Abstract
Chylomicronemia caused by a deficiency in lipoprotein lipase (LPL) or GPIHBP1 (the endothelial cell protein that transports LPL to the capillary lumen) is typically diagnosed during childhood and represents a serious, lifelong medical problem. Affected patients have high plasma triglyceride levels (>1500 mg/dl) and a high risk of acute pancreatitis. However, chylomicronemia frequently presents later in life in the absence of an obvious monogenic cause. In these cases, the etiology for the chylomicronemia is presumed to be “multifactorial” (involving diabetes, drugs, alcohol, or polygenic factors) but on a practical level the underlying cause generally remains a mystery. Here, we describe a 15-year-old female with chylomicronemia caused by GPIHBP1 autoantibodies (which abolish LPL transport to the capillary lumen). Remarkably, chylomicronemia in this patient was intermittent, interspersed between periods when the plasma triglyceride levels were normal. GPIHBP1 autoantibodies were easily detectable during episodes of chylomicronemia but were undetectable during periods of normotriglyceridemia. During the episodes of chylomicronemia (when GPIHBP1 autoantibodies were present), plasma LPL levels were low, consistent with impaired LPL transport into capillaries. During periods of normotriglyceridemia, when GPIHBP1 autoantibodies were absent, plasma LPL levels normalized. Because the chylomicronemia in this patient was accompanied by debilitating episodes of acute pancreatitis, the patient was ultimately treated with immunosuppressive drugs, which resulted in disappearance of GPIHBP1 autoantibodies and normalization of plasma triglyceride levels. GPIHBP1 autoantibodies need to be considered in patients who present with unexplained acquired cases of chylomicronemia.
Introduction
Deficiencies in lipoprotein lipase (LPL), GPIHBP1, apolipoprotein CII, apolipoprotein AV, or lipoprotein maturation factor 1 (LMF1) cause lifelong and unremitting chylomicronemia (“Familial Chylomicronemia Syndrome”), with very high plasma triglyceride levels (often >1,500 mg/dl) and a substantial risk of acute pancreatitis.1, 2 Patients with familial forms of chylomicronemia typically come to medical attention in early childhood, and genetic testing typically provides a definitive diagnosis.3 However, patients with chylomicronemia often present later in life in the absence of a prior history of hyperlipidemia. In these cases, the cause for chylomicronemia is said to be “multifactorial” (involving genetic predisposition, diabetes, drugs, or alcohol) but on a practical level the underlying cause often remains unclear. Autoimmune causes for chylomicronemia are frequently not considered in the differential diagnosis, even by very experienced physicians.4 Here, we describe an adolescent female with intermittent chylomicronemia resulting from waxing and waning levels of GPIHBP1 autoantibodies.
Case Report
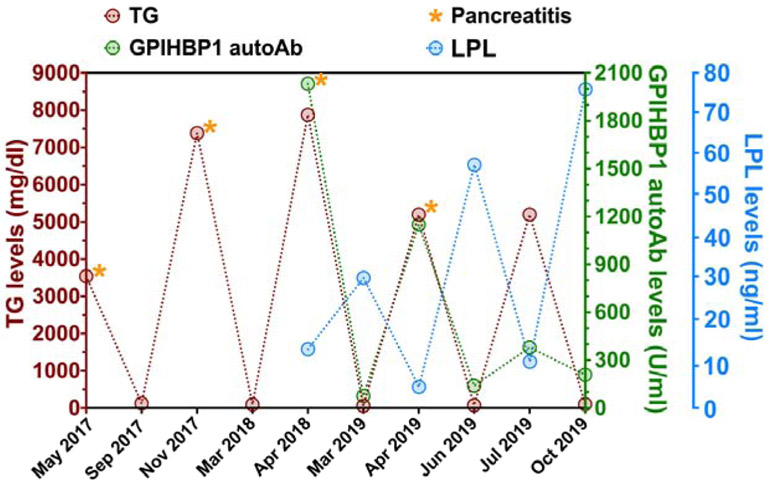
A previously healthy 15-year-old African-American girl presented with acute pancreatitis and lipemic plasma in May 2017 (Figure 1). The patient had a weight of 86 kg (98th percentile), height of 164 cm (60th percentile), and a body mass index of 32.4 kg/m2. She had no organomegaly or features of lipodystrophy. She had normal thyroid function, a normal serum albumin, and a normal serum glucose and hemoglobin A1C. The triglyceride and total cholesterol levels were 3,539 and 329 mg/dl, respectively, and the HDL cholesterol level was 17 mg/dl. The patient was given fenofibrate (145 mg/day) and advised to adhere to a diet low in fats (<15 g of fat/day). By September 2017, the plasma triglyceride levels normalized (124 mg/dl). However, chylomicronemia (plasma triglycerides, 7384 mg/dl) and acute pancreatitis recurred in late November 2017. In early March 2018, the plasma triglycerides had normalized (95 mg/dl). Five weeks later, in April 2018, the patient presented with acute pancreatitis, and the plasma triglycerides were 7865 mg/dl. At that time, the hematocrit was 29, and anisopoikilocytosis was observed on the blood smear. Coombs tests (warm and cold) were positive, raising the possibility of autoimmune hemolytic anemia.
Figure 1.
Triglyceride (TG), LPL, and GPIHBP1 autoantibody (autoAb) levels in the patient’s plasma over a two-year period. This graph demonstrates that when GPIHBP1 autoAb levels were high (green circles, right y-axis), LPL plasma levels plummeted (blue circles, right y-axis) and plasma TGs (brown circles, left y-axis) rose sharply. It also shows that when GPIHBP1 autoAb levels were low, LPL and TG plasma levels normalized. Episodes of acute pancreatitis are indicated by orange asterisks.
Targeted exome sequencing of the patient's genomic DNA5 revealed no pathogenic variants in LPL, APOC2, APOC3, APOA5, APOE, LMF1, GPIHBP1, GCKR, CREB3L3, or GPD1.
To explore the possibility of an autoimmune cause for the chylomicronemia, a plasma sample from the April 2018 hospitalization was tested for autoantibodies against LPL and GPIHBP1. No LPL autoantibodies were present, but GPIHBP1 autoantibodies were easily detectable. GPIHBP1 autoantibodies were detected with a solid-phase immunoassay (testing immunoglobulin binding to recombinant human GPIHBP1).6 GPIHBP1 autoantibodies were confirmed by a second laboratory with a similar immunoassay that used independent reagents.7
GPIHBP1 autoantibodies block the ability of the GPIHBP1 on endothelial cells to capture LPL within the subendothelial spaces and shuttle it to the capillary lumen.8 Impaired delivery of LPL to the capillary lumen disrupts the lipolytic processing of triglyceride-rich lipoproteins, resulting in chylomicronemia. In the initial report of chylomicronemia caused by GPIHBP1 autoantibodies,6 the plasma LPL levels in affected patients were low (reflecting reduced delivery of LPL into capillaries). In the current patient, the plasma LPL level (measured with a monoclonal antibody–based immunoassay9) during the April 2018 episode of chylomicronemia was very low, 14 ng/ml (levels of LPL in pre-heparin plasma are typically >40 ng/ml). The patient continued fenofibrate and was advised to adhere to a low-fat diet.
By early March 2019, the plasma triglycerides normalized (61 mg/dl); GPIHBP1 autoantibodies were undetectable; and the LPL level increased to 31 ng/ml. Six weeks later, in late April 2019, the patient developed acute pancreatitis, and the plasma triglycerides were >5200 mg/dl. GPIHBP1 autoantibodies were easily detectable, and the LPL level was extremely low (5 ng/ml). With the recurrence of GPIHBP1 autoantibodies and chylomicronemia, immunosuppressive treatment was initiated with mycophenolate mofetil (1 gram BID) and prednisolone (60 mg/day, tapering to 40 mg/day). By late June 2019, the plasma triglycerides fell to 77 mg/dl; GPIHBP1 autoantibodies were very low; and the plasma LPL level normalized (58 ng/ml). The patient was not compliant with the immunosuppressive drug regimen, and by mid-July the plasma triglycerides increased to >5200 mg/dl. GPIHBP1 autoantibodies were easily detectable although lower than in April 2019. The plasma LPL level was very low (11 ng/ml). In September 2019, the patient was given two intravenous infusions of rituximab (1 gram) along with Solu-Medrol (1 gram); the patient continued to take mycophenolate mofetil (500 mg BID). By October 2019, the triglyceride levels normalized (105 mg/dl); GPIHBP1 autoantibodies were undetectable; and the plasma LPL levels were normal (76 ng/ml).
In this patient, the plasma LPL levels were low when GPIHBP1 autoantibodies were present but were within the normal range when GPIHBP1 autoantibodies were absent. In contrast, the plasma levels of hepatic triglyceride lipase and endothelial lipase (lipase family members that do not interact with GPIHBP1) were within normal limits during the entire course of the disease.
Discussion
This case demonstrates that intermittent episodes of chylomicronemia, accompanied by low plasma levels of LPL, can be caused by GPIHBP1 autoantibodies. On three occasions and in the absence of immunosuppressive drugs, the plasma triglyceride levels returned to normal. During the remissions, GPIHBP1 autoantibodies disappeared and plasma levels of LPL increased to within the normal range.
The mechanism by which GPIHBP1 autoantibodies cause chylomicronemia is straightforward.6 GPIHBP1 autoantibodies block the ability of GPIHBP1 to capture LPL and transport it across endothelial cells to the capillary lumen. The reduced amount of LPL in the capillary lumen impairs LPL-mediated processing of triglyceride-rich lipoproteins, leading to markedly elevated plasma triglyceride levels. In the absence of GPIHBP1 autoantibodies, LPL transport to the capillary lumen is unimpeded, allowing processing of triglyceride-rich lipoproteins by LPL to proceed.
This case illustrates that GPIHBP1 autoantibodies are not always persistent but instead can be intermittent. Autoantibodies against the insulin receptor sometimes disappear, resulting in normalization of glucose tolerance and insulin sensitivity.10 Also, autoantibodies against the M-type phospholipase A2 receptor in patients with membranous glomerulopathy can disappear, causing remission of disease.11 In the current case, we demonstrated that levels of GPIHBP1 autoantibodies can wax and wane, resulting in intermittent chylomicronemia. In the past, there have been hints that plasma triglyceride levels can fluctuate widely in patients with the GPIHBP1 autoantibody syndrome. For example, substantial variations in plasma triglyceride levels were observed in a 26-year-old Algerian male in whom the GPIHBP1 autoantibody syndrome was diagnosed using an archived sample of lipemic plasma.6, 12 In another case, chylomicronemia caused by GPIHBP1 autoantibodies apparently resolved in the absence of immunosuppressive drugs.13 In these cases, however, changes in plasma triglyceride levels were not linked to changes in GPIHBP1 autoantibody levels. The current case report demonstrates that remissions of chylomicronemia can result from disappearance of GPIHBP1 autoantibodies.
Chylomicronemia from the GPIHBP1 autoantibody syndrome often occurs in the setting of other autoimmune diseases, but in some patients GPIHBP1 autoantibodies and chylomicronemia are the sole manifestation of autoimmune disease.6 In the current case, the patient was noted to have a positive Coombs test, raising the possibility of autoantibodies against erythrocytes.
Experience in treating GPIHBP1 autoantibody patients with immunosuppressive drugs is extremely limited. In one patient, the chylomicronemia resolved after several infusions of rituximab,6 a CD20-specific monoclonal antibody that is often used to treat autoantibody diseases (e.g., pemphigus vulgaris, idiopathic thrombocytopenic purpura).14-16 In another patient, the chylomicronemia resolved with mycophenolate mofetil, a drug that is often used to prevent organ transplant rejection.6 However, in these cases, there was no laboratory evidence that the immunosuppressive drugs led to reduced levels of GPIHBP1 autoantibodies. In the current case, prednisolone and mycophenolate mofetil levels lowered GPIHBP1 autoantibodies and normalized the triglyceride levels, but that remission was cut short by incomplete compliance with the treatment regimen. Later, GPIHBP1 autoantibodies and chylomicronemia resolved after infusions of rituximab and resumption of mycophenolate mofetil treatment. The current case provides the best evidence to date that immunosuppressive drug therapy can lead to the disappearance of GPIHBP1 autoantibodies, recovery of GPIHBP1 function, and normalization of LPL levels and plasma triglyceride concentrations.
The decision to institute drug therapy for the GPIHBP1 autoantibody syndrome should be made in consultation with physicians who are familiar with these drugs and their side effect profiles. The risk of immunosuppressive drugs needs to be weighed against the morbidity and mortality risks associated with chylomicronemia-driven episodes of acute pancreatitis.
The GPIHBP1 autoantibody syndrome is often overlooked when managing patients with chylomicronemia.4 We believe that the GPIHBP1 autoantibody syndrome needs to be considered in all unexplained cases of acquired chylomicronemia, particularly when there is clinical or serologic evidence for autoimmune disease.6 Making the diagnosis of the GPIHBP1 autoantibody syndrome is simple and straightforward.6, 7 Moreover, making the diagnosis of the GPIHBP1 autoantibody syndrome opens the door to considering a targeted therapy (i.e., immunosuppressive drug therapy).
Highlights.
Intermittent chylomicronemia can be caused by intermittent GPIHBP1 autoantibodies.
GPIHBP1 autoantibodies result in low plasma levels of lipoprotein lipase.
Chylomicronemia from GPIHBP1 autoantibodies can be successfully treated.
GPIHBP1 autoantibodies should be considered in any case of acquired chylomicronemia
Acknowledgments
Funding Source: Supported by grants (HL090553, HL087228, and HL125335) from the National Heart, Lung, and Blood Institute and a Transatlantic Network Grant (12CVD04) from the Leducq Fondation.
Abbreviations:
- LPL
lipoprotein lipase
- GPIHBP1
Glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1.
Footnotes
Conflict of Interest: No additional conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Kazuya Miyashita is an employee of Immunobiologic Laboratories and holds stock in that company. Author Katsuyuki Nakajima holds stock in Immunobiologic Laboratories and serves as a consultant for Skylight and Sysmex. The other authors have no relevant financial relationships to disclose.
References
- 1.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27(5):459–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong LG, Young SG, Beigneux AP, Bensadoun A, Oberer M, Jiang H, et al. GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol Metab. 2016;27(7):455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rios JJ, Shastry S, Jasso J, Hauser N, Garg A, Bensadoun A, et al. Deletion of GPIHBP1 causing severe chylomicronemia. J Inherit Metab Dis. 2012;35(3):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chait A, Eckel RH. The chylomicronemia syndrome is most often multifactorial: A Narrative Review of Causes and Treatment. Ann Intern Med. 2019;170(9):626–634. [DOI] [PubMed] [Google Scholar]
- 5.Johansen CT, Dube JB, Loyzer MN, MacDonald A, Carter DE, McIntyre AD, et al. LipidSeq: a next-generation clinical resequencing panel for monogenic dyslipidemias. J Lipid Res. 2014;55(4):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, et al. Autoantibodies against GPIHBP1 as a cause of hypertriglyceridemia. N Engl J Med. 2017;376(17):1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita K, Fukamachi I, Machida T, Nakajima K, Young SG, Murakami M, et al. An ELISA for quantifying GPIHBP1 autoantibodies and making a diagnosis of the GPIHBP1 autoantibody syndrome. Clin Chim Acta. 2018;487:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young SG, Fong LG, Beigneux AP, Allan CM, He C, Jiang H, et al. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 2019;30(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida T, Miyashita K, Sone T, Tanaka S, Nakajima K, Saito M, et al. Determination of serum lipoprotein lipase using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2015;442:130–135. [DOI] [PubMed] [Google Scholar]
- 10.Flier JS, Bar RS, Muggeo M, Kahn CR, Roth J, Gorden P. The evolving clinical course of patients with insulin receptor autoantibodies: spontaneous remission or receptor proliferation with hypoglycemia. J Clin Endocrinol Metab. 1978;47(5):985–995. [DOI] [PubMed] [Google Scholar]
- 11.Diaz M, Agraz I, Soler MJ. Anti-phospholipase A2 receptor antibody and spontaneous remission in membranous nephropathy. Clin Kidney J. 2019;12(1):33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charriere S, Peretti N, Bernard S, Di Filippo M, Sassolas A, Merlin M, et al. GPIHBP1 C89F neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. J Clin Endocrinol Metab. 2011;96(10):E1675–1679. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Dallinga-Thie GM, Hovingh GK, Chang SY, Sandoval NP, Dang TLP, et al. GPIHBP1 autoantibodies in a patient with unexplained chylomicronemia. J Clin Lipidol. 2017;11(4):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bylsma LC, Fryzek JP, Cetin K, Callaghan F, Bezold C, Mehta B, et al. Systematic literature review of treatments used for adult immune thrombocytopenia in the second-line setting. Am J Hematol. 2019;94(1):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacIsaac J, Siddiqui R, Jamula E, Li N, Baker S, Webert KE, et al. Systematic review of rituximab for autoimmune diseases: a potential alternative to intravenous immune globulin. Transfusion. 2018;58(11):2729–2735. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):882–894. [DOI] [PubMed] [Google Scholar]