Abstract
Background:
Mitochondria play a fundamental role in the pathogenesis of Alcoholic Liver Disease (ALD). The preservation of functional mitochondria during toxic alcohol insults is essential for cell survival and is maintained by key processes known as mitochondrial dynamics, including fragmentation and fusion, which are regulated by mitochondria-shaping proteins (MSP). We have shown mitochondrial dynamics to be distorted by alcohol in cellular and animal models, but the effect in humans remains unknown.
Methods:
Hepatic gene expression of the main MSP involved in the mitochondrial fusion and fragmentation pathways was evaluated in patients with alcoholic hepatitis by DNA microarray (n=15) and RT-PCR (n=32). The activation of Dynamin-1-like protein (Drp1) was also investigated in mitochondria isolated from liver biopsies of ALD patients (n=8). The effects of alcohol on mitochondrial dynamics and on MSP protein expression were studied in human precision cut liver slices (PCLS) exposed for 24 hours to increasing doses of ethanol (50–250mM).
Results:
A profound hyperactivation of the fragmentation pathway was observed in alcoholic hepatitis patients, with a significant increase in the expression of Drp1 and its adapters/receptors. The translocation of Drp1 to the mitochondria was also induced in patients with severe ALD and was affected in the PCLS with short-term exposure to ethanol but only mildly. The fusion pathway was not altered in ALD and this was confirmed in the PCLS model.
Conclusions:
The present study reveals the role of mitochondrial dynamics in human ALD, confirming our previous observations in animal and cell culture models of ALD. Taken together, we show that alcohol has a significant impact on the fragmentation pathway, and we confirm Drp1 as a potential therapeutic target in severe ALD.
Keywords: Alcoholic Liver Disease, Mitochondrial Fragmentation, Dynamin-1-like protein, Precision-Cut Liver Slices, Alcoholic Hepatitis
Introduction
Alcoholic Liver Disease (ALD) is associated with a broad range of clinical presentations. The effects of harmful drinking initially manifest with mild and reversible hepatic fat accumulation, but after decades of alcohol misuse this can induce increasing levels of inflammation (steatohepatitis) that drive the development of fibrosis and cirrhosis (O’Shea et al., 2010). One of the most severe and life-threatening presentations is alcoholic hepatitis (AH), which is characterised by systemic inflammation, high susceptibility to bacterial infection, hepatocyte injury, steatosis and high short-term mortality (20–50% within 3 months) (Lucey et al., 2009). ALD remains a ‘treatment enigma’ and abstinence represents the cornerstone of intervention for all stages of this disorder (Chokshi, 2018). However, once end-stage liver disease is established, transplantation whilst controversial, may be the last available possibility. In AH, steroids administration to dampen the relentless inflammation remains the only pharmacological option but clinical studies have questioned their efficacy and challenged their short-term beneficial effects (Thursz et al., 2015). New treatments are therefore urgently needed and a better understanding of the pathogenesis of ALD may be the only route for the development of novel therapeutic interventions.
The pathogenesis of ALD is multifaceted and includes both direct and indirect factors, such as hepatocyte toxicity derived from ethanol metabolism, oxidative stress, DNA damage, metabolic alterations and inflammation. Central to these disease processes is mitochondrial dysfunction which has been shown to be critical during both the onset and progression of ALD (Zhong and Lemasters, 2018). Mitochondria are essential organelles for the preservation of cell homeostasis. They play key roles in several pathways ranging from energy conversion, regulation of apoptotic signalling, cell death and metabolism. In order to fulfil cellular demands and to promptly react during challenging conditions, mitochondria can move across the cell and adjust their shape with cycles of fusion (the union of separate organelles to form an elongated organelle) and fragmentation (division of a single mitochondrion into two smaller individual entities). These processes are referred to as mitochondrial dynamics and this plasticity in shape represents one of the main strategies to maintain organelle quality control to sustain a functional mitochondrial pool and to react to cellular stressors (Eisner et al., 2018). Dysfunctional mitochondria can undergo fragmentation to be then cleared by auto(mito)phagy (Twig et al., 2008) or promote the apoptotic cascade in case of severe insults (Frank et al., 2001). Conversely, fusion processes can allow sharing of metabolites, enzymes and mitochondrial gene products throughout the entire mitochondrial network to allow full functionality of damaged mitochondria and the maintenance of energetic homeostasis (Liesa and Shirihai, 2013).
Critically, the balance between fusion and fragmentation determines the shape, and ultimately the function, of the mitochondrion and this is tightly controlled by the activity of several dynamin-related proteins called mitochondria shaping proteins (MSP). All the main MSP share a highly conserved GTPase domain and present the ability to self-assemble, hydrolyze GTP and remodel membranes. Mitofusin-1 and −2 (MFN1, MFN2) and Optic atrophy-1 (OPA1) are the proteins involved in the process of organelle fusion acting on the outer and inner mitochondrial membranes. Allied to this, mitochondrial fission is orchestrated by the mitochondrial recruitment and assembly of cytosolic dynamin-1-like protein (Drp1 or DNM1L) into oligomers at sites of scission. Mitochondrial translocation of Drp1 is regulated by a remarkable number of post-translational modifications, including phosphorylation, ubiquitylation and SUMOylation, whereas its targeting to fission sites is facilitated by adapter proteins, including mitochondrial fission 1 protein, mitochondrial fission factor, mitochondrial dynamics proteins of 49 and 51 kDa (Breitzig et al., 2018).
Considering the importance of mitochondrial activity for the maintenance of cell integrity and the strict correlation between mitochondrial morphology and function is expected that alterations in the mitochondrial dynamics can be a causative and/or exacerbating feature in the pathogenesis and progression of several disorders (Chan, 2019). It has been shown that mitochondrial dynamics are affected during hepatotoxicity, including experimental models of drug induced liver injury (Li et al., 2019) and viral hepatitis (Kim et al., 2013, Kim et al., 2014). Moreover, previous studies on animal models have described a striking mitochondrial adaptation in response to chronic alcohol toxicity including effects such as slower mitochondrial movements and fewer fusion events in ethanol-fed rats compared to the controls (Das et al., 2012) or increased diversity of mitochondrial shape with a hyper-fragmented phenotype in livers of alcohol-fed mice (Han et al., 2012). In a recent study from our laboratory, we have further investigated the effects of alcohol on mitochondrial dynamics and confirmed that changes in mitochondrial shape are closely correlated with alcohol toxicity in in vitro cell culture and animal models of ALD (Palma et al., 2019b), we have also identified Drp1 as the main protein which mediates these alterations and suggested that MSPs represent druggable targets. Whether this phenomenon is true in humans is unknown and is the aim of the current investigation.
Materials and methods
Human precision cut liver slices (PCLS):
As previously described (Palma et al., 2019a), human liver tissue was obtained from patients who underwent partial hepatectomy (Table 1 for baseline characteristics, Research Ethics Committee reference 17/NE/0340). Immediately after surgical resection, the healthy portion of the liver specimen was harvested and kept on ice in sterile University of Wisconsin solution (ViaSpan; Bridge to Life Ltd, London, UK) until slicing. Time from harvest to culture was kept to a minimum (3–10 hours) and the preparation of PCLS was performed as previously described (de Graaf et al., 2010). Each slice was placed in a 12-well plate and cultured in Williams medium E supplemented with 2g/L glucose (Sigma, Gillingham, UK), penicillin and streptomycin (Life technologies, Paisley, UK), with the addition of insulin-transferrin-selenium (Life technologies, Paisley, UK), epidermal growth factor 1nM (Life technologies, Paisley, UK), corticosterone 1μM (Sigma, Gillingham, UK), glucagon 100nM (Sigma, Gillingham, UK) and human AB serum 5% (Gemini Bio-product, West Sacramento, CA). The medium was saturated with carbogen (95% O2/5% CO2) and the plates kept in sealed chambers at 37°C in a shaking incubator. After an initial pre-incubation of 2 hours to allow the recovery after the cut, each slice was maintained in culture for 24 hours with or without the addition of ethanol (50mM, 100mM or 250mM) and the medium was replaced every day. Cell viability was evaluated in 3 PCLS per patient by measuring the content of ATP with the ATP Bioluminescence Assay Kit CLS II (Roche, Basel, Switzerland), normalized against the total amount of proteins quantified by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Basingstoke, UK), as previously described (de Graaf et al., 2010).
Table 1.
Baseline characteristics of liver donors utilized for Precision Cut Liver Slices.
| Baseline Characteristics | N=6 Median (25–75 IQR) |
|---|---|
| Age (y) | 56 (51–61) |
| Male n (%) | 2 (33%) |
| BMI | 24.5 (24.4–24.6) |
| Laboratory and hemodynamic parameters | |
| ALT (U/L) | 24 (16.75–33) |
| Serum bilirubin (mg/dL) | 0.5 (0.1–0.7) |
| Pathological conditions | |
| Metastatic colorectal adenocarcinoma n (%) | 4 (67%) |
| Focal nodular hyperplasia n (%) | 1 (17%) |
| Metastatic ductal breast adenocarcinoma n (%) | 1 (17%) |
| Background liver histology | |
| Mild steatosis n (%) | 5 (83%) |
| Fibrosis n (%) | 0 (0%) |
Abbreviations: BMI, body mass index.
All patients received chemotherapy prior to resection.
Research Ethics Committee reference 17/NE/0340- IRAS Project ID 222302
Clinical cohorts:
For all the cohorts the protocols were approved by the local Research Ethics Committee and all patients gave written informed consent. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
GEO Cohorts: Data available in Gene Expression Omnibus (GEO) (Reference Series: GSE28619) (Affo et al., 2013). This publicly available dataset includes transcriptomic data from liver samples in 15 patients with AH and 7 healthy individuals.
Barcelona Cohorts: Thirty-two patients with clinical, analytical and histological features of ALD of varying severity (Table 2 for baseline characteristics) admitted at the Hospital Clinic of Barcelona, Spain (2007–2010), prospectively included according to previously described criteria for AH (Dominguez et al., 2008, Dominguez et al., 2009, Colmenero et al., 2007) and as controls, specimens of normal liver tissue obtained from cadaveric liver donors (n=4) or from patients having resection of liver metastases (n=4) as previously described (Affo et al., 2013).
Brussels Cohorts: Eight patients with advanced ALD were recruited from the Clinique d’Hépatologie, Hopital Erasme, Brussels (Table 3 for baseline characteristics). As controls we utilised the normal portion of liver tissue obtained from donors having a partial hepatectomy (n=6; Table 1 for baseline characteristics).
Table 2.
Baseline characteristics of patients included in Barcelona cohorts.
| Baseline Characteristics | N= 32 Median (25–75 IQR) |
|---|---|
| Age (y) | 51 (45–56) |
| Male n (%) | 24 (75%) |
| Megamitochondria n (%) | 21 (65%) |
| Corticosteroids n (%) | 13 (40%) |
| SIRS+ (%) | 11 (34%) |
| Laboratory and hemodynamic parameters | |
| Hemoglobin (g/dL) | 12 (9–13) |
| Leukocyte count x109/L | 8.1 (5.7–11.4) |
| Platelet count x109/L | 121 (97–134) |
| AST (U/L) | 116 (65–190) |
| ALT (U/L) | 43 (23–60) |
| Serum albumin (g/dL) | 2.7 (2.4–3.2) |
| Serum creatinine (mg/dL) | 0.8 (0.6–0.9) |
| Serum bilirubin (mg/dL) | 4.7 (2.0–13.4) |
| International normalized ratio | 1.4 (1.3–1.7) |
| HVPG (mmHg) | 19 (14–21) |
| Alcoholic hepatitis severity scores at admission | |
| Fibrosis stage n (%) F1 F2 F3 F4 |
2 (6%) 0 (0%) 5 (16%) 25 (78%) |
| MELD score | 17 (13–23) |
| ABIC score | 7.1 (6.6–8.1) |
| Maddrey score | 56 (49–68) |
| Severe AH n (%)* | 22 (69%) |
| Clinical decompensations during hospitalization | |
| AKI n (%)** | 10 (31%) |
| Infection n (%) | 11 (34%) |
| Mortality at 90 days n (%) | 6 (19%) |
Abbreviations: SIRS, Systemic Inflammatory Response Syndrome; HVPG, Hepatic Venous Pressure Gradient; MELD, Model for End Stage Liver disease; ABIC, Age-Bilirubin-INR-Creatinine; AH, Alcoholic Hepatitis; AKI, Acute Kidney Injury.
SIRS was defined as 2 or more of the following variables: temperature > 38°C (100.4°F) or < 36°C (96.8°F), heart rate > 90 x’, respiratory >20 x’ or PaCO2<32mm Hg and Leucocytes >12,000/mm3 or < 4,000/mm3.
Defined as: ABIC score > 6.71 or MELD score >21 points.
AKI was defined as an abrupt (within 48 hours) reduction in kidney function currently defined as an absolute increase in serum creatinine of more than or equal to 0.3 mg/dl (≥ 26.4 μmol/l), a percentage increase in serum creatinine of more than or equal to 50% (1.5-fold from baseline) based on AKIN (acute kidney injury network) criteria.
Table 3.
Baseline characteristics of patients included in Brussels cohort.
| Baseline Characteristics | N= 8 Median (25–75 IQR) |
|---|---|
| Age (y) | 60 (51–62) |
| Male n (%) | 4 (50%) |
| Megamitochondria n (%) | 2/5 (40%) |
| Corticosteroids n (%) | 2 (25%) |
| Laboratory and hemodynamic parameters | |
| Platelet count x109/L | 92.5 (77.5–141.75) |
| AST ( U/L) | 66 (54–86) |
| ALT (U/L) | 30 (26–35) |
| Serum albumin (g/dL) | 25 (22–36) |
| Serum creatinine (mg/dL) | 1 (0.7–1.5) |
| Serum bilirubin (mg/dL) | 3 (1.1–10.4) |
| International normalized ratio | 2 (1.3–1.9) |
| HVPG (mmHg) | 13 (12–14.5) |
| Alcoholic hepatitis severity scores at admission | |
| Fibrosis stage F4 n (%) | 8 (100%) |
| MELD score | 16 (10–21) |
| Severe AH n (%)* | 2 (25%) |
| Mortality at 90 days n (%) | 1 (12.5%) |
Abbreviations: HVPG, Hepatic Venous Pressure Gradient; MELD, Model for End Stage Liver disease; AH, Alcoholic Hepatitis.
Defined as: MELD score >21 points.
Electron Microscopy:
The PCLS were fixed with 2% paraformaldehyde and 1.5% glutaraldehyde in 0.1M cacodylate buffer and then fixed in 1% OSO4/ 0.1M cacodylate buffer pH7.3 at 3˚C. After the staining with 0.5% uranyl acetate, the specimens were dehydrated in a graded ethanol-water series and infiltrated with Agar 100 resin mix. Serial 1μm sections were cut and stained with 1% toluidine blue for light microscopy. Ultrathin sections were cut at 70–80 nm using a diamond knife on a Reichert ultracut microtome, collected on 300 mesh copper grids and stained with uranyl acetate and lead citrate. Then samples were viewed in a Joel 1010 transition electron microscope and images recorded using a Gatan Orius camera. After image collection, 50–100 cells were blindly analysed to quantify for the presence of megamitochondria (length, width >1μm).
Mitochondrial isolation:
PCLS and human liver tissue (Brussels cohorts) were homogenized in isolation buffer (250mM sucrose, 10mM TRIS, 1mM EGTA, pH 7.4) using the Precellys®24 grinder (Bertin Technologies, Montigny le Bretonneux, France) with ceramic bead-vials. Mitochondria were then isolated by standard differential centrifugations as previously described (Frezza et al., 2007): homogenates were spun at 900g for 10 minutes; the supernatant was recovered and further spun at 9000g for 10 minutes in order to obtain the mitochondrial fraction, which was then washed and centrifuged again at 9000g for 10 minutes. Mitochondrial protein concentration was quantified by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Basingstoke, UK).
Western Blot Analysis:
Protein extracts were obtained from the mitochondrial fraction isolated from PCLS or liver fragments (Brussels cohorts) and separated by 12% SDS-PAGE, transferred onto nitrocellulose membranes (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and analysed as previously described (de Brito and Scorrano, 2008). The following antibodies were used: anti-Drp1, anti-Tom20 (BD Bioscience, Oxford, UK); anti-MiD51 (SMCR7L/MID51) (ProteinTech Europe, Manchester, UK); anti-Mitofusin-1, anti-Mitofusin-2, anti-actinin (Santa Cruz biotechnology, Heidelberg, Germany); anti-VDAC1/Porin (Abcam, Cambridge, UK).
MSP analysis using Real-Time Reverse Transcription Polymerase Chain Reaction Analysis (RT-PCR):
Total RNA was extracted from liver tissue (Barcelona cohorts) using Tri-Reagent (Life technologies, Paisley, UK) following the standard procedure recommended by the manufacturer. Quantitative real-time PCR reactions were carried out in a StepOnePlus™ Real-Time PCR System using commercial primer-probe pairs (Applied Biosystems, Foster City, CA). mRNA levels for human DRP1 (gene name DNM1L), OPA1, MFN1 and MFN2 were measured. 18S RNA was used as the endogenous control. Gene expression values were calculated based on the ΔΔCt method. The results were expressed as 2-ΔΔCt referred as fold increase compared with the mean expression quantified on normal livers.
Statistical analysis:
Continuous variables were expressed as mean ± standard error of the mean (SEM) and categorical variables were described as means of counts and percentages. Comparisons were performed using independent or paired non-parametric tests (Mann-Whitney or Wilcoxon tests) and Friedman’s analysis of variance for repeated measures, when multiple treatment groups were analysed simultaneously. Statistics were calculated using GraphPad Prism 6 (GraphPad, La Jolla, CA). Statistical significance was set at P≤0.05.
Results
The mitochondrial fragmentation pathway is significantly activated during severe Alcoholic Hepatitis.
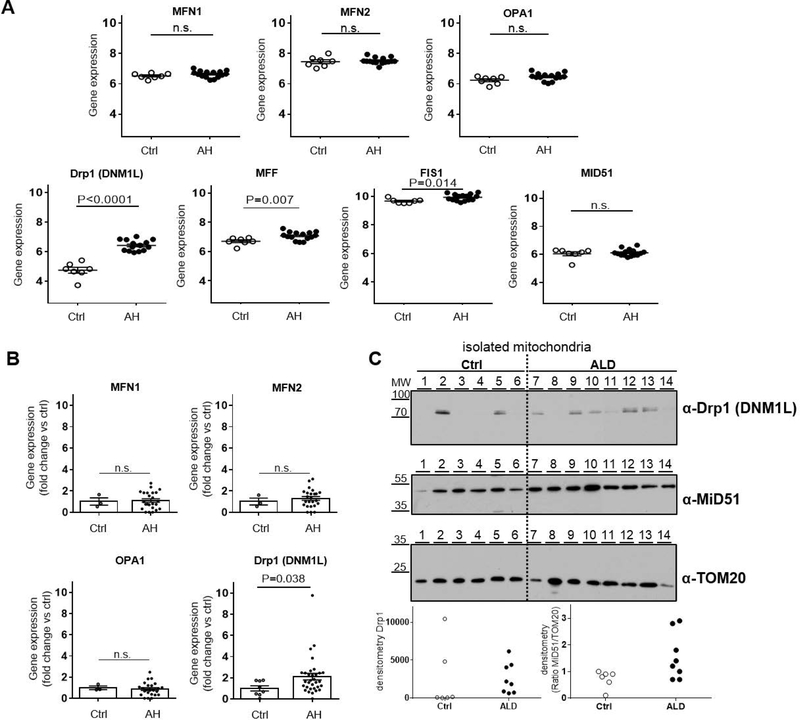
The hepatic gene expression of a broad range of proteins involved in mitochondrial dynamics was screened in the GEO Dataset reference series GSE28619. In this cohort, liver samples from 15 patients with AH were compared to tissue from 7 healthy individuals. The expression levels of the main MSP regulating mitochondrial fusion (MFN1, MFN2, OPA1) were found to be similar between AH patients and healthy controls (Fig. 1- Panel A; P>0.05), confirming our previous findings in cell culture and animal experimental models of ALD (Palma et al., 2019b). The analysis of the mitochondrial fragmentation pathway showed instead an important change with ALD. In AH patients, the expression of Drp1 and of some of its adapters was significantly increased compared to the levels of these genes quantified in normal livers (Fig. 1- Panel A; P<0.05).
Figure 1. Hepatic expression and activation of the mitochondria-shaping proteins involved in mitochondrial fragmentation are induced during alcoholic hepatitis.
(A) Hepatic gene expression assessed by DNA microarray in liver biopsies from patients with alcoholic hepatitis (AH, n=15) and healthy controls (Ctrl, n=7); GEO reference series GSE28619. Fusion pathway: Mitofusin-1 (MFN1), Mitofusin-2 (MFN2), Optic-atrophy-1 (OPA1) expression was found no different between AH and Ctrl (mean ± SEM; n.s. P>0.05). Fragmentation pathway: Dynamin-1-like protein(Drp1 gene name DNM1L) and its adapters/receptors Mitochondrial fission factor (MFF) and Mitochondrial fission 1 protein (FIS1), but not Mitochondrial dynamics protein of 51KDa (MiD51), were significantly induced in AH (mean ± SEM; P<0.05, n.s. P>0.05).
(B) Hepatic gene expression assessed by RT-PCR in liver biopsies from patients with alcoholic hepatitis (AH, n=32) admitted at the Hospital Clinic of Barcelona, see Table 2 for baseline characteristics, and healthy controls (Ctrl, n=8). Fusion pathway: mRNA levels of MFN1, MFN2 and OPA1 shown no difference between livers of AH and Ctrl (n=3) (mean ± SEM; n.s. P>0.05). Fragmentation pathway: mRNA levels of Drp1 (gene name DNM1L) were increased in AH patients compared to Ctrl (n=8) (mean ± SEM; P<0.05).
(C) Protein analysis in alcoholic hepatitis patients (AH, n=8) admitted at the Clinique d’Hépatologie, Brussels, see Table 3 for baseline characteristics and healthy controls (n=6). Mitochondria isolated from liver tissue of healthy controls (lines 1–6) and AH patients (lines 7–14) were subjected to Western blot analysis (representative pictures, TOM20 as loading control and densitometry of Drp1 and ratio MiD51/TOM20, individual values). Drp1 was found increased in the mitochondrial fraction of the majority of AH samples compared to ctrl.
The results on the changes in the transcriptome derived by DNA microarray were then verified by RT-PCR in liver specimens from 32 AH patients and 8 healthy individuals (Table 2 for baseline characteristics). In line with the findings shown in the GEO Dataset the mitochondrial fusion pathway was not affected during ALD and the hepatic expression of MFN1, MFN2 and OPA1 did not differ in AH patients when compared to healthy controls (Fig.1- Panel B; P>0.05). On the other hand, the upregulation of the fragmentation pathway was confirmed also in this cohort of patients with Drp1 significantly overexpressed in the livers of AH patients (Fig. 1- Panel B; P<0.05). We also found a direct correlation (P<0.05; r=0.356) between Drp1 mRNA levels in the liver biopsies of AH patients and the blood concentration of Aspartate aminotransferase (AST).
Besides Drp1 gene expression, its activation was also explored in ALD. A fundamental step to initiate mitochondrial fragmentation is the translocation of Drp1 from the cytosol to the outer mitochondrial membrane (Smirnova et al., 2001). The amount of Drp1 localised on the organelles which indicates its activation was quantified by Western blot in the mitochondrial fraction isolated from liver specimens from patients with severe stage of ALD (Brussels cohort, n=8, Table 3 for baseline characteristics) and compared to mitochondria isolated from normal livers (n=6, Table 1 for baseline characteristics). Most ALD samples (60%) showed a higher amount of Drp1 bound to the organelles suggesting its increased translocation compared to the healthy controls, where only 2/6 (30%) showed this increase (Fig. 1- Panel C) confirming the impact on the mitochondrial fragmentation pathway in ALD.
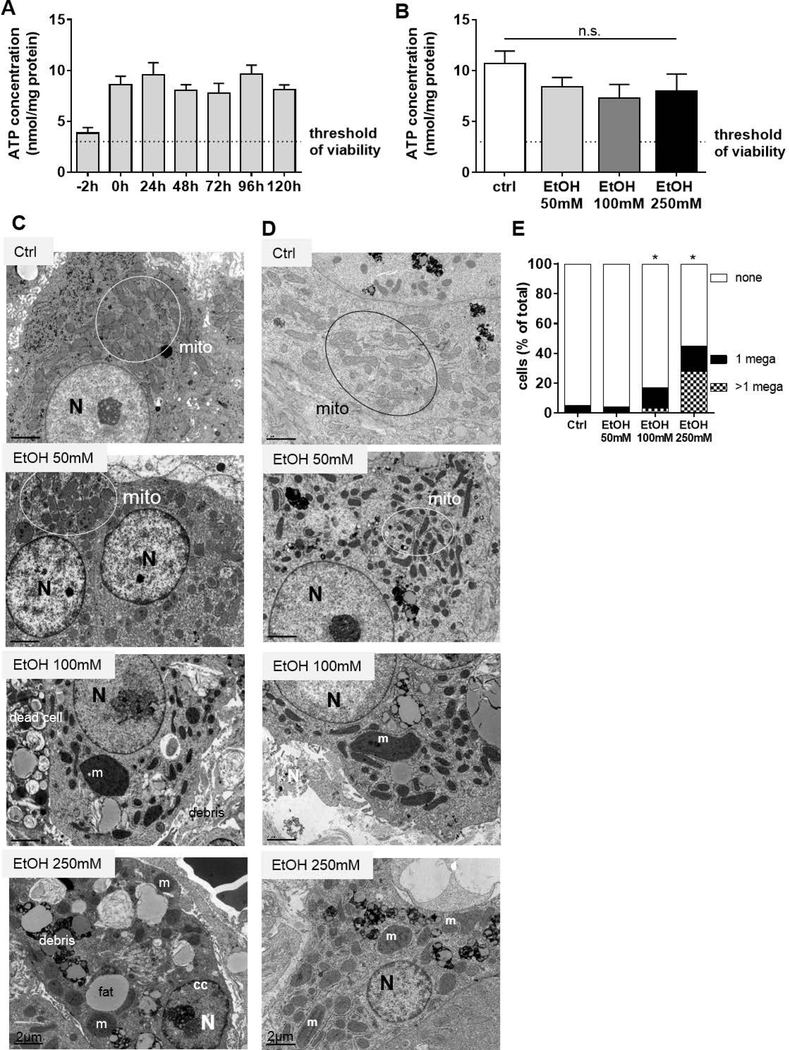
Human liver slices treated with ethanol as a model of early stage of ALD.
Precision cut slices derived from healthy human liver were cultured for 24 hours with increasing doses of ethanol (50, 100 and 250mM). Toxic effects were evaluated by quantification of ATP levels, as previously reported for the PCLS (Hadi et al., 2013). In the untreated slices, the ATP content was always above the accepted criteria for viability (ATP 3–12 nmol/mg of protein as reported in (de Graaf et al., 2010)) and stable for up to 5 days with a standard deviation of 1.31 (Fig. 2- Panel A). After exposure to ethanol, levels of ATP tended to drop for all the doses tested when compared to the untreated slices (Fig. 2- Panel B; P>0.05), but the overall ATP differences were moderate and not statistically significant when compared to the controls. Moreover, the levels of transaminases and lactate dehydrogenase in the supernatants of PCLS did not increase following ethanol treatment (data not shown). The evaluation of the intracellular structure by electron microscopy (Fig. 2- Panel C) revealed signs of increased cell injury during alcohol exposure with the presence of karyorrhexis (fragmented nuclei and chromatin disintegration) and prominent cytoplasmic vacuolation with a loss of structural integrity of the all membranes. These characteristics were detected in almost 30% of samples treated with ethanol (5/18 PCLS). The EM analysis showed an important fat droplet accumulation in the hepatocytes of the PCLS treated with ethanol (Fig. 2- Panel D) and a striking appearance of very enlarged mitochondria (megamitochondria). The incidence of megamitochondria increased during ethanol exposure in a dose-dependent manner as shown in representative pictures (Fig. 2- Panel D) and in the quantitative analysis (Fig. 2- Panel E). Megamitochondria were consistently induced by ethanol treatment in approximately 70% of the specimens examined (10/15 PCLS) and in 10 to 50% of the cells analysed at each concentration (>200 cells analysed in total). The characteristics observed in the PCLS resemble the clinical presentation of ALD patients at an early stage of disease supporting the validity of this model for the study.
Figure 2. Human Precision Cut Liver Slices (PCLS) as a model of early stage of Alcoholic Liver Disease.
(A) Viability of PCLS over 5 days in culture. The culture system was validated assessing the viability by quantifying the ATP content in 3 slices per patient normalized against the total protein amount. The timepoint −2h indicates the ATP measurement immediately after cutting and the timepoint 0h shows the ATP content after 2 hours of slice recovering in complete oxygenized medium, as described in (de Graaf et al., 2010). The ATP levels were found consistent and stable over the culturing time (5 days) and above the threshold of viability (3nmol/mg of protein). Bars show the mean ± S.E.M.; n=6 patients, 3 slices per patient.
(B) Viability of PCLS exposed to ethanol. ATP concentration was measured after 24 of ethanol (EtOH) exposure at the indicated doses (mean ± SEM, n=6 patients, 3 slices per patient for each condition, P>0.05).
(C) Mild hepatocyte damage induced by ethanol in PCLS. Representative electron micrographs of PCLS after 24 hours of ethanol (EtOH) exposure at 50, 100 and 250mM shown a mild cell injury of hepatocytes and apoptotic signatures such as chromatin condensation in the nucleus at the highest dose utilised (Abbreviations: mito = mitochondria, N = nucleus, fat = fat accumulation, M = megamitochondria (width, length>1μm), cc = chromatin condensation).
(D) Megamitochondria development in PCLS exposed to ethanol. Representative electron micrographs of PCLS after 24 hours of ethanol (EtOH) exposure at 50, 100and 250mM shown the development of enlarged megamitochondria (width, length >1μm) (Abbreviations: mito = mitochondria, N = nucleus, M = megamitochondria).
(E) Megamitochondria incidence in PCLS exposed to ethanol. The percentage of cells presenting one or more megamitochondria (width, length >1μm) was quantified in untreated slices (ctrl) and slices exposed for 24 hours to the indicated dose of ethanol (EtOH) and the difference was significant at the higher doses (>300 cells analysed in total, *P<0.05).
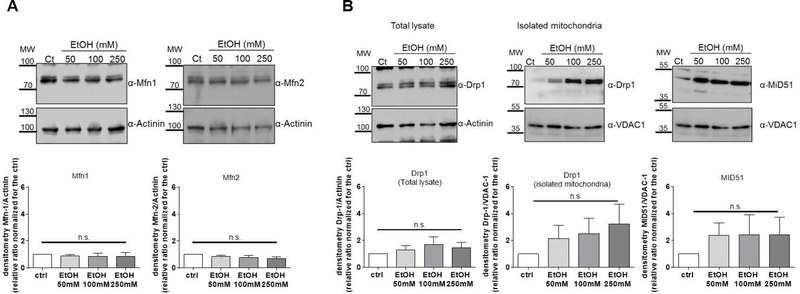
The expression of mitochondria-shaping proteins is not profoundly altered in the early stage of ALD.
Liver biopsies from early stage of ALD are difficult to justify clinically, and so the impact of early exposure to ethanol on mitochondrial dynamics was evaluated in the PCLS model. The mitochondrial fusion pathway did not show any change and the protein expression of the main MSP involved (MFN1, MFN2) was very similar in ethanol-treated slices compared to the untreated ones (Fig. 3- Panel A; P>0.05), in line with the results obtained in AH patients (Fig. 1). The effect of ethanol on the fragmentation pathway was only mild compared to the striking alterations observed in AH patients. The amount of Drp1 detected in the mitochondrial fraction isolated from slices exposed to alcohol showed a trend of increasing translocation of Drp1 to the organelles in comparison with the untreated slices, but the difference was not significant (Fig. 3- Panel B; P>0.05). There was also a slight but not significant increase in the protein expression of one of Drp1 receptors, while the protein expression of Drp1 was very similar between control and ethanol-treated slices (Fig. 3- Panel B; P>0.05).
Figure 3. Protein expression of mitochondria-shaping proteins is not severely affected by 24 hours of ethanol exposure in human PCLS.
(A) Fusion pathway in PCLS treated with ethanol (EtOH). The protein expression of Mitofusin-1 (Mfn1) and Mitofusin-2 (Mfn2) was the same in untreated slices and after 24 hours of ethanol at the indicated doses (P>0.05).
(B) Fragmentation pathway in PCLS treated with ethanol (EtOH). The protein expression of Dynamin-1-like protein (Drp1) and Mitochondrial dynamics protein of 51KDa (MiD51), and the localisation of Drp1 on mitochondria was analysed by Western Blot in PCLS treated with the indicated doses of EtOH (P>0.05).
In all panels representative western blots are shown along with the densitometric analysis (mean ± SEM, n>3).
Discussion
The aim of this study was to investigate the impact on mitochondrial dynamics and MSP in ALD human samples. One of the main findings was that in the severe stages of the disease the regulators of mitochondrial morphology, especially of the fragmentation process, are significantly altered, suggesting a skewed balance in the equilibrium between fusion and fission during alcohol-related liver injury. Variations in the expression of MSP have been previously shown in several disorders, primarily neurodegenerative diseases including Alzheimer’s (Zhu et al., 2013) and Parkinson’s (Pozo Devoto and Falzone, 2017), cardiovascular diseases (Vasquez-Trincado et al., 2016) and various types of cancer (Maycotte et al., 2017), metabolic conditions like type II diabetes or obesity (Zorzano et al., 2009) and very recently in Non-Alcoholic Fatty Liver Disease (Hernandez-Alvarez et al., 2019). This is the first time that the MSP have been studied in patients affected by alcoholic hepatitis and their expression shown to be modified in livers from patients with ALD.
The gene expression and protein activation of MSPs in liver biopsies from 55 patients with severe ALD demonstrated that the mitochondrial fragmentation pathway was hyper-activated and among all the MSP, DRP1 was found consistently over-expressed in patients affected by AH. Other studies have shown important effects on mitochondrial dynamics and on Drp1 during liver injury, but these observations are limited to in vitro or animal models (Hasnat et al., 2018, Li et al., 2019, Ramachandran et al., 2013, Yang et al., 2017). A lack of confirmation of experimental results in more relevant models (whole human tissue) and especially in patients hinders the translation of knowledge clinically and is mainly due to the difficulty in obtaining such samples. One of the most important achievements of this study is the validation of the relationship between ALD, mitochondrial dynamics and MSP in a cohort of patients.
As liver biopsies from patients with mild or early stage ALD are not clinically indicated, we employed the human PCLS as an ex vivo model of the early stage of the disease. The aim of this was to investigate whether a direct alcohol exposure or ongoing injury were contributing to the changes in MSP and mitochondrial dynamics. Firstly, we show this model recapitulates the characteristics of ‘early’ stage chronic alcohol-related hepatotoxicity and tissue stress (megamitochondria) but without severe hepatocyte injury. We found that the fusion pathway was not perturbed in this model, in agreement with the observations in patients with severe ALD. We observed mild changes in Drp1 translocation and activation of the fission machinery in the ethanol-treated human liver slices, whilst these were not significantly different from the control, there was a trend of hyper-activation of the Drp1 pathway. In support of this, we found a positive correlation between Drp1 hepatic gene expression in AH patients and levels of AST, revealing that significant hyper-activation of the fragmentation pathway is associated with a higher severity of disease and hepatocyte damage. This is further corroborated by other studies, where increased levels of Drp1 are associated with cell injury and augmented apoptosis (Li et al., 2019). These results support the evidence that mitochondria have a striking capacity to react to stress and that the balance between fusion and fission plays a prominent role in the physiological or pathological response of the cell to toxic insults (Eisner et al., 2018). An initial increased fragmentation triggered by ethanol intoxication can be beneficial for the cell and coordinate with mitophagy to clear the cell from dysfunctional organelles, however when the insult becomes chronic this can overcome the adaptive mechanisms and lead to a pathological perturbation of the balance between mitochondrial dynamics (Lemasters and Zhong, 2018). We believe that this study confirms the key role for Drp1 in ALD and in particular during alcoholic hepatitis but further studies are needed to investigate the controversial regulatory mechanisms of this protein, such as the interaction with its receptors/adapters or its several posttranslational modifications (Palmer et al., 2011, Palmer et al., 2013).
Despite the global burden of ALD, the therapeutic options are very limited and inadequate. The need to develop new treatment strategies to reduce alcohol-induced liver damage is critical, especially as the rates of ALD continue to escalate both in United States and Europe (Kim et al., 2018, Pimpin et al., 2018). In recent years, key advancements in understanding mitochondrial dynamics and the molecular mechanisms regulating mitochondrial fusion and fragmentation both physiologically and in pathological settings have raised interest in the possibility of targeting these pathways therapeutically. The present study demonstrates that the mitochondrial fragmentation pathway is hyper-activated during human ALD and suggests a role for Drp1 inhibition as potential therapy to re-establish a balance between mitochondrial dynamics. The protective effects of Drp1 inhibition have been reported in several studies, in neurodegenerative diseases, including multiple sclerosis (Lou et al., 2012), Parkinson’s disease (Rappold et al., 2014, Su and Qi, 2013), Huntington’s disease (Guo et al., 2013, Song et al., 2011), cardiac injury (Disatnik et al., 2013, Ong et al., 2010) and cancer (Xie et al., 2015). Interestingly Galloway et al showed that the decrease of mitochondrial fragmentation induced by Drp1 inhibition was associated with a reduction of steatosis and oxidative stress in a mouse model of non-alcoholic fatty liver disease (Galloway et al., 2014). In a recent study from our laboratory, we demonstrated that the genetic inactivation of Drp1 had a beneficial effect against alcohol-induced hepatotoxicity in two experimental models. In the VL-17A cells (hepatoma cell line able to metabolise alcohol) the expression of an inactive mutant of Drp1 prevented the growth impairment caused by ethanol exposure and liver-specific knock out mice for Drp1 exposed to a chronic-binge drinking diet (NIAA diet) shown reduced liver injury compared to wild type animals exposed to the same diet. It is noteworthy that the lack of Drp1 in the liver of these mice was also associated with an increased formation of megamitochondria and this effect was correlated with lower hepatotoxic levels. Different clinical studies support the hypothesis that megamitochondria development is a beneficial adaptive response to alcohol and have shown that megamitochondria detection in liver biopsies is one of four clinical and histological parameters associated with a better outcome in terms of survival at 90 or 180 days in patients with AH (Altamirano et al., 2014, Andrade et al., 2016, Kim 2017).
In summary, our previous findings in cell culture and animal models of ALD (Palma et al., 2019b) together with the data in the current study, both in human biopsies of severe ALD and in human precision cut liver slice model of early ALD, demonstrate a key role for mitochondrial shaping protein Drp1 in the pathogenesis of ALD and highlight the potential therapeutic utility of targeting Drp1 in ALD.
Acknowledgments
We thank Geny Groothuis (University of Groningen, NL) and Mark Turmaine (University of London, UK) for the technical support.
Financial support: this study was supported by the Foundation for Liver Research. RB was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAA, 1U01AA021908–01 and 1U01AA020821). RB and JC were supported by the Instituto de Salud Carlos III (ISCIII) (FIS PI17/00673 and PI12/01265 respectively). GO received a grant from the Asociacion Espanola para el Estudio del Higado and is partially funded by 1U01AA020821. JA wishes to express his gratitude to the Mexican National Council of Science and Technology (CONACyT, Mexico City, Mexico) for partially supporting his predoctoral stay at IDIBAPS.
Abbreviations:
- ALD
Alcoholic Liver Disease
- AH
Alcoholic Hepatitis
- MSP
Mitochondria-Shaping Protein
- MSP
Mitochondria-shaping proteins
- MFN
Mitofusin
- OPA
Optic atrophy-1
- Drp1 or gene name DNM1L
Dynamin-1-like protein, Dynamin related protein-1
- PCLS
Precision Cut Liver Slices
- GEO
Gene Expression Omnibus
- RT-PCR
Real-Time Reverse Transcription Polymerase Chain Reaction
- SEM
Standard Error of the Mean
Footnotes
Disclosures: None
Reference
- Affo S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millan C, Loaeza-del-Castillo A, Altamirano J, Garcia-Pagan JC, Arroyo V, Gines P, Caballeria J, Schwabe RF, Bataller R (2013) Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, Augustin S, Mookerjee RP, Michelena J, Smyrk TC, Buob D, Leteurtre E, Rincon D, Ruiz P, Garcia-Pagan JC, Guerrero-Marquez C, Jones PD, Barritt AS, Arroyo V, Bruguera M, Banares R, Gines P, Caballeria J, Roskams T, Nevens F, Jalan R, Mathurin P, Shah VH, Bataller R (2014) A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 146:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade P, Silva M, Rodrigues S, Lopes J, Lopes S, Macedo G (2016) Alcoholic hepatitis histological score has high accuracy to predict 90-day mortality and response to steroids. Dig. Liver Dis 48:656–660. [DOI] [PubMed] [Google Scholar]
- Breitzig MT, Alleyn MD, Lockey RF, Kolliputi N (2018) A mitochondrial delicacy: dynamin-related protein 1 and mitochondrial dynamics. Am J Physiol Cell Physiol 315:C80–C90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2019) Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol. [DOI] [PubMed] [Google Scholar]
- Chokshi S (2018) Can we reliably predict response to corticosteroid treatment in severe alcoholic hepatitis? Hepatol Commun 2:625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballeria J, Gines P (2007) Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology 132:687–697. [DOI] [PubMed] [Google Scholar]
- Das S, Hajnoczky N, Antony AN, Csordas G, Gaspers LD, Clemens DL, Hoek JB, Hajnoczky G (2012) Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflugers Arch 464:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605–610. [DOI] [PubMed] [Google Scholar]
- de Graaf IA, Olinga P, de Jager MH, Merema MT, De KR, van de Kerkhof EG, Groothuis GM (2010) Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc 5:1540–1551. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D (2013) Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc 2:e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, Arroyo V, Gines P, Caballeria J, Bataller R (2009) Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 136:1639–1650. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, Garcia-Pagan JC, Fernandez R, Moreno M, Banares R, Arroyo V, Caballeria J, Gines P, Bataller R (2008) A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am. J. Gastroenterol 103:2747–2756. [DOI] [PubMed] [Google Scholar]
- Eisner V, Picard M, Hajnoczky G (2018) Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol 20:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1:515–525. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc 2:287–295. [DOI] [PubMed] [Google Scholar]
- Galloway CA, Lee H, Brookes PS, Yoon Y (2014) Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am. J. Physiol Gastrointest. Liver Physiol 307:G632–G641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X (2013) Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J Clin Invest 123:5371–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi M, Westra IM, Starokozhko V, Dragovic S, Merema MT, Groothuis GM (2013) Human precision-cut liver slices as an ex vivo model to study idiosyncratic drug-induced liver injury. Chem. Res. Toxicol 26:710–720. [DOI] [PubMed] [Google Scholar]
- Han D, Ybanez MD, Johnson HS, McDonald JN, Mesropyan L, Sancheti H, Martin G, Martin A, Lim AM, Dara L, Cadenas E, Tsukamoto H, Kaplowitz N (2012) Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: biogenesis, remodeling, and functional alterations. J. Biol. Chem 287:42165–42179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnat M, Yuan Z, Naveed M, Khan A, Raza F, Xu D, Ullah A, Sun L, Zhang L, Jiang Z (2018) Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: a novel mechanism in triptolide-induced hepatotoxicity. Cell Biol Toxicol. [DOI] [PubMed] [Google Scholar]
- Hernandez-Alvarez MI, Sebastian D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, Plana N, Veiga SR, Hernandez V, Vasconcelos N, Peddinti G, Adrover A, Jove M, Pamplona R, Gordaliza-Alaguero I, Calvo E, Cabre N, Castro R, Kuzmanic A, Boutant M, Sala D, Hyotylainen T, Oresic M, Fort J, Errasti-Murugarren E, Rodrigues CMP, Orozco M, Joven J, Canto C, Palacin M, Fernandez-Veledo S, Vendrell J, Zorzano A (2019) Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 177:881–895 e817. [DOI] [PubMed] [Google Scholar]
- Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, Ahmed A (2018) Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology 155:1154–1163 e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Khan M, Quan J, Till A, Subramani S, Siddiqui A (2013) Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathog 9:e1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Syed GH, Khan M, Chiu WW, Sohail MA, Gish RG, Siddiqui A (2014) Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. U. S. A 111:6413–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WC, Y.-I. Joo SK Jung YJ (2017) Alcoholic hepatitis histological scores predict short-term survival in Asian patients with biopsy-proven alcoholic hepatitis. J Hepatol 66:S118. [Google Scholar]
- Lemasters JJ, Zhong Z (2018) Mitophagy in hepatocytes: Types, initiators and role in adaptive ethanol metabolism. Liver Res 2:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhou J, Li Y, Sun K, Chen J (2019) Mitochondrial Damage and Drp1 Overexpression in Rifampicin- and Isoniazid-induced Liver Injury Cell Model. J Clin Transl Hepatol 7:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA (2012) Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One 7:e33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey MR, Mathurin P, Morgan TR (2009) Alcoholic hepatitis. N Engl J Med 360:2758–2769. [DOI] [PubMed] [Google Scholar]
- Maycotte P, Marin-Hernandez A, Goyri-Aguirre M, Anaya-Ruiz M, Reyes-Leyva J, Cortes-Hernandez P (2017) Mitochondrial dynamics and cancer. Tumour Biol 39:1010428317698391. [DOI] [PubMed] [Google Scholar]
- O’Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G (2010) Alcoholic liver disease. Hepatology 51:307–328. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121:2012–2022. [DOI] [PubMed] [Google Scholar]
- Palma E, Doornebal EJ, Chokshi S (2019a) Precision-cut liver slices: a versatile tool to advance liver research. Hepatol Int 13:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Ma X, Riva A, Iansante V, Dhawan A, Wang S, Ni HM, Sesaki H, Williams R, Ding WX, Chokshi S (2019. b) Dynamin-1-Like Protein Inhibition Drives Megamitochondria Formation as an Adaptive Response in Alcohol-Induced Hepatotoxicity. Am J Pathol 189:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Elgass KD, Parton RG, Osellame LD, Stojanovski D, Ryan MT (2013) Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J. Biol. Chem 288:27584–27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Stojanovski D, Ryan MT (2011) The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 23:1534–1545. [DOI] [PubMed] [Google Scholar]
- Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N, Committee EHS (2018) Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 69:718–735. [DOI] [PubMed] [Google Scholar]
- Pozo Devoto VM, Falzone TL (2017) Mitochondrial dynamics in Parkinson’s disease: a role for alpha-synuclein? Dis Model Mech 10:1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H (2013) Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58:2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ, Tieu K (2014) Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 5:5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med 17:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YC, Qi X (2013) Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet 22:4545–4561. [DOI] [PubMed] [Google Scholar]
- Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O’Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH, Trial S (2015) Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 372:1619–1628. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594:509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, Ferguson AN, Kashatus DF, Bao S, Rich JN (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang H, Ni HM, Xiong A, Wang Z, Sesaki H, Ding WX, Yang L (2017) Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice. Redox Biol 12:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Lemasters JJ (2018) A Unifying Hypothesis Linking Hepatic Adaptations for Ethanol Metabolism to the Proinflammatory and Profibrotic Events of Alcoholic Liver Disease. Alcohol Clin Exp Res 42:2072–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Perry G, Smith MA, Wang X (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 33 Suppl 1:S253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Palacin M (2009) Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch Physiol Biochem 115:1–12. [DOI] [PubMed] [Google Scholar]