Abstract
The vitamin D receptor (VDR) and its ligand 1,25(OH)2D3 (1,25D) impact differentiation and exert anti-tumor effects in many tissues, but its role in salivary gland has yet to be defined. Using immunohistochemistry (IHC), we have detected strong VDR expression in murine and human salivary gland ducts. Compared to normal gland, VDR protein expression was retained in differentiated human pleomorphic adenoma (PA) but was undetectable in undifferentiated PA and in carcinomas, suggesting deregulation of VDR during salivary cancer progression. To gain insight into the potential role of VDR in salivary cancer, we assessed the effects of vitamin D in vivo and in vitro. Despite the presence of VDR in salivary gland, chronic dietary vitamin D restriction did not alter morphology of the salivary epithelium in C57/Bl6 mice. The localization of VDR in ductal epithelium prompted us to examine the effects of 1,25D in an established cell line (mSGc) derived from normal murine submandibular gland (SMG). This previously characterized cell line consists of multiple stem, progenitor and differentiated cell types as determined by mutually exclusive cellular expression of basal, ductal and myoepithelial markers. We demonstrated VDR expression and regulation of VDR target genes Vdr and Postn by 1,25D in mSGc, indicating functional ligand-mediated transcriptional activity. The effect of VDR signaling on epithelial differentiation markers was assessed by qPCR and IHC in mSGc cells treated with 1,25D. We found that 1,25D reduced mRNA expression of the basal cell progenitor marker keratin 5 (K5) and increased expression of the differentiated ductal cell marker keratin 7 (K7). Further, we found that 1,25D significantly decreased the number of proliferating cells, including proliferating K5+ cells. Characterization of cell cycle by Muse cytometry indicated 1,25D treatment decreased cells in S, G2, and M phase. The inhibition of K5+ cell proliferation by 1,25D is of particular interest because K5+ basal cells contribute to a wide variety of salivary tumor types. Our studies suggest that 1,25D alters cancer-relevant progenitor and differentiation markers in the salivary gland.
Keywords: Vitamin D, VDR, salivary gland, cancer
Introduction
The active metabolite of vitamin D, 1,25 dihydroxycholecalciferol (1,25D), signals through the vitamin D receptor (VDR), a nuclear receptor that acts as a transcription factor. VDR signaling is best known for calcium homeostasis and maintenance of bone1,2; however, the importance of VDR signaling extends beyond skeletal health. It is well established that vitamin D deficiency enhances tumorigenesis in multiple organs3–5 and that 1,25D decreases proliferation, promotes cellular differentiation and displays additional anti-tumorigenic properties6–8,9–14 in diverse cell types. In the murine submandibular salivary gland (SMG), epithelial progenitor cell populations are highly positive for VDR transcript15 and in human gland, VDR localizes to epithelial cells16. Initial studies using ligand radio-labeling show nuclear localization of VDR ligand to salivary ductal epithelial cells of multiple species17,18, suggesting a functional and conserved role. Although one study reported inhibitory effects of 1,25D on salivary carcinoma cells in vitro19, VDR actions have not been extensively explored in salivary gland cells or salivary tumors.
Salivary cancer is most often diagnosed in late stages of disease, leading to dismal outcomes and significantly reduced survival rates20–22. In multiple large patient cohorts, evidence of nodal involvement, facial nerve invasion and metastasis significantly reduce survival to less than 50% at five years20,23,24. Current therapy for salivary gland cancer is surgical resection, followed by elective neck dissection, radiation and/or chemotherapy. While there are many types of salivary cancers, those derived from basal cells are consistently associated with poor patient prognosis and high rates of recurrence due to lack of effective therapies25.
Basal ductal cells in the salivary gland are cytokeratin 5-positive (K5+) and differentiate into mature ductal cells during development26–31. Recent research has highlighted similarities between mammary and salivary squamous tumors derived from K5+ basal cells32,33. The majority of primary salivary tumors are positive for K5 mRNA34 and expression of K5 mRNA has been detected in nodes of patients with malignant salivary cancers. Because VDR expression in the mammary gland has been correlated with K5+ cell fate transition10 and VDR null mice are highly sensitive to carcinogen induced tumorigenesis3, we have initiated a preliminary examination of VDR localization and expression in the salivary gland using diverse murine and human model systems.
In the studies reported here, we compared VDR localization in human and mouse SMG tissue. Additionally, we examined VDR protein localization in four types of human salivary neoplasias, including benign pleomorphic adenoma (PA) and three metastatic types, adenoid cystic carcinoma (AdCC), squamous cell carcinoma (SCC) and mucoepidermoid carcinoma (MEC). As VDR signaling promotes differentiation in many cell types including breast epithelium, skeletal muscle, oligodendrocytes and osteoblasts12,14,35,36, we hypothesized a similar function in the salivary gland. To test this hypothesis, we utilized vitamin D deficient diets in vivo and 1,25D treatment in vitro to characterize the impact of the VDR ligand on mouse SMG37. Our data support an anti-proliferative and pro-differentiating role for VDR in normal salivary gland and provide rationale for further studies.
Materials and Methods
Human and Murine Salivary Gland Samples
Deidentified, formalin fixed, paraffin embedded (FFPE) sections of normal human salivary gland and various human salivary cancers were purchased from Pantomics (Richmond, CA) in both single section (SAL01, SAL02, SAL03) and in tissue microarray format (HNT961). All human samples were surgically harvested from both male and female patients, with an age range of 17–79 years old, and assessed by a clinical pathologist prior to receipt. The tissue microarray included all parotid salivary gland tumor samples including pleomorphic adenoma (n=8 patients, benign ungraded), squamous cell carcinoma (n=1 patient T2N0M0), and adenoid cystic carcoma (n=3 patients, all T1N0M0). These tumor samples were processed in technical duplicate, with a minimum of four fields of view examined. Information on glandular origin of the mucoepidermoid carcinoma was not available (n=1 patient) and was examined in technical quadruplicate. Normal human salivary gland samples were also obtained from Pantomics (n=2 patients, examined in technical duplicate). Murine FFPE samples were harvested from adult female WT C57Bl6 mice in accordance with approved institutional IACUC protocols. Only submandibular glands from female mice were examined as they are more histologically similar to normal human glands than glands from male mice16.
FFPE Immunohistochemistry
All FFPE samples were deparaffinized as described in Gervais et al.38 with the substitution of xylene for Histochoice. Permeabilization was completed with 0.3% Triton in PBS for 10 minutes. Hot citrate antigen retrieval was performed for fifteen minutes prior to blocking in 0.3% bovine serum albumin in PBS (BSA-PBS) for a minimum of one hour at room temperature. Primary antibodies (Supp. Table 1) were diluted in BSA-PBS and incubated overnight at 4°C. Slides were washed three times in PBS prior to secondary antibody application (Supp. Table 1) in BSA-PBS. Slides were incubated in secondary antibody for a minimum of one hour at room temperature, followed by two PBS washes. After soaking in DAPI (1ug/mL), slides were rinsed twice in PBS and mounted using a glycerol based mounting media.
Dietary Vitamin D Studies
Female weanling C57/Bl6 mice (Taconic) were housed in the animal facility at the Cancer Research Center, University at Albany. Pups were reared on AIN93G diet containing 1000IU vitamin D3/kg (SUFF diet, obtained from Research Diets, New Brunswick, NJ for eight weeks, after which they were randomly allocated to remain on the SUFF diet or switched to AIN93 diet devoid of vitamin D3 (DEF diet). All diets were fed ad libitum and mice were weighed weekly. After four weeks on SUFF or DEF diets, tissues were collected and immediately frozen for cryosection or fixed in 10% neutral buffered formalin for paraffin embedding. All animal care procedures were performed in accordance with approved institutional IACUC protocols.
Cryosection Immunohistochemistry
Murine SMG tissue was submerged in optimal cutting temperature (OCT) media (electron microscopy services) and flash frozen in liquid nitrogen. Tissues were stored in OCT at −80°C prior to sectioning 10μm sections at −20°C, followed by fixation in 4% paraformaldehyde for 18 minutes. Tissues were washed twice in PBS and dried prior to storage at −80°C. Cryosections were blocked and stained as described above without antigen retrieval.
mSGc Culture
Previously characterized murine SMG cells (mSGc) were a kind gift from Dr. Rose Romano (SUNY Buffalo)37. Cells were maintained in salivary cell growth media including DMEM/F12 (Sigma D8900) with 20ng/mL of human epithelial growth factor (Sigma E9644), 20ng/mL of human fibroblast growth factor (R&D 233-FB), 10μg/mL human insulin (Sigma I9278), 1μM dexamethasone (Sigma D4902), 5mL N2 supplement (Gibco 17502), and 5mL Pen/Strep (Sigma P4333).
Cells for RNA extraction were plated at 500,000 cells in 10cm3 dishes, incubated for 48 hours to facilitate attachment, then treated with 100nM 1,25D solubilized in 100% ethanol or vehicle for 24 hours. Cells for flow cytometry were plated at 225,000 cells in a 6 well plate and harvested for analysis as described below. Cells for immunocytochemistry (ICC) were plated at 100,000 cells/well in 24 well plates on glass coverslips and treated with 100nM 1,25D or vehicle control. Cells on coverslips were photographed during culture using a brightfield Nikon Eclipse TS100 with a 20×PlanFluor 0.45NA lens. For Western blotting, cells were plated at 100,000 cells in 10cm3 dishes and treated for 72 hours with either 100nM 1,25D or vehicle control. A supraphysiological dose of 100nM 1,25D was chosen with the aim of receptor saturation.
qPCR
RNA was isolated from cell pellets using RNEasy Plus isolation kits (Qiagen). RNA quality and concentration were measured on a NanoDrop spectrophotometer and 0.05μg/μL was used for cDNA synthesis reactions with random hexamers and Taqman Multiscribe Reverse Transcriptase. PowerUp Sybr Green (ABI), 12μM cDNA, and primers (Supp. Table 1) were used for qPCR assays on a QuantStudio 12k Flex System (ThermoFisher). qPCR data was expressed relative to 18s RNA, and fold changes between 1,25D treated and control samples were calculated as ΔΔCT. The data shown represent the average fold change of three cell passages (biological replicates) +/− SD.
Flow Cytometry
Cells were trypsinized and resuspended in equal volumes of 1%FBS-PBS to wash and pellet. Cells were fixed overnight in 90% EtOH at −20°C and spun down the following day. Cells were analyzed using the Muse Cell Cycle kit (Millipore MCH100106). Briefly, cells were resuspended in 0.2%BSA-PBS to wash, spun down, resuspended in equal volumes of Muse reagent containing propidium iodide, and incubated for 30 minutes on ice. Flow cytometry was completed using the Muse “Cell Cycle” program and data was exported to Excel.
Western Blot
Cells were lysed with 0.3% Triton in PBS, scraped with a sterile scraper and stored at −20°C until use. Samples were thawed on ice and briefly sonicated prior to addition of sample buffer (150mM Tris 25% glycerol, 12% sodium dodecyl sulfate, 0.05% bromophenol blue and 6% β-mercaptoethanol). Samples were mixed 1:1 with sample buffer and denatured at 95°C for ten minutes prior to separation on a 4% stacking and 12% running SDS-PAGE acrylamide gel. Separation was performed in Tris buffered Glycine with Sodium dodecyl sulfate (TGS: 25mM Tris, 192mM glycine, 0.1% SDS, pH8.3) and transferred on ice to PVDF membranes (GE Healthcare) in cold Tris/glycine buffer (25mM Tris, 192mM glycine) at 110V for 1.5 hours. Blocking was performed for 1 hour at room temperature, oscillating, using 5% milk in 0.5% Tween Tris buffered saline pH7.6 (TBS-T). Primary antibody directed against VDR (Supp Table 1) was applied in 3% milk TBS-T at 4°C overnight, rolling. The membrane was washed four times for ten minutes while oscillating in TBS-T prior to HRP conjugated secondary antibody (GE Healthcare) application in 3% milk TBS-T for 1.5 hours at room temperature, rolling. The membrane was washed an additional four times in TBS-T for ten minutes while oscillating prior to ECL application (Super Signal, ThermoFisher). Imaging was performed on an iBright CL1000 imager (ThermoFisher).
Immunocytochemistry
Cells on coverslips in 24 well plates were fixed with 4% paraformaldehyde in PBS for 10 minutes and stored in PBS at 4°C. Plates were gradually returned to room temperature, permeabilized with 0.3% Triton in PBS for 10 minutes and subsequently rinsed with PBS. After blocking with BSA-PBS for one hour at room temperature, primary antibody (Supp. Table 2) diluted in BSA-PBS was applied overnight at 4°C. Coverslips were rinsed three times in PBS, followed by application of secondary antibody (Supp. Table 2) diluted in BSA-PBS. Coverslips were rinsed twice in PBS, incubated with DAPI (1ug/mL, 10 minutes) and rinsed in PBS followed by distilled water prior to mounting on Superfrost Plus slides (Fisher Scientific) using Fluro-Gel (Electron Microscopy Sciences) prior to imaging.
Image Processing
Brightfield images of H&E stained sections were captured on a Zeiss Axioskop 2 using a 10× Achroplan 0.25NA lens and an Axiocam. Fluorescent images were obtained on an Olympus IX81 scope using a 20×UPlanApo 0.75NA lens and a Q Imaging Retiga-4000DC camera run using Metamorph software. Images were obtained at matching exposure for each antigen. FIJI (ImageJ) was used to process all images. Images for qualitative comparison and presentation were subjected to a 50-pixel rolling ball background subtraction. Primary quantification of vessels was completed manually by visual identification of vessels and ducts on H&E stained images using the loop select tool (n=6 mice per group). Secondary quantification was performed using CD31/αSMA/DAPI fluorescent overlay images (n=3 mice per group). Merged images were created using the “color merge” function. Images for quantification were not adjusted for color. Images for presentation were equally adjusted using the “adjust color” function. Immunocytochemistry overlays of Ki67, K5 and DAPI were created in FIJI using the “color merge” function on unadjusted images obtained at equal exposure. Manual cell counts were performed using these overlay images for Ki67/K5/DAPI using the “cell counter” function in FIJI (Supp Fig. 1). A minimum of three biological replicates were counted. Three representative fields per replicate were chosen based on DAPI confluence, imaged for Ki67 and K5 signal, merged per field, counted and averaged. Images shown are representative for n=3 passages with n=3 fields examined. FIJI cell counter function was used to manually mark four populations of cells and auto-calculate the numbers of cells positive for each color mark (Supp Fig. 1).
Graphing and Statistics
Tabulation, calculation and graphing were performed in Excel. Statistics were performed using freeware VassarStats. Statistical comparison of two groups was completed using a Student’s unpaired two-tailed t-test. Comparison of more than two groups was completed using an unweighted ANOVA with Tukey’s HSD. Methods used for each data set are detailed in the figure legends.
Results
VDR localization in human and murine salivary glands
VDR mRNA expression has been documented in both human16 and mouse15 salivary glands. However, no data on VDR protein expression or localization in salivary tissue has been published. We conducted IHC with a primary antibody previously validated in tissue from VDR null mice39–41 to localize VDR in FFPE sections of human salivary gland. As shown in Fig 1A, VDR staining was detected in the human salivary gland and was located almost exclusively in ductal cells. Subcellular localization was mainly cytoplasmic, however nuclear expression was also observed. For comparison, VDR expression in FFPE sections of SMG from C57Bl6 wild type mice was annotated. VDR localization in mouse SMG was similar to that in human salivary gland, with signal primarily in ductal epithelial cells (Fig. 1B). In normal human salivary gland, little to no colocalization of VDR and the basal progenitor marker K5 was observed (Fig. 1C).
Fig. 1. Localization of VDR in human and murine salivary glands.
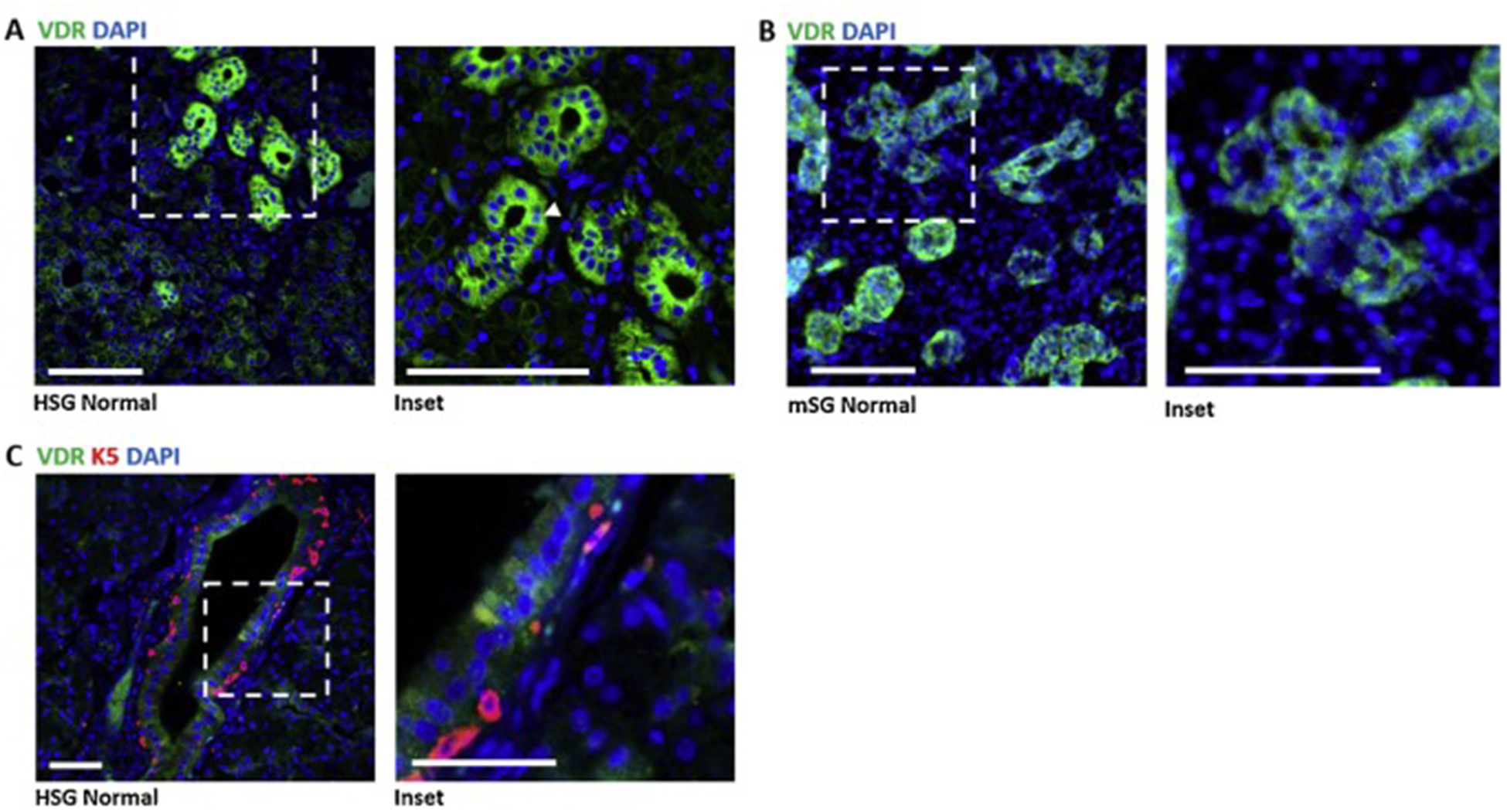
A) Immunohistochemistry of 4μm thick FFPE sections of normal human salivary gland shows VDR (green) reactivity primarily in ductal cells but also in other cell types. Expression appears mainly cytoplasmic, however nuclear expression marked by DAPI (blue) was also observed (inset, arrowheads, n=2 patients). Scale bars 100μm. B) Immunohistochemistry of 8μm thick FFPE sections of normal adult mouse SMG tissue shows similar localization for VDR (green), staining mainly in the ducts. Scale bars 100μm. C) Normal human parotid salivary gland immunostained for VDR (green) and basal cell marker K5 (n=2 patients). Scale bars 50μm.
VDR localization in human salivary tumors
In the mammary gland and other tissues, VDR expression is frequently altered (usually reduced, but occasionally increased) during tumorigenesis5,7,8,42. To determine if VDR expression changes in human salivary cancer, we used IHC to examine protein expression and localization in one type of benign and in three types of metastatic salivary cancers. In benign pleomorphic adenoma, we observed more intense VDR signal (green) compared to normal gland (Fig 2) in five of eight patient samples; however, in areas of these tumors that displayed significant dysplasia, VDR signal was notably absent (Supp Fig. 2A). In PA tumors from three patients, we observed a clear loss of VDR signal and a lack of cellular architecture (Supp Fig. 2B). Consistent with the reduction in VDR signal in dysplastic tissue, VDR signal was nearly absent in all malignant salivary cancers examined, including cystic areas of adenoid cystic carcinoma (AdCC), squamous cell carcinoma (SCC) and mucoepidermoid carcinoma (MEC) (Fig. 2B). Note that normal gland as well as all tumor types were positive for the basal progenitor marker K5 (red staining in Fig 1C, Fig. 2, Supp Fig. 2).
Fig. 2. VDR expression is altered in human salivary cancer.
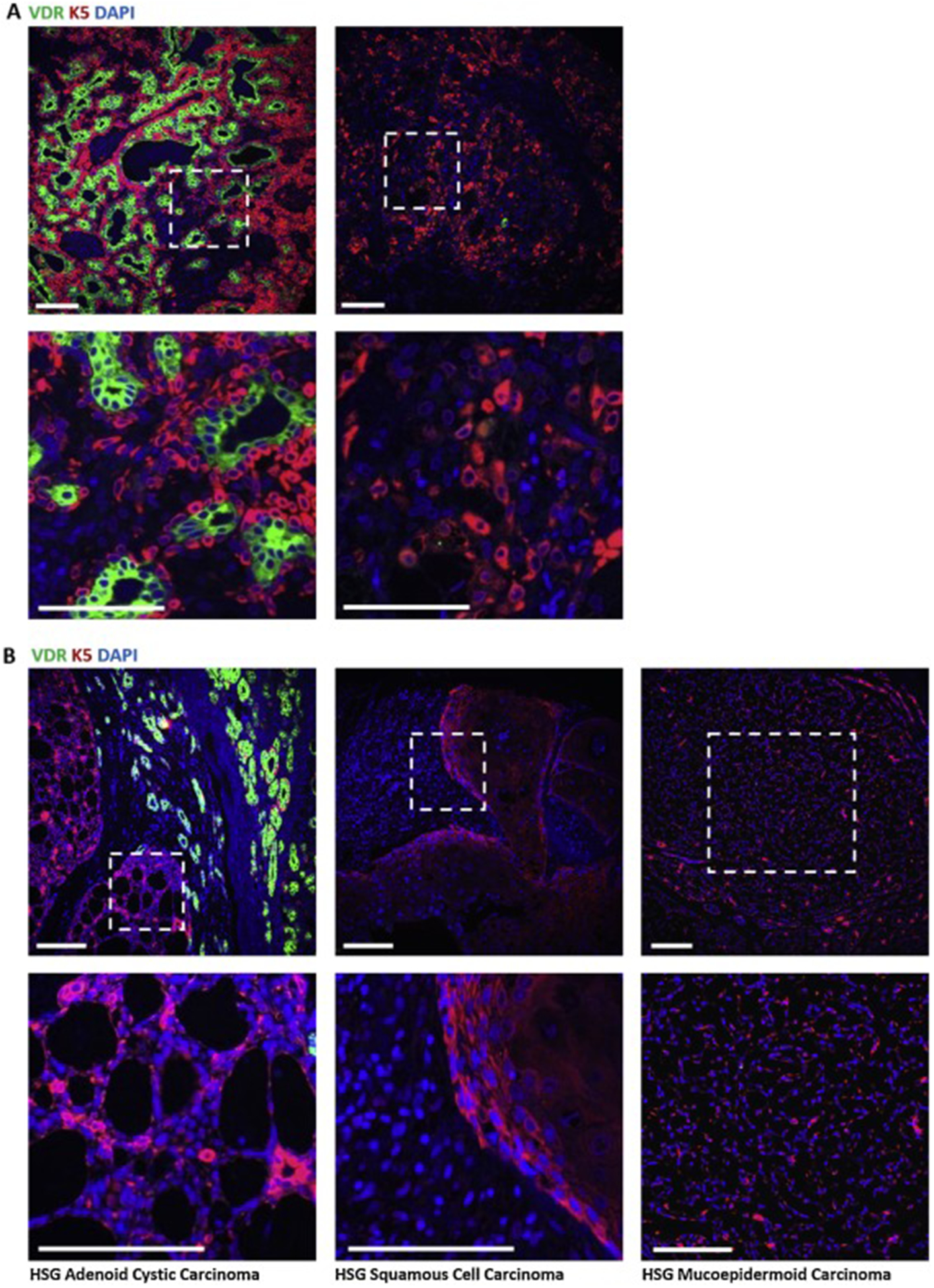
A) In benign pleomorphic adenoma (PA), VDR staining (green) is intense in areas retaining glandular structure yet is absent in areas lacking structural elements (n=8 patients, see Supp Fig. 2). B) Immunostaining for VDR in three types of metastatic salivary cancer, adenoid cystic carcinoma (AdCC), squamous cell carcinoma (SCC), and mucoepidermoid carcinoma (MEC). AdCC samples show positive staining for VDR outside of cystic areas and loss of VDR signal within cystic areas (n=3 patients). SCC and MEC do not show appreciable VDR signal (n=1 patient each). Scale bars 100μm.
Dietary vitamin D deficiency does not significantly alter salivary gland architecture
After confirming VDR expression in normal salivary ducts, we employed a mouse model to determine the impact of dietary vitamin D deficiency on the salivary gland. Female C57Bl6 mice reared on vitamin D sufficient AIN93G diets were randomized to continue on the vitamin D sufficient (SUFF) diet or were switched to the AIN93G diet devoid of vitamin D (DEF). After 4 weeks, submandibular salivary glands were harvested for histological analysis. These diets significantly reduced serum 25D as expected (SUFF: 39.0±8.4 ng/ml; DEF:18.3±4.5 ng/ml; mean±SD; n=4) but did not significantly alter body weight (SUFF: 26.3±2.9g; DEF: 25.3±2.0g, mean±SD; n=6). Histological examination of hematoxylin and eosin stained sections indicated that epithelial tissue architecture was similar in glands from DEF and SUFF mice (Fig. 3A). We did note moderate increases in the size of arterioles and venules in glands from DEF compared to SUFF mice (Supp Fig. 3 B,C). To follow up on these subtle changes in vasculature of DEF mice detected in H&E images (Supp Fig. 3A,B), we performed IHC for alpha-smooth muscle actin (α-SMA), which marks the smooth muscle component of the arteriole tunica externa, and CD31, which marks the endothelial cells in the tunica interna. Similar distribution and pattern for both CD31 and SMA were observed in the vasculature of glands from SUFF and DEF mice, indicating no gross differences in the arteriole layers associated with dietary vitamin D deficiency (Supp Fig. 3C,D,E).
Fig. 3. Effect of vitamin D depletion on murine submandibular salivary gland.
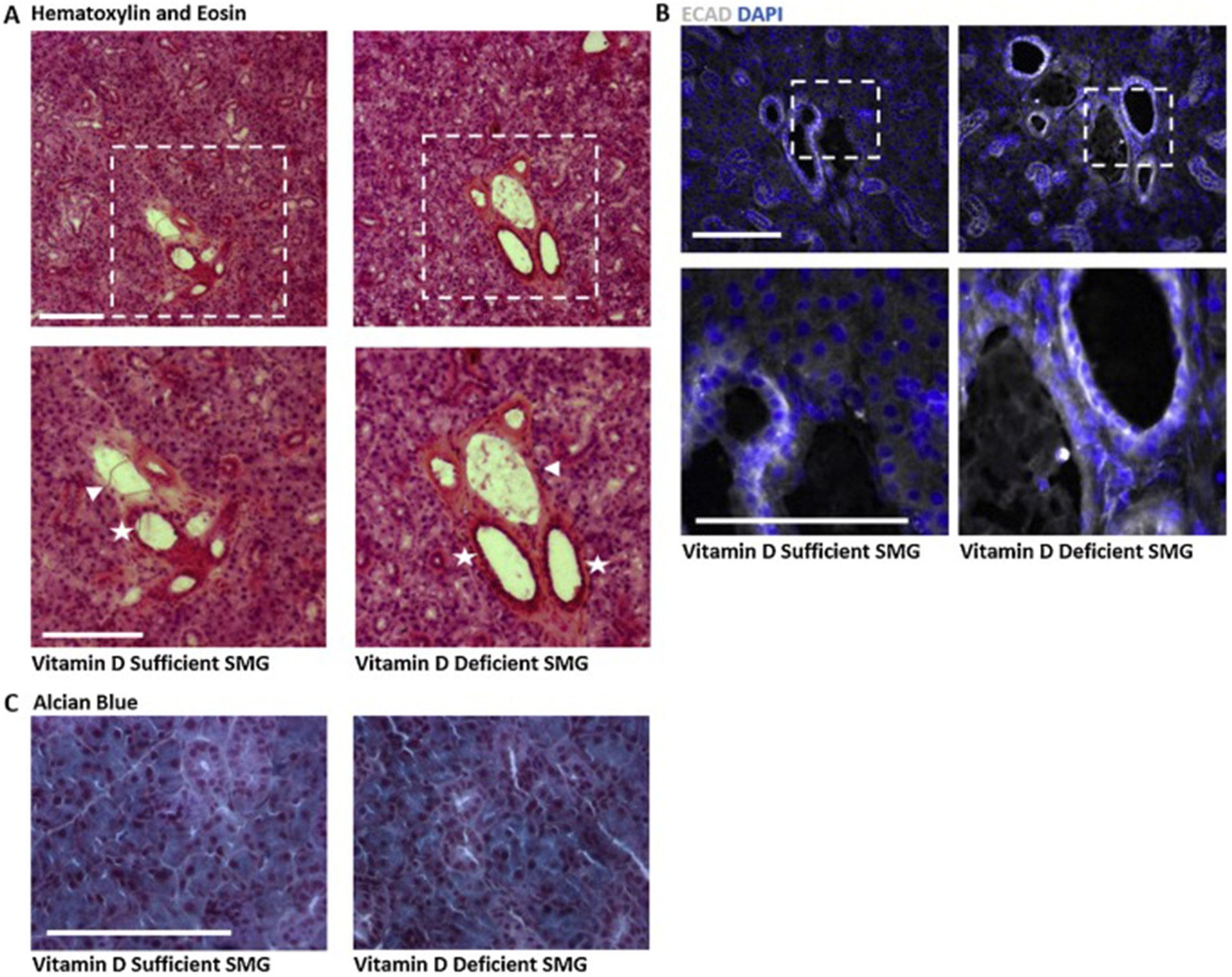
Submandibular glands were harvested, frozen and sectioned for histological analysis. Images representative of 3–6 mice are shown. A) Hematoxylin and eosin staining of SMGs highlights increased intensity of eosin surrounding large ducts (stars) and accompanying vasculature (arrows) in glands from vitamin D deficient compared to vitamin D sufficient mice. Scale bar 100μm. B) IHC for epithelial cadherin (ECAD) shows no visible change in the epithelial compartment in glands from vitamin D deficient mice compared to controls. Scale bar 50μm. C) Alcian blue staining showing secretory mucins in salivary glands from vitamin D deficient mice as compared to controls. Scale bar 100μm.
To specifically investigate the effect of dietary vitamin D on the salivary epithelium, we performed IHC for E-cadherin (ECAD), which marks epithelial integrity and is frequently reduced in carcinomas. No reduction in ECAD signal was observed in glands from DEF versus SUFF mice (Fig. 3B), suggesting maintenance of epithelial integrity despite reduced serum 25D in DEF mice. To assess secretory capacity, we performed Alcian blue staining to highlight mucins and again observed no changes associated with dietary deficiency (Fig. 3C).
Murine salivary gland cells express VDR and respond to 1,25D
To more clearly define the effects of vitamin D in the salivary gland, we utilized a recently characterized heterogenous cell line (mSGc) derived from mouse SMG.37 We first verified the presence of VDR by Western blot. As shown in Fig 4A, the VDR antibody identified a strong immunoreactive band in mSGc lysates at approximately 48kDa, the predicted size of murine VDR. qPCR was used to assess the impact of 1,25D on expression of Vdr and periostin (Postn), a gene strongly repressed by VDR ligands43,44. Treatment with 1,25D for 48 hours decreased expression of both Vdr and Postn (Fig. 4B) suggesting that VDR is transcriptionally active in mSGc. When mSGc were plated in media containing 1,25D, changes in cellular morphology were observed. Time lapse imaging after 24, 48, and 72 hours of exposure to 1,25D indicated enrichment of cells with spindle-like projections relative to vehicle treated cultures (Fig. 4C). Supplementation of mSGc media with 1,25D also significantly decreased cell number (Fig. 4D,E,F). Taken together, this data confirms that mSGc cultures express VDR and respond to 1,25D with changes in gene expression, altered morphology and reduced growth.
Fig. 4. VDR expression and function in murine salivary gland cell culture.

A) Western blot for VDR in murine submandibular salivary gland cells (mSGc) demonstrates VDR expression in vitro. B,C) RNA isolated from mSGc cultures treated with 100nM 1,25D or vehicle for 24 hrs was used for qPCR analysis with primers specific for mVdr and mPostn. Data were normalized to 18S mRNA and expressed as fold change in 1,25D treated samples relative to control values, shown +/− SD. VDR transcript was not significantly altered in 1,25D treated cells (Student’s unpaired two-tailed t test, p=0.16, mean +/− SD, n=3). Periostin (Postn) was significantly decreased in cells treated with 1,25D. (Student’s unpaired two-tailed t test, p<0.0001, mean +/− SD, n=3). D) Phase contrast images show morphology of mSGc treated with 100nM 1,25D or vehicle for 24, 48 and 72 hrs. Whereas vehicle treated cells were flat and round, 1,25D treated cells appeared raised and formed spindle-like projections (arrows). Scale bar 100μm. E, F) Cells were stained with DAPI nuclear dye and manually counted after 72h treatment with 100nM 1,25D or vehicle. Three representative fields were counted and averaged per sample. 1,25D decreased total cell number by approximately 50% compared to control cultures. (Student’s unpaired two-tailed t test, p=0.008, mean ± SEM, n=3 biological replicates, 3 representative fields counted and averaged for each sample). Scale bars 50μm.
1,25D increases differentiation markers and decreases proliferation of mSGc
To further explore the changes in mSGc morphology induced by 1,25D we assessed the epithelial marker ECAD by IHC. Since vitamin D promotes differentiation in many tissue types9,10,12,14,45, we expected that the changes in morphology induced by 1,25D might be associated with upregulation of ECAD. As shown in Fig 5A, Cdh1 mRNA expression was increased in mSGc within 24 hours of exposure to 1,25D and a corresponding increase in ECAD protein was detected after 72h exposure by IHC (Fig. 5B). As stratified epithelial tissues can be further characterized by keratin distribution, we also assessed K5 (Krt5) and K7 (Krt7) expression, as markers of basal progenitor cells and differentiated ductal cells respectively. By qPCR, we observed a non-significant decrease in Krt5 gene expression and a significant increase in Krt7 gene expression after 24 hours exposure to 1,25D (Fig. 5C). Using IHC, we examined whether prolonged treatment (72 hours) with 1,25D altered K5 protein expression. Although Krt5 mRNA was slightly decreased after 24 hours of 1,25D treatment, the relative percentage of K5+ cells per field was unchanged after 72 hours of treatment (Fig. 5D). This finding suggests that 1,25D does not alter the percentage of K5+ cells, but further studies are needed to determine if such cells gain additional differentiation markers in the presence of 1,25D.
Fig. 5. 1,25D increases differentiation markers and decreases proliferation of mSMGc in vitro.

A) RNA isolated from mSGc cultures treated with 100nM 1,25D or vehicle for 24 hrs was used for qPCR analysis with primers specific for mCdh1. Data were normalized to 18S mRNA and expressed as fold change in 1,25D treated samples relative to control values, shown +/− SD. Epithelial cadherin (Cdh1) expression increases with 100nM 1,25D after 24 hours exposure in culture. (Student’s unpaired two-tailed t test, p=0.18, mean +/− SD, n= 3 biological replicates). B) Immunocytochemistry for ECAD (green) after 72 hours suggests increased ECAD protein in 1,25D treated samples (n= 3 biological replicates). Scale bar 50μm. C) Quantitative PCR using RNA isolated from mSGc cultures treated with 100nM 1,25D or vehicle for 24 hrs with primers specific for mKrt5 or mKrt7. Data were normalized to 18S mRNA and expressed as fold change in 1,25D treated samples relative to control values, shown +/− SD. Krt5 and Krt7 indicate basal and mature ductal cells respectively, with analysis showing decreased Krt5 expression with increased Krt7 expression (Unweighted ANOVA with Tukey’s HSD, ** p<0.01, +/− SD, n= 3). D) Immunocytochemistry for Ki67 (white) in K5 positive cells (green) shows a global decrease in proliferation in 1,25D treated cells. Scale bar 50μm. E) Cell count of Ki67+ cells shows a significant decrease in percent of Ki67+ cells per area after 72 hours in culture with 100nM 1,25D (Student’s unpaired two-tailed t test, * p=0.02, +/− avg SEM, n= 3 independent biological replicates, with 3 representative fields counted, normalized to total DAPI+ cells and averaged per sample). F) Further characterization of Ki67+ cells co-positive for K5 indicates 1,25D significantly decreases the percentage of Ki67+K5+ cells per field. (Student’s unpaired two-tailed t test, * p=0.02, +/− avg SEM, n= 3 independent biological replicates, with 3 representative fields counted, normalized to total K5+ cells and averaged per sample). Scale bars 50μm. G) Flow cytometry was used to identify the percentage of cells in G1/G0 versus S+M phases of the cell cycle. 1,25Ddecreased the percentage of cells in S+M phases relative to control samples. (Unweighted ANOVA with Tukey’s HSD, ** p≤0.01, +/− avg SEM, n=4 biological replicates with n=2 averaged technical replicates each)
Differentiation is typically coupled to cell cycle exit46, and in other cellular contexts 1,25D decreases proliferation9. Thus, ICC was used to quantitate Ki67, a protein only present in actively cycling cells regardless of cell cycle phase47. The number of Ki67+ mSGc decreased after 72 hours of 1,25D treatment (Fig. 5D,E). Interestingly, the number of cells positive for both Ki67 and K5 also significantly decreased with 1,25D (Fig. 5F). To specifically examine the effects of 1,25D on mSGc cell cycle, cells were stained with PI and the percentage of cells in each phase of thecell cycle was quantitated by flow cytometry. Treatment with 1,25D increased the percentage of cells in G1/G0 and decreased the percentage of cells in G2/S/M as compared to vehicle controls (Fig. 5G). Together, these data suggest that 1,25D triggers cell cycle exit and differentiation in mSGc.
Discussion
Reduced VDR signaling, via dietary vitamin D deficiency or genetic deletion of the receptor itself, alters development and contributes to increased tumorigenesis and metastasis in many tissues9,48,49. The data presented here provides the first evidence that vitamin D signaling may also contribute to salivary gland homeostasis. This conclusion is based on analysis of VDR expression in normal gland and in four types of human salivary neoplasias as well as in vitro studies of 1,25D actions in a murine salivary epithelial cell line. Of particular interest with respect to salivary cancer, our preliminary study supports the concept that 1,25D alters salivary differentiation and that VDR expression is lost in aggressive salivary cancers.
Salivary gland cancers are relatively rare (3–6% of all cancers)24,50,51 and extremely heterogeneous, with multiple tumor types classified by cell type of origin. Unfortunately, most salivary cancers are diagnosed after invasion of surrounding tissue, leading to low overall survival rates20,50. The most aggressive types of salivary cancers are derived from basal cells, and these exhibit poor prognosis and high recurrence rates due to lack of definitive therapies25. Three of the most prevalent malignant salivary cancers are mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (AdCC), and squamous cell carcinoma (SCC)50,52. Although VDR protein was present in ductal cells of normal parotid salivary gland and in differentiated areas of benign lesions, we observed little to no VDR signal in cystic areas of AdCC, SCC or MEC tissue suggesting VDR down-regulation during tumor progression and metastasis. VDR expression pattern in pleomorphic adenoma, a relatively indolent tumor, was mixed, with higher than normal expression in differentiated areas and reduced expression in dysplastic areas. Although usually benign at diagnosis, pleomorphic adenomas can undergo progression to “carcinoma ex pleomorphic adenoma” which is usually fatal. More study is necessary to identify VDR targets in PA and to determine whether vitamin D status impacts the transition to more aggressive disease. Understanding the function of the VDR in these unique salivary tumors could have clinical significance.
In the murine adult salivary gland, the ductal epithelium is maintained by K5+ basal progenitor cells found in the pseudostratified layers of the large and intercalated ducts30,53. A recent lineage tracing study shows the flexibility of K5+ basal cells, with K5+ cells contributing to mature ductal cells during homeostasis and also to other cell types during times of stress and injury30. In both the human salivary gland and murine SMG, VDR expression was restricted to ductal cells, including intercalated and small ducts, consistent with previous data indicating radio-labeled ligand uptake in salivary ductal epithelium in the mouse, rat and hamster17. In human salivary gland tumors, we demonstrate that loss of VDR expression is associated with increased K5 staining, supporting previous investigations demonstrating high K5 positivity in MEC tissues54,55.
To further explore the role of VDR in the salivary gland, we used dietary vitamin D manipulation in adult mice, subjecting 12 week old female mice to four weeks of vitamin D deficiency prior to examination of SMG histology. Surprisingly, although the deficient diet reduced serum 25D to approximately 50% of control values, we observed only subtle differences in histology of glands from vitamin D sufficient and deficient mice. Interestingly, we initially observed a change in the salivary vasculature of vitamin D deficient mice, yet upon examination of vascular smooth muscle and endothelial cell layers, we observed a wide variation in samples. Consistent with previous studies showing no change in secretory capacity of the rat submandibular salivary gland with vitamin D deficiency56, our results show no visible defect in the epithelial compartment or in secretory mucins as assessed by Alcian blue staining. These data suggest that either VDR signaling is not essential for maintenance of adult salivary gland, or that the timing and magnitude of the dietary deficiency was not sufficient to compromise VDR function in the gland.
Vitamin D signaling decreases proliferation and promotes differentiation in many tissues9,14,45 and is necessary for normal mammary ductal development36. Since our initial investigations indicated VDR localization in ductal cells of human and murine salivary glands, we examined the effects of 1,25D in vitro using mSGc, an established murine salivary cell line previously characterized to contain heterogeneous ductal cell populations37. After confirming the presence of VDR by Western blot, we demonstrated functional VDR signaling in mSGc cultures treated with 1,25D. Treatment with 1,25D altered Vdr and Postn expression, indicating active signaling through VDR. Overexpression of periostin, an extracellular matrix protein, is correlated with tumorigenesis57–59 and 1,25D has been shown to reduce Postn in a VDR dependent manner43,44. It is therefore possible that loss of VDR in salivary tumors may correlate with increased Postn; however, the role of POSTN in salivary gland cellular differentiation and tumorigenesis has not been studied. 1,25D treatment of mSGc also reduced cell numbers, inhibited proliferation/cell cycles and altered phenotype. These observations are consistent with a previous study indicating that 1,25D triggered differentiation in HSG-3AZA salivary cell culture60. To assess differentiation, we first verified that 1,25D did not decrease epithelial characteristics in mSGc using both qPCR and ICC for Cdh1 and ECAD respectively. Epithelial differentiation is commonly marked by expression of specific keratins, particularly K5 (basal) and K7 (differentiated ducts). We observed significantly increased expression of K7 and a trend towards decreased K5 in 1,25D treated cells, suggesting altered differentiation. As differentiation is linked to cell cycle exit46,61 and vitamin D signaling is known to reduce proliferation in multiple cell types9, we examined cell number, Ki67 expression and DNA content. Our results indicate that 1,25D administration to mSGc cultures significantly decreases proliferation in all cells, including K5+ cells. As 1,25D reduced the total number of cells and the rate of proliferation, the observed morphological changes (cellular projections) could be secondary to decreased cell density. For example, mature ductal cells are cuboidal in vivo, but may assume a myoepithelial morphology at low density. Further investigation to clarify the specific aspects of 1,25D on mSGc morphology and differentiation are warranted. Collectively, our data suggest that loss of vitamin D signaling secondary to VDR downregulation may promote proliferation and K5+ cell expansion during salivary tumorigenesis.
Although we observed significant effects of 1,25D on proliferation and differentiation in normal salivary cells in vitro, our dietary study failed to detect defects in glandular morphology in adult mice fed vitamin D deficient diets. It remains possible that earlier or more severe vitamin D deficiency would alter glandular architecture. SMG development and differentiation is largely complete by post-natal day 1 (P1)31 and in the adult SMG, only a small percentage of cells are actively cycling unless the gland is injured or stressed30,62. It is therefore also likely that dietary vitamin D deficiency would result in a stronger phenotype in stressed or neoplastic tissues composed of a higher percentage of proliferating cells.
In conclusion, these studies provide insight into the role of the vitamin D pathway in salivary gland and form the basis for future work to address VDR function in additional developmental and pathological contexts.
Supplementary Material
Highlights.
VDR is expressed in ductal epithelium of human and mouse salivary gland
VDR is down regulated during salivary cancer progression
Cultured salivary cells express VDR and respond to 1,25-dihydroxyvitamin D
1,25-dihydroxyvitamin D induces cell cycle arrest and alters progenitor and differentiation markers in cultured salivary cells
Acknowledgements:
The authors thank Dr. Douglas Conklin for use of his Olympus IX80 microscope system. The authors are grateful to Dr. Rebecca Sinnott DeVaux for her critical reading and feedback on this manuscript. This research was partially funded by NIH grant CA194500 (to JW), and NIH NRSA fellowship, F32DE027868 (to KD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors declare no financial or non-financial conflicts of interest.
References
- 1.van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr 2001;11:199–226. [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D and Bone. Curr Osteoporos Rep 2012;10:151–9. 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol 2005;97:153–64. 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan AV, Trump DL, Johnson CS, Feldman D. The Role of Vitamin D in Cancer Prevention and Treatment. Endocrinol Metab Clin North Am 2010;39:401–contents 10.1016/j.ecl.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh J. Vitamin D and breast cancer: Past and present. J Steroid Biochem Mol Biol 2018;177:15–20. 10.1016/j.jsbmb.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci 2009;46:190 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018;50:. 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborti CK. Vitamin D as a promising anticancer agent. Indian J Pharmacol 2011;43:113–20. 10.4103/0253-7613.77335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev 2008;66:S116–124. 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F. Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res BCR 2012;14:211 10.1186/bcr3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente AG, Errea O, van Wijngaarden P, Gonzalez GA, Kerninon C, Jarjour AA, et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol 2015;211:975–85. 10.1083/jcb.201505119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang G-X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 2015;98:240–5. 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson K, Saini A, Strömberg A, Alam S, Lilja M, Rullman E, et al. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016;157:98–111. 10.1210/en.2015-1685. [DOI] [PubMed] [Google Scholar]
- 14.Braga M, Simmons Z, Norris KC, Ferrini MG, Artaza JN. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr Connect 2017;6:139–50. 10.1530/EC-17-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Salivary Gland Gene Expression Atlas n.d.
- 16.The Human Protein Atlas n.d.
- 17.Stumpf WE, Hayakawa N. Salivary glands epithelial and myoepithelial cells are major vitamin D targets. Eur J Drug Metab Pharmacokinet 2007;32:123–9. 10.1007/BF03190474. [DOI] [PubMed] [Google Scholar]
- 18.Stumpf WE. Vitamin D and the digestive system. Eur J Drug Metab Pharmacokinet 2008;33:85–100. 10.1007/BF03191025. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Liu Y, Huang Z, Li H, Gan X, Shen Z. 1,25-Dihydroxyvitamin D3 alleviates salivary adenoid cystic carcinoma progression by suppressing GPX1 expression through the NF-κB pathway. Int J Oncol 2016;48:1271–9. 10.3892/ijo.2016.3341. [DOI] [PubMed] [Google Scholar]
- 20.Jang JY, Choi N, Ko Y-H, Chung MK, Son Y-I, Baek C-H, et al. Treatment outcomes in metastatic and localized high-grade salivary gland cancer: high chance of cure with surgery and post-operative radiation in T1–2 N0 high-grade salivary gland cancer. BMC Cancer 2018;18:672 10.1186/s12885-018-4578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt NC, Sharma A, Gilbert MR, Kim S. Early T Stage Salivary Duct Carcinoma: Outcomes and Implications for Patient Counseling. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg 2015;153:795–8. 10.1177/0194599815601659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh J-L, Lee J-I, Choi S-H, Nam SY, Kim S-O, Cho K-J, et al. Prognostic factors and oncologic outcomes of 56 salivary duct carcinoma patients in a single institution: high rate of systemic failure warrants targeted therapy. Oral Oncol 2014;50:e64–66. 10.1016/j.oraloncology.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA, et al. Salivary gland carcinoma in Denmark 1990–2005: Outcome and prognostic factors: Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2012;48:179–85. 10.1016/j.oraloncology.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Hocwald E, Korkmaz H, Yoo GH, Adsay V, Shibuya TY, Abrams J, et al. Prognostic Factors in Major Salivary Gland Cancer: The Laryngoscope 2001;111:1434–9. 10.1097/00005537-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer Stem Cells in Breast. Pathobiol J Immunopathol Mol Cell Biol 2008;75:75–84. 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emmerson E, Knox SM. Salivary gland stem cells: A review of development, regeneration and cancer. Genes N Y N 2000 2018;56:e23211 10.1002/dvg.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knox SM, Lombaert IMA, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010;329:1645–7. 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon HR, Nelson DA, DeSantis KA, Morrissey JM, Larsen M. Endothelial cell regulation of salivary gland epithelial patterning. Dev Camb Engl 2017;144:211–20. 10.1242/dev.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeSantis KA, Stabell AR, Spitzer DC, O’Keefe KJ, Nelson DA, Larsen M. RARα and RARγ reciprocally control K5+ progenitor cell expansion in developing salivary glands. Organogenesis 2017;13:125–40. 10.1080/15476278.2017.1358336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng P-L, Aure MH, Maruyama T, Ovitt CE. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep 2018;24:1464–1470.e3. 10.1016/j.celrep.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knosp WM, Knox SM, Hoffman MP. Salivary gland organogenesis. Wiley Interdiscip Rev Dev Biol 2012;1:69–82. 10.1002/wdev.4. [DOI] [PubMed] [Google Scholar]
- 32.Boecker W, Stenman G, Loening T, Andersson MK, Bankfalvi A, von Holstein S, et al. K5/K14-positive cells contribute to salivary gland-like breast tumors with myoepithelial differentiation. Mod Pathol Off J U S Can Acad Pathol Inc 2013;26:1086–100. 10.1038/modpathol.2013.45. [DOI] [PubMed] [Google Scholar]
- 33.Boecker W, Stenman G, Loening T, Andersson MK, Berg T, Lange A, et al. Squamous/epidermoid differentiation in normal breast and salivary gland tissues and their corresponding tumors originate from p63/K5/14-positive progenitor cells. Virchows Arch Int J Pathol 2015;466:21–36. 10.1007/s00428-014-1671-x. [DOI] [PubMed] [Google Scholar]
- 34.McDonald LA, Walker DM, Gibbins JR. Cervical lymph node involvement in head and neck cancer detectable as expression of a spliced transcript of type II keratin K5. Oral Oncol 1998;34:276–83. 10.1016/S1368-8375(98)80008-0. [DOI] [PubMed] [Google Scholar]
- 35.Meyer MB, Benkusky NA, Lee C-H, Pike JW. Genomic determinants of gene regulation by 1,25-dihydroxyvitamin D3 during osteoblast-lineage cell differentiation. J Biol Chem 2014;289:19539–54. 10.1074/jbc.M114.578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Dev Camb Engl 2002;129:3067–76. [DOI] [PubMed] [Google Scholar]
- 37.Min S, Song E-AC, Oyelakin A, Gluck C, Smalley K, Romano R-A. Functional characterization and genomic studies of a novel murine submandibular gland epithelial cell line. PLoS ONE 2018;13:. 10.1371/journal.pone.0192775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gervais EM, Desantis KA, Pagendarm N, Nelson DA, Enger T, Skarstein K, et al. Changes in the Submandibular Salivary Gland Epithelial Cell Subpopulations During Progression of Sjögren’s Syndrome-Like Disease in the NOD/ShiLtJ Mouse Model. Anat Rec Hoboken NJ 2007 2015;298:1622–34. 10.1002/ar.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Becklund BR, DeLuca HF. Identification of a highly specific and versatile vitamin D receptor antibody. Arch Biochem Biophys 2010;494:166–77. 10.1016/j.abb.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis 2004;25:2361–72. 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 41.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol Baltim Md 2004;18:2208–23. 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 42.Brożyna AA, Jóźwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res 2014;34:2735–43. [PMC free article] [PubMed] [Google Scholar]
- 43.LaPorta E, Welsh J. Modeling vitamin D actions in triple negative/basal-like breast cancer. J Steroid Biochem Mol Biol 2014;144PA:65–73. 10.1016/j.jsbmb.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terao M, Yang L, Matsumura S, Yutani M, Murota H, Katayama I. A vitamin D analog inhibits Th2 cytokine- and TGFβ -induced periostin production in fibroblasts: a potential role for vitamin D in skin sclerosis. Dermatoendocrinol 2015;7:. 10.1080/19381980.2015.1010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim IM, Norris KC, Artaza JN. Vitamin D and Cardiac Differentiation. Vitam Horm 2016;100:299–320. 10.1016/bs.vh.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Wainwright LJ, Lasorella A, Iavarone A. Distinct mechanisms of cell cycle arrest control the decision between differentiation and senescence in human neuroblastoma cells. Proc Natl Acad Sci U S A 2001;98:9396–400. 10.1073/pnas.161288698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311–22. . [DOI] [PubMed] [Google Scholar]
- 48.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26:662–87. 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 49.Hossain S, Beydoun MA, Beydoun HA, Chen X, Zonderman AB, Wood RJ. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin Nutr ESPEN 2019;30:170–84. 10.1016/j.clnesp.2018.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin HH, Limesand KH, Ann DK. Current State of Knowledge on Salivary Gland Cancers. Crit Rev Oncog 2018;23:139–51. 10.1615/CritRevOncog.2018027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA, et al. Salivary gland carcinoma in Denmark 1990–2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2011;47:677–82. 10.1016/j.oraloncology.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis 2002;8:229–40. 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 53.Nelson DA, Manhardt C, Kamath V, Sui Y, Santamaria-Pang A, Can A, et al. Quantitative single cell analysis of cell population dynamics during submandibular salivary gland development and differentiation. Biol Open 2013;2:439–47. 10.1242/bio.20134309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pires FR, Chen S-Y, da Cruz Perez DE, de Almeida OP, Kowalski LP. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol 2004;40:545–51. 10.1016/j.oraloncology.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Butler RT, Spector ME, Thomas D, McDaniel AS, McHugh JB. An Immunohistochemical Panel for Reliable Differentiation of Salivary Duct Carcinoma and Mucoepidermoid Carcinoma. Head Neck Pathol 2013;8:133–40. 10.1007/s12105-013-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peterfy C, Tenenhouse A, Yu E. Vitamin D and parotid gland function in the rat. J Physiol 1988;398:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Idolazzi L, Ridolo E, Fassio A, Gatti D, Montagni M, Caminati M, et al. Periostin: The bone and beyond. Eur J Intern Med 2017;38:12–6. 10.1016/j.ejim.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Huang Z, Cui D, Ouyang G. The Multiaspect Functions of Periostin in Tumor Progression. Adv Exp Med Biol 2019;1132:125–36. 10.1007/978-981-13-6657-4_13. [DOI] [PubMed] [Google Scholar]
- 59.Cui D, Huang Z, Liu Y, Ouyang G. The multifaceted role of periostin in priming the tumor microenvironments for tumor progression. Cell Mol Life Sci CMLS 2017;74:4287–91. 10.1007/s00018-017-2646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azuma M, Kawamata H, Kasai Y, Yanagawa T, Yoshida H, Sato M. Induction of cells with a chondrocyte-like phenotype by treatment with 1 alpha,25-dihydroxyvitamin D3 in a human salivary acinar cell line. Cancer Res 1989;49:5435–42. [PubMed] [Google Scholar]
- 61.Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016;15:196–212. 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 2015;33:231–7. 10.1016/j.devcel.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


