Abstract
Objective:
To determine the characteristics of children with fetal alcohol spectrum disorders (FASD) and their mothers in a Midwestern city.
Methods:
Case control samples were drawn from two separate first grade cohorts (combined N=4,047) in every city school using different methods. In Cohort Sample One, all consented small children (≤25th centile on height, weight and/or head circumference) entered the study along with a random sample from all enrolled students. Cohort Sample Two was drawn totally at random. Child growth, dysmorphology, and neurobehavior were assessed using Collaboration on FASD Prevalence (CoFASP) criteria, and mothers were interviewed.
Results:
For the samples combined, 891 children received dysmorphology exams, and 692 were case conferenced for final diagnosis. Forty-four children met criteria for FASD. Total dysmorphology scores differentiated diagnostic groups: fetal alcohol syndrome (FAS), 16.7; partial fetal alcohol syndrome (PFAS), 11.8; alcohol-related neurodevelopmental disorder (ARND), 6.1; and typically-developing controls, 4.2. Neurobehavioral tests distinguished children with FASD from controls, more for behavioral problems than cognitive delay. Children with ARND demonstrated the poorest neurobehavioral indicators. An adjusted regression model of usual pre-pregnancy drinking indicated that maternal reports of three drinks per drinking day (DDD) significantly were associated with a FASD diagnosis, (p=0.020, OR= 10.1, 95%CI=1.44–70.54), as were five or more DDD (p<.001, OR=26.47, 95%CI=4.65–150.62). Other significant maternal risk factors included: self-reported drinking in any trimester; smoking and cocaine use during pregnancy; later pregnancy recognition, later and less prenatal care; lower maternal weight, BMI, and head circumference; and unmarried status. There was no significant difference in FASD prevalence by race, Hispanic ethnicity, or socioeconomic status at this site, where the prevalence of FASD was 14.4–41.2 per 1,000 (1.4–4.1%).
Conclusion:
This city displayed the lowest prevalence of FASD of the four CoFASP sites. Nevertheless, FASD were common, and affected children demonstrated a common, recognizable and measurable array of traits.
Keywords: fetal alcohol spectrum disorders, alcohol use and abuse, women, prenatal alcohol use, prevalence, children with FASD
INTRODUCTION
Recent, meta-analytic literature reviews have indicated that there is a general lack of adequate empirical research on the epidemiology of fetal alcohol spectrum disorders (FASD) throughout the world (Lange et al., 2017; Roozen et al., 2016b). Especially lacking is research linking detailed alcohol exposure data and other maternal characteristics to specific FASD outcomes (Roozen et al., 2018).
Active Case Ascertainment
Since the diagnosis of fetal alcohol syndrome (FAS) was first described (Jones and Smith, 1973), surveillance systems, prenatal clinic-based studies, and special referral clinics have proven inadequate for determining the prevalence of FAS or other FASD (May et al., 2009). Active case ascertainment (ACA) in schools has successfully determined the prevalence and characteristics of FASD in communities in South Africa, Italy, and Croatia (May et al., 2000, 2006, 2007, 2011, 2013, 2016a, 2016b, 2017; Petkovic and Barisic, 2010, 2013; Urban et al., 2008, 2015; Viljoen et al., 2001, 2005). ACA is also a robust method for linking maternal characteristics to child outcomes (May and Gossage, 2001; Roozen et al., 2018, 2016a; Stratton et al., 1996). In ACA studies, physical examinations and neurobehavioral testing are provided to children to determine their characteristic traits (Adnams et al., 2001; Aragon et al., 2008; Kalberg et al., 2013; Kodituwakku et al., 2006), and mothers are interviewed about alcohol use and other health-related behavior during the index pregnancy (Ceccanti et al., 2014; May et al., 2005, 2008, 2010, 2013; Viljoen et al., 2002). Therefore, ACA studies provide a platform for profiling traits of children with FASD and maternal risk factors.
Previous ACA studies in the U.S.
Four ACA community studies of FASD prevalence were published for U.S. communities prior to the Collaboration on FASD Prevalence (CoFASP) initiative. The first study reported on FAS prevalence of 3.1 per 1,000 in one county in Washington State; no summary of child or maternal risk traits was presented (Clarren et al., 2001). Burd et al (1999) and Poitra et al (2003) reported on screening methods and prevalence among children in a head start program, and the prevalence of FAS was 4.3–5.9 per 1,000. A third study summarized child and maternal traits for FAS and partial fetal alcohol syndrome (PFAS) in a Rocky Mountain city. The prevalence was 11–25 per 1,000 for FAS and PFAS combined (May et al., 2015). Finally, a manuscript reported on the prevalence of full continuum of FASD in a Midwestern city, use the diagnostic criteria of (Hoyme et al., 2005). The prevalence of total FASD was 24 to 48 per 1,000 (May et al., 2014). In each of these previous studies, the reporting of significant child and maternal risk characteristics was somewhat limited.
This Study Community
The characteristics of two samples of first grade children who qualified for a diagnosis on the continuum of FASD in this Midwestern U.S. City were studied 2010–2015. Samples were supported by the NIAAA-funded Collaboration on FASD Prevalence (CoFASP), which represented a first major, multi-sample, multi-site effort to examine the population-based prevalence of the full continuum of FASD and maternal risk factors in the U.S.
While in the previous Midwestern City publication, we summarized diagnoses and traits found in the first child cohort (Sample 1) using different diagnostic criteria (May et al., 2014), this manuscript presents results when CoFASP criteria and cut-off standards are applied to Cohort Sample 1 and also reports the results combined with Cohort Sample 2, which used only random methods.
This city had 172,000 residents in 2015 and a robust economy which relied on banking, medical care, agriculture, manufacturing, and higher education (Table 1). The population grew three times more rapidly than the U.S. as a whole from 2010 to 2015. Racial and ethnic composition of the city is primarily White non-Hispanic (85%) (“U.S. Census Bureau QuickFacts: United States,” 2015). Median age, 34.5 years, is younger than the overall U.S. population, and mean household value is less than the U.S. average. More people graduated from high school and college in this city than the general U.S. population, and per capita income and median household income are comparable to the U.S. average. In this state, 79% of adults identify themselves as Christian, 5% fewer people are unaffiliated with a formal religion (“nones”) when compared to the U.S. average, and most people report that religion is very/somewhat important in their lives (Pew Research Center, 2015). The state health rank falls between 15th and 19th of the 50 states (America’s Health Rankings Annual Report, 2015). Binge alcohol use and excessive drinking are slightly more common in this state and county than in the U.S. population. State per capita alcohol consumption was higher than the U.S. (2.62 vs. 2.30 gallons) (LaVallee and Yi, 2011); but other regions of this state had higher rates of heavy drinking than this city. Excessive drinking in this county is 18%, while the U.S. average is 16.8% (“CDC - BRFSS,” 2013). Over the past decade, this city demonstrated greater prosperity and more rapid growth than most medium-sized Midwestern cities.
Table 1.
Demographic Indicators for the Midwestern City Compared to the United States
| Demographic Indicator | Midwestern City | United States |
|---|---|---|
| Population (7/2015)1 (percentage of US population) |
171,544 (0.05%) |
321,418,820 (100%) |
| Population change (%) since 20101 | 11.4% | 4.1% |
| Race/Hispanic Ethnicity (2010)1 | ||
| White, non-Hispanic | 84.9% | 63.7% |
| Black, non-Hispanic | 4.2% | 12.6% |
| American Indian and Alaskan Native | 2.7% | 0.9% |
| Asian | 1.8% | 4.8% |
| Two or more races | 2.5% | 2.9% |
| Hispanic or Latino | 4.4% | 16.3% |
| Foreign born persons1 | 7.1% | 13.1% |
| Age – years (median) | 34.5 | 37.2 |
| Housing1 | ||
| Median household value | $155,200 | $176,700 |
| Education1 | ||
| High School graduate or higher, % ages ≥25 years | 90.8% | 86.3% |
| Bachelor’s degree or higher, % ages ≥25 years | 32.5% | 29.3% |
| Economy1 | ||
| Per capita income in past 12 months (2014 dollars) | $28,120 | $28,555 |
| Median household income | $52,607 | $53,482 |
| Persons in poverty | 11.8% | 14.8% |
| Religion5 | ||
| Composition | ||
| Christian | 79% | 70.6% |
| Non-Christian | 3% | 5.9% |
| Unaffiliated (nones) | 18% | 22.8% |
| Importance of Religion | ||
| Very important | 57% | 58% |
| Somewhat important | 24% | 24% |
| Not too important/not at all | 18% | 16% |
| Health Behavior | Median 25 | |
| Overall state health Rank in US2 | 15–19 | (Range 1–50) |
| Alcohol Use | ||
| Binge drinking^ state %, (US rank)2 | 17.4% (35) | 16.8% (25) |
| Excessive drinking+, state % (US rank)2 | 18.3% (30) | Median = 17.4% (25) |
| Excessive drinking, county3 | 18.0% | Mean = 16.8% |
| Heavy drinking#, city3 | 6.4% | 5.0% |
| State per capita ethanol consumption (2009), volume per person 14 years and older4 | 2.62 gallons 9.91 liters |
2.30 gallons 8.71 liters |
Sources:
US Census, 2015
United Health Foundation, America’s Health Rankings, 2015; comprised of scores on behaviors, community and environment, policy and clinical care; scores are ranked for each of the 50 states with better scores resulting in a higher rank among the 50 states; ranges indicate that different rankings are provided for each of the four domains named above
Behavioral Risk Factor Surveillance System (BRFSS) 2013 data of the CDC. Reported in local city and county statistical reports
LaVallee and Yi, 2011. NIAAA Surveillance Report #92
Pew Research Center. America’s Changing Religion Landscape, 2015. Online. www.pewresearch.org.
Binge drinking defined as: during the past 30 days, the consumption of 5 or more drinks for men or 4 or more drinks for females on an occasion
Heavy drinking is defined as males having more than two drinks per day and females having more than one drink per day
Excessive drinking of alcohol is defined as both binge drinking (above) and chronic drinking also referred to as heavy drinking (above)
METHODS
Protocols and consent forms were approved by The University of New Mexico School of Medicine and the University of North Carolina. Active consent for children to participate was obtained from parents and separately from mothers for maternal interviews.
Diagnostic Criteria
CoFASP studies utilized Revised Institute of Medicine (IOM) diagnostic guidelines for FASD (Hoyme et al., 2005), with revised cut-off values set by investigator teams and the CoFASP advisory group (Hoyme et al., 2016). The continuum of FASD has four specific diagnoses: fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD) (Hoyme et al., 2016). Each diagnostic category was utilized in this study, yet ARBD is rare in any population (May et al., 2016b, 2016a, 2015, 2014, 2011a). The diagnosis of FAS and PFAS can be made without a confirmed history of alcohol exposure with revised IOM criteria (Hoyme et al., 2005, 2016; Stratton et al., 1996). Many women may under-report alcohol consumption during pregnancy (Alvik et al., 2006; Bakhireva et al., 2017; Wurst et al., 2008); yet in some populations prenatal drinking is reported quite accurately (Fortin et al., 2017; May et al., 2018a). In epidemiology studies, the diagnosis of any FASD is rarely made without direct maternal reports of significant alcohol use during pregnancy, prior to pregnancy recognition or collateral reports of alcohol-related behavior. An ARND diagnosis always requires direct confirmation of prenatal alcohol use in the index pregnancy. Other recognizable malformation syndromes were ruled out by medical geneticists/dysmorphologists prior to making a diagnosis on the FASD continuum. Final diagnoses were made in formal, data-driven case conferences.
Cohort One Sampling: A Census of Growth Deficient Children and Random Sample Entry
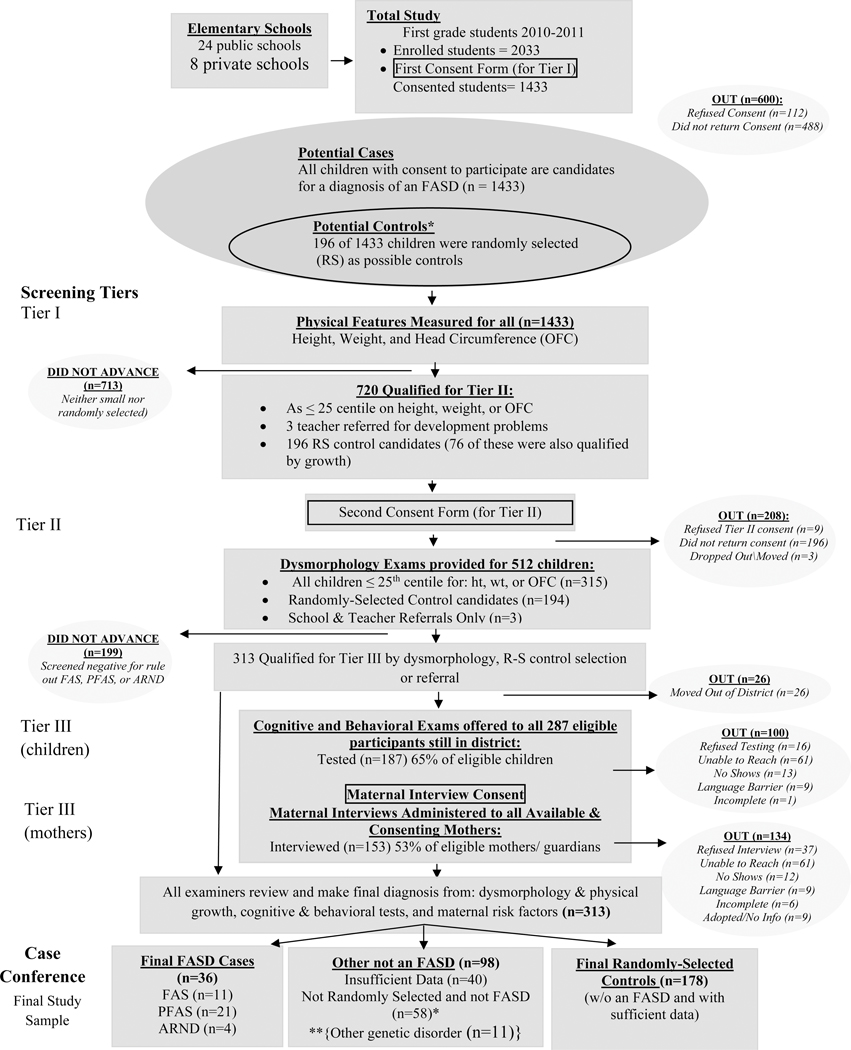
Two sampling methods were used in two independent cohorts of first grade children enrolled in all public (n=24) and private schools (n=8) in this city. In 2010, Cohort Sample 1 was initiated. Consent forms were sent to parents of all first grade students (n=2,033) enrolled in schools that year; 1,545 forms were returned (76%), 112 of the forms were refusals, leaving 1,433 (70.5%) children for this sample (see Figure 1). In Sample 1, children entered the study via: 1) oversampling of every consented child who was ≤ 25th centile on height, weight and/or head circumference, or 2) selection by a simple, random sample drawn from consented children in the entire first grade class rolls. The random sample provided a comparison (control) group that was representative of children in this population, and a vehicle for estimating the true prevalence of FASD (May et al., 2018a). All small children were provided full dysmorphology exams, and if there were indications of FASD traits or another known anomaly, they were referred on to neurobehavioral tests and behavioral checklists. All randomly-selected children were also provided dysmorphology examinations and all referred on for full testing. Three additional, consented children entered the study by referral, due to developmental concerns expressed by parents and/or teachers. Children entering via non-random selection routes and found not to qualify for a diagnosis within the FASD continuum (or another known malformation syndrome), did not default to the control group. Identical exams and testing were performed on all children who participated through all tiers of the study (Figure 1). If possible, each child’s mother was interviewed in person.
Figure 1.
Sampling Methodology for Prevalence of FASD in Midwestern City: Sample 1
*If a child was randomly selected and found to have an FASD or another known genetic or teratogenic disorder, he/she was classified appropriately and removed from the control group.
**11 were not FASD, with other genetic disorders
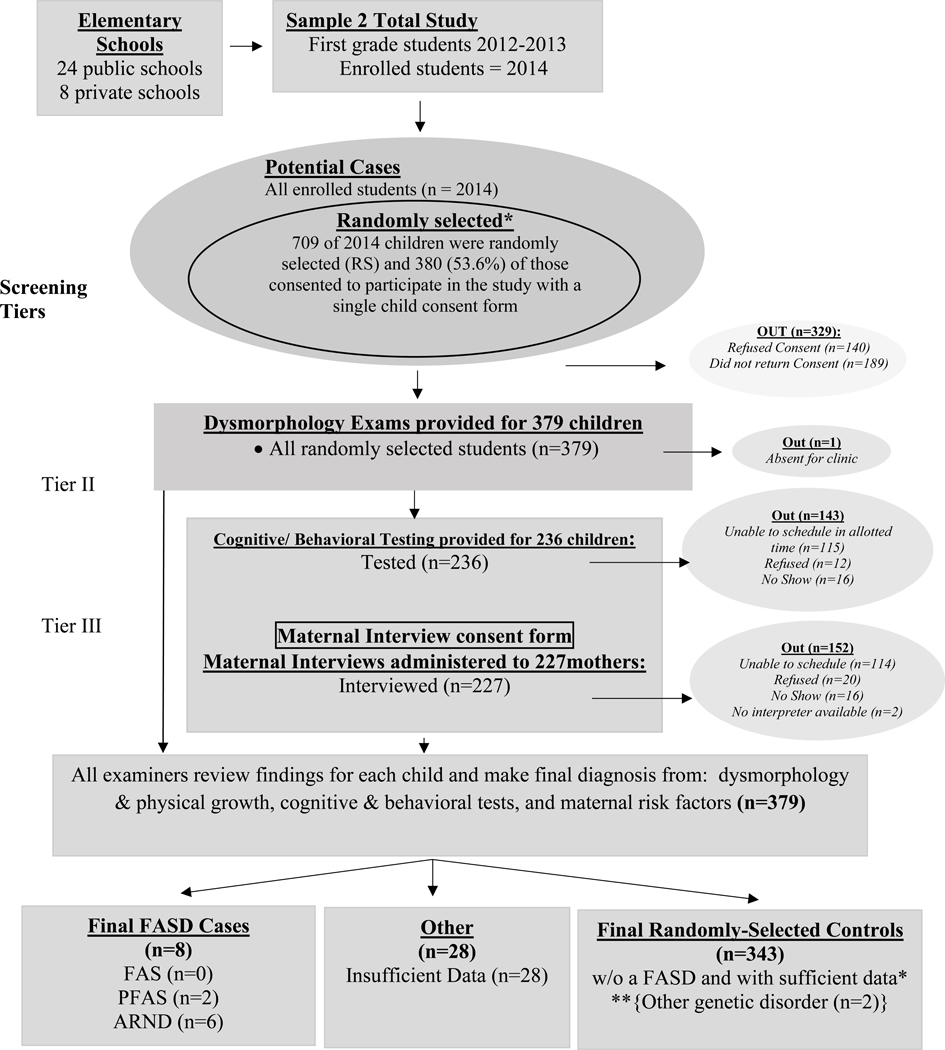
Cohort Two Sampling: A Simple Random Sample
Cohort Sample 2 was drawn by a simple random sample from the 2,014 children enrolled in the same schools, two years (Fall of 2012) after cohort Sample 1. Seven-hundred and nine (709) unique numbers were chosen randomly by computer, and as before, parents were provided information and consent forms through the take-home folder system utilized by schools (Figure 2). Five-hundred and twenty (520) consent forms (73%) were returned with 380 (53.6%) providing consent to participate. Because every child in Sample 2 was selected randomly, each child was to be assessed for neurobehavior and their mothers interviewed for maternal risk.
Figure 2.
Sampling Methodology for Prevalence of FASD in Midwestern City: Sample 2
*If a child was randomly selected and found to have an FASD or another known genetic or teratogenic disorder, he/she was classified appropriately and removed from the control group.
** 2 were not FASD, but had other genetic disorders
The Study Process-Three Tiered Assessment Used for Both Samples
All consented children in both samples were measured for height, weight, and occipitofrontal (head) circumference (OFC) in Tier I: in Sample 1 to oversample small children and in Sample 2 to begin the dysmorphology exam. In Tier II of Sample 1, all consented small children, three children referred by teachers, and all randomly-selected children were provided dysmorphology exams by pediatric dysmorphologists/medical geneticists. Teams measured each child’s height and weight, took facial photographs (frontal and 45-degree angle), and provided structured, standardized dysmorphology examinations blinded from any background information on children or mothers. Exams assessed growth, facial measurements, and minor or major anomalies of the craniofacies, body, and heart for the presence or absence of specific features of FASD (Hoyme et al., 2016). Each child was assigned a “dysmorphology score,” an objective research tool quantifying growth deficiency and structural anomalies that correlates well with outcomes from maternal drinking (Ervalahti et al., 2007). Inter-rater reliability, for specific trait measurements, has been tested for this team, producing Cronbach’s alpha coefficients of: 0.993 for OFC, 0.957 for inner canthal distance (ICD), 0.951 for palpebral fissure length (PFL), and 0.928 for philtrum length (May et al., 2011b) and acceptable reliability on other correlation measures (May et al., 2000; Viljoen et al., 2005).
After reviewing dysmorphology findings for each child, the dysmorphologist assigned a preliminary diagnosis: a) not-FASD, b) FAS or PFAS (based on growth deficiency and dysmorphology alone), c) diagnosis deferred (rule out a specific FASD diagnosis pending developmental/behavioral testing and maternal interview), or d) another genetic or malformation syndrome. All randomly-selected children and those in categories b and c were advanced to Tier III.
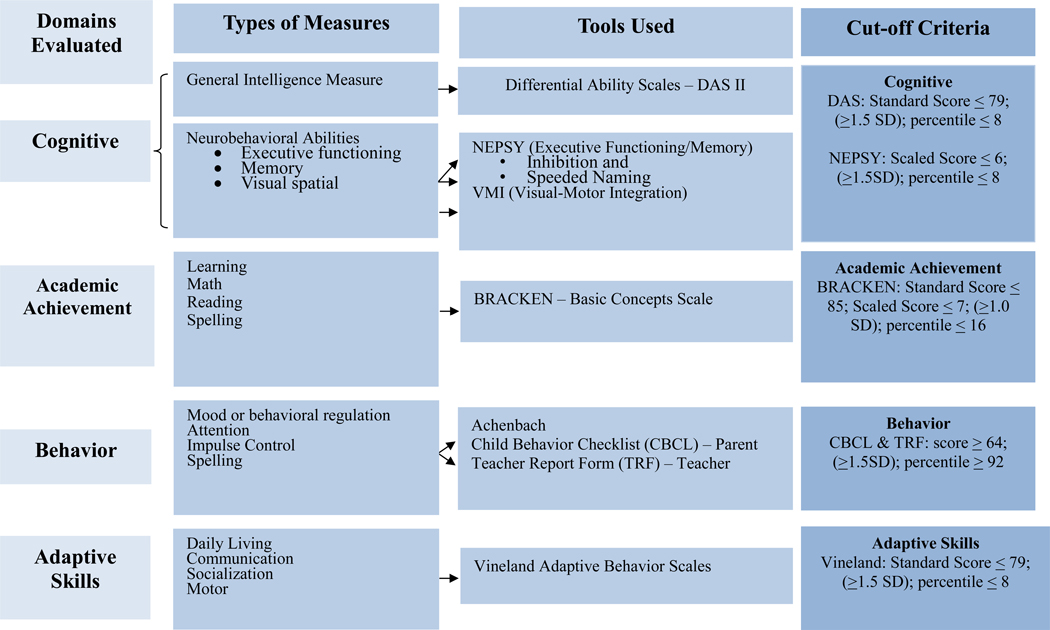
In Tier III, the CoFASP battery was provided by school psychologists (Figure 3) to evaluate cognition, academic achievement, behavior and adaptive skills. The instruments included were the Differential Abilities Scale-2nd Edition (Elliott, 2007) to assess general intelligence; the NEPSY-II (Korkman et al., 2007) for executive functioning, memory, and visual spatial integration; the Developmental Test of Visual-Motor Integration (VMI) (Beery and Beery, 2004) for eye-hand coordination; and the Bracken Basic Concepts Scale-Revised (Bracken, 1998) to measure basic concept development in math, reading and spelling. Checklists were employed to assess behavior: the Achenbach Child Behavior Checklist (CBCL) was completed by a parent and teachers (TRF) to assess behavior (Achenbach and Rescorla, 2001). The Vineland Adaptive Behavior Scales (Sparrow et al., 2005) were also completed by teachers and parents.
Figure 3.
CoFASP Cut-Off Criteria Set for all Domains: Neurobehavioral Testing Battery
Consenting mothers of Tier III participants were interviewed, in-person, by project staff. Maternal questions were sequenced to maximize accurate reporting of general health, childbearing, nutrition, alcohol and other drug use, and socioeconomic status (SES) variables. Drinking questions employed a timeline, follow-back sequence (Sobell et al., 2001, 1988) and Vessels alcohol product methodology for accurate calibration of alcohol units (Kaskutas and Graves, 2001, 2000; Kaskutas and Kerr, 2008). Current alcohol consumption for the week preceding the interview was embedded in dietary intake questions (King, 1994) to aid accurate calibration of drinking quantity, frequency, and gestational timing of alcohol use (Alvik et al., 2006; May et al., 2013a, 2008, 2005). The mother was asked about alcohol use before and during the index pregnancies. Retrospective reports of alcohol use have been found to be generally accurate in several populations (Czarnecki et al., 1990; Fortin et al., 2017; Hannigan et al., 2010). The accuracy of self-report data produced by this approach has been confirmed by biomarker results in one population (May et al., 2018b).
Maternal risk data were gathered in both samples for a total of 380 mothers. The American standard drink unit definition was used, where one drink equals consuming 14g of absolute alcohol: 12oz (350mL) of beer at (5% alcohol by volume); 5oz (150mL) of wine (12% by volume); or 1.5oz (44mL) of liquor (40% by volume) (“What Is A Standard Drink? | National Institute on Alcohol Abuse and Alcoholism (NIAAA),” n.d.). Drinking during pregnancy was confirmed if at least one of the following criteria was met: a) six or more standard drinks per week for two or more weeks during pregnancy; b) three or more drinks per occasion two or more times during pregnancy; or c) documentation of alcohol-related social or legal problems in proximity to the index pregnancy (e.g. treatment for alcohol abuse or driving under the influence). These CoFASP criteria were deemed sufficient evidence of drinking during the index pregnancy (Hoyme et al., 2016).
Multidisciplinary Case Conferences: Assignment of Accurate Final Diagnoses
After all data were collected, summarized and reviewed, diagnoses were assigned in structured, multidisciplinary case conferences. All children who qualified for Tier II screening by dysmorphology findings, random selection entry into the study, or teacher referral were conferenced. Each child’s findings for each domain were discussed while digital photographic images of each child (frontal and 45° profile views) were projected on a screen. After discussion of specific findings and assessment of sufficient evidence in each domain, final diagnoses were assigned by team consensus. Consistency and quality assurance were enhanced by strict application of CoFASP criteria in the examinations and conference proceedings. Final diagnoses were later double-checked for consistency and accuracy by data management teams at UNC, UCSD, and UNM were triple-checked by reciprocal exchange of all child diagnostic data for all FASD cases and a sample of non-cases. Each team was blinded to the other team’s classifications and determined whether criteria had been applied accurately.
Statistical Analysis
Data analyses were performed with Excel (“Microsoft Excel,” 2016) and SPSS (IBM, 2017). Case control findings for child measures were compared across diagnostic groups using chi square and Fisher’s exact tests for categorical variables, t-tests, one-way analysis of variance (ANOVA) and post-hoc Dunnett’s correction pairwise comparisons with α=.05. Bonferroni-adjusted alpha values are indicated in each table.
Partial correlation analysis, controlling for maternal drug use, was employed to determine associations between maternal drinking and pregnancy recognition with select child outcomes. In calculating partial correlations, transformations were undertaken for most measures due to skewness. Logarithmic transforms were applied to the usual number of drinks per drinking day (DDD) before pregnancy, number of weeks before mother’s recognition of the index pregnancy, and the teacher’s reports of rule-breaking and attention problems. Square root transformations were applied to the child’s total dysmorphology and general abilities scores. Although highly unbalanced, transformations could not be applied to “yes/no” items: reported maternal drinking during pregnancy trimesters, and the covariate, whether mother had used drugs during the index pregnancy. Use of pairwise deletion ensured that all available data were included. A statistical criterion of p<.0017 was set to control for Type I familywise error rate in partial correlation analyses. Partial correlations may be attenuated due to non-normality remaining after transformation and to highly unbalanced frequencies in dichotomous categories.
Logistic regression predicting FASD as a function of number of drinks per drinking day (DDD) prior to pregnancy was performed, while controlling for tobacco and drug use during pregnancy. Varying amounts of data were missing on the measures, but there was no more than 5% missing for any one variable and only 6% missing data overall. Due to the small number of children with diagnoses, multiple imputations were undertaken to stabilize the estimates. No transformations were applied to tobacco or drug use during pregnancy, and number of DDD was treated as a categorical variable. SPSS MI (Multiple Imputation) function imputed 25 complete datasets, and pooled results over the 25 imputations are presented in the table.
The site prevalence of FASD was calculated from the average of the rates using CoFASP criteria from the two independent, individual cohort samples published previously (May et al., 2018a). The lower rates for FASD represent the minimum (lower bound) prevalence possible given the number of children meeting CoFASP-revised-IOM guidelines (numerator) in combined site samples divided by the total children enrolled in first grade classes in both cohorts. The higher rates employed a conservative, weighted correction factor for each diagnosis based on the proportion of diagnoses made within the subsamples of randomly-selected entrants. Weighted correction was applied to the unconsented first grade students for all FASD diagnoses and additionally to not-FASD small students for ARND estimated cases among those who entered the study through growth qualification alone. The calculation of rates is described fully in the e-appendix of the CoFASP prevalence summary paper (May et al., 2018a).
RESULTS
FASD cases by racial and ethnic distribution are presented in Table 2. Whether measured across specific diagnoses of FASD or measured by FASD vs. not FASD, the rates of FASD are not significantly different by race or Hispanic ethnicity.
Table 2.
Distribution of FASD Case and Randomly-Selected Controls by Racial Categories: Midwestern City
| FAS | PFAS | ARND | RS controls |
χ2 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| White | 440 | 77.9 | 8 | 72.7 | 20 | 87.0 | 8 | 80.0 | 404 | 77.5 | 6.692 | 0.669 |
| Hispanic | 30 | 5.3 | 0 | 0.0 | 1 | 4.3 | 0 | 0.0 | 29 | 5.6 | ||
| African American | 43 | 7.6 | 1 | 9.1 | 2 | 8.7 | 0 | 0.0 | 40 | 7.7 | ||
| Other | 52 | 9.2 | 2 | 18.2 | 0 | 0.0 | 2 | 20.0 | 48 | 9.2 | ||
| FASD | RS controls | χ2 | p-value | |||||||||
| n | % | n | % | n | % | |||||||
| White | 440 | 77.9 | 36 | 81.8 | 404 | 77.5 | 0.964 | 0.810 | ||||
| Hispanic | 30 | 5.3 | 1 | 2.3 | 29 | 5.6 | ||||||
| African American | 43 | 7.6 | 3 | 6.8 | 40 | 7.7 | ||||||
| Other | 52 | 9.2 | 4 | 9.1 | 48 | 9.2 | ||||||
Child Physical Traits
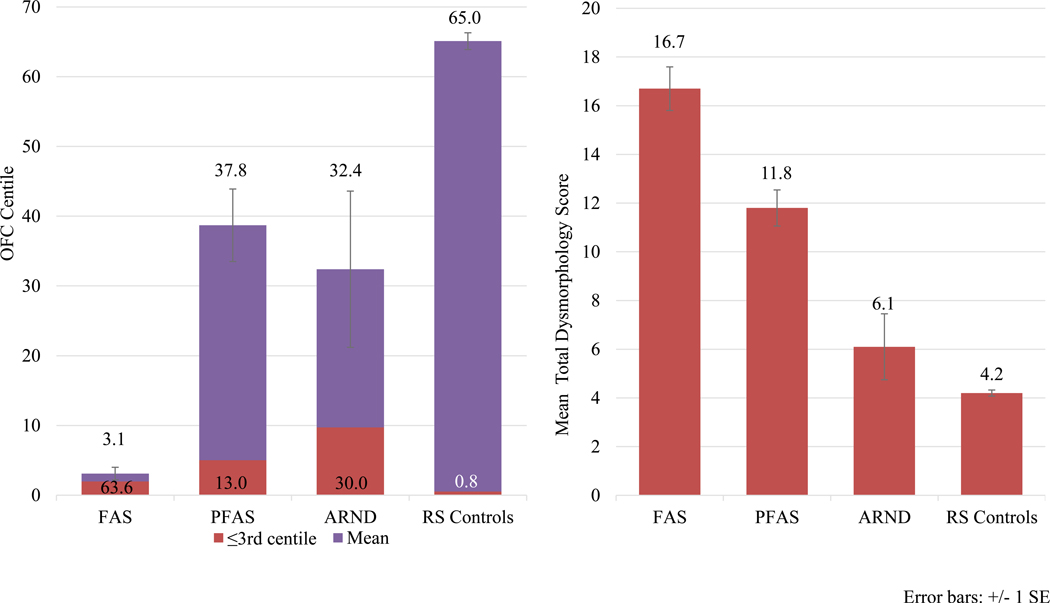
Forty-four (44) cases on the continuum of FASD were diagnosed, 36 from Sample 1 and 8 from Sample 2. In the combined data, 25% of children had FAS, 52% had PFAS, and 23% had ARND. There were 521 randomly-selected children diagnosed as typically-developing controls after full examinations and neurobehavioral assessment. Children with FASD differed significantly (Bonferroni-adjusted) from controls on all variables in Table 3 with two exceptions: age and sex. On all other growth, cardinal facial features, and other minor anomalies, group differences were statistically significant; appropriate classification and specificity were achieved for child physical traits using IOM-CoFASP diagnostic criteria. Average total dysmorphology scores were: FAS=16.7; PFAS=11.8; ARND=6.1; and controls=4.2. Post-hoc analyses indicate significant bi-variate group differentiation by dysmporphology except for children with ARND vs. controls by definition, are likely to differ due to growth restriction or minor anomalies. OFC differed significantly across groups (Figure 4). Mean OFC for controls was 65th centile, and the PFAS group had the largest OFC of the FASD groups, 39th centile. Sixty-three percent of the FAS children were ≤3rd centile of OFC, and 40% of the children with ARND had an OFC ≤ 10th centile. Children with ARND had a lower average BMI centile than controls. Other minor anomalies and growth deficiency were more prevalent in all FASD diagnostic groups than controls. The mandibular and maxillary arc measurements were depressed in all FASD groups over controls. Hypoplastic midface was more common in FASD groups than controls, as were camptodactyly, other aberrant palmar creases, and heart malformations in the PFAS group.
Table 3.
Demographic, Physical Growth, Cardinal FAS Features, Other Minor Anomalies, and Total Dysmorphology Scores from a Midwestern City: 2010–2014
| Children with FAS (n=11) |
Children with PFAS (n=23) |
Children with ARND (n=10) |
Randomly-Selected Control Children (n=521) |
Test-score | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth and Cardinal Features | ||||||||||
| Sex (% Male) | 36.4 | 60.9 | 40.0 | 52.6 | χ2=2.420 | .490 | ||||
| Current Age (in months) – Mean (SD) | 82.4 | (6.5) | 84.7 | (4.1) | 82.3 | (4.9) | 81.8 | (4.8) | F=2.792 | .040E |
| Height Percentile – Mean (SD) | 7.0 | (6.3) | 32.3 | (28.3) | 56.5 | (35.6) | 60.3 | (27.6) | F=20.514 | <.001A,B,C,E |
| Weight Percentile – Mean (SD) | 13.7 | (11.5) | 34.2 | (25.0) | 42.3 | (31.0) | 61.2 | (27.0) | F=19.476 | <.001A,C,E |
| Child’s BMI Percentage – Mean (SD) | 40.3 | (34.7) | 44.6 | (28.6) | 28.6 | (27.5) | 58.6 | (27.7) | F=6.854 | <.001F |
| Occipitofrontal Circumference (OFC) Percentile – Mean (SD) | 3.1 | (3.1) | 38.7 | (24.9) | 32.4 | (35.3) | 65.0 | (27.3) | F=29.051 | <.001A,C,E |
| OFC centile ≤3rd centile | 63.6 | 13.0 | 30.0 | 0.8 | χ2=180.414 | <.001 | ||||
| OFC centile ≤10th centile | 100.0 | 21.7 | 40.0 | 4.8 | χ2=154.190 | <.001 | ||||
| Palpebral Fissure Length (PFL) Percentile – Mean (SD) | 10.9 | (14.4) | 12.2 | (12.5) | 22.3 | (11.5) | 29.1 | (16.0) | F=13.365 | <.001C,E |
| PFL centile ≤3rd centile | 36.4 | 39.1 | 10.0 | 5.4 | χ2=50.268 | <.001 | ||||
| PFL centile ≤10th centile | 72.7 | 56.5 | 10.0 | 10.7 | χ2=71.575 | <.001 | ||||
| Smooth Philtrum (% Yes) | 90.9 | 87.0 | 30.0 | 14.0 | χ2=116.289 | <.001 | ||||
| Narrow Vermilion (% Yes) | 81.8 | 82.6 | 10.0 | 17.9 | χ2=79.246 | <.001 | ||||
| Other Minor Anomalies | ||||||||||
| Maxillary Arc (in cm) – Mean (SD) | 23.2 | (1.2) | 24.1 | (.8) | 24.2 | (1.2) | 24.8 | (1.0) | F=12.326 | <.001C,E |
| Mandibular Arc (in cm) – Mean (SD) | 23.7 | (1.3) | 25.1 | (.8) | 25.0 | (1.3) | 25.9 | (1.2) | F=14.703 | <.001A,C,E |
| Hypoplastic midface (% Yes) | 54.5 | 47.8 | 50.0 | 25.0 | χ2=13.116 | .004 | ||||
| Camptodactyly (% Yes) | 18.2 | 0.0 | 10.0 | 2.3 | χ2=13.226 | .004 | ||||
| Other aberrant palmar creases (% Yes) | 9.1 | 17.4 | 10.0 | 2.1 | χ2=20.810 | <.001 | ||||
| Heart malformations (% Yes) | 0.0 | 4.3 | 0.0 | 0.2 | χ2=10.863 | .012 | ||||
| Total Dysmorphology Score – Mean (SD) |
16.7 | (3.0) | 11.8 | (3.6) | 6.1 | (4.3) | 4.2 | (2.9) | F=111.075 | <.001A,B,C,D,E |
Post-hoc significant difference between:
FAS & PFAS;
FAS & ARND;
FAS & Controls;
PFAS & ARND;
PFAS & Controls;
ARND & Controls.
Bonferroni adjusted significance level for Growth and Cardinal Features = 0.005; for other minor anomalies = 0.007
Figure 4.
Occipitofrontal Circumference (OFC) and Total Dysmorphology Score by FASD Diagnosis, Midwestern City
Neurobehavioral Profiles by Diagnostic Group
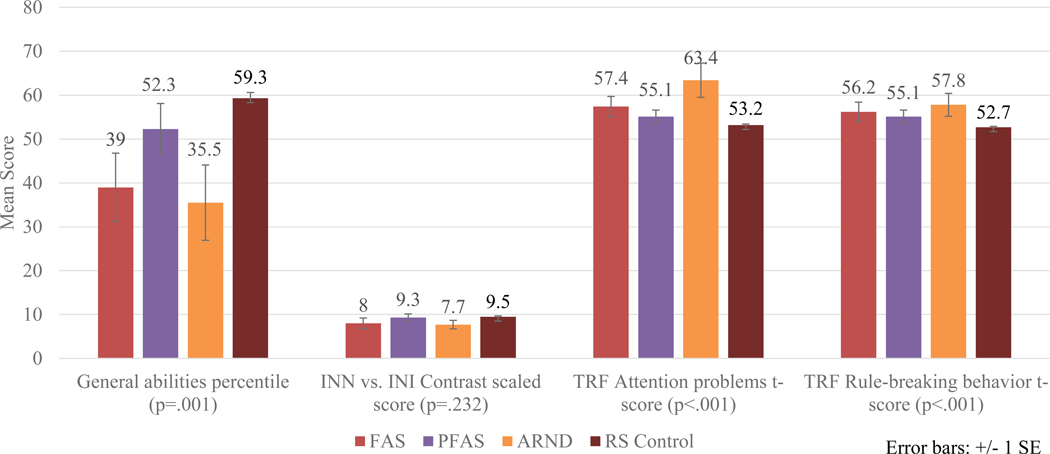
The CoFASP battery was effective in distinguishing diagnostic groups across the FASD spectrum (Appendix Table E1). Figure 5 highlights neurobehavioral results by specific diagnoses. In the intellectual domain, children with PFAS scored the highest of the FASD groups on the General Abilities Test, whereas children with ARND had the lowest scores on most executive function items as evidenced by the INN vs. INI (name vs inhibition) contrast scaled score, although this difference across groups was not statistically significant. On most behavioral variables (mood regulation, attention, impulse control, and adaptive function), there were consistent and significant differences across groups, and in Figure 5 attention problems and rule breaking behavior are significantly different across all diagnostic groups. Children with ARND had the poorest scores (See also Appendix E1). In Table 4 all neurobehavioral test scores for the entire CoFASP Battery are presented comparing children with FASD to controls. The FASD group was significantly differentiated from controls on all neurobehavioral measures, mostly at Bonferroni-adjusted significance levels, with the exception of: non-verbal reasoning, INN vs INI (name vs inhibition) contrast score, learning, visuomotor precision combined scaled score, CBCL rule-breaking behavior t-score, oppositional defiant t-scores, and Vineland standard scores of communication and daily living skills as reported by the teacher and socialization as reported by the parent.
Figure 5.
Selected Cognitive and Behavioral Measures by Specific FASD Diagnoses, Midwestern City
Table 4.
Neurobehavioral Findings Among Children with FASD and Randomly-Selected Controls from a Midwestern City: 2010–2014
| Children with FASD (n=37) |
Randomly-Selected Control Children (n=331) |
t-test | p-value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Intellectual Domain | (n=37) | (n=331) | ||
| General Abilities Percentile | 44.9 (25.7) | 59.3 (23.7) | −3.494 | .001** |
| Verbal Cluster Percentile | 40.4 (25.6) | 60.1 (25.0) | −4.486 | <.001** |
| Nonverbal Reasoning Cluster Percentile | 42.3 (25.5) | 50.3 (24.5) | −1.878 | .061 |
| Spatial Cluster Percentile | 54.9 (25.5) | 63.2 (22.3) | −2.113 | .035* |
| Executive Function | (n=37) | (n=330) | ||
| INN (Naming) combined scaled score | 7.9 (3.5) | 10.1 (3.5) | −3.680 | <.001** |
| INN vs. INI Contrast Scaled Score | 8.6 (3.4) | 9.5 (3.3) | −1.599 | .111 |
| INI (Inhibition) combined scaled score | 7.8 (3.1) | 9.6 (3.4) | −3.059 | .002** |
| INS (Switching) combined scaled score | 7.9 (2.6) | 10.0 (3.1) | −3.665 | <.001** |
| Speeded Naming Combined scaled score | 7.8 (2.1) | 9.3 (2.7) | −3.312 | .001** |
| Learning1 | (n=8) | (n=221) | ||
| BBCS School Readiness Composite Scaled Score | 11.5 (2.4) | 12.4 (2.1) | −1.235 | .218 |
| BBCS Readiness Composite Standard Score | 107.0 (13.3) | 112.3 (10.4) | −1.405 | .161 |
| Visual Spatial | (n=37) | (n=331) | ||
| VMI Standard Score | 93.9 (8.6) | 97.2 (7.4) | −2.542 | .011** |
| Visuomotor Precision Combined scaled score | 9.1 (2.9) | 9.8 (2.9) | −1.527 | .128 |
| Mood Regulation2 | ||||
| CBCL Anxious/depressed t-score | 56.3 (7.6) | 53.1 (4.8) | 2.400 | .022* |
| TRF Anxious/depressed t-score | 55.1 (6.7) | 51.9 (3.6) | 3.087 | .004** |
| CBCL Withdrawn/depressed t-score | 56.9 (7.5) | 53.1 (5.0) | 2.803 | .008* |
| TRF Withdrawn/depressed t-score | 56.2 (7.8) | 52.2 (4.2) | 3.318 | .002** |
| CBCL Internalizing Problems t-score | 54.3 (10.6) | 48.1 (8.9) | 3.156 | .003** |
| TRF Internalizing Problems t-score | 52.1 (10.8) | 45.0 (7.9) | 4.175 | <.001** |
| CBCL Externalizing Problems t-score | 53.5 (10.6) | 49.4 (9.5) | 2.298 | .022* |
| TRF Externalizing Problems t-score | 53.5 (9.6) | 48.5 (7.8) | 3.935 | <.001** |
| CBCL Affective problems t-score | 56.7 (7.1) | 53.2 (5.1) | 2.646 | .012* |
| TRF Affective problems t-score | 55.6 (6.6) | 52.0 (3.9) | 3.496 | .001** |
| CBCL Anxiety problems t-score | 57.3 (7.6) | 53.4 (5.4) | 2.769 | .009* |
| TRF Anxiety problems t-score | 56.5 (7.2) | 51.9 (4.0) | 4.051 | <.001** |
| Attention2 | ||||
| CBCL Attention problems t-score | 59.9 (10.4) | 54.4 (6.4) | 2.945 | .006** |
| TRF Attention problems t-score | 57.6 (9.2) | 53.0 (4.8) | 3.189 | .003** |
| CBCL Attention deficit/hyperactivity problems t-score | 58.2 (8.5) | 54.0 (6.2) | 2.756 | .009** |
| TRF Attention deficit/hyperactivity problems t-score | 58.0 (9.9) | 53.6 (5.3) | 2.848 | .007** |
| Impulse Control2 | ||||
| CBCL Rule-breaking behavior t-score | 54.6 (5.7) | 53.6 (4.9) | 1.109 | .268 |
| TRF Rule-breaking behavior t-score | 56.0 (7.0) | 52.7 (4.8) | 2.948 | .005** |
| CBCL Aggressive behavior t-score | 57.0 (7.4) | 53.9 (6.0) | 2.292 | .028* |
| TRF Aggressive behavior t-score | 55.5 (7.0) | 52.6 (4.7) | 2.627 | .012* |
| CBCL Oppositional defiant problems t-score | 56.9 (7.0) | 54.9 (6.0) | 1.580 | .123 |
| TRF Oppositional defiant problems t-score | 54.2 (6.3) | 52.5 (5.0) | 1.695 | .097 |
| CBCL Conduct problems t-score | 56.9 (7.1) | 53.8 (5.7) | 2.802 | .005** |
| TRF Conduct problems t-score | 56.3 (7.9) | 52.5 (4.8) | 3.125 | .003** |
| Adaptive Function3 | ||||
| Vineland (Parent) VABS Communication Standard Score | 98.0 (14.1) | 106.2 (12.7) | −3.430 | .001** |
| Vineland (Teacher) VABS Communication Standard Score | 94.3 (16.2) | 98.1 (13.0) | −1.634 | .103 |
| Vineland (Parent) VABS Daily Living Skills Standard Score | 99.6 (16.0) | 103.5 (11.5) | −1.364 | .181 |
| Vineland (Teacher) VABS Daily Living Skills Standard Score | 96.6 (14.4) | 99.3 (13.2) | −1.157 | .248 |
| Vineland (Parent) VABS Socialization Standard Score | 100.1 (17.0) | 104.8 (13.4) | −1.810 | .071 |
| Vineland (Teacher) VABS Socialization Standard Score | 96.1 (15.4) | 103.8 (15.0) | −2.836 | .004** |
Children less than 7 at time evaluation did not complete a BRACKEN.
For CBCL: n=32 for FASD; n=298 for Controls. For TRF: n=42 for FASD; n=444 for Controls.
For Vineland (Parent): n=32 for FASD; n=292 for Controls.
For Vineland (Teacher): n=35 for FASD; n=437 for Controls.
Significant at <0.05
Significant at the Bonferroni-adjusted level of significance: Intellectual: 0.0125; Executive Function: 0.01; Learning: 0.025; Visual Spatial: 0.025; Mood Regulation: 0.004; Attention: 0.0125; Impulse Control: 0.006; Adaptive Function: 0.008
Maternal Risk Data-Proximal and Distal Variables
All maternal risk variables that significantly differentiated mothers of children with FASD from control mothers are included in Tables 5 and 6 with a selected few that did not. Table 5 presents proximal causal variables of drinking and other drug use. Ninety-six percent (96%) of mothers of children with FASD drank prior to pregnancy, compared to 60% of controls. The mean number of DDD prior to pregnancy was 4.4 for mothers of children with FASD and 2.5 for mothers of controls, and DDD reporting was highest among mothers of children with ARND (mean, 7 drinks; median, 6.5 drinks, Table E1). Pre-pregnancy frequency per week was higher among mothers of FASD, for 67% reported drinking at least weekly compared to 32% for controls; 15% of the mothers of FASD children reported daily drinking vs. 3% for controls. More mothers of children with FASD (52%) reported exceeding their usual daily amounts of alcohol than mothers of controls (17%). Mothers of children with FASD were more likely to report drinking (yes/no) in the first trimester (42%) compared to mothers of controls (5.3%). Fewer mothers of controls reported drinking in the second and third trimesters. Only 2–3% of mothers of controls reported drinking in the latter trimesters. Virtually all quantity measures of drinking during pregnancy appear to have been underreported (Table 5), especially in the FAS and PFAS group (Table E1).
Table 5.
Proximal Maternal Risk Factors for FASD: Alcohol and Drug Use in Midwestern City
| Children with FASD (n=31) |
Randomly-Selected Control Children (n=305) |
χ2
or t-test |
p-value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Alcohol Use – Before and During Pregnancy | ||||
| Drank before pregnancy (% Yes) | 96.3 | 60.1 | 13.918 | <.001** |
| # of drinks consumed on usual drinking day before pregnancy1 | 4.4 (3.0) | 2.5 (2.3) | 3.481 | .001** |
| Mdn = 3.5 | Mdn = 2.0 | -- | -- | |
| Usual frequency – before pregnancy1 | ||||
| Everyday or almost everyday | 14.8 | 2.8 | ||
| 3–4 times per week | 11.1 | 4.5 | ||
| 1–2 times per week | 40.7 | 24.3 | ||
| 2–3 times per month | 11.1 | 23.2 | ||
| 1 time per month or less | 22.2 | 45.2 | 16.426 | .002** |
| Days drank more than usual – before pregnancy (% Yes)1 | 51.9 | 17.1 | 18.782 | <.001** |
| Drank in 1st trimester (% Yes) | 41.9 | 5.3 | 47.845 | <.001** |
| # of drinks on usual drinking day1 −1st | 3.2 (3.4) | 4.1 (4.6) | −0.478 | .638 |
| Usual frequency1 – 1st | ||||
| Everyday or almost everyday | 25.0 | 28.6 | ||
| 3–4 times per week | 0.0 | 14.3 | ||
| 1–2 times per week | 25.0 | 0.0 | ||
| 2–3 times per month | 0.0 | 0.0 | ||
| 1 time per month or less | 50.0 | 57.1 | 4.714 | .194 |
| Drank in 2nd trimester (% Yes) | 20.0 | 3.3 | 16.643 | <.001** |
| # of drinks on usual drinking day1 - 2nd | 1.0 (0.0)B | 2.7 (3.9) | −0.747 | .472 |
| Usual frequency1 – 2nd | ||||
| Everyday or almost everyday | 0.0 | 14.3 | ||
| 3–4 times per week | 0.0 | 14.3 | ||
| 1–2 times per week | 0.0 | 0.0 | ||
| 2–3 times per month | 33.3 | 0.0 | ||
| 1 time per month or less | 66.7 | 71.4 | 3.197 | .362 |
| Drank in 3rd trimester (% Yes) | 20.0 | 2.6 | 20.426 | .001A,** |
| # of drinks on usual drinking day1 - 3rd | 1.0 (0.0)B | 1.9 (1.9) | −0.759 | .464 |
| Usual frequency1 – 3rd | ||||
| Everyday or almost everyday | 0.0 | 16.7 | ||
| 3–4 times per week | 0.0 | 0.0 | ||
| 1–2 times per week | 0.0 | 0.0 | ||
| 2–3 times per month | 0.0 | 0.0 | ||
| 1 time per month or less | 100.0 | 83.3 | .381 | .537 |
| Alcohol Use - Current | ||||
| Drink in past 30 days (% Yes) | 73.1 | 65.7 | .589 | .443 |
| Binge 5+ in past month (% Yes) | 4.2 | 2.5 | .243 | .485A |
| Why usually drink: because others drink | 12.5 | 19.3 | .674 | .588A |
| Why usually drink: to feel less anxious | 12.5 | 8.4 | .478 | .450A |
| Why usually drink: to forget worries (% Yes) | 20.8 | 7.0 | 5.759 | .033A,* |
| Reported current drinking problem at interview (% Yes) | 12.5 | 2.3 | 9.419 | .014A,** |
| Recovering drinker (% Yes) | 9.7 | 4.7 | 1.461 | .204A |
| Drug Use | ||||
| Used tobacco – during pregnancy (% Yes) | 25.0 | 8.9 | 8.008 | .011A,** |
| Used any drugs in pregnancy (% Yes) | 15.6 | 4.3 | 7.190 | .021A,* |
| Abused prescription – during pregnancy | 0.0 | 1.4 | 5.030 | 1.00A |
| Used marijuana – during pregnancy (% Yes) | 6.3 | 3.9 | .513 | .362A |
| Used marijuana & alcohol – during pregnancy (% Yes) | 6.5 | 1.4 | 4.053 | .103A |
| Used cocaine - during pregnancy (% Yes) | 3.2 | 0.7 | 14.810 | .002A,** |
| Used methamphetamine – pregnancy (% Yes) | 2.7 | 1.1 | 2.032 | .160A |
| Used tobacco | ||||
| Yes, within last 30 days | 37.5 | 24.3 | ||
| Yes, in lifetime | 18.8 | 33.6 | ||
| Never | 43.8 | 42.1 | 6.790 | .034 |
| Used any drug in lifetime (% Yes) | 40.6 | 27.2 | 2.580 | .147A |
| Used marijuana – in lifetime (% Yes) | 29.0 | 24.4 | .320 | .662A |
| Used methamphetamine – in lifetime (% Yes) | 13.3 | 5.9 | 2.418 | .124A |
| Used heroin – in lifetime (% Yes) | 0.0 | 0.3 | .100 | 1.00A |
| Used club drugs – in lifetime (% Yes) | 0.0 | 2.3 | .190 | 1.00A |
| Used crack/cocaine – in lifetime (% Yes) | 16.1 | 4.3 | 7.730 | .018A,** |
| Abused pain killers – in lifetime (% Yes) | 12.5 | 2.3 | 3.050 | .199A |
Among women who drank in that specific time period.
A collateral interview confirmed alcohol consumption but with unknown quantity and/or frequency.
Fisher exact test
All respondents (n=3) reported the same value; therefore, there is not standard deviation
Significant at 0.05
Significant at the Bonferroni-adjusted level: Alcohol use before and during pregnancy = 0.003; current alcohol use = 0.007; drug use = 0.003.
Table 6.
Distal Maternal Risk Factors for FASD: Physical, Health, Childbearing, Prenatal Care, Postnatal Variables, and Demographic Variables from a Midwestern City.
| Children with FASD (n=31) | Randomly-Selected Control Children (n=304) | χ2 or t-test | p-value | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Physical | ||||||
| Height at interview (cm) | 165.9 | (8.6) | 166.2 | (6.6) | −0.220 | .860 |
| Weight at interview (kg) | 67.1 | (18.8) | 78.4 | (20.7) | −2.921 | .004** |
| Body Mass Index | 24.5 | (6.0) | 28.2 | (7.2) | −2.705 | .007** |
| Head circumference | 54.0 | (1.9) | 55.1 | (1.4) | −2.567 | .001** |
| Weight before pregnancy (in kg) | 60.7 | (14.2) | 69.1 | (16.7) | −2.480 | .014* |
| Stomach ulcers – in lifetime (% Yes) | 12.5 | 7.5 | .996 | .306A | ||
| Liver problems / hepatitis – in lifetime | 6.3 | 1.6 | 3.027 | .136A | ||
| Depression – in lifetime (% Yes) | 53.3 | 35.2 | 3.860 | .049* | ||
| Childbearing | ||||||
| Gravidity | 3.0 | (1.7) | 3.3 | (1.6) | −0.931 | .352 |
| Parity | 2.6 | (1.4) | 2.7 | (1.1) | −0.523 | .601 |
| Miscarriages | 0.5 | (0.8) | 0.5 | (1.0) | −0.167 | .868 |
| Abortions | 0.1 | (0.3) | 0.0 | (0.2) | 0.662 | .508 |
| Stillbirths | 0.0 | (0.0) | 0.0 | (0.2) | −0.669 | .504 |
| Birth order of index child | 1.7 | (0.8) | 1.9 | (1.1) | −1.299 | .195 |
| Pregnancy recognition (in weeks) | 8.5 | (8.3) | 5.2 | (2.5) | 4.949 | <.001** |
| Prenatal Care | ||||||
| Once knew pregnant, take vitamins (% Yes) | 83.3 | 94.0 | 4.819 | .045A,* | ||
| # of times seen by healthcare provider | ||||||
| Never | 6.5 | 0.0 | ||||
| 1–5 times | 6.5 | 1.7 | ||||
| More than 5 times | 87.1 | 98.3 | 12.654 | .001** | ||
| When first seen by healthcare provider | ||||||
| 1st trimester | 78.1 | 95.7 | ||||
| 2nd trimester | 12.5 | 3.3 | ||||
| 3rd trimester | 6.3 | 0.7 | ||||
| Delivery only | 3.1 | 0.3 | 14.610 | .002** | ||
| Premature labor (% Yes)2 | 33.3 | 12.5 | 5.810 | .028A,* | ||
| Other health problems during pregnancy | 41.9 | 22.3 | 5.933 | .018* | ||
| Accidents/injury – during pregnancy (% Yes) | 6.3 | 7.6 | 0.073 | 1.00A | ||
| Postpartum depression (% Yes) | 20.0 | 15.9 | .338 | .603 | ||
| Postnatal Variables | ||||||
| Child’s Weight (grams) at birth | 2941 | (612) | 3287 | (619) | −3.008 | .003** |
| Child’s Estimated gestation age at birth (in weeks) | 37.9 | (2.1) | 38.5 | (2.5) | −1.365 | .173 |
| Breastfed (% Yes)1 | 62.5 | 77.2 | .933 | .393 | ||
| Consumed alcohol in breastfeeding period1,3 | 60.0 | 21.5 | 4.114 | .077A | ||
| Pump and dump5 (%Yes) among mothers who breastfed | 60.0 | 16.6 | 6.182 | .041A,* | ||
| Child lives with biological mother (% Yes) | 80.6 | 95.7 | 11.896 | .004A,** | ||
| Child lives with: | ||||||
| Foster/Adopted/Relative | 9.7 | 3.3 | ||||
| Biological mother | 22.6 | 19.8 | ||||
| Biological father | 9.7 | 1.0 | ||||
| Biological mother and father | 58.1 | 75.9 | 11.980 | .005* | ||
| Father ever had a drinking problem | ||||||
| Never | 38.5 | 61.9 | ||||
| In the past, but not currently | 61.5 | 31.3 | ||||
| Currently | 0.0 | 1.7 | ||||
| Both past and currently | 0.0 | 5.1 | 8.512 | .028* | ||
| Years of Education completed1 | 14.5 | (2.0) | 15.0 | (2.8) | −.0478 | .633 |
| Household yearly income - during pregnancy | 64200 | (53342) | 62362 | (41555) | 0.206 | .837 |
| Marital Status - current | ||||||
| Married | 56.7 | 78.9 | ||||
| Divorced/Widowed/Separated/Single | 43.3 | 17.5 | ||||
| Living with partner | 0.0 | 3.6 | 12.038 | .005A,** | ||
| Marital Status - current | ||||||
| Married | 56.3 | 77.0 | ||||
| Divorced/Widowed/Separated/Single | 25.0 | 11.8 | ||||
| Living with partner | 18.8 | 11.2 | 6.878 | .029A | ||
| Spirituality: none [0] to high [10]1 | 4.1 | (3.4) | 6.2 | (2.7) | −1.987 | .157 |
Fisher Exact Test
Includes only MW sample II
Includes only MW sample I
Among women who breastfed.
Among women who breastfed and consumed alcohol in the breastfeeding period.
Pump and dump is the colloquial name for expressing breastmilk after drinking alcohol and disposing of it.
Significant at 0.05
Significant at the Bonferroni-adjusted value: physical variables = 0.006; childbearing variables = 0.007; prenatal care = 0.007; postnatal variables = 0.004.
Concerning current drinking, many of the mothers of children with a FASD reported the same or less drinking than before and during the index pregnancy. Current drinking is only significantly different among groups on one variable: 12.5% of mothers of children with FASD reported a drinking problem compared to 2.3% of controls.
Co-morbid use of other drugs is more likely among mothers of children with FASD than controls; they are more likely to report using tobacco, cocaine, and “any drugs” during pregnancy. During pregnancy, children diagnosed with FASD were reported to have been exposed to tobacco (25%), any drugs (16%), marijuana (6%), cocaine (3%), and methamphetamine (3%). Mothers of children with FASD are overall more likely to report lifetime use of tobacco, methamphetamine, cocaine, and painkillers with only exposure to crack cocaine significantly higher. Mothers of children with ARND are most likely to report co-morbid drug use (Table E2). Simultaneous use of alcohol and marijuana during pregnancy was reported by 6.5% of mothers of children with FASD compared to 1.4% of controls. This difference was not statistically significant.
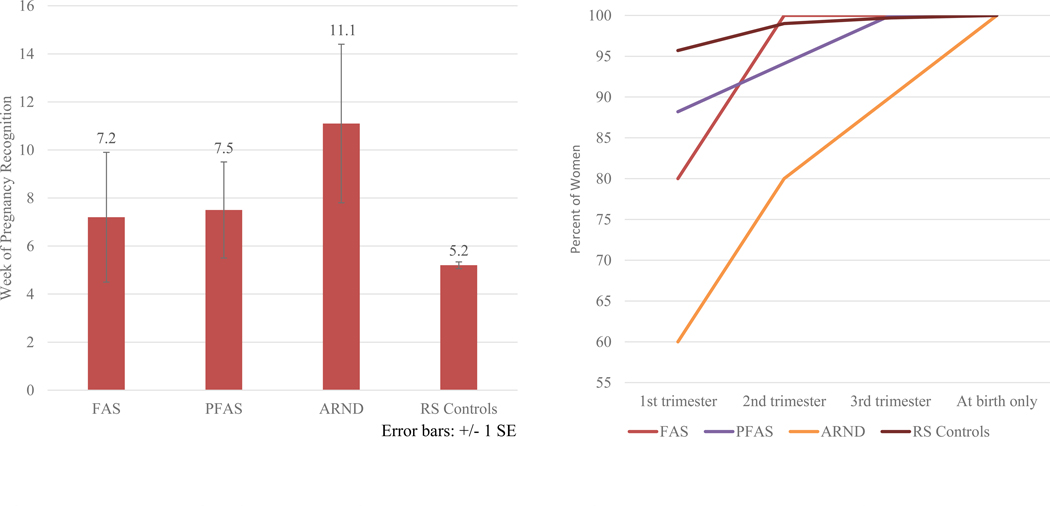
In Table 6, mothers of children with FASD weighed less, had smaller heads, and a lower average BMI than controls. Health indicators were not significant, although depression in one’s lifetime and lower weight before pregnancy approached significance. The only significant childbearing variable among groups is week of pregnancy recognition. Mothers of children with FASD report significantly later recognition of pregnancy than controls (8.5 vs. 5.2 weeks). This is true in all FASD groups (Figure 6 and Table E3). Mothers of children with FASD have these risk indicators: they are somewhat less likely to take prenatal vitamins; they visit providers significantly less for prenatal care; they make their first prenatal visit significantly later; and report more premature labor (especially mothers of children with ARND, 100%).
Figure 6.
A. Week When Pregnancy Was First Recognized, Midwestern Site
B. Timing of First Visit to Healthcare Provider by Trimester, Midwestern site
Children with FASD were significantly lighter at birth, less likely to live with his/her biological mother or married parents. Approaching significance is that mothers of children with FASD (PFAS and ARND especially) report consuming alcohol during the breastfeeding period (Tables 6 and E2). Expressing and disposing of breastmilk (“Pump and Dump”) was practiced by more mothers of children with FASD. Fathers of children with FASD were more likely to have had a drinking problem than controls, children with FASD were more likely to live in foster or adoptive placement and their parents were more likely to be divorced, separated or living with a partner. There is no significant difference among the groups by maternal education completed or yearly income; socio-economic status does not discriminate maternal risk at this site. Finally, not in Table 6 or Table E3, there was no statistically significant difference reported among groups for regularity of institutional religious attendance or professed formal religion.
Correlation Analysis
Partial correlation analysis measured associations between maternal variables and child cognitive/behavioral measures, FASD diagnosis, and total dysmorphology scores after adjusting for whether mother had used drugs during the index pregnancy (Table E4). Maternal reports of drinking during second or third trimester correlated significantly with teacher reports of attention problems, but neither of these correlations were particularly strong once adjusted for drug use. With absolute values of r equal to .18 and .20, each variable accounted for no more than about 4% of the variance in the child’s behavior. There were also suggested, but not statistically significant, links between first trimester drinking and attention and usual DDD before pregnancy with lower total dysmorphology score and lower probability of a FASD diagnosis.
Further correlation analysis was undertaken via binary logistic regression. Adjustments were made to control for any tobacco use and any illicit drugs during pregnancy. Table 7 presents adjusted results for the relationship of reported DDD three months prior to pregnancy to a FASD diagnosis. Reporting three DDD prior-to-pregnancy is significantly related to a FASD diagnosis (p=0.020) with an odds ratio of 10.1 (95%CI=1.4–70.5). The likelihood of a FASD diagnosis in this community is 10 times greater for a woman who reports drinking three DDD prior to pregnancy. Furthermore, likelihood increases to 26.5 times greater (95%CI=4.7–150.6) with usual DDD of five or more prior to the index pregnancy.
Table 7.
Adjusted Binary Logistic Regression Analysis of FASD Diagnosis as a Function of Usual Number of Drinks per Drinking Day 3 Months Prior to Pregnancy: Over 25 Imputations – Midwestern City (n=337)
| S.E. | Sig. | Odds Ratio Exp(B) | Lower | Upper | Fraction Missing Info. | Relative Increase Variance | Relative Efficiency | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 drink per drinking day | 1.399 | 0.962 | 0.147 | 4.051 | 0.610 | 26.888 | 0.290 | 0.398 | 0.989 |
| 2 drinks per drinking day | 1.355 | 0.888 | 0.128 | 3.876 | 0.677 | 22.195 | 0.246 | 0.319 | 0.990 |
| 3 drinks per drinking day | 2.311 | 0.990 | 0.020 | 10.089 | 1.443 | 70.536 | 0.222 | 0.280 | 0.991 |
| 4 drinks per drinking day | 2.191 | 1.095 | 0.046 | 8.945 | 1.037 | 77.153 | 0.280 | 0.381 | 0.989 |
| 5 drinks or more per drinking day | 3.276 | 0.885 | 0.000 | 26.472 | 4.653 | 150.615 | 0.228 | 0.290 | 0.991 |
| Covariates | |||||||||
| Used tobacco during pregnancy | 0.651 | 0.588 | 0.268 | 1.918 | 0.606 | 6.072 | 0.024 | 0.025 | 0.999 |
| Used any illicit drugs during pregnancy | −0.066 | 0.733 | 0.929 | 0.936 | 0.222 | 3.941 | 0.053 | 0.055 | 0.998 |
| Constant | −3.919 | 0.766 | 0.000 | 0.020 | 0.004 | 0.090 | 0.273 | 0.368 | 0.989 |
Reference group: non-drinkers
Estimated Prevalence of FASD from the Combined Data
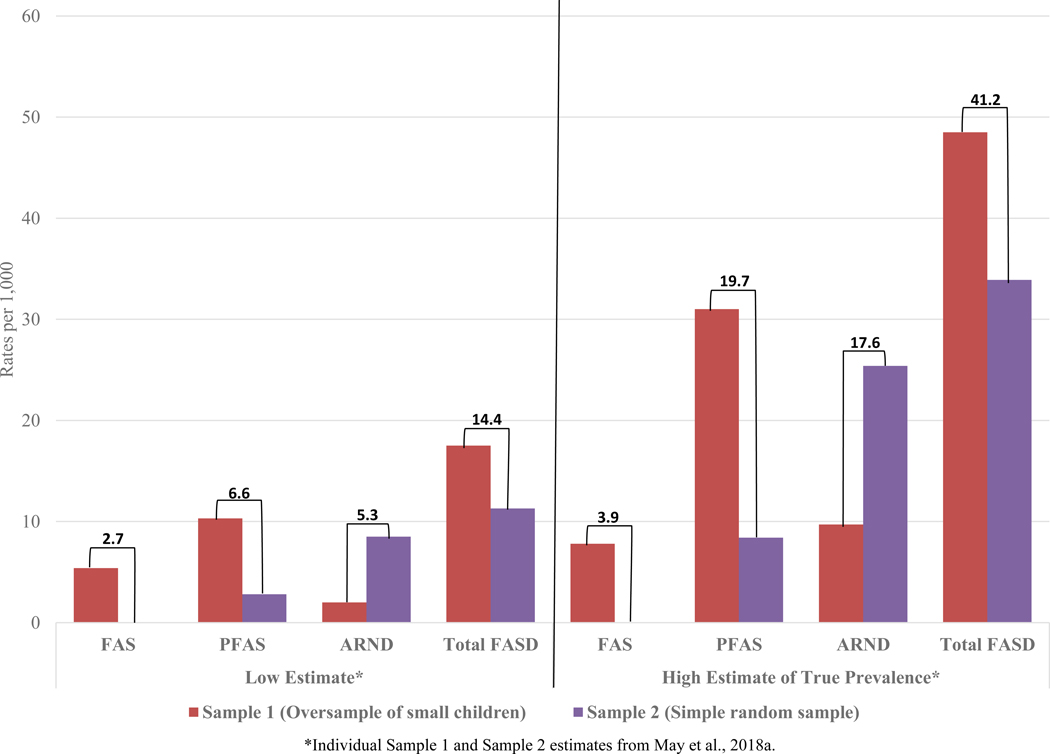
In Figure 7, the combined sample prevalence of FASD diagnoses are presented. The rate of FAS was no lower than 2.7 per 1,000 children, and using a conservative site-specific weighting formula (May et al., 2018a) estimated to be 3.9 per 1,000. FASD prevalence is no lower than 14.4 per 1,000 and weighted rate was 41.2 per 1,000 or 4.1% of the first grade population.
Figure 7.
Combined Sample Site Prevalence of FASD Diagnoses in the Midwestern City: Cohort Samples 1 and 2 and Mean Prevalence
*Individual Sample 1 and Sample 2 estimates from May et al., 2018a.
DISCUSSION
This city in the Midwestern United States is a prosperous, rapidly growing, midsize city with many economic and social indicators similar to U.S. averages. There were no differences in FASD prevalence rates by race and Hispanic ethnicity, a finding consistent across three of the CoFASP sites. The estimated prevalence of FASD in this community is 1.4 to 4.1%, which is higher than would have been predicted from common estimates of FASD prevalence from the past (May et al., 2009), but not surprising given increases in alcohol use among females in the past two decades (Grant et al., 2017). Nevertheless, this site had the lowest FASD prevalence of any of the four CoFASP sites (May et al., 2018a). There was no difference in FASD prevalence by racial and ethnic categories, but the results may be due, in part, to low statistical power from the relatively few cases of FASD.
Overall, the study data describe the variety of consequences of maternal drinking in the prenatal period for first grade children. Drinking three DDD or more prior to pregnancy is reported by many mothers of children with FASD, and this pattern may be continued by many individuals until relatively late pregnancy recognition and later visits to a healthcare provider than with other mothers. Prenatal alcohol exposure causes multiple problems for many of the children who are exposed at various levels and times during pregnancy, and it may also be exacerbated by co-morbid exposure with other drugs (Brown et al., 2017; Fish et al., 2019; Hingson et al., 1982; Volkow et al., 2017). Children diagnosed with any of the diagnoses on the continuum of FASD manifested a variety of physical and neurobehavioral deficits not experienced by the typically-developing children at age seven.
Physical Traits and Dysmorphology
Dysmorphology, growth, and physical traits were well distinguished and categorized into groups by the revised IOM diagnostic methods using CoFASP-specified cut-off criteria (Hoyme et al., 2016). There were multiple (facial and other physical) sizes and appearances among and within the FASD diagnostic groups. This variation is due in part to quantity, frequency, and timing of alcohol exposure to the fetus (Lipinski et al., 2012; May et al., 2013a; Parnell et al., 2013; Sulik et al., 1981) and possibly to other co-morbid drug exposures. Nevertheless, while we found variable appearances and neurobehaviors from one child to the next, both within and among FASD diagnostic groups, each child met strict phenotypic criteria for a diagnosis within the FASD continuum. If one were searching for children with FAS or PFAS, let alone ARND, by focusing primarily upon the classic FAS facial phenotype described in the early pediatric literature (Sulik et al., 1981), or a single behavioral phenotype (Mattson et al., 2013, 2010), few children with FASD would be found in ACA, population-based studies, or diagnosed correctly (Chasnoff et al., 2015).
Neurobehavioral Performance
Variations in performance by individual children on a variety of cognitive and executive functioning tests was also demonstrated among and within the various FASD groups and consistently between the children with FASD vs. controls. Neurobehavioral performance was consistently poor for all the FASD diagnostic groups, but consistently the poorest for children with ARND. Neurobehavioral deficits in cognition, executive functioning, memory, and visual spatial abilities have been shown in studies of prenatally alcohol-exposed children (Coles et al., 2010; Mattson et al., 1999, 2013, 2019; Mattson and Riley, 2011; Rasmussen, 2005; Ware et al., 2012). The results of the overall cognitive, visual spatial, and executive functioning assessments with this cohort of children revealed highly significant differences between those diagnosed with FASD and control children (See Table 4). Cognitive deficits often are secondary in severity to the behavioral deficits seen in this population. Behavioral issues studied empirically with prenatally-exposed populations have shown more significant deficits and greater numbers of deficits specifically in areas of attention, impulse control, attention deficit, hyperactivity, mood disorders and self-regulation (Coles et al., 2009, 1997; Kodituwakku et al., 1995; O’Connor and Paley, 2009; Pei et al., 2011). Behavioral differences between the children with FASD and controls in this study showed similar behavioral patterns. Highly significant differences were seen in the following behavioral areas on both the CBCL and TRF: internalizing problems; attention problems and attention deficit/hyperactivity problems; conduct problems (See Table 4). Skills of daily living or adaptive functioning are skills necessary for one to function independently in the daily activities of life: daily living, social abilities and motor development. Although adaptive skills have been shown to be impaired in children exposed to alcohol (Fagerlund et al., 2012; Kalberg et al., 2006; Streissguth et al., 1991; Thomas et al., 1998), recently Doyle et al (2019) have shown that prenatally-exposed children with higher intellectual functioning had a decreased level of adaptive abilities, and that the lag in adaptive skills was heavily impacted by communication abilities. At this site two areas of significance in adaptive functioning emerged between the children with FASD and the control children: communication skills reported by the parents and socialization skills reported by the teacher.
Maternal Risk Assessment
Mothers of children with FASD, especially the mothers of children with ARND, report more frequent and heavier use of alcohol before pregnancy than do the mothers of controls. Mothers of children with FASD also report some drinking in the first, second, and third trimesters, although the frequency and amounts reported seem to be low. Drinking prior to pregnancy proved to be the most forthright reports of quantity of drinking per episode. The regression model indicated a 10-fold greater likelihood of a FASD diagnosis when a mother reported usual DDD of three pre-pregnancy over that of those who reported no drinking. Furthermore, the odds of a FASD diagnosis increased to 26 times with reports of five or more DDD. Large confidence intervals in this analysis are concerning, but likely due to few cases in some drinking categories. Therefore, reported odds ratios should be interpreted as approximate. First trimester drinking may be of prime importance for causing long-lasting physical and neurobehavioral deficits in children. Mothers of children with FASD report more problems with alcohol use and recovery and co-morbidity with other drug use, especially tobacco and marijuana. At this site the difference in co-morbid alcohol and marijuana use reported between mothers of FASD children and controls was not statistically significant, but substantial (6.5 vs. 1.4%). Given recent pre-clinical study findings (Fish et al., 2019), and the likelihood of underreporting, this is a concerning finding. Maternal alcohol use correlates significantly, but not strongly, with physical anomalies, poorer growth, and some neurobehavioral outcomes in this study and other studies (May et al., 2016b, 2016a, 2013b).
Multiple proximal and distal variables also influence these outcomes both prenatally and throughout the early years of life. On average, mothers of children with FASD recognized their pregnancy later than control mothers, visited a healthcare provider later in pregnancy, and the number of prenatal visits was fewer for the FASD group. The combination of significant pre-pregnancy drinking and late pregnancy recognition and/or confirmation, are of major significance in many of the FASD cases, especially PFAS and ARND. Therefore, in this Midwestern site, reports of significant drinking three months prior to pregnancy, late recognition of pregnancy, and later seeking of prenatal healthcare produce a high-risk profile. If reliable alcohol use biomarkers are not feasible or available, pre-pregnancy drinking levels have substantial predictive value.
Strengths and Limitations
The combined-sample consent rate was higher at this site (62%) than any other CoFASP site. Consent was 70.5%, the oversample of growth deficient children, and lower (54%) for the simple random sample. Key school administrators and a majority of teachers and school personnel were enthusiastic and invested in facilitating this study, especially in Sample 1 where the small children were oversampled. With the simple random sample, Sample 2, the representativeness of the more dysmorphic forms of FASD (FAS and PFAS) is likely an undercount, since there were no children diagnosed with FAS and only two with PFAS. Alternatively, Sample 1, which oversampled small children, may have produced an underestimate of ARND, since the rate of ARND was one-third that of Sample 2. A second limitation of this study is an underreporting of prenatal drinking. The research staff successfully interviewed 89.8% of mothers of children who completed all three tiers of the study. However, none of the five mothers of children with FAS directly reported drinking during any trimester, and prenatal drinking was confirmed in only three of these individuals (due to “no shows”) by co-lateral sources during clinical pediatric follow-up. Similarly, only 53% of mothers of children with PFAS directly reported drinking in the first trimester. Finally, stricter cut-off criteria for evidence of alcohol use employed by the CoFASP group reduced the number of ARND cases diagnosed in Sample 1 from that reported in a previous publication from this sample (May et al., 2014). Therefore, it is unknown how underreporting of alcohol has affected the maternal risk sample values and cases diagnosed, and its impact is greatest on ARND rates. Third, since the rate of ARND from Sample 1 was likely underestimated even with employing weighted correction techniques, one would suspect the combined sample rate of ARND is an underestimate. The true rate of FASD may also be somewhat higher than reported here. Identifying ARND cases via random samples of children from a general population may be the most accurate approach for determining the prevalence of ARND if accurate alcohol exposure information can be obtained. Oversampling of small children, on the other hand, is best for determining the accurate prevalence of FAS and PFAS.
CONCLUSION
Growth, dysmorphology, and neurobehavioral traits of children with FASD were described in detail in this Midwestern City. Children who qualify for a diagnosis on the continuum of FASD have a variety of phenotypes both within and across diagnostic categories and neurobehavioral performance levels. Behavioral problems were more likely to qualify children for a diagnosis on the spectrum of FASD than cognitive ability. Many of the behaviors and skills of children with FASD provide additional challenges and burdens for schools, teachers and parents. Significant maternal risk factors reported were substantial drinking prior to pregnancy recognition, co-morbid use of other drugs prior to and during pregnancy, late recognition of pregnancy and prenatal care, and usual pre-pregnancy drinking levels in the form of binge drinking three or more DDD. The prevalence of total FASD in this community is 4.1%. While significantly higher than previously accepted estimates of 1%, this rate was the lowest of any site of the four CoFASP communities. Only the Southeastern Site had a similarly low rate. There were no significant differences in FASD prevalence at this site by race, ethnicity or socioeconomic status, which is an indication that FASD risk is distributed across various strata of this community.
Supplementary Material
What’s Known on this Subject:
There are few population based studies in the United States of the characteristics of children with specific diagnoses on the FASD continuum and their mothers. Most studies of FASD prevalence and maternal and child characteristics have been undertaken using passive methods of case ascertainment or are selective in nature, thereby underestimating rates of FASD. Furthermore, most clinical and epidemiological studies of FASD do not provide a detailed overview of physical and neurodevelopmental traits of children fully diagnosed FASD and associated maternal risk factors in comparison with typically-developing children and their mothers in the same population.
What this Study Adds:
Using active case ascertainment methods among children in a representative, middle class county in a Midwestern City in the United States, traits of children with FAS and total FASD and their mothers are described and compared to characteristics of normally-developing children and their mothers in the same community. The results of two such studies in two cohorts of first grade students from the same city are presented here. The traits provide clear differentiation of diagnostic groups within FASD, and the prevalence of the complete continuum using strict CoFASP criteria and cut-off points was found to be substantially higher than most older estimates for the general U.S. population.
ACKNOWLEDGEMENTS
This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grants RO1 AA15134-04S1, UO1 AA019894. Marcia Scott, Ph.D., Kenneth Warren, Ph.D., Faye Calhoun, D.P.A., and the late T-K Li, M.D. of NIAAA provided intellectual guidance, encouragement, and support for prevalence studies of FASD for many years. We extend our deepest gratitude and thank you to Dr. Pamela Homan, Superintendent of the public schools, Dr. Tom Lorang, Superintendent of the Catholic schools, the Boards of Education, administrators, principals, teachers, and psychologists of the public and private school systems in the study community who have hosted and assisted the investigators in the research process over the years. Their professional support, guidance, and facilitation have been vital to the success of this study. We are especially grateful to Marie Rickert and Patti Pannell who were the study champions in the public school system and facilitated the study in many ways throughout its duration. We are also grateful for the advice and participation in the initiation, planning and completion of the project by the CoFASP Advisory Committee members who were led by Marcia Scott, Ph.D., NIAAA Project Officer, and Judith Arroyo, Ph.D., Michael Charness, M.D., William Dunty, Ph.D., Daniel Falk, Ph.D., Dale Herald, M.D., Ph.D., and Edward Riley, Ph.D. We dedicate this paper to our deceased colleague, Jason Blankenship, Ph.D., who was excited about this project and invested much effort into initiating it before his premature death in October 2013.
Funding Source: This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894 as part of the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP) consortium. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosure: The authors have no financial relationship relevant to this article to disclose.
Abbreviations:
- ARBD
alcohol-related birth defects
- ARND
alcohol-related neurodevelopmental disorder
- BMI
Body Mass Index
- CDC
Centers for Disease Control and Prevention
- DDD
drinks per drinking day
- FASD
fetal alcohol spectrum disorders
- FAS
fetal alcohol syndrome
- ICD
inner canthal distance
- IPD
inter pupillary distance
- IOM
Institute of Medicine
- OFC
occipitofrontal (head) circumference
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PFAS
partial fetal alcohol syndrome
- PFL
palpebral fissure length
- SES
socioeconomic status
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Achenbach T, Rescorla L (2001) Manual For The ASEBA School-Age Forms And Profiles. Burlington, VT, University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA (2001) Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcohol Clin Exp Res 25:557–562. [PubMed] [Google Scholar]
- Alvik A, Haldorsen T, Lindemann R (2006) Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Paediatr 95:686–693. [DOI] [PubMed] [Google Scholar]
- America’s Changing Religious Landscape | Pew Research Center (n.d.) Available at: https://www.pewforum.org/2015/05/12/americas-changing-religious-landscape/ Accessed November 15, 2019.
- America’s Health Rankings Annual Report (2015). Minnetonka, Minnesota. [Google Scholar]
- Aragon AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, Mitchell ER, May PA (2008) Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 32:1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC (2017) Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol Clin Exp Res 41:1004–1011. [DOI] [PubMed] [Google Scholar]
- Beery KE, Beery NA (2004) The Beery-Buktenica Development Test of Visual-Motor Integration, Fifth Edit ed. San Antonio, TX, Pearson Assessment. [Google Scholar]
- Bracken BA (1998) Braken Basic Concept Scale - Revised. San Antonio, Texas. [Google Scholar]
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS (2017) Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA 317:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Cox C, Poitra B, Wentz T, Ebertowski M, Martsolf JT, Kerbeshian J, Klug MG (1999) The FAS Screen: a rapid screening tool for fetal alcohol syndrome. Addict Biol 4:329–336. [DOI] [PubMed] [Google Scholar]
- CDC - BRFSS (n.d.) Available at: https://www.cdc.gov/brfss/ Accessed November 15, 2019.
- Ceccanti M, Fiorentino D, Coriale G, Kalberg WO, Buckley D, Hoyme HE, Gossage JP, Robinson LK, Manning M, Romeo M, Hasken JM, Tabachnick B, Blankenship J, May PA (2014) Maternal risk factors for fetal alcohol spectrum disorders in a province in Italy. Drug Alcohol Depend 145:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L (2015) Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics 135:264–70. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM (2001) Screening for fetal alcohol syndrome in primary schools: A feasibility study. Teratology 63:3–10. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E (2009) Math Performance and Behavior Problems in Children Affected by Prenatal Alcohol Exposure: Intervention and Follow-Up. J Dev Behav Pediatr 30:7–15. [DOI] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC (2010) Verbal and Nonverbal Memory in Adults Prenatally Exposed to Alcohol. Alcohol Clin Exp Res 34:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE (1997) A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 21:150–61. [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D (1990) Five-year reliability of self-reported alcohol consumption. J Stud Alcohol 51:68–76. [DOI] [PubMed] [Google Scholar]
- Doyle LR, Coles CD, Kable JA, May PA, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD (2019) Relation between adaptive function and IQ among youth with histories of heavy prenatal alcohol exposure. Birth Defects Res 111:812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (2007) Differential Ability Scales-II (DAS-II). San Antonio, Texas, Harcourt Assessment. [Google Scholar]
- Ervalahti N, Korkman M, Fagerlund Å, Autti-Rämö I, Loimu L, Hoyme HE (2007) Relationship between dysmorphic features and general cognitive function in children with fetal alcohol spectrum disorders. Am J Med Genet Part A 143A:2916–2923. [DOI] [PubMed] [Google Scholar]
- Fagerlund Å, Åse F, Autti-Rämö I, Ilona A-R, Kalland M, Mirjam K, Santtila P, Pekka S, Hoyme HE, Eugene HH, Mattson SN, Sarah MN, Korkman M, Marit K (2012) Adaptive behaviour in children and adolescents with foetal alcohol spectrum disorders: a comparison with specific learning disability and typical development. Eur Child Adolesc Psychiatry 21:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE (2019) Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction. Sci Rep 9:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Muckle G, Jacobson SW, Jacobson JL, Belanger RE (2017) Alcohol use among Inuit pregnant women: Validity of alcohol ascertainment measures over time. Neurotoxicol Teratol 64:73–78. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013. JAMA Psychiatry 74:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V (2010) A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 44:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Alpert JJ, Day N, Dooling E, Kayne H, Morelock S, Oppenheimer E, Zuckerman B (1982) Effects of maternal drinking and marijuana use on fetal growth and development. Pediatrics 70:539–46. [PubMed] [Google Scholar]
- Hoyme H, May P, Kalberg W, Kodituwakku P, Gossage J, Trujillo P, Buckley D, Miller J, Aragon A, Khaole N, Viljoen D, Jones K, Robinson L (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM (2017) IBM SPSS Statistics for Windows. [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet (London, England) 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, May PA, Blankenship J, Buckley D, Gossage JP, Adnams CM (2013) A Practical Testing Battery to Measure Neurobehavioral Ability among Children with FASD. Int J Alcohol Drug Res 2:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Eugene Hoyme H, Trujillo PM, Buckley D, Aragon AS, May PA (2006) Comparison of Motor Delays in Young Children With Fetal Alcohol Syndrome to Those With Prenatal Alcohol Exposure and With No Prenatal Alcohol Exposure. Alcohol Clin Exp Res 30:2037–2045. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2001) Pre-pregnancy drinking: how drink size affects risk assessment. Addiction 96:1199–1209. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2000) An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse 12:67–78. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Kerr WC (2008) Accuracy of Photographs to Capture Respondent-Defined Drink Size. J Stud Alcohol Drugs 69:605–610. [DOI] [PubMed] [Google Scholar]
- King AC (1994) Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health 84:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragon AS, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, May PA (2006) Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: Preliminary results. Alcohol Clin Exp Res 30:1551–1561. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD (1995) Specific Impairments in Self-Regulation in Children Exposed to Alcohol Prenatally. Alcohol Clin Exp Res 19:1558–1564. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007) NEPSY (NEPSY-II), Second. ed San Antonio, Texas, Pearson Assessment. [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee RA, Yi H (2011) Surveillance Report #92: Apparent per alcohol consumption: national, state, and regional trends, 1977–2009. [Google Scholar]
- Lipinski RJ, Hammond P, O’Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, Budin F, Parnell SE, Suttie M, Godin EA, Everson JL, Dehart DB, Oguz I, Holloway HT, Styner MA, Johnson GA, Sulik KK (2012) Ethanol-Induced Face-Brain Dysmorphology Patterns Are Correlative and Exposure-Stage Dependent. PLoS One 7:e43067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle LR (2019) Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated With Prenatal Alcohol Exposure. Alcohol Clin Exp Res 43:acer.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP (1999) Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 23:1808–15. [PubMed] [Google Scholar]
- Mattson SN, Riley EP (2011) The Quest for a Neurobehavioral Profile of Heavy Prenatal Alcohol Exposure. Alcohol Res Heal 34:51. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP (2010) Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 34:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, De Vries M, Marais A-S, Kalberg W, Buckley D, Adnams C, Hasken J, Tabachnick B, Robinson L, Manning M, Bezuidenhout H, Adam M, Jones K, Seedat S, Parry C, Hoyme H (2017) Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa. Int J Environ Res Public Health 14:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and Characteristics of Fetal Alcohol Spectrum Disorders. Pediatrics 134:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S (2013a) Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend 133:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D (2000) Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health 90:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Daniel F, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018a) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA - J Am Med Assoc 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, Jones KL, Hoyme D, Seedat S, Parry CDH, Hoyme HE (2016a) The continuum of fetal alcohol spectrum disorders in four rural communities in south africa: Prevalence and characteristics. Drug Alcohol Depend 159:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Eugene Hoyme H, Aragón AS, Buckley D, Stellavato C, Phillip Gossage J, Robinson LK, Jones KL, Manning M, Ceccanti M (2011a) Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: New estimated rates are higher than previous estimates. Int J Environ Res Public Health 8:2331–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Phillip Gossage J, Kalberg WO, Eugene Hoyme H, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M (2006) Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res 30:1562–1575. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP (2001) New data on the epidemiology of adult drinking and substance use among American Indians of the northern states: Male and female data on prevalence, patterns, and consequences. Am Indian Alaska Nativ Ment Heal Res 10:1–26. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL (2005) Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health 95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15:176–192. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL (2007) The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend 88:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL (2008) Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res 32:738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Smith M, Tabachnick BG, Robinson LK, Manning M, Cecanti M, Jones KL, Khaole N, Buckley D, Kalberg WO, Trujillo PM, Hoyme HE (2010) Population differences in dysmorphic features among children with fetal alcohol spectrum disorders. J Dev Behav Pediatr 31:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, De Vries MM, Marais AS, Stegall JM, Marsden D, Parry CDH, Seedat S, Tabachnick B (2018b) A utilitarian comparison of two alcohol use biomarkers with self-reported drinking history collected in antenatal clinics. Reprod Toxicol 77:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Gossage JP, Robinson LK, Manning M, Hoyme HE (2015) Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend 155:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, Adnams CM, Barnard R, Joubert B, Cloete M, Tabachnick B, Robinson LK, Manning MA, Jones KL, Bezuidenhout H, Seedat S, Parry CDH, Hoyme HE (2016b) The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend 168:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning M, Buckley D, Hoyme HE (2011b) Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend 119:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning MA, Blankenship J, Buckley D, Hoyme HE, Adnams CM (2013b) Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr 34:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Excel (2016).
- O’Connor MJ, Paley B (2009) Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev 15:225–234. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Holloway HT, O’Leary-Moore SK, Dehart DB, Paniaqua B, Oguz I, Budin F, Styner MA, Johnson GA, Sulik KK (2013) Magnetic resonance microscopy-based analyses of the neuroanatomical effects of gestational day 9 ethanol exposure in mice. Neurotoxicol Teratol 39:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C (2011) Mental health issues in fetal alcohol spectrum disorder. J Ment Heal 20:473–483. [DOI] [PubMed] [Google Scholar]
- Petkovic G, Barisic I (2013) Prevalence of fetal alcohol syndrome and maternal characteristics in a sample of schoolchildren from a rural province of Croatia. Int J Environ Res Public Health 10:1547–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic G, Barisic I (2010) FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod Toxicol 29:237–241. [DOI] [PubMed] [Google Scholar]
- Poitra BA, Marion S, Dionne M, Wilkie E, Dauphinais P, Wilkie-Pepion M, Martsolf JT, Klug MG, Burd L (2003) A school-based screening program for fetal alcohol syndrome. Neurotoxicol Teratol 25:725–729. [DOI] [PubMed] [Google Scholar]
- Rasmussen C (2005) Executive Functioning and Working Memory in Fetal Alcohol Spectrum Disorder. Alcohol Clin Exp Res 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Roozen S, Black D, Peters G-JY, Kok G, Townend D, Nijhuis JG, Koek GH, Curfs LMG (2016a) Fetal Alcohol Spectrum Disorders (FASD): an Approach to Effective Prevention. Curr Dev Disord Reports 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Koek G, Curfs L (2018) Systematic literature review on which maternal alcohol behaviours are related to fetal alcohol spectrum disorders (FASD). BMJ Open 8:e022578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L (2016b) Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res 40:18–32. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M (2001) Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse 36:313–331. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI (1988) The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol 49:225–232. [DOI] [PubMed] [Google Scholar]
- Sparrow SA, Cicchetti D V, Balla DA (2005) Vineland Adaptive Behavior Scales, Second. ed Bloomington, Minnesota. [Google Scholar]
- Stratton K, Howe C, Battaglia F (1996) Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington DC. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF (1991) Fetal alcohol syndrome in adolescents and adults. JAMA 265:1961–7. [PubMed] [Google Scholar]
- Sulik K, Johnston M, Webb M (1981) Fetal alcohol syndrome: embryogenesis in a mouse model. Science (80- ) 214:936–938. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP (1998) Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res 22:528–33. [PubMed] [Google Scholar]
- U.S. Census Bureau QuickFacts: United States (n.d.) Available at: https://www.census.gov/quickfacts/fact/table/US/PST045218 Accessed November 15, 2019.
- Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA (2002) Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol 63:6–17. [PubMed] [Google Scholar]
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole NCO, Kodituwakku P, Asante KO, Findlay R, Quinton B, Marais A-S, Kalberg WO, May PA (2005) Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol 66:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, Blanco C (2017) Marijuana Use During Stages of Pregnancy in the United States. Ann Intern Med 166:763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN (2012) Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 36:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- What Is A Standard Drink? | National Institute on Alcohol Abuse and Alcoholism (NIAAA) (n.d.) Available at: https://www.niaaa.nih.gov/what-standard-drink Accessed November 18, 2019.
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundstr??m Poromaa I (2008) Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT-a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol 198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.