Dement. 11:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattsson N, Insel PS, Nosheny R, Tosun D, Trojanowski JQ, Shaw LM, et al. (2014): Emerging beta-amyloid pathology and accelerated cortical atrophy. JAMA Neurol. 71:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Insel PS, Hansson O, Mackin RS, Weiner M, Mattsson N, Alzheimer’s Disease Neuroimaging I (2018): Amyloid pathology in the progression to mild cognitive impairment. Neurobiol Aging. 64:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. (2016): A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 87:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. (2006): Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 46.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. (2008): Frequent Amyloid Deposition Without Significant Cognitive Impairment Among the Elderly. Archives of Neurology. 65:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. (1988): Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 23:138–144. [DOI] [PubMed] [Google Scholar]
- 48.Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. (2016): PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. (2016): Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontecorvo MJ, Devous MD Sr., Navitsky M, Lu M, Salloway S, Schaerf FW, et al. (2017): Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, et al. (2016): Evaluation of Tau Imaging in Staging Alzheimer Disease and Revealing Interactions Between beta-Amyloid and Tauopathy. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Small SA, Duff K (2008): Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 60:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jagust WJ, Mormino EC (2011): Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends in Cognitive Sciences. 15:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan CL, Bauman K (2016): Educational attainment in the United States: 2015. Washington, DC: Department of Commerce, U.S. Census Bureau. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.