Abstract
Introduction:
There is a paucity of information on the clustering of cardiometabolic risk factors in young adults and how this clustering may vary based on whether or not they perform sufficient levels of physical activity.
Methods:
We analyzed baseline data from 346 young adults (23.3±4.4 y) participating in the Healthy Body Healthy U (HBHU) clinical trial from 2015–2018. Cardiometabolic risk factors were measured according to standard procedures and moderate-to-vigorous intensity physical activity (MVPA) was determined by accelerometry. A cardiometabolic clustering score (CCS; ranging from 0 to 5) was created from five biomarkers according to whether or not a standard clinical risk cut point was exceeded (0=no; 1=yes): abdominal circumference [>102 cm (men) or >88 cm (women)]; HbA1c (≥ 5.7%); HDL-C [<40 mg/dL (men) or <50 mg/dL (women)]; SBP (≥ 130 mmHg); and DBP (≥85 mmHg). Cardiometabolic dysregulation (CD) was defined as a CCS ≥ 3. Multiple logistic regression determined the independent association between level of MVPA and CD, while adjusting for sex, race/ethnicity, sedentary time, and smoking,
Results:
The prevalence of CD was 18% (22% in men; 17% in women). We observed a non-linear graded association between MPA and CD. Participants performing 150–300 min/week of MVPA significantly lowered their odds of CD by 66% (OR=0.34; 95% CI=0.16, 0.75), while those exceeding 300 min/week lowered their odds by 61% (OR=0.39; 95% CI: 0.18, 0.86), compared with those performing <150 min/week, independent of obesity and the other covariables.
Conclusion:
Recommended levels of moderate-intensity physical activity is significantly associated with a lower odds of CD and thus may prevent or diminish the need for expensive pharmaceutical treatment over the remainder of the lifespan.
Keywords: body weight, chronic disease, metabolic syndrome, physical activity
Introduction
Metabolic syndrome is a clustering of biological risk factors comprising excess abdominal adiposity, dysregulated glucose, triglycerides and high-density lipoprotein cholesterol (HDL-C) concentrations, and hypertension (1). Between 1988 and 2012 the prevalence of the metabolic syndrome increased from 25.3% to 34.2% in the United States (US) (2). Several of the components of the metabolic syndrome are evident in childhood (3) and continue to increase in prevalence through adulthood (1, 2). National Health and Nutrition Examination Survey (NHANES) data from 2007–2012 indicate that among non-obese adults, the prevalence of metabolic syndrome was 23.2% among those 18–29 years of age and increased to 35.1% in adults 30–49 years old (2). In adult populations, risk factor clustering contributes to accelerated atherosclerotic processes, type 2 diabetes and cardiovascular disease (4, 5). There is some evidence, however, that the clustering pattern of these various components of the metabolic syndrome varies by age (6, 7), with younger adults having a lower prevalence of HDL-C dysregulation, glucose intolerance, and hypertension compared with older adults (7). The stability of the metabolic syndrome definition also has been questioned during the transition period from adolescence to young adulthood, thereby resulting in the American Academy of Pediatrics calling for a more inclusive approach of focusing on cardiometabolic risk factor clustering, rather than on the metabolic syndrome per se (8).
The prevalence of obesity in US adults aged 20–39 years old increased from 31% in 2010 to 36% in 2016 (9), and 2018 National College Health Assessment (NCHA) data indicate that almost 37% of undergraduates surveyed were overweight or obese (10). Moreover, data from the Longitudinal Study of Adolescent Health indicate that obesity prevalence doubles between adolescence and the early 20s, and then doubles again from the early 20s to the early 30s (11). Cardiometabolic risk factor clustering increases markedly among adolescents and young adults with overweight (body mass index [BMI] 25–29.9 kg/m2) or obesity (BMI ≥30 kg/m2), compared with those having a healthy (BMI 18.5–24.9 kg/m2) body weight (12–15). These parallel increasing trends in metabolic dysregulation and in obesity in young adults is clinically significant, as these conditions increase the risk of more serious chronic diseases and premature mortality later in adulthood (16).
Regular physical activity is effective in preventing excessive weight gain and in the treatment of obesity-related co-morbidities (17, 18). Moreover, recent evidence suggests that regular physical activity improves cardiometabolic health in adolescents and young adults independent of their weight status (19, 20). The Physical Activity Guidelines for Americans, 2nd Edition recommend that adults achieve 150 min/week of moderate-intensity physical activity, 75 min/week of vigorous-intensity activity, or a combination of both (18). Despite the well-known benefits of moderate-to-vigorous intensity physical activity only 21% of US adults (≥18 years) meet these Guidelines (21). In college-aged populations, the prevalence of physical inactivity (i.e., not meeting the Guidelines) is between 36 and 50% (22).
The transition from adolescence to young adulthood is a pivotal time of major life changes and time-consuming obligations that often disrupt the ability to be physically active. Unhealthy behaviors adopted at this time become more difficult to alter through early adulthood, thus increasing the risk of weight gain and metabolic dysregulation before middle-age (23, 24). Unfortunately, there is a paucity of information on the clustering of metabolic risk factors in this age group and how this clustering may vary based on whether or not young adults perform sufficient levels of physical activity. Although previous cross-sectional studies have reported that between 20–40% of young adults had at least one component of the metabolic syndrome (7, 12, 13), none of those studies examined the role of physical activity in mitigating that risk factor profile. Thus, the purpose of this study was to determine the association between moderate-to-vigorous intensity physical activity (MVPA) and cardiometabolic risk factor clustering in young adults enrolled in a weight management randomized controlled trial. We hypothesized that level of MVPA would be significantly and inversely associated with the odds of cardiometabolic dysregulation, independent of weight status.
Methods
Study Participants
Study participants (N=460) were enrolled in the Healthy Body Healthy U (HBHU) randomized controlled trial that tested digital intervention strategies to promote weight loss and management between 2015 and 2018. Main eligibility criteria were: 1) 18–35 years of age and enrolled in a college or university in the District of Columbia or Boston area; 2) A BMI of 25 to 45 kg/m2; 3) an active Facebook user (logged in within the last month) with regular text message access; and 4) generally healthy enough to participate in physical activity. For a full list of eligibility criteria, see Napolitano, et al. (25). Participants were included in these analyses if they had at least at least 4 days with 10 hours of ActiGraph wear time and complete cardiometabolic risk factor data, leaving a final sample size of 346 participants for the analysis. Study participants provided written informed consent and all study procedures were approved by the Institutional Review Board of the George Washington University.
Clinical Measures
Weight and height were measured in duplicate using a digital scale (Seca Model 769) and standard portable stadiometers. Weight was recorded to the nearest 0.2 kg, while height was recorded to the nearest 0.1 cm, and BMI was calculated as [weight (kg)/height (m2)]. Abdominal circumference was measured in triplicate at the level of the umbilicus using a cloth tape measure. Each measurement was recorded upon exhale, ensuring that the tape measure remained parallel to the floor and untwisted. Measurements were rounded to the nearest 0.1 cm and averaged.
Blood pressure measurements were taken in triplicate using a digital blood pressure monitor (OMRON HEM-907XL), with an appropriately sized cuff (bladder length encircling 80–100% of participant’s upper arm). Participants sat quietly for five minutes prior to measurement, and the average systolic (SBP) and diastolic (DBP) blood pressures were calculated from the three measurements. Capillary blood samples were obtained after an overnight fast of at least 8 hours for the measurement of fasting glucose (OneTouch® Ultra® 2) and hemoglobin A1c (HbA1c; A1cNow+, PTS Diagnostics).
A cardiometabolic clustering score (CCS) was created from the variables abdominal circumference, HbA1c, HDL-C, SBP, and DBP, according to whether a standard clinical risk cut point was exceeded (0=no; 1=yes). These cut points were: abdominal circumference [>102 cm (men) or >88 cm (women)]; HbA1c (≥ 5.7%); HDL-C [<40 mg/dL (men) or <50 mg/dL (women)]; SBP (≥ 130 mmHg); and DBP (≥85 mmHg) (4, 26). Scores for each risk factor were summed across all five factors to derive the CCS, which ranged from 0 to 5. We considered a CCS score ≥3 to be associated with a higher odds of cardiometabolic dysregulation (CD).
Physical Activity
Prior to study randomization, physical activity was measured objectively by accelerometry (ActiGraph, wGT3X-BT) for seven consecutive days. Participants were instructed to wear the accelerometer on the right side of the waist at the tip of the iliac crest. Valid wear time was defined as ≥600 min/day (waking hours) for at least four of the seven days (27) and was validated by the ActiLife software. Non-wear was defined as an interval of at least 60 consecutive minutes of zero activity counts, with allowance for 1–2 minutes of counts between 0 and 100 counts per minute (cpm). Thresholds of accelerometer counts for adults were based on established cut-points by Freedson, et al. (28) to define physical activity intensity. Sedentary time (min/day) was defined as accelerometer counts <100 cpm, while MVPA (min/week) was defined as ≥1952 cpm. Vigorous-intensity physical activity (min/week) was defined as counts between 5725 and 9498 cpm. To account for variability in number of wear days, weekly MVPA was calculated by multiplying the average daily total by seven.
Statistical Analysis
Univariate analyses (means±SD, frequencies (%)) first were generated on all study variables in order to determine their distributions within the study population. We assessed collinearity and associations among the study variables using correlation and chi-square tests. We used logistic regression to evaluate the simple associations of each study variable and covariable on the odds of CD. Pre-specified multivariable logistic regression models were then used to determine the independent association between weekly MVPA and the odds of CD while adjusting for sex, race/ethnicity, self-reported smoking, and sedentary time. These covariables were chosen because of their demonstrated association with both MPA and cardiometabolic risk in the literature. We considered MVPA first as a continuous variable in the modeling and also then categorized it to approximate the Physical Activity Guidelines for Americans, 2nd Edition (<150 min/week; 150–300 min/week; and >300 min/week) (18). Odds ratios (OR) and 95% confidence intervals (CI) are reported from the final logistic regression models to describe the odds of CD within each level of MVPA, relative to the referent group (<150 min/week). Analyses were performed in SAS, 9.4 at an alpha level of p<0.05.
Results
The mean age of the study participants was 23.3±4.4 years. Of the 346 participants, most were women (78%) and non-smoking (93%). About 49% of the sample identified as Non-Hispanic White and 40% identified as Non-White. On average, participants spent 309±161 min/week in MVPA, with only 23.6±39.4 min/week being of vigorous intensity. Only 15% engaged in <150 min/week of MVPA, while 38% performed between 150–300 min/week, and 47% exceeded 300 min/week. Participants were sedentary for approximately 9.2±1.3 h/day. Table 1 displays mean risk factor and MVPA levels for women and men separately. As indicated, both men and women were obese and had excess abdominal adiposity. Women had significantly higher HbA1c concentrations compared with men, while men had a significantly higher SBP, DBP, and MVPA levels compared with women.
Table 1 -.
Levels of cardiometabolic risk factors and weekly MVPA for women and men (n=346)
| Risk Factor | Women (n=269) | Men (n=77) | p-value* |
|---|---|---|---|
| BMI (kg/m2) | 31.0±4.4 | 31.3±4.6 | 0.56 |
| Abdominal Circ (cm) | 97.6±11.2 | 104.5±12.3 | <0.0001 |
| HbA1c (%) | 5.3±0.43 | 5.2±0.45 | 0.03 |
| SBP (mmHg) | 112±10 | 125±11 | <0.0001 |
| DBP (mmHg) | 73±9 | 75±9 | 0.03 |
| HDL-C (mg/dl) | 50.5±11.1 | 41.5±9.1 | <0.0001 |
| MVPA (min/week) | 297±159 | 350±165 | 0.01 |
Data are mean (±SD); MVPA=moderate-to-vigorous intensity physical activity;
Sex-differences determined by independent t-tests.
The prevalence of CD was 18% (22% in men; 17% in women, p=0.32). The majority (89%) had between one and three risk factors for cardiometabolic disease, while the proportion of those having four or more was negligible (Figure 1). The most prevalent risk factors were excess abdominal adiposity (75%), followed by low HDL-C (47%), and HbA1c concentrations ≥ 5.7% (19%). Approximately 10% of participants had isolated SBP hypertension, while 11% had isolated diastolic hypertension, and 3% had both. Agreement between systolic and diastolic hypertension was low (kappa=0.23; p<0.67), suggesting a distinction between these risk factors in young adults.
Figure 1.
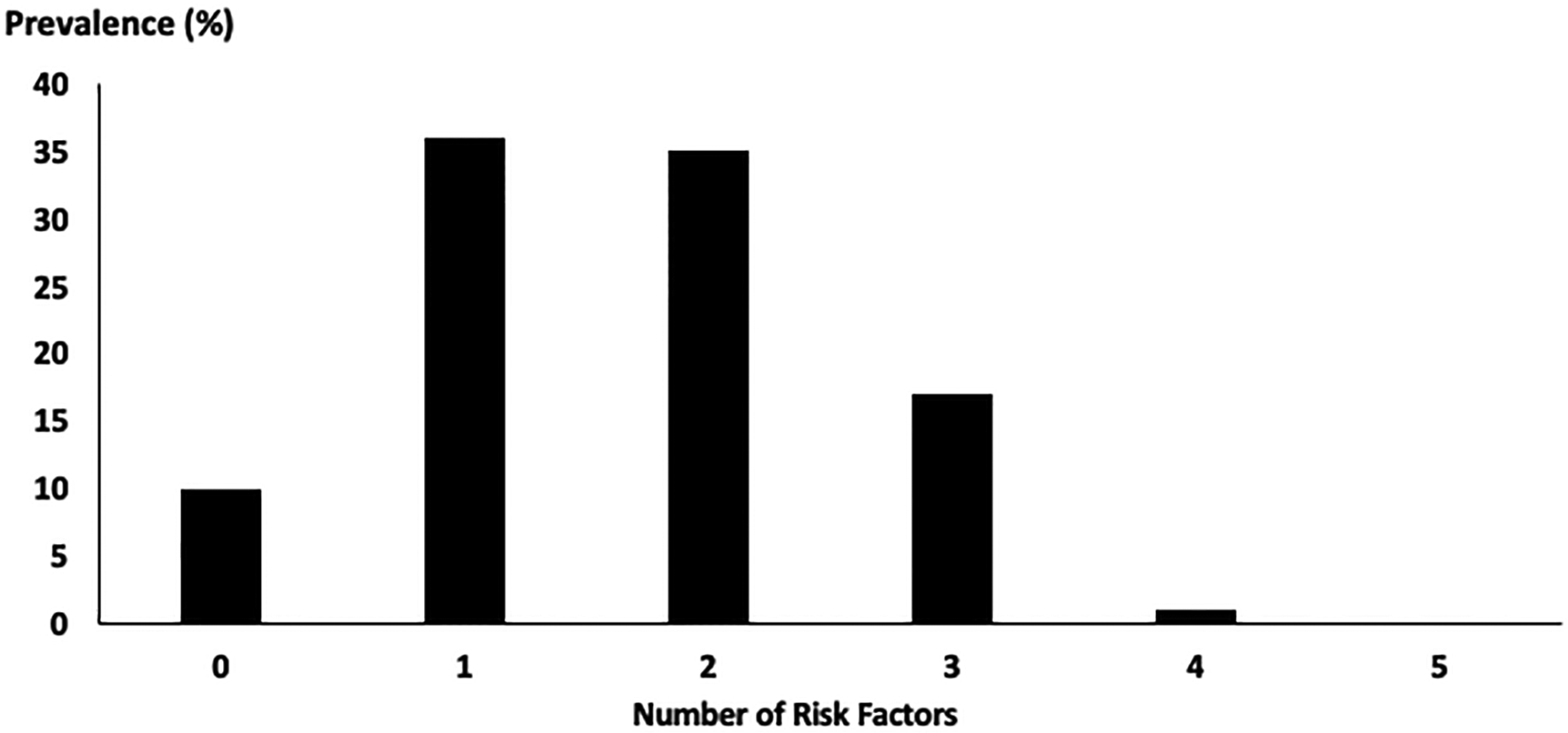
Clustering of Cardiometabolic Risk Factors in the HBHU Cohort (N=346).
The mean BMI in those participants engaging in <150 min/week of MVPA was 32.6±4.6 kg/m2; in those performing 150–300 min/week, BMI was 31.0±4.0 kg/m2; and was 30.7±4.6 kg/m2 in those performing > 300 min/week of MVPA (p<0.05). Although this latter finding confirms the inverse-graded association usually observed between level of physical activity and BMI, it is important to note that in this young adult sample, obesity was present even at the highest level of MVPA.
We observed only a marginally significant association between the continuous measure of MVPA and CD in the multivariable analysis (OR=0.90; 95% CI: 0.80, 1.02), presumably due to the non-linear nature of this relationship (Table 2). Indeed, the unadjusted graded curve in Figure 2, indicates a steep drop in risk early on, with some leveling off of benefits at higher levels of MVPA. The results of the multivariable logistic regression modeling using the categorical MVPA measure also are shown in Table 2. Compared with participants who did not achieve the minimum levels of MVPA (<150 min/week), those who performed 150–300 min/week had a 66% lower odds of CD (OR=0.34; 95% CI=0.16, 0.75), while those exceeding 300 min/week lowered their odds by 61% (OR=0.39; 95% CI: 0.18, 0.86). Participants identifying as Non-White experienced a two-fold higher odds of CD compared with Non-Hispanic Whites (OR=2.01; 95%CI=1.08, 3.72), and men had a 70% higher odds of CD compared with women, although this later finding failed to reach statistical significance (OR=1.74; 95%CI=0.89, 3.42). These results were not influenced by sedentary time or by smoking.
Table 2.
Logistic regression models predicting cardiometabolic risk (N=346).
| Variable | Model 1a | Model 2b, e | Model 3c, e | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | aORd | 95% CI | aORd | 95% CI | |
| MVPA (continuous; hrs/week) | 0.90 | 0.80, 1.00 | 0.90 | 0.80, 1.02 | -- | -- |
| MVPA (category) | ||||||
| <150 min/week (n=51) | REF | REF | ||||
| 150–300 min/week (n=132) | 0.41 | 0.20, 0.88 | --- | --- | 0.34 | 0.16, 0.75 |
| >300 min/week (n=163) | 0.42 | 0.20, 0.86 | 0.39 | 0.18, 0.86 | ||
| Sedentary Time (hrs/week) | 1.18 | 0.96, 1.46 | 1.10 | 0.88, 1.37 | 1.14 | 0.91, 1.44 |
| Race/Ethnicity | ||||||
| Non-Hispanic White (n=168) | REF | REF | REF | |||
| Non-White (n=137) | 2.08 | 1.16, 3.74 | 1.96 | 1.06, 3.61 | 2.01 | 1.08, 3.72 |
| Unknown (n=41) | 1.08 | 0.41, 2.86 | 1.02 | 0.38, 2.78 | 0.90 | 0.32, 2.48 |
| Sex | ||||||
| Female (n=269) | REF | REF | REF | |||
| Male (n=77) | 1.37 | 0.74, 2.57 | 1.74 | 0.90, 3.38 | 1.74 | 0.89, 3.42 |
| Regular smokere | ||||||
| No (n=320) | REF | REF | REF | |||
| Yes (n=23) | 0.40 | 0.09, 1.78 | 0.39 | 0.09, 1.76 | 0.36 | 0.08, 1.67 |
Model 1: Unadjusted; each predictor entered into a separate model
Model 2: All covariates entered simultaneously; MVPA entered as a continuous variable
Model 3: All covariates entered simultaneously; MVPA entered as a categorical variable
aOR: adjusted odds ratio
n=343 because of 3 participants with missing smoking history
Figure 2.
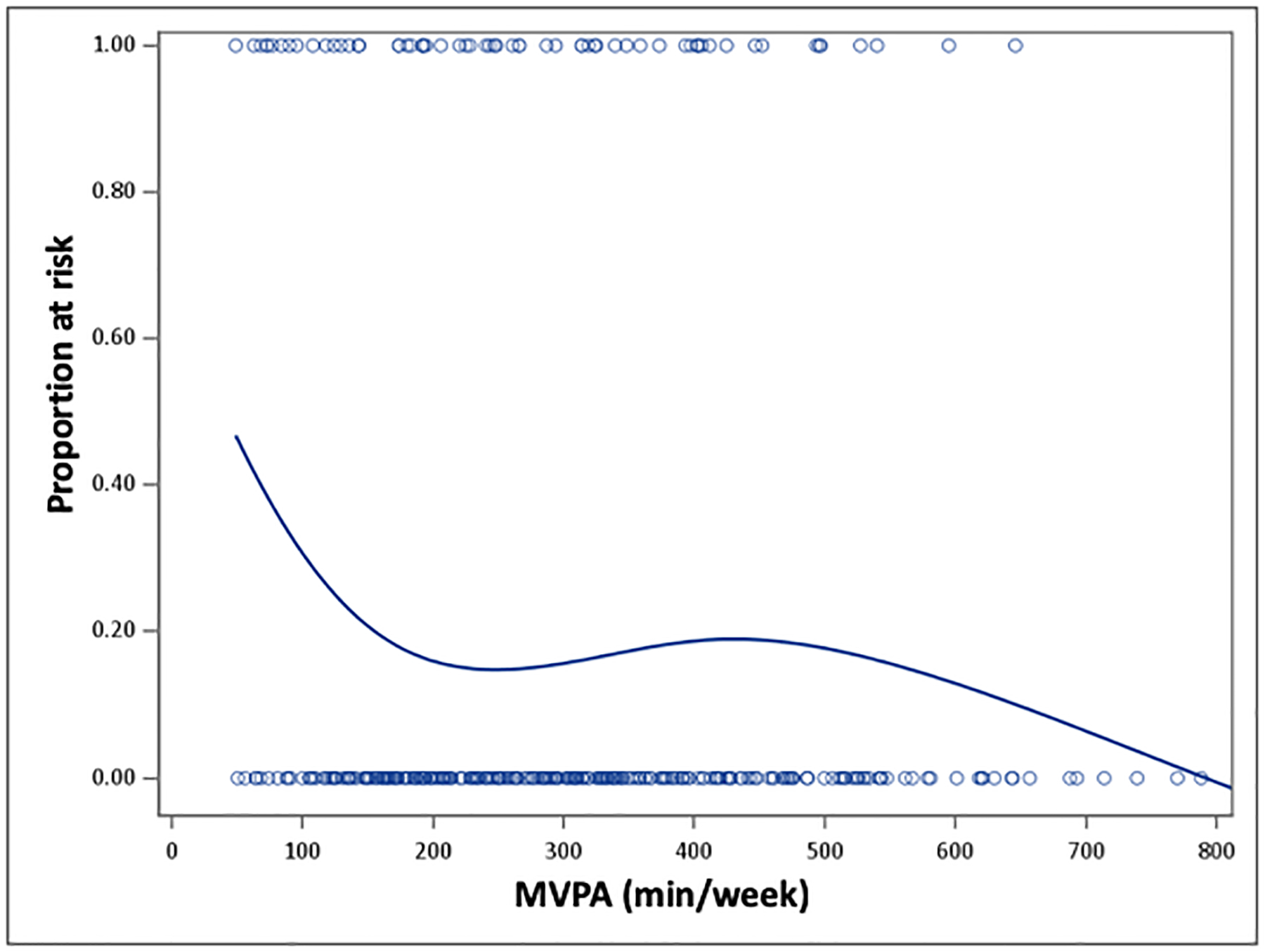
The Smoothed Dose-Response Association between MVPA and Cardiometabolic Risk. The curve is derived from the unadjusted logistic regression model using a penalized b-spline curve. MVPA=moderate-to-vigorous intensity physical activity.
Discussion
The prevalence of the metabolic syndrome has increased substantially in the United States (2) and globally (29) over the last several decades, and this increasing trend has become especially apparent in young adults. Data from the Cardiovascular Risk in Young Finns Study (29) indicate a significant secular trend in 24 year-old Finns between 1986 and 2001, with the prevalence of metabolic syndrome increasing from 1.0% to 7.5% (p<0.0001) over these 15 years. Among the Finnish men in this cohort, there was a six-fold increase in the prevalence of metabolic syndrome (from 4% to 25.2%; p<0.0001) between the ages of 24–39 years, with most of the age-related increase in prevalence due to increases in obesity and serum triglyceride levels.
This current study did not focus on the metabolic syndrome per se; rather, we captured cardiometabolic risk by quantifying the clustering of its components (8). The clustering of risk factors for cardiometabolic conditions across the lifespan has been examined less frequently than the metabolic syndrome, and there is some evidence that this clustering may be greater than expected in younger adults (25–44 years) compared with older adults (≥65 years) (6), thereby suggesting that metabolic syndrome differs in younger versus older people. This notion is further supported by data from NHANES indicating a differential distribution of risk components for young versus older adults (7). We observed that 18% of this younger adult sample had three or more risk factors related to the metabolic syndrome. This prevalence of CD is considerably higher than the 5–7% prevalence of metabolic syndrome reported from a pooled analysis of 26,609 young adults from 17 countries (30). This discrepancy is likely because the analysis by Nolan and colleagues (30) used the standard biomarkers for defining metabolic syndrome (waist circumference, fasting glucose, triglyceride, and HDL-C concentrations, and hypertension) and we did not. Moreover, our study subjects all had prevailing overweight or obesity at baseline, thereby making them more susceptible to multiple other cardiometabolic risk factors. Also, data from the National Health and Nutrition Examination Survey indicate that isolated diastolic hypertension is the most common hypertension subtype among young adults (31). Therefore, we were interested in the isolated contribution of diastolic (from systolic) hypertension on the cardiometabolic clustering score and did not combine these two blood pressure variables.
The overwhelming contributor to CD in our population was excess abdominal adiposity (75%), which is consistent with what has been observed in obese medical school students living in India (59%)(32), in 25–44 year old adults living in Australia (58%)(6), and 18–29 year old adults in the US (86%)(7). Excess overall and abdominal adiposity, especially in the visceral depot, secretes various pro-inflammatory factors (e.g., cytokines and adipokines) that are linked to the pathophysiology of cardiovascular disease. Chronic, low grade inflammation leads to hyperglycemia, dyslipidemia, hypertension, and insulin resistance, thus initiating the metabolic syndrome (33). That this potent risk factor for cardiometabolic disease was observed at such a young age in our study sample is cause for concern due to the relation of chronic inflammation and cardiometabolic dysregulation to early mortality (34).
We observed a non-linear graded association between level of MVPA and the likelihood of having CD among young adults with prevailing obesity. A longitudinal analysis over 20 years among young adults in the Coronary Artery Risk Development in Young Adults (CARDIA) study (35) indicated that physical fitness was inversely associated with the development of metabolic syndrome. Similar to studies linking MVPA or cardiorespiratory fitness to all-cause mortality (17, 36), the largest drop in the odds of CD in our study was observed between those engaging in <150 min/week of MVPA and those achieving 150–300 min/week, with a leveling off of benefits at higher levels of MVPA. Indeed, over 60% of the benefits of MVPA to cardiometabolic health were observed within a volume that approximates the target range 150–300 min/week of moderate-intensity activity from the Physical Activity Guidelines for Americans, 2nd Edition (18). Since our participants engaged in an average of only about 24 min/week of vigorous-intensity physical activity, we feel confident about reporting our results within the framework of the moderate-intensity physical activity cut-points used in the national guidelines.
Findings from other countries (6, 28) indicate a higher prevalence of metabolic syndrome in men, compared with women, with the exception of the 24 year old age group in the Finnish cohort, in which the prevalence of metabolic syndrome was higher in women (29). In contrast, NHANES data from 1988 to 2012 indicate a similar prevalence for men and women (2). We observed a slightly higher prevalence of CD in men compared with women; however, this difference was not statistically significant in the presence of race, sedentary time, and cigarette smoking. We observed a higher prevalence of CD in non-white versus white participants, and this is consistent with data from both NHANES (2) and CARDIA (35). Moore and colleagues (2) report that the greatest increase in prevalence of metabolic syndrome between 1988 and 2012 was observed in non-Hispanic black men (55%), whereas the increase was 31% in non-Hispanic white men. Collectively, these findings underscore the importance of surveillance within various at-risk demographic subgroups in order to improve preventive, management, and treatment approaches to cardiometabolic disease.
We note the limitations to our study. The cross-sectional nature of these baseline data limit our ability to establish temporality between MVPA and cardiometabolic risk and thus, our ability to make causal inferences is compromised. Even though MVPA was objectively measured using accelerometry, some of the higher values may not represent regular MVPA habits; however, we do not expect that any potentially-inflated MVPA behavior to be differential by weight status or by CD level. Also, we used the abdominal (rather than the waist) circumference as a marker for excess abdominal adiposity and this may explain our higher prevalence of elevated abdominal circumference compared with others (6, 32). We previously have observed a strong correlation (r = 0.80–0.83; p< 0.001) between the abdominal circumference and the visceral fat area measured by computed tomography (37). Finally, our study population comprised a self-selected cohort of college students, who may not represent the general population of younger adults. Although this self-selection may compromise the generalizability of the findings, we propose that the benefits of MVPA to cardiometabolic risk may be even greater in less robust younger people with lower educational attainment and socioeconomic status and higher levels of obesity.
Findings from this study, coupled with larger observational studies indicate an increasing prevalence of cardiometabolic risk factors among young adults. Many young adults may not be aware of their cardiometabolic risk status (38) or may think they are less susceptible to cardiometabolic disease due to their age (39). This highlights the need for targeted risk communication to young adults, as well as to providers treating this population. Our data also suggest that MVPA may be an effective strategy to reverse or delay the onset of cardiometabolic disease – even in young people with obesity. Moreover, MVPA may prevent or diminish the need for expensive pharmaceutical treatment of cardiometabolic disease over the lifespan. Given the growing prevalence of metabolic syndrome at younger and younger ages, any effort to avoid drug therapies for as long as possible into middle- and older-age has substantial economic and public health implications.
Acknowledgements
The authors would like to acknowledge the valuable contributions from the HBHU participants and student research assistants.
This study was funded by NIH/NIDDK R01 DK100916 to Dr. Napolitano. The results of this study do not constitute endorsement by ACSM, and are presented clearly, honestly, and without fabrication, falsification, or inappropriate manipulation. The contents of this manuscript have not been previously published elsewhere.
Footnotes
Conflicts of Interest
The results of this study do not constitute endorsement by ACSM, and are presented clearly, honestly, and without fabrication, falsification, or inappropriate manipulation. The contents of this manuscript have not been previously published elsewhere. No financial disclosures or conflicts of interest have been reported by the authors of this manuscript.
References
- 1.Ford ES, Giles WH, and Dietz WH. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA. 2002; 287:356–359, doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Moore JX, Chaudhary N, and Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017; 14: E24–E24, doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Bao W, Begum S, Elekasabany A, Srinivasan SR and Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. 2000;49:1042–1048, doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the Metabolic Syndrome. Circulation. 2009; 120:1640–1645, doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GB, McFarlane PA, et al. Cardiometabolic risk in Canada: a detailed analysis and position paper by the cardiometabolic risk working group. Can J Cardiol. 2011;27:e1–e33, doi: 10.1016/j.cjca.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Devers MC, Campbell S, and Simmons D. Influence of age on the prevalence and components of the metabolic syndrome and the association with cardiovascular disease. BMJ Open Diabetes Research & Care. 2016; 4: e000195, doi: 10.1136/bmjdrc-2016-000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner AD, Sardi GL, and Reed JF III. Components of the metabolic syndrome differ between young and old adults in the US population. The Journal of Clinical Hypertension. 2012; 14: 502–506, doi: 10.1111/j.1751-7176.2012.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magge SN, Goodman E, Armstrong SC, AAP COMMITTEE ON NUTRITION, SECTION ON ENDOCRINOLOGY, SECTION ON OBESITY. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140:e20171603. [DOI] [PubMed] [Google Scholar]
- 9.Hales CM, Fryar CD, Carroll MD, Freedman DS, and OgdenCL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018; 319: 1723–1725, doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College Health Association. American College Health Association–National College Health Assessment II: Undergraduate students reference group executive summary fall 2018. (Hanover, MD, 2018). [Google Scholar]
- 11.Gordon-Larsen P, The NS, and Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity. 2010;18: 1801–1804, doi: 10.1038/oby.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang TT, Kempf AM, Strother ML, Li C, Lee RE, Harris KJ, Kaur H. Overweight and components of the metabolic syndrome in college students. Diabetes Care. 2004:27:3000–3001, doi: 10.2337/diacare.27.12.3000. [DOI] [PubMed] [Google Scholar]
- 13.Huang T, Shimel A, Lee RE, Delancey W, Srother ML. Metabolic risks among college students: prevalance and gender differences. Metab Syndr Relat Disord. 2007;5:365–372, doi: 10.1089/met.2007.0021. [DOI] [PubMed] [Google Scholar]
- 14.Skinner AC, Mayer ML, Flower K, and WeinbergerM. Health status and health care expenditures in a nationally representative sample: how do overweight and healthy-weight children compare? Pediatrics. 2008;121: e269–e277, doi: 10.1542/peds.2007-0874. [DOI] [PubMed] [Google Scholar]
- 15.Renders CM. et al. Identifying metabolic syndrome without blood tests in young adults—The Terneuzen Birth Cohort. European Journal of Public Health. 2008; 18:656–660, doi: 10.1093/eurpub/ckn056. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005; 28: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 17.U. S. Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. (Office of Disease Prevention and Health Promotion, Washington, DC: ), 2018. [Google Scholar]
- 18.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018; 320:2020–2028, doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colberg SR, Sigal RJ, Yardley JE, Riddel MC, Dunstan DW, Dempsey PC, Horton ES, et al. Physical activity/exercise and diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065, doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wennberg P, Gustafsson PE, Dunstan DW, Wennberg M, and Hammarström A. Television viewing and low leisure-time physical activity in adolescence independently predict the metabolic syndrome in mid-adulthood. Diabetes Care. 2013;36:2090–2097, doi: 10.2337/dc12-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Facts about Physical Activity, <https://www.cdc.gov/physicalactivity/data/facts.htm> (2018). Accessed February 18, 2019.
- 22.Keating XD, Guan J, Piñero JC, and Bridges DM. A meta-analysis of college students’ physical activity behaviors. Journal of American College Health. 2005;54:116–126, doi: 10.3200/JACH.54.2.116-126. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CE, Jacobs DR Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA Study. Am J Epidemiol. 2000;151:1172–1181, doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 24.Tucker JM, Welk GJ, and Beyler NK. Physical activity in U.S. adults: compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40:454–461, doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Napolitano MA, Whiteley JA, Mavredes MN, Faro J, DiPietro L, Hayman LL, et al. Using social media to deliver weight loss programming to young adults: Design and rationale for the Healthy Body Healthy U (HBHU) trial. Contemp Clin Trials. 2017;60:1–13, doi: 10.1016/j.cct.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norberg M, Eriksson JW, Lindahl B, Andersson C, Rolandsson O, Stenlund H, Weinehall L. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J Intern Med. 2006;260;263–271, doi: 10.1111/j.1365-2796.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 27.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 28.Freedson PS, Melanson E, and Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. [DOI] [PubMed] [Google Scholar]
- 29.Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, and Raitakari OT. The prevalence of the metabolic syndrome in young adults. The Cardiovascular Risk in Young Finns Study. J Int Med. 2007;26:159–169, doi: 10.1111/j.1365-2796.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 30.Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, and Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Preventive Medicine Reports. 2017;7:2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, and Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on the National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 32.Usha SMR, Chandrika N, Shetty HV, and Reena R. A study of the components of metabolic syndrome in young adults. Biomedical Research. 2014;25:45–50. [Google Scholar]
- 33.Shoelson SE, Lee A, and Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. [DOI] [PubMed] [Google Scholar]
- 35.Poon VTW, Kuk JL and Ardern CI. Trajectories of metabolic syndrome development in young adults. PLoS ONE 9(11): e111647 10.1371/journal.pone.0111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekelund U, Steene-Johannessen J, Brown WJ, Wang Fagerland M, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. The Lancet. 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 37.DiPietro L, Katz LD and Nadel ER. Excess abdominal adiposity remains correlated with altered lipid concentrations in healthy older women. Int J Obesity. 1999;23:432–436. [DOI] [PubMed] [Google Scholar]
- 38.Sarpong DF, Curry IY, and Williams M. Assessment of knowledge of critical cardiovascular risk indicators among college students: does stage of education matter? International Journal of Environmental Research and Public Health. 2017;14:250. doi: 10.3390/ijerph14030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skøt L, Nielsen JB, and Leppin A. Who perceives a higher personal risk of developing type 2 diabetes? A cross-sectional study on associations between personality traits, health-related behaviours and perceptions of susceptibility among university students in Denmark. BMC Public Health. 2018;18:972, doi: 10.1186/s12889-018-5884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


