Abstract
Proper neurite formation is essential for appropriate neuronal morphology to develop and defects at this early foundational stage have serious implications for overall neuronal function. Neuritogenesis is tightly regulated by various signaling mechanisms that control the timing and placement of neurite initiation, as well as the various processes necessary for neurite elongation to occur. Kinases are integral components of these regulatory pathways that control the activation and inactivation of their targets. This review provides a comprehensive summary of the kinases that are notably involved in regulating neurite formation, which is a complex process that involves cytoskeletal rearrangements, addition of plasma membrane to increase neuronal surface area, coupling of cytoskeleton/plasma membrane, metabolic regulation, and regulation of neuronal differentiation. Since kinases are key regulators of these functions during neuromorphogenesis, they have high potential for use as therapeutic targets for axon regeneration after injury or disease where neurite formation is disrupted.
Keywords: Axonal outgrowth, Neurodevelopmental disorder, Protein modification, Autism spectrum disorder, Neurite outgrowth
Introduction
Importance of studying neurite formation
Neurite formation is a fundamental, yet poorly understood, step in neurodevelopment that can be categorized into two sequential cellular events: neurite initiation followed by neurite elongation. The formation of neurites marks the initial break in the morphological symmetry of the cell, driving neuronal polarization. At early stages preceding neuritogenesis, neurons appear round with a uniform smooth surface. Actin-rich filopodia and lamellipodia emerge and are stabilized by microtubules to become neurites. As cellular polarity develops and cells take on a mature neuronal morphology, neurites differentiate into an axon and multiple dendrites (Fig. 1) [1, 2]. The maturation of neurites has important functions in establishing neuronal morphogenesis. The early pivotal stage of neurite formation affects later stages of morphogenesis such as arborization and synapse formation, which can impact the establishment of appropriate connectivity in the brain [3, 4]. The highly polarized morphology of a mature neuron requires all stages of neuronal morphogenesis to occur properly. Neuritogenesis, although often overlooked, is perhaps the most crucial stage of neuronal morphogenesis because it lays the foundation upon which other stages rely.
Fig. 1.
Schematic of neuritogenesis. Immature neurons appear round with a uniform smooth surface before neuritogenesis occurs. Internal cellular asymmetry is generated by signaling mechanisms that determine the locations of neurite initiation. Actin-rich filopodia and lamellipodia emerge and are stabilized by microtubules to become primitive neurites, breaking the morphological symmetry of the cell and driving neuronal polarization. During neurite elongation, processes such as plasma membrane addition, intracellular transport, and protein synthesis are crucial, as the neuron extends its neurites and adds surface area. As mature neuronal morphology develops, neurites differentiate into an axon and multiple dendrites. MT microtubule. Figure created with BioRender.com
The purpose of this review is to highlight the importance of the relatively uninvestigated process of neurite formation and to summarize what is known concerning the regulation of neuritogenesis via protein kinases (Table 1). Kinases play integral roles in neurite formation regulatory pathways by phosphorylating various substrates, allowing for control over the activation and deactivation of components of the signaling pathway. Since phosphorylation status of key regulators controls many cellular processes including cell cycle regulation, proliferation, metabolism, and apoptosis, a focus on the role of kinases in neurite formation allows for a comprehensive review of various regulatory mechanisms (Table 2) [5–8]. Additionally, we consider the potential use for protein kinases as currently untapped therapeutic targets to promote axon regeneration.
Table 1.
Summary table of all kinases reviewed
| Name | Type | Family | Regulation of neurite formation | Pathways and downstream targets | Citations |
|---|---|---|---|---|---|
| Akt | Serine/threonine kinase | Akt | Elongation | PI3K–Akt–GSK3β | [68, 76, 94, 115] |
| AMPK | Serine/threonine kinase | AMPK | Initiation and elongation | Akt or mTOR | [70–79] |
| Aurora kinase A | Serine/threonine kinase | Aurora kinase | Elongation | NDEL1 | [97, 98] |
| CaMK II | Serine/threonine kinase | Calmodulin-dependent protein kinase | Initiation and elongation | cAMP levels | [46, 71, 72, 116–119] |
| Cdk5 | Serine/threonine kinase | Cdk | Elongation | GPCR signaling, Cdc42, MAPK/ERK | [100, 101] |
| FAK | Non-receptor tyrosine kinase | FAK | Initiation and elongation | Arp2/3 complex | [48–54] |
| JNK | Serine/threonine kinase | MAPK | Initiation and elongation | Rac–cdc42–JNK | [22, 60, 61, 66–70, 120] |
| MAPK/ERK | Serine/threonine kinase | MAP | Initiation and elongation | Ras–Raf–MEK–ERK | [22, 60–65, 101, 111, 115, 121, 122] |
| mTOR | Serine/threonine kinase | Phosphatidyl-inositol 3-kinase-related kinase | Elongation | PI3K–Akt–mTOR | [78, 79, 94, 114] |
| PI3K | Lipid kinase | PI3K | Initiation and elongation | RTK or GPCR or Trk–PI3K–Akt | [34, 40, 68, 76, 82–94, 115] |
| PKA | Serine/threonine kinase | PKA | Initiation and elongation | cAMP–PKA–CREB | [55–57, 111, 112] |
| PKC | Serine/threonine kinase | PKC | Initiation and elongation | PLC–PKC–PKD | [56, 58, 59] |
| Rho kinase | Serine/threonine kinase | Rho | Elongation | ROCK/ROK | [89, 93, 102–104, 113, 123–125] |
Table 2.
Summary table designating which of the reviewed kinases regulate each of the key processes involved in neurite formation
| Key process during neurite formation | ||||
|---|---|---|---|---|
| Generation of initial internal cellular asymmetry | Cytoskeletal dynamics and cytoskeletal–membrane coupling | Differentiation and survival | Unknown significance | |
| Regulated by | PI3K | FAK | AMPK | PKA |
| MAPK/ERK | MAPK/ERK | PKC | ||
| CaMK II | JNK | |||
| PI3K | ||||
| Akt | ||||
| mTOR | ||||
| Aurora kinase A | ||||
| Cdk5 | ||||
| Rho | ||||
Clinical implications of neurite formation
Neurite formation is a critical early step in neuronal development upon which later stages rely. The fidelity of this process is crucial in ensuring proper synaptogenesis, axon guidance, and neuronal function. Improper neuritogenesis underlies a variety of neurodevelopmental disorders such as schizophrenia [9], Down syndrome [10], and Autism spectrum disorder (ASD) [11–13]. Certain genes that are causatively mutated in ASD also have roles in neuritogenesis. Alterations to neuritogenesis effecting neurite length, number, and other structural abnormalities in developing neurons are closely linked to the manifestation of certain behavioral symptoms present in ASD [11]. For example, mutations in the purinergic receptor KIAA2022 lead to impaired neurite outgrowth resulting in stunted growth of both axons and dendrites in cultured rat hippocampal neurons, and detailed analysis of patients with the same mutations showed manifestation of intellectual disability accompanying ASD [11, 14]. Another molecular mechanism underlying neuritogenesis defects and ASD pathology is altered SLC25A12 expression [12]. Increased expression of SLC25A12 has been found in the prefrontal cortex of post-mortem brains of patients diagnosed with ASD, and overexpression of SLC25A12 in mouse embryonic cortical neurons revealed increased apical dendritic growth, altered mitochondrial trafficking, and impaired dendritic spine morphology [12]. Unsurprisingly, protein kinases are also responsible for driving many of these defective pathways. For example, cyclin-dependent kinase-like 5 (CDKL5) is a serine/threonine kinase that when mutated is associated with X-linked neurodevelopmental disorders, ASD, and Rett syndrome [15]. One of CDKL5’s main functions is positively regulating neurite outgrowth and dendritic arborization. CDKL5 knockout mice have several severe defects including failure of dendritic maturation due to disrupted protein kinase B (Akt) and glycogen synthase kinase 3 beta (GSK3β) signaling which is important for neuritogenesis as discussed in this review [15, 16]. CDKL5 knockout mice also have several behavioral defects akin to the human disorder such as hypoactivity, limb clasping, and abnormal eye tracking [17]. Furthermore, examples of neurite formation defects in animal models of schizophrenia show decreased neurite extension [9]. Laminin, which is known for its favorable effects in neurite outgrowth, becomes toxic when it forms deposits. These deposits and subsequent toxic neurite sprouting are characteristic of both Down syndrome and Alzheimer’s disease, eventually resulting in neurodegeneration [10]. Abnormal neurite formation underlying diseases includes both increased and decreased neurite growth, emphasizing the necessity of precisely regulated control over the mechanisms responsible. Continued investigation of the mechanisms of neuritogenesis will benefit these patients by offering new avenues for therapeutic innovation to either restore neurite formation or trim back aberrant neurite formation.
Spinal cord injury and neurodegenerative disorders that result in axonal injury or degeneration of neurites are also conditions that will benefit from the study of the mechanisms that drive neurite formation. For example, spinal cord injury often results in axonal damage, and one of the biggest barriers to treatment of spinal cord injury is failure of axon regeneration. If it is possible to identify a master regulator responsible for driving neuritogenesis during development, that regulator could be a useful therapeutic target to activate and drive axon regeneration following injury or degeneration. As of 2019, forty-eight pharmaceuticals that target protein kinases are already FDA approved for treatment of a variety of disorders, particularly for cancer treatment [18]. Protein kinases represent versatile, easily manipulated targets that allow for control over multiple pathways simultaneously creating enormous therapeutic benefit, but also extreme complexity. Many protein kinases that are targets of these approved therapeutics contribute to mechanisms that drive neurite formation during development, such as mammalian target of rapamycin (mTOR), cyclin-dependent kinases (CDK), and protein kinase A (PKA) all of which will be highlighted in this review [18]. Repurposing these therapeutics in a targeted and specific manner to drive axon regeneration following spinal cord injury is currently a popular research topic, the potential benefits of which cannot be understated [18]. However, the identification of therapeutic targets is difficult because the mechanisms underlying neurite formation remain relatively unstudied. A detailed elucidation of these mechanisms is crucial to gain a better understanding of neurodevelopmental diseases and spinal cord injury, as well as to identify potential targets for therapeutic innovation. This review concludes by highlighting certain kinase-driven pathways important during neuritogenesis during development that are being manipulated to drive axon regeneration following injury or disease.
Model systems for neuritogenesis
A variety of tools and model systems exist to study neurite formation, and each has its strengths and weaknesses. This section is intended to serve as a tool for the reader to better understand, compare, and interpret the results of the model systems discussed in the literature reviewed in this article.
Adrenal pheochromocytoma (PC12) cell line
PC12 cells have been widely used as a model system for neurobiological studies since the original isolation of the cell line in 1976 from the adrenal medulla of a rat [19]. This cell line responds reversibly to nerve growth factor (NGF), which arrests cell division and causes extension of processes similar to those produced by sympathetic neurons in primary cell culture [19]. NGF-induced generation of neurite-like outgrowths is a slow RNA synthesis-dependent process that begins after a lag of at least 24 h after treatment and typically takes 2–7 days [20, 21]. In contrast, regeneration of neurites, defined as NGF-dependent regrowth of neurites by re-plating within 24 h after subculture of NGF-treated PC12 cells, is RNA synthesis-independent since this has been shown to occur in the presence of high concentrations of RNA synthesis inhibitors [21]. PC12 cells that are subjected to exposure to NGF for a week, which is also referred to as ‘NGF-priming’, acquire the ability to rapidly regenerate neurites [20]. This suggests that NGF stimulates neurite outgrowth via both RNA synthesis-dependent and -independent pathways. The effects of NGF on PC12 cells can be reversed upon return to NGF-free medium, where about 75% of cells will lose their processes within 24 h and cell division will resume within 3 days [19].
While the signaling mechanism of neuronal differentiation in PC12 cells is not completely understood, it is well established that NGF activates at least three mitogen-activated protein (MAP) kinase pathways which each converge on either MAPK/ERK, JNK, or p38 [22]. While PC12 cell response to NGF has been the most extensively studied, PC12 cells also respond to other neurotrophic and growth factors. Many of the same mechanisms that drive PC12 neuronal differentiation play a role in neurite formation in primary neurons.
Advantages of using PC12 cells to investigate neurite formation include their versatility for pharmacological manipulations and ease of culture. Additionally, the proliferation and differentiation of this cell line have been well characterized, allowing for a high degree of control in experiments. Critics of the use of non-neuronal PC12 cells to study neuritogenesis have concerns about the representativeness of this model of true neurite formation. While PC12 cells are not neurons they have many neuron-like characteristics including the synthesis, storage, and release of neurotransmitters, expression of many neuron-specific proteins, neuron-like secretion from varicosities, and a differentiated phenotype that resembles a neuronal phenotype [19, 23–26]. It will be difficult to ultimately silence concerns regarding use of non-neuronal PC12 cells to study a process considered to be unique to neurons. However, there is convincing evidence that this cell line is an appropriate and representative model of neurite formation.
Neuroblastoma Neuro2a (N2a) cell line
N2a cells are similar to PC12 cells in that they are non-neuronal cells capable of differentiating into a neuron-like phenotype, including the extension of neurite-like processes. Unlike PC12 cells, N2a cells are derived from mouse neuroblastoma. Differentiation of N2a cells may be induced via depletion of serum in cell culture medium or treatment with retinoic acid [27, 28]. Differentiated N2a cells display a neuron-like phenotype which includes a decrease in proliferation rate, extension of long neurite-like processes, and expression of neuron-specific proteins [27, 29]. The method by which neuronal differentiation is induced impacts the timeline of neurite formation, which is advantageous for experimental manipulations. For example, it has been reported that neurite initiation begins only minutes after removal of fetal bovine serum (FBS) from culture media and that multiple long dendritic-like processes can be seen after 2 h when differentiation is induced in this manner [30]. This is ideal for experiments that can be done quickly, such as live imaging experiments designed to study neurite initiation and elongation. However, this approach would not be suitable for experiments requiring multiple days in culture, since cells will begin to die due to lack of FBS. An option for these types of experiments would be to induce neuronal differentiation of N2a cells via transfer of the cells from culture media supplemented with 10% FBS to neurobasal medium supplemented with a defined serum-free supplement (B27), which will cause differentiation to occur within 24–72 h [29]. Differentiation induced by retinoic acid treatment has a longer timeline. It has been reported that a 48-h incubation period with retinoic acid will cause neuronal differentiation and will allow the N2a cells to be cultured for 4 or 5 days [27, 31]. While the mechanism by which N2a cells achieve neuronal differentiation is incompletely understood, several important factors for neurite formation have been identified that seem to act via multiple kinase-dependent pathways [31].
N2a cells provide an advantageous model for studying neurite formation for many of the same reasons as PC12 cells: they are simple to maintain in culture and the rate of neurite formation can be easily controlled. During neurite formation most N2a cells remain in sync or at the same phase of neurite formation, allowing for high-throughput experiments to be performed. The degree of control the experimenter has over the timing of neurite formation allows cells to be collected or imaged at specific time points during neurite formation, easily allowing for the separation between neurite initiation and neurite elongation. Critics of N2a cells may take issue with the use of a non-neuronal cell to study neurite formation, despite the multiple advantages such models have over the alternatives.
Primary neurons
The major primary neuronal cell types used to study neuritogenesis in vitro discussed in this review are rodent embryonic hippocampal and cortical pyramidal neurons. These primary neuronal models are more representative of neurite formation than PC12 and N2a cell lines, which need to be induced to differentiate and do not use neuronal culture. While neurons will have begun extending neurites in vivo, they will retract these processes and begin neurite formation again upon being plated in vitro. Depending on the neuronal cell type used it is possible that neurite formation will occur in varying stages and timescales, and likely involve different underlying mechanisms because the final neuronal morphology differs by cell type. This could lead to cell-type specific involvement of different mechanisms, and divergence of the effects of different molecules. Hippocampal neurons will extend lamellipodium shortly after plating and attach to the substrate, which is commonly polylysine and laminin, and formation of multiple neurites will occur between 12 and 24 h [32]. Cortical neurons follow a similar timeframe.
Practical considerations for primary neuronal models of neurite formation are the need to isolate cells for each experiment, as opposed to storing an immortalized cell line, and difficulty of transfection. Because genetic manipulation is a useful tool for manipulating the activity of kinases, the ability to transfect a particular cell type is an important factor in evaluating model systems. Another consideration when working with transfected primary neurons is that re-plating neurons after the transfection has taken effect may be necessary to study neurite initiation, specifically. If neurons are transfected upon plating, there will be a delay between neurite initiation and when the genetic manipulation takes effect. This is satisfactory if the focus of the experiment is neurite elongation. However, it may be best to re-plate the neurons allowing neurite initiation to begin after the genetic manipulation has taken full effect. This prompts another question: does neuritogenesis occur via the same mechanism(s) each time a neuron polarizes? This is a concern for all primary neuronal cultures since upon plating the neurons will begin to polarize for the second time after being isolated in an already partially polarized state in the brain.
Neurite formation
Neurite initiation and elongation
Neurite formation is regulated by myriad pathways that converge on common targets, allowing a developing neuron to extend and stabilize new processes adding to its surface area. Cytoskeletal rearrangement and coordination between actin and microtubules, addition to the plasma membrane, cell adhesion, protein synthesis, and coordination between the cytoskeleton and plasma membrane are all crucial functions during neuritogenesis [33–35]. Neurite initiation marks the earliest stage of neuronal morphogenesis. Neurite initiation is primarily responsible for the break in the morphological symmetry of the initially spherical neuron and stabilizing immature neurite precursors, while neurite elongation is more concerned with the addition of membrane and further extension of these stabilized processes.
Before neurite initiation can begin, an immature neuron must first generate internal asymmetry to designate protrusion sites for future neurites to extend from [36]. Generation of internal asymmetry relies on intracellular signaling and external cues, such as tension on the soma surface, to designate specific regions within the cell where neurites will extend from [37, 38]. This early stage of neuronal differentiation is poorly understood. It is known that redistribution of cortical actin into large aggregates precedes neuritogenesis and determines the sites of neurite initiation [39]. The signaling mechanism initiating actin aggregation to promote neuritogenesis involves local clustering of phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2), which is necessary and sufficient for neurite initiation [40].
Neurite initiation begins with extension of actin-rich filopodia and lamellipodia that develop into mature neurites. It is unclear precisely how the actin cytoskeleton organizes to form these initial structures. Proposed mechanisms for neurite initiation include the de novo filament model and the convergent elongation model. The de novo filament model proposes that nucleation and elongation of actin fibers in a single direction, followed by crosslinking into filopodial actin bundles, is responsible for the formation of filopodia [41]. The convergent elongation model suggests that branched actin filament networks within lamellipodia are elongated by anti-capping proteins and bundled into filopodia [42]. The two models do not appear to be exclusive, thus both mechanisms may occur depending on the specific cell type and/or environmental factors. The actin-rich precursor structures are relatively dynamic and must be stabilized via microtubule invasion, which prevents the collapse of the structure and is followed by rapid condensation and extension as the processes mature into a neurite [13].
Elongation of neurites is crucial for establishment of circuitry in the developing brain. Neuronal growth cones on primitive stabilized neurites lead developing neurites to their respective targets. These growth cones exist at the distal ends of all neurites and are specialized, dynamic areas of movement composed of actin-rich lamellipodia and filopodia [43, 44]. Various stimuli can trigger outgrowth of neurites by stimulating calcium influx through channels along the surface of established neurites [45]. This calcium influx and signaling is a critical regulatory component of the cytoskeletal dynamics that must take place for neurite elongation to occur [46]. The dynamicity of elongating neurites is crucial for synaptic plasticity, synaptic pruning, and synapse formation, as well as correct pathfinding [47].
In the following sections of this review, we discuss protein kinases important in regulating neurite initiation and elongation. A kinase is involved specifically in neurite initiation if disruption or knockout causes a change in the number of neurites formed, while kinases that cause a change in neurite length upon disruption or knockout are involved specifically in neurite elongation. Phosphorylation status is crucial for regulating the activity of many molecules in key regulatory pathways, and, therefore, it is unsurprising that multiple kinases are involved in the mechanisms that drive both neurite initiation and neurite elongation.
Kinases
Focal adhesion kinase (FAK)
FAK is a non-receptor tyrosine kinase that is strongly activated by integrin-mediated signaling, which leads to FAK association with proteins such as paxillin and Src to result in focal adhesion assembly [48]. FAK is highly expressed in the nervous system, particularly in the cortex and hippocampus [49, 50]. Functional roles for FAK include directly conveying extracellular signals by shuttling between focal adhesions and the nucleus and mediating the interactions with the extracellular matrix [51, 52]. However, the most important function of FAK in terms of neurite initiation is linking extracellular cues to cytoskeletal reorganization and coordination with the plasma membrane, which is crucial as the surface area of the cell increases during neurite initiation. FAK is necessary for neuritogenesis in primary cortical neurons in the presence of laminin [53]. FAK couples exocytic and cytoskeletal machinery to drive neuritogenesis by promoting coordinated activity of the actin-related proteins (Arp2/3) complex and vesicle-associated membrane protein 7 (VAMP7)-mediated exocytosis [53]. Further evidence of FAK’s role in neurite initiation is provided by a study using the small molecule inhibitor Y15 to block FAK activity, which leads to a decrease in the total number of neurites in mouse hippocampal neurons [54]. FAK appears to promote neurite initiation mainly by coupling extracellular cues to membrane and cytoskeletal rearrangements.
Protein kinase A (PKA) and protein kinase C (PKC)
PKA is a cyclic AMP (cAMP)-dependent protein kinase highly expressed in nervous tissue which functions in neuronal differentiation and cytoskeletal protein phosphorylation [55]. In SH-SY-5Y human neuroblastoma cells, PKA activation induces neurite outgrowth while PKA inhibition prevents neurite outgrowth [56]. Treatment of cells with forskolin or dbcAMP leads to increased intracellular cAMP levels and increased PKA activity, which about tripled the percentage of cells with neurites [56]. The PKA inhibitor, N-(2-guanidinoethyl)-5-isoquinolinesulfonamide (HA 1004), prevented the increase in neurites in cells treated with forskolin or dbcAMP [56]. Neurite formation induced via PKA activation is dependent on microtubule polymerization [56]. A study using the AS583-8 neuronal progenitor cell line, derived from fetal rat brain, showed that cAMP-mediated neuritogenesis requires PKA activation [57]. PKA is an important regulator and promoter of neurite formation, and future investigations of PKA could help elucidate the role of cAMP in neuritogenesis. Studies of cAMP-mediated neuritogenesis would be beneficial due to the high versatility of cAMP as a second messenger impacting many signaling pathways.
PKC is a phospholipid-dependent protein kinase that is involved in many of the same processes as PKA, including neuritogenesis, in which the function of PKC opposes that of PKA [56]. In SH-SY-5Y human neuroblastoma cells, PKC activation restricts neurite outgrowth and leads to retraction of newly formed neurites [56]. Additionally, PKC inhibition via l-(5-isoquinolinesulfonyl)-2-methylpiperazine dihydrochloride (H7) and staurosporine treatment promotes neurite outgrowth, suggesting that PKC is a negative regulator of neuritogenesis [56]. To investigate the regulation of PKC during neurite formation, SH-SY-5Y human neuroblastoma cells were simultaneously treated with PKC and calpain inhibitors [58]. Calpain is a known regulator of PKC, and treatment with both calpain inhibitors and PKC did not produce additive or synergistic effects on neurite outgrowth [58]. This suggests that PKC and calpain function in the same pathway, as inhibition of two separate pathways would have likely resulted in additive or synergistic effects. These results suggest that PKC functions downstream of calpain during neurite formation [58]. In contrast, PKC has also been implicated as a positive regulator in a PC12 model of neuritogenesis [59]. Down-regulation of PKC by phorbol ester or incubation with PKC-selective inhibitors GF109203X and calphostin C has been shown to effectively block neurite formation in PC12 cells [59]. Differences between these studies may be the result of variations between different model systems or mechanisms used to induce neurite formation. However, an important role for PKC in neuritogenesis is likely, as modulation of its activity has resulted in interesting phenotypes during neurite formation in multiple studies.
Mitogen-activated protein kinase pathways: MAPK/ERK
Mitogen-activated protein kinases (MAPKs) are extracellular regulated kinases (ERK) that produce a signaling cascade activated by a variety of ligands and receptors involved in many processes including cellular differentiation. MAPKs are involved in three major pathways in mammalian cells that control the activities of MAPK/ERK, c-Jun N-terminal kinase (JNK), and p38 MAPK [60, 61]. MAPK/ERK signaling is essential for all stages of neuronal morphogenesis: initiation, elongation, branching, axon growth, targeting and synapse formation [22]. MAPK/ERK is a regulator of cell fate and its signaling contributes to a cell’s commitment to either proliferation or differentiation [62]. MAPK/ERK’s role in neuronal differentiation lies in its contribution to the signaling mechanisms that regulate neurite initiation.
MAPK/ERK signaling is involved in the mechanism responsible for promoting neurite formation in PC12 cells treated with curcuminoids, which have neurotrophic qualities [63]. MAPK/ERK’s role in neuritogenesis has also been studied in a variant PC12 cell line, PC12-N1, which spontaneously extends processes and displays an increased sensitivity to NGF. NGF treatment of PC12-N1 cells leads to an activation of MAPK/ERK, and subsequent neurite extension and elongation [22]. Different molecular pathways are activated in response to NGF-induced neurite formation in PC12 cells, but MAPK/ERK signaling exclusively has been shown to be necessary and sufficient for both neurite initiation and neurite elongation [22]. Spontaneous activation of MAPK/ERK without NGF treatment also causes cells to extend neurites. Another variant of PC12 cells, PC12m3 cells, respond to the environmental stimulus d-limonene to promote neurite outgrowth via the MAPK pathways [64]. The mechanism of d-limonene to induce neuritogenesis involves p38 MAPK since treatment with the p38 MAPK inhibitor SB203580 inhibited neurite outgrowth in response to d-limonene treatment [64].
The importance of MAPK/ERK in neuritogenesis has been demonstrated in other cell lines as well. In a study using alpha-lipoic acid (LA) to induce neurite formation in N2a cells it was shown that LA treatment significantly increased MAPK/ERK phosphorylation and subsequent activation levels, and that inhibition of MAPK/ERK activation completely abolished LA-induced neurite outgrowth [65]. This suggests that LA stimulates neurite outgrowth through the activation of MAPK/ERK signaling, providing more evidence that MAPK/ERK play an important role in initiating neuritogenesis. LA-stimulated neurite growth in N2a cells is mediated in a reactive oxygen species (ROS)-dependent manner [65]. LA administration transiently produces ROS to serve as a signaling molecule, and an optimized production of ROS is essential for neurite outgrowth and differentiation in N2a cells [65]. Wang et al. repeated N2a experiments in primary hippocampal neurons and found similar results, that treatment with LA promotes neurite outgrowth and increase neurite length via MAPK/ERK signaling in a ROS-dependent manner [65]. Inhibition of MAPK/ERK signaling abolished neurite outgrowth, and inhibition of ROS production prevented both neurite outgrowth and MAPK/ERK activation. These results in multiple model systems provide strong evidence that the MAPK/ERK signaling pathway is essentially integrated into multiple aspects and mechanisms of neuritogenesis.
c-Jun N-terminal kinase (JNK)
While JNK has several different isoforms, JNK3 is the primary neuronal isoform [66]. JNK regulates a range of biological processes implicated in neurodegenerative disorders [67]. JNK is a negative regulator of neurotrophic processes such as neurite formation. This is exemplified in spiral ganglion (SG) neurons in the cochlea, in which the number of neurites is regulated by brain-derived neurotrophic factor (BDNF). BDNF exerts opposing effects on neuritogenesis by activating different pathways to either promote or inhibit neurite initiation, via p38 and Akt signaling or JNK, respectively [68]. Inhibition of upstream regulators of JNK modestly enhanced BDNF-induced neurite formation, while activation of Rac/cdc42/JNK signaling by BDNF may reduce the formation of neurites [68].
Conflicting data show that JNK signaling is crucial for neurite initiation and elongation during axonal regeneration in dorsal root ganglion (DRG) neurons. A lack of JNK2 and JNK3, but not JNK1, delayed neuritogenesis while pharmacologically inhibiting JNK caused dramatic neurite retraction [69]. This neurite retraction function results from a combined impact on JNK1 and JNK2 activities [69]. Additionally, JNK1 and JNK2 have been shown to regulate the phosphorylation state of microtubule-associated protein 1B (MAP1B), which has a known role in neurite formation [70]. Therefore, the role of JNK in neurite initiation is controversial. Some of these conflicting data points might be explained by differences between model systems or focus on different JNK isoforms; however, the role of JNK in neuritogenesis is complex and may be dependent on various other signaling factors.
Ca2+/calmodulin-dependent protein kinase II (CaMK II)
Calcium signaling plays a crucial role in changes in cell shape, synapse formation, neuritogenesis, and many other cellular processes. Ca2+/calmodulin-dependent protein kinase II (CaMK II) is a major mediator of calcium signaling in the nervous system and works in these dynamic processes by engaging the cytoskeleton [46]. CaMK II is highly expressed in the brain and is a key regulator of many hippocampal and cortical processes with a suggested role in both neurite initiation and elongation [46]. CaMK II has both α and β isoforms which are exclusively expressed in the brain although the α isoform has been more extensively studied in the context of neurite formation. CaMK II has both traditional calcium-dependent kinase activity as well as less well-studied calcium-independent activity. The calcium-independent activity of CaMK II occurs via the following mechanism: brief calcium signals activate CaMK II and stimulate autophosphorylation of Thr-286, which allows CaMK II to remain in its active state even when calcium levels return to basal levels. It is the calcium-independent activity of CaMK II that has been implicated in neuritogenesis [46].
N2a cells expressing the α isoform of CaMK II showed immediate neurite formation, without serum deprivation, within 15 min of plating [46]. This is unique because N2a cells do not typically form neurites unless the culture media is depleted of fetal bovine serum (FBS). The calcium-independent activity of CaMK II was measured in N2a cells to assess its role in neurite formation [46]. Immediately after plating, CaMK II’s calcium-independent activity rapidly increased with a peak at 30 min and then a gradual decrease over the course of 2 h [46]. This initially would suggest that CaMK II activity is critical for neurite initiation, but authors noted that its activity remained high compared to basal levels for up to 4 h following plating [46]. This remaining activity suggests that following neurite initiation, CaMK II continues to play a role in neurite elongation. Authors then mutated CaMK II to prevent the auto-phosphorylation necessary to perform calcium-independent activity, and found that cells had less and much shorter neurites, implicating specifically the calcium-independent activity of CaMK II in both neurite initiation and elongation [46]. Further support for the role of CaMK II in neurite initiation comes from an experiment involving the CaMK II inhibitor, KN62, which reduced the number of dendrites per cultured embryonic rat hippocampal pyramidal neuron [71]. The β isoform of CaM kinase II has also been investigated and shown to promote neurite outgrowth and a greater change in cellular morphology than the α isoform [72]. There is convincing evidence that CaMK II is a critical regulator of neurite initiation, as modification of CaMK II in various model systems produces a marked phenotype.
Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)
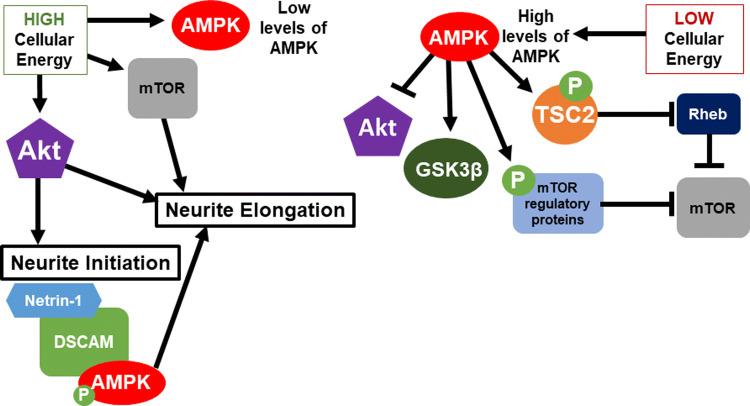
AMPK is a critical regulator of cellular homeostasis, functions as an energy sensor of the cell, and is a master regulator of many metabolic pathways [73]. Neurite formation is a highly energy-dependent process since it requires increased protein and membrane synthesis and high levels of intracellular transport. The role of AMPK as an energy sensor allows it to also be a crucial regulator of both neurite initiation and neurite elongation. Cellular energy levels must be optimal to allow neurite formation to occur and AMPK can act in all stages to allow the cell to continue or halt neurite formation to optimize efficient energy expenditure. When AMPK activity is altered during early stages of neuronal development, axon initiation and neuronal polarization in both cultured embryonic hippocampal neurons and embryonic cortical brain slices are defective [74, 75]. This suggests that the metabolic state of a neuron during neurite initiation may have implications for the extent to which the cell will commit to the energetically expensive process of morphological polarization. The cell may not commit to neuronal morphogenesis if it is not in an energy-abundant state, implicating AMPK signaling as crucial at these early time points. AMPK immunoreactivity was detected in hippocampal neurons as early as 6 h after plating, providing support that AMPK is important at early developmental time points [76]. AMPK regulation could be an integral factor in determining whether a cell will begin neuritogenesis based on its metabolic state.
During later stages of neurite elongation, when AMPK increases due to energetic stress there is inhibition of dendritic growth and arborization [76]. Wakita et al. showed that inhibition of AMPK promotes dopaminergic neurite outgrowth by activating the mTOR signaling pathway, which will be discussed in more detail in later sections. In this way, AMPK seems to be a negative regulator of neurite extension and branching, specifically in neurites that continue to develop into dendrites [77]. Other researchers have shown that when activated, AMPK suppresses regulators of neurite elongation, dendritic growth, and branching both directly and indirectly [76, 78]. Specifically, AMPK inhibits the mTOR pathway indirectly by phosphorylating tumor suppressor TSC2 and directly by phosphorylating mTOR regulatory-associated proteins [78]. Phosphorylation of TSC2 inhibits Rheb, a small GTPase that when bound to mTOR greatly increases mTOR activity [79]. Phosphorylation of mTOR regulatory-associated proteins prevents mTOR’s ability to phosphorylate its targets [78]. AMPK also indirectly inhibits PI3K signaling, which is required for both neurite initiation and neurite elongation [76]. AMPK achieves this by decreasing the phosphorylation levels of downstream effectors Akt and GSK3β, which disrupts neurite elongation as will be discussed in later sections [76]. Other researchers have also shown that regulators of AMPK-dependent pathways can control neurite outgrowth via lowering cellular energy levels, directly inhibiting AMPK, or indirectly inhibiting AMPK-dependent pathways [77, 80]. Their results are supportive of what has been presented thus far, with lowering energy levels or activating AMPK leading to a decrease in neurite elongation, and inhibition of AMPK leading to an increase in dendritic growth [77, 80]. These results suggest that AMPK acts as a negative regulator of neurite elongation by sensing low cellular energy levels and inhibiting two crucial pathways, mTOR and PI3K, involved in neurite elongation (Fig. 2).
Fig. 2.
Summary of the energy hypothesis and energy-independent hypothesis of AMPK in neurite formation. The energy hypothesis for AMPK states that depending on the available cellular energy, levels of AMPK change to affect the activation or inhibition of certain pathways important for neuritogenesis. When AMPK levels are low, there is activation of mTOR and Akt pathways to promote both neurite initiation and elongation. When AMPK levels are high, AMPK inhibits neurite formation by both directly and indirectly inhibiting Akt and mTOR signaling. The energy-independent hypothesis of AMPK states that Netrin-1 activates DSCAM leading to phosphorylation and activation of AMPK, which promotes neurite elongation
There is competing evidence, however, that AMPK activation is also crucial for correct neuronal development and neurite elongation. There is compelling evidence that AMPK interacts with Down syndrome cell adhesion molecule (DSCAM), which is a netrin-1 receptor, and facilitates netrin-1-induced neurite outgrowth in mouse primary cortical neurons [81]. There is evidence that netrin-1 activates AMPK via phosphorylation, AMPK interacts with DSCAM, and that inhibition of AMPK activity blocks netrin-1-mediated neurite elongation [81]. These findings suggest that during development, AMPK actually acts as a positive regulator of neurite elongation and the role of AMPK may not be as clear cut as previously hypothesized. This alternate role of AMPK also suggests that AMPK may have a more direct role in neurite formation that is not necessarily linked to the energy state of the cell.
Important differences to note between these studies are the cell type used and attention to AMPK subunit composition, which could potentially explain the variation in results. Collogues that reported AMPK as a negative regulator of neurite elongation used primary rat embryonic hippocampal neurons [76], primary rat embryonic cortical neurons [80], and primary rat embryonic dopaminergic mesencephalic neurons [77]. Cell type-specific functions of AMPK in neuritogenesis may exist in hippocampal and dopaminergic neurons when compared to cortical neurons. Two groups both used primary cortical neurons and found opposing results, which suggest a more complicated explanation than simply a difference in cell type-specific function of AMPK. AMPK has multiple subunit isoforms that are tissue specific, cell type specific, and have expression and functional changes throughout development [76]. AMPK has two α subunit isoforms (1 and 2), two β subunit isoforms (1 and 2), and three γ subunit isoforms (1, 2a, and 2b) [76]. The γ subunit of AMPK interacts with DSCAM promoting neurite elongation via netrin-1 signaling [81], while activation of AMPK inhibits neurite elongation through either α1 or α2 subunits [81]. While both of the reviewed studies that came to opposing conclusions used primary cortical neurons, one study used cortical cultures prepared from embryonic 18 (E18) rats, while the other used cortical cultures prepared from E15 mouse embryos [75, 81]. It is possible that the subunit isoform composition of AMPK is slightly different in cortical cultures depending on the age at dissection and animal model used. This would support the hypothesis that variation in specific cell types and developmental stage of the cells impacts kinase activity. The remaining collogues that found support for AMPK as a negative regulator used a general AMPK activator (berberine) and a general AMPK inhibitor (staurosporine) that do not have specificity for AMPK depending on subunit isoform composition [77, 80]. It is possible that the subunit isoform composition of AMPK is what determines whether AMPK acts as a positive or negative regulator of neurite elongation and these opposing results warrant continued investigation. There is also opposing evidence for how PI3K/Akt/mTOR pathways regulate neurite elongation which will be discussed, and this could also contribute to the conflicting evidence seen with AMPK’s effect on neurite elongation.
Phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt)–mammalian target of rapamycin (mTOR)
It is almost impossible to read about different cellular processes without encountering a role for PI3K, Akt, and mTOR signaling, and neuritogenesis is no different. These three kinases commonly act either in the same pathways or in highly interconnected pathways to regulate neurite initiation and elongation (Fig. 3). PI3K is known to regulate membrane and cytoskeletal rearrangements in response to growth factor stimulation [82–84]. Activation of PI3K is required for the initiation of NGF-stimulated neurite formation in PC12 cells which can be demonstrated by treatment with the PI3K inhibitor wortmannin, which causes complete inhibition of morphological responses to NGF in PC12 cells [85]. Further evidence for an important role for PI3K in neurite initiation can be found from the neonatal cochlear spiral ganglion (SG) explant model, which strongly depends on input from neurotrophins like BDNF to survive. Treatment of neonatal SG explants with BDNF has been shown to increase neurite number, but not length, suggesting that BDNF is important for regulating specifically neurite initiation in SG neurons [86, 87]. Inhibition of PI3K, or its downstream target Akt, reduced or eliminated BDNF-mediated increases in number of neurites [68]. This implicates PI3K/Akt signaling in mediating the neurite initiation-promoting effects of BDNF in SG neurons.
Fig. 3.
Schematic of signaling pathways regulated by PI3K/Akt/mTOR that promote neuritogenesis. A variety of ligands lead to neurite initiation and elongation through PI3K/Akt/mTOR signaling. Signaling through both TrkA/TrkB receptors leads to activation of PI3K and subsequent phosphorylation and activation of Akt. Akt then activates Cdc42 and Rac1 which modify the cytoskeleton and lead to neurite initiation. Signaling through RTKs and GPCRs as well as inhibition of ROCK1 activates PI3K. Subsequent phosphorylation and activation of Akt leads to phosphorylation and inhibition of GSK3β, which decreases the phosphorylation of MAP2 increasing microtubule polymerization. Inhibition of GSK3β also leads to activation of APC which leads to microtubule assembly. Together, these downstream targets of GSK3β lead to neurite elongation. Akt also activates Tau which increases microtubule stability and results in neurite elongation. Akt also phosphorylates mTOR which promotes neurite elongation and forms a complex with Sin1 and Rictor which together feedback onto Akt allowing it to phosphorylate its targets with maximum efficiency. RTK receptor tyrosine kinase, GPCR G-protein coupled receptor, ATF3 activating transcription factor 3, Atorvastatin inducer of neurite formation, WISP2/CCN5 WNT1- inducible-signlaing pathway protein 2
Furthermore, PI3K has a role in mediating neurite initiation in other models of neurotrophin-driven neuritogenesis. One model proposes that neurotrophin signaling via NGF activates TrkA, a tyrosine receptor kinase that activates PI3K, subsequently leading to the activation of Rac1 and Cdc42 in PC12 cells [88, 89]. Rac1 and Cdc42 are upstream targets of several cytoskeleton-modifying proteins such as the Arp2/3 complex [90, 91]. Live imaging of chicken DRG axons showed that treatment with BDNF can cause PI3K to be activated via TrkB, resulting in another neurite initiation-promoting pathway that leads to the accumulation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) which co-localizes with actin patches that underlie sites of filopodia protrusion [92]. This model outlines a signaling mechanism initiated by NGF/BDNF to activate TrkA/TrkB and the downstream target PI3K, leading to increased local PIP3, Cdc42, and Rac which have well-established roles in neurite initiation [34]. NGF activation of TrkA/PI3K/Rac1 has been shown to inhibit RhoA during PC12 differentiation, and expression of a dominant negative RhoA leads to an increase in neurite initiation [89, 93]. These studies provide further evidence that PI3K is involved in numerous signaling pathways with many downstream targets important for neurite initiation.
Many groups have elucidated Akt’s involvement in neurite elongation and branching, and most agree that it has a positive effect on neurite elongation in many different primary cell types such as hippocampal neurons, dorsal root ganglion cells, hypoglossal neurons, superior nerve ganglion neurons, and PC12 cells [94]. Akt can exert its effects under different stimulators of neurite elongation such as BDNF, hepatocyte growth factor, and NGF [94]. Akt is a downstream effector of PI3K. PI3K activation leads to phosphorylation and activation of Akt, which then phosphorylates and inhibits GSK3β [94]. Inhibition of GSK3β leads to a decrease in phosphorylation of its target, MAP2, which when dephosphorylated allows for increased microtubule polymerization and neurite elongation, specifically of neurites that will become dendrites [94]. GSK3β inhibition also increases elongation of the neurite that will become the axon via microtubule plus end binding protein adenomatous polyposis coli (APC), which promotes microtubule assembly along the growing neurite [94]. Akt also phosphorylates tau which promotes neurite elongation via microtubule interactions. The PI3K–Akt pathway is a well-known regulator of mTOR, and mTOR is a direct substrate of Akt [94]. Phosphorylation and subsequent activation of mTOR by Akt has been shown to promote neurite growth and branching in primary hippocampal neurons [94]. mTOR has also been shown to be the primary effector of dendritic branching. Once activated mTOR associates with Sin1 and Rictor which as a complex then feeds back onto Akt to provide additional phosphorylation. This additional phosphorylation allows for Akt to reach its maximal activity levels [94]. In this way PI3K–Akt acts as a master regulator whose activity allows for precise complete control of neurite formation and maturation of all neurites from the dendrites to the axon, establishment of correct neuronal polarity, and branching of mature neurites.
Like with AMPK, there are several competing hypotheses for how the PI3K–Akt pathway affects neurite elongation. Evidence discussed thus far supports the theory that PI3K–Akt activation increases neurite elongation in many primary cell types through a variety of interconnected mechanisms. However, studies in PC12 cells have shown that activation of PI3K–Akt signaling does not result in neurite outgrowth but instead may directly be linked to cell survival [94]. Others have found evidence that PI3K inhibitors showed negligible effects on neurite elongation [94]. There is also some evidence that Akt negatively regulates neurite elongation by overriding the growth arresting effects of neurite elongation promoting factors [94]. These competing studies all took place in PC12 cells, and it may be the virtue of the model system used that lead to the conflicting evidence of Akt’s actions in neurite elongation. These differences are most likely due to potential cell type-specific activity of Akt in primary neurons versus PC12 cells, which is common to many of the kinases discussed in this review (Table 3). Primary neurons isolated from tissue, depending on the developmental day, have already halted cell division and undergone neuritogenesis in vivo and upon plating begin neuritogenesis in vitro. PC12 cells are an immortalized cell line that maintains the ability to actively divide in vitro until reversibly induced by growth factors to undergo neuritogenesis. The PI3K–Akt signaling pathway plays different roles in PC12 cells depending on whether the cell is in a differentiated or undifferentiated state, specifically activation of Akt signaling has been shown to override the growth arresting effects necessary for neuritogenesis to allow the cell cycle to continue [94, 95]. Akt also functions in cell proliferation of neuronal precursor cells, but like PC12 cells, once neuronal differentiation begins its role changes to promote neuritogenesis [96]. Because PC12 cells continuously actively divide in vitro, it is possible that there is competition or crosstalk between Akt’s differential signaling pathways, one of which has the ability to override the growth factor induction of neuritogenesis, explaining the conflicting results seen in these studies.
Table 3.
Summary of cell-type specificity of kinases in neuritogenesis and regeneration
| Primary cortical neurons | Primary hippocampal neurons | Primary dopaminergic neurons | Neuroblastoma cells | PC12 cells | Spiral ganglion neurons | Dorsal root ganglion neurons | Conclusion | Citations | |
|---|---|---|---|---|---|---|---|---|---|
| Akt | N/A | (+) elongation | N/A | N/A | (+) elongation | (+) initiation | (+) elongation | Cell type specific | [94–96, 114] |
| (-) elongation | (+) regeneration | ||||||||
| AMPK | (+) elongation | (−) elongation | (−) elongation | N/A | N/A | N/A | N/A | Cell type specific, subunit composition specific | [70–79] |
| (−) elongation | (−) initiation | ||||||||
| Aurora kinase A | (+) elongation | N/A | N/A | N/A | N/A | N/A | (+) elongation | Not cell type specific | [98] |
| CaMK II | N/A | (+) elongation | N/A | (+) initiation | N/A | N/A | N/A | Cell-type specific | [46, 71] |
| (+) elongation | |||||||||
| Cdk5 | (+) elongation | N/A | N/A | N/A | N/A | N/A | N/A | More studies in different model systems must be performed | [99–101] |
| FAK | (+) initiation | (+) initiation | N/A | N/A | N/A | N/A | N/A | Not cell type specific | [53, 54] |
| JNK | N/A | N/A | N/A | N/A | N/A | (+) initiation | (+) initiation | Cell-type specific, isoform-type specific, pathway specific | [68–70] |
| (+) regeneration | |||||||||
| (−) initiation | |||||||||
| MAPK/ERK | N/A | (+) initiation | N/A | (+) initiation | (+) initiation | N/A | (+) regeneration | Cell type specific | [22, 63–65, 114] |
| (+) elongation | |||||||||
| (+) elongation | |||||||||
| mTOR | N/A | (+) elongation | N/A | N/A | N/A | N/A | (+) regeneration | More studies in different model systems must be performed | [94, 114] |
| PI3K | N/A | N/A | N/A | N/A | (+) initiation | (+) initiation | (+) initiation | Not cell type specific | [85–87, 114] |
| (+) regeneration | |||||||||
| PKA | N/A | N/A | N/A | (+) initiation | N/A | (+) elongation | N/A | Cell type specific | [56, 57, 111, 112] |
| (−) elongation | |||||||||
| (+) regeneration | |||||||||
| PKC | N/A | N/A | N/A | (−) initiation | N/A | N/A | N/A | More studies in different model systems must be performed | [56, 58, 59] |
| Rho kinase | (+) elongation | N/A | N/A | N/A | (+) elongation | N/A | N/A | Dose dependent* | [93, 102–104, 113] |
| (−) elongation | |||||||||
| (−) regeneration | |||||||||
| (−) elongation |
Kinases are designated as cell-type specific if they either produce opposite or additional effects in different model systems
N/A not assessed, (+) elongation/initiation/regeneration positive effector of neurite elongation, initiation, or regeneration, (−) elongation/initiation/regeneration inhibits neurite elongation, initiation, or regeneration
*Rho kinase is designated as “dose dependent” because depending on the level of inhibition used it produces different effects on neurite formation in multiple cell types
Mitotic protein kinases
Neurons undergo neuritogenesis as they transition from an undifferentiated to a differentiated, mature state. As a result, many protein kinases have roles in both stages as the cell transitions. Cell cycle and mitotic protein kinases are beginning to be investigated for activities outside of their more traditional roles, particularly in post-mitotic cells, for this very reason. For example, Aurora Kinase A, which traditionally serves as a microtubule reorganizer during cell division, has recently been shown to play a critical role in neuronal migration in the developing cortex [97]. Aurora kinase A has also been shown to be an essential regulator for neurite elongation in dorsal root ganglion cells and primary cortical neurons [98]. Using a mechanism similar to how it functions during mitosis, Aurora kinase A is phosphorylated, activated, and serves to reorganize microtubules to promote neurite elongation [98]. The mechanism specific to neurite elongation has been elucidated by Mori et al., protein kinase C-ζ (aPKC) phosphorylates and activates Aurora kinase A which then accumulates at the neurite hillock, Aurora kinase A phosphorylates NDEL1 at the same location where aPKC, Aurora A and NDEL1 then form a complex which becomes a microtubule organizing center that regulates microtubule invasion and projection into the elongating neurites [98]. Although the core function of Aurora kinase A remains the same in undifferentiated and differentiated neurons, interacting with microtubules and phosphorylating target proteins, this function produces very different cellular outcomes in mitotic neurons as compared to postmitotic neurons.
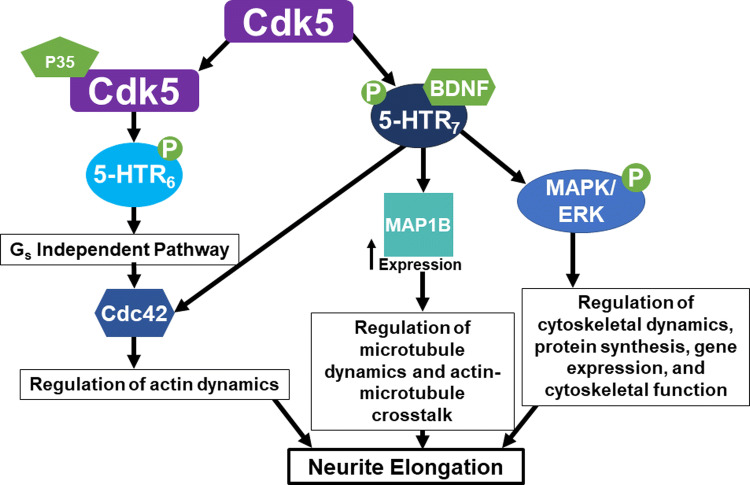
Another family of proteins that has recently gained notoriety in the field of neurite formation, specifically neurite elongation, for acting outside of their traditional mitotic roles is the cyclin-dependent kinases. Cyclin-dependent kinase 5 (Cdk5) is the only cyclin-dependent kinase that is silent during the cell cycle and becomes activated upon induction of neuronal differentiation [99]. Once Cdk5 becomes activated, it acts through multiple serotonin receptors to facilitate neurite elongation [100, 101]. Serotonin receptor 5-HTR6 is a G protein-coupled receptor (GPCR) which acts through Gs-coupled signaling. Gs is a subtype of Gα subunit, which along with the Gβ and Gγ subunits make up a complete heterotrimeric G protein. This serotonin receptor recruits Cdk5–p35 which activates Cdk5. Cdk5 then phosphorylates the receptor, leading to Gs-independent signaling which acts through Cdc42, a Rho GTPase involved with the regulation of the actin cytoskeleton, which is crucial for neurite growth. This Cdk5–5-HTR6-induced neurite growth can occur in the absence of a ligand for the serotonin receptor. The second serotonin receptor that Cdk5 can act through to promote neurite elongation is 5-HTR7, a receptor tyrosine kinase [101]. In contrast to 5-HTR6, 5-HTR7-induced neurite elongation requires ligand binding, such as BDNF, and subsequent 5-HTR7 activation. Cdk5 is recruited to the receptor, phosphorylates the receptor, and then activates MAPK/ERK by phosphorylation as well [101]. 5-HTR7 activation and subsequent downstream effector activation of Cdk5 and MAPK/ERK leads to a series of intracellular changes in cytoskeletal dynamics, protein synthesis, gene expression, and cytoskeletal function [101]. There is evidence that 5-HTR7 signaling also acts through Cdc42 to regulate actin dynamics [100]. There is also a significant, striking increase in MAP1B expression following 5-HTR7 activation [101]. MAP1B is not only an essential regulator of microtubule dynamics, but also of actin–microtubule cross-talk during neurite elongation [100]. These results elucidate a clear mechanism by which Cdk5 signaling, acting alongside different serotonin receptors, regulates two key components of the cytoskeleton, actin and microtubules, as well as their crosstalk to efficiently produce neurite elongation (Fig. 4). It is important to note that Cdk5 specifically acts on neurite elongation, as manipulations showed no change in numbers of neurites from the soma, providing evidence that neurite initiation and elongation truly are separate processes although many kinases and other proteins can play similar roles in both phenomena.
Fig. 4.
Schematic of Cdk5 signaling mechanisms to promote neurite elongation. Cdk5 acts through two serotonin receptors, 5-HTR6 and 5-HTR7, to promote neurite elongation. 5-HTR6 recruits p35–Cdk5 and activates Cdk5. Cdk5 then phosphorylates and activates 5-HTR6 signaling through a Gs-independent mechanism to activate Cdc42 to regulate the actin cytoskeleton and promote neurite elongation. 5-HTR7 signaling requires ligand binding, such as BDNF, to become activated. Once activated 5-HTR7 recruits Cdk5 which phosphorylates it. Phosphorylated 5-HTR7 then activates MAPK/ERK via phosphorylation which has a variety of downstream effects to promote neurite elongation. 5-HTR7 can also activate Cdc42 to regulate actin dynamics to promote neurite elongation. Finally, 5-HTR7 phosphorylation also leads to an increase in MAP1B expression which promotes neurite elongation via multiple cytoskeletal linked mechanisms
Rho kinase
Stimulation of the RhoA/Rho-associated protein kinase (ROCK/ROK) pathway by NGF application causes neurite outgrowth in PC12 cells and primary cortical neurons [102]. ROCK is a downstream effector of RhoA signaling, and there are different inhibitors that can be used to study the effects of intervening at different levels of this signaling pathway. ROCK is a serine/threonine kinase and activates a series of Ezrin–radixin–moesin (ERM) proteins via phosphorylation, mediating the function of ERM proteins in neuritogenesis and neuronal morphology [103]. Whether ROCK directly phosphorylates ERM proteins is controversial in different cell types, but evidence suggests that Rho kinase does mediate the activation of these proteins in primary cortical neurons, although there may also exist Rho-independent activation [103]. Different levels of ROCK inhibition have different effects on neurite length and neuronal morphology. For example, treatment with Rho kinase inhibitor II at low concentrations increases neurite length whereas treatment at high concentrations decreases neurite length [103]. Other groups have found evidence that RhoA inhibition with ibuprofen is efficient at significantly increasing neurite length at high concentrations of between 100 and 500 μM although ROCK inhibition using multiple ROCK inhibitors Y-27632 and Fasudi significantly increased neurite length at concentrations as low as 1 μM [104]. This group used human NT2 cells which are neuronal precursor cells that can be driven to differentiation. The mechanism of this increase in neurite elongation is still unknown, but researchers hypothesize that it is the level cellular molecules such as ERM proteins and other targets of ROCK existing in the cytoplasm that dictates the balance between increasing and decreasing neurite length [103].
Protein kinases and neurite formation in neurological disease
Disrupted neurite formation is currently gaining notoriety as a contributing pathogenic mechanism for a variety of neurodevelopmental disorders as well as other neurological diseases. The roles of protein kinases that drive the signaling pathways responsible for either proper or aberrant neurite formation are currently being elucidated. For example, dystrophic neurites, neurite shortening, and neurite degeneration are hallmarks of many neurodegenerative disorders such as Alzheimer’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease (PD) [105, 106]. There are several genes that have been causatively linked to the development of PD, the most common of which is leucine-rich repeat kinase 2 (LRRK2) [105]. Neuronal cultures harvested from LRRK2 mutant mice had significantly decreased neurite length, which was exacerbated by knockdown of the protein responsible for phosphorylating LRRK2, 14-3-3. Inhibition of LRRK2 activity reversed the toxic neurite shortening in primary neuronal cultures. 14-3-3 proteins regulate LRRK2 in many important ways such as increasing or decreasing its kinase activity. It has been shown in rodent PD models and human PD models that 14-3-3 expression is reduced, which could potentially increase LRRK2 kinase activity and subsequently increase the neurodegenerative process by contributing to neurite shortening and neurotoxicity [105]. This study makes altering the activity of LRRK2 an attractive therapeutic target to combat neurite degeneration in PD.
The LIM kinases are important for several complex cellular mechanisms and are linked to neuritogenesis via their roles in actin and microtubule dynamics. Disruptions to LIM kinase activities have been reported in many neurological disorders such as intellectual disability, Williams–Beuren syndrome, schizophrenia, Alzheimer’s disease, and Parkinson’s disease [107, 108]. Mutations to LIM kinase genes present in patients with intellectual disability have been shown to prevent proper neurite outgrowth and neurite arborization [107, 108]. In Alzheimer’s disease, increased expression of LIM kinase 1 leads to neuritic dystrophy and subsequent pathology [107]. Because the LIM kinases have indispensable roles in actin and microtubule dynamics which are the foundation for the integrity of neuritogenesis and neurite maintenance, it would be unsurprising if the other mentioned disorders in which LIM kinases are linked also had disrupted neuritogenesis.
Neurite formation deficits have also been linked to neuropsychiatric disorders, specifically to bipolar disorder (BPD) [109]. Many studies have linked GSK3ß with BPD, with significant decreases in expression in the prefrontal and temporal cortex [110]. As outlined in this review, GSK3ß forms an integral component of the PI3K–Akt signaling pathway that allows proper neurite elongation to occur. GSK3ß is a negative effector of neurite elongation; however, some level of GSK3ß expression is necessary for proper neurite formation to occur [109]. In cultured neurons, complete deficiency of GSK3ß leads to a significant increase in neurite complexity and protrusions on neurites; however, loss of GSK3ß in vivo leads to decreased dendritic complexity and spine density [109]. These results indicate that GSK3ß may play different roles in neuritogenesis in vitro compared to in vivo, but that the proper level of GSK3ß expression is necessary for proper neuronal morphogenesis. The same study showed that the functional and anatomical aberrations, including neurite formation, due to GSK3ß knockout were correlated with mania-like behavior that is present in BPD [109]. Future studies should aim to more directly link defective neuritogenesis to functional and behavioral consequences present in the discussed disorders.
Protein kinases in axon regeneration
As extensively outlined in this review paper, there are many different kinase-dependent pathways that contribute to neurite formation. These pathways work in conjunction during different stages of neurite formation to allow for the tight regulation and control necessary for proper neuronal development of morphology, circuitry, and ultimately function. Until recently, these pathways have been explored mainly in the context of development, but they clearly provide a variety of potential therapeutic targets for the adult nervous system in response to injury or disease where neurite formation is disrupted. This section will focus on the current work that is harnessing the potential of the discussed targets and mechanisms to drive neuritogenesis, leading to axon regeneration and recovery.
cAMP/PKA
Cyclic AMP (cAMP) and cAMP-dependent protein kinase (PKA) play extensive roles in neurite initiation during development. cAMP has also provided a promising target for regeneration because it can override the inhibitory effect of myelin-associated proteins and inhibitory factors [111]. cAMP promotes regeneration in a variety of neuron types, and usually signals through PKA which then causes an increase in gene expression of ERK1/2 [111]. The mechanism of action of MAPK/ERK in neurite elongation has been extensively discussed, and now has been shown to play a role in cAMP-mediated regeneration in dorsal root ganglion neurons [111]. cAMP-mediated neurite regeneration has also been studied in spiral ganglion neurons (SGN) [112]. SGNs provide afferent fibers that relay auditory signals from the cochlea to the cochlear nucleus in the brainstem, and researchers identified that activation of cAMP signaling in this cell type potentially represents a strategy to enhance SGN fiber elongation following cochlear implants to improve patient outcomes [112]. Deafness can be caused due to loss of hair cells following many different assaults such as noise trauma, drugs, aging, or genetic disorders [112]. The SGN cells innervating the lost hair cells lose trophic support and begin to degenerate, which can create issues even after cochlear implants are placed [112]. Increasing cAMP levels and subsequent activation of PKA by either infusion of molecules into the cochlea, exogenous gene transfer, or activation of calcium-dependent signals enhanced regrowth and regeneration of SGNs across glial boundaries in vitro [112]. As seen with many of these mechanisms, optimization of the amount of activation must occur to produce efficient elongation, as very elevated levels of cAMP and PKA activation can actually lead to decreases in neurite length [112]. The presented research on cAMP highlights an important point that studying the pathways of neurite formation presents potential therapeutic targets that will extend beyond the field of spinal cord injury research and has the potential to help other populations, such as those with neuronal degeneration following deafness.
ROCK
Controversial evidence for ROCK’s role in neurite formation has been discussed, and it is being debated whether under developmental conditions ROCK works to positively or negatively regulate neurite elongation. Despite these debates, there is clear evidence that inhibition of ROCK at some concentration, whether that concentration is low or high is debatable, results in a strong increase of neurite outgrowth, length, and an increase in number of neurons extending neurites [104]. Researchers have shown that activation of RhoA, the upstream activator of ROCK, leads to impairment of neurite regeneration in post-mitotic PC12 cells that have been scraped and re-plated in the presence of NGF to prime neurite re-initiation and re-elongation [93]. Control cells were able to successfully regenerate their neurites [93]. This provides more evidence for RhoA and ROCK being negative regulators of neurite elongation. More recent work has shown that RhoA and ROCK are expressed in adult peripheral sensory neurons specifically in the axon growth cone, and that RhoA levels are increased after axotomy [113]. When ROCK inhibitors are applied to cultured sensory neurons results showed increased neurite formation, and most strikingly ROCK inhibition applied at low levels in vivo increased numbers of axons and Schwann cells regenerating beyond the injury site and increased the overall distance that the axons grew [113]. These are very exciting findings that corroborate previous evidence that ROCK inhibition at low doses can increase neurite formation during development and we can apply this evidence to an adult peripheral injury model in vivo [102, 103].
JNK
JNK’s role in neurite initiation has been elucidated, but its role in elongation during development remains unclear. However, JNK signaling has been proven essential for neurite initiation and elongation during axonal regeneration [69]. There are three JNK isoforms, JNK1, JNK2, and JNK3 and they are all expressed in adult DRG neurons and each play distinct roles during axon regeneration. JNK2 and JNK3 are essential for neurite re-initiation, and JNK1 and JNK2 are involved with neurite re-elongation, with JNK1 being crucial for elongation of already existing neurites [69]. JNK2 and JNK3 can translocate to the nucleus and are involved in regulation of regeneration associated genes, and JNK exerts its elongating actions by phosphorylating microtubule associated proteins such as MAP1B and MAP2. JNK2 also phosphorylates MAP1B during neurite re-elongation [69]. The effects of knocking out these different JNK isoforms were only seen in adult cells, and knockout of JNK had no effect on neurite elongation during development. Authors suggested that this may be due to compensation during development by other signaling pathways, kinases, or other JNK isoforms that were not specifically knocked out [69]. The different roles of JNK isoforms discussed are specific to neurite regeneration in adult cells following injury. Studies of JNK in axon regeneration highlight another important point, which is that researchers aiming to harness developmental mechanisms to drive regeneration should consider not only proteins that are crucial for neurite elongation but to also consider those that are essential for neurite initiation as their roles may change in the adult nervous system.
MAPK/ERK and PI3K–Akt
MAPK/ERK and PI3K–Akt signaling pathways are indispensable throughout multiple stages of neuritogenesis. Methylcobalamin (MeCbl) is a vitamin B12 analog that has been used to treat peripheral nerve disorders such as Bell’s palsy, diabetic neuropathy, and carpal tunnel syndrome [114]. MeCbl has also been shown to promote neurite outgrowth and neuron survival in cervical ganglion neurons and DRGs in vitro [114]. The proposed mechanism behind MeCbl’s actions is methylation of Ras, which promotes its function. Ras is upstream of MAPK/ERK and PI3K–Akt, both of which promote neurite elongation themselves and activate mTOR which also promotes neurite elongation. It has also been shown that Akt activation of mTOR leads to increased regeneration following axotomy [94]. Okada et al. provided evidence for this mechanism in vitro [114]. To analyze these results in vivo, Okada et al. used a rat sciatic nerve injury model to observe MeCbl’s effects. They found that administration of MeCbl following sciatic nerve injury improved recovery of sensory function, motor function, and axon regeneration but also increased regeneration of myelin which had inhibitory effects on terminal latency of the sciatic nerve [114]. MeCbl is evidenced as a promising therapeutic target for not only peripheral nerve injury but also potentially central nerve injury due to its mechanistic effects on PI3K–Akt, MAPK/ERK, and mTOR which provide positive regulation of neurite elongation.
These studies provide compelling evidence for kinases as therapeutic targets to promote axon regeneration in a variety of injury models. It is well understood that immature, developing axons in many organisms have a much higher intrinsic capacity for growth and regeneration when compared to mature axons. Manipulating kinases in the adult nervous system to act in a similar fashion as they do during neuritogenesis has thus far been a successful attempt to harness the intrinsic capacity of developing axons for regrowth. Researchers are yielding exciting results by looking into pharmacological agents that are already FDA approved for various usages that target protein kinases, for example, vitamin B12 and ibuprofen, and applying them to axon regeneration [104, 114]. Many other kinase inhibitors that are already FDA approved are marketed for anticancer treatment, and these papers suggest a potential alternate therapeutic use for them to promote axon regeneration. Future studies should take advantage of the wealth of existing kinase inhibiting and activating therapeutics available for application in axon regeneration.
Conclusion
Protein kinases are indispensable components of many stages of neuritogenesis, and their roles as regulators present them as attractive targets for therapeutic innovation in diseases or injuries where neurite formation is disrupted. This comprehensive review highlights many kinases whose pathways have been elucidated, but there is more room for discovery. Evidence for how pathways or individual kinases act during different stages of neurite formation in different model systems is contradictory in several cases, thus further investigation is warranted.
Acknowledgements
This review has been supported by a research grant from the NINDS (NS096098).
Author contributions
SAB, SMB and TS wrote the initial draft of the manuscript. SAB and SMB edited it and created the figures. KT edited and finalized it.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 3.Reese D, Drapeau P. Neurite growth patterns leading to functional synapses in an identified embryonic neuron. J Neurosci. 1998;18(15):5652–5662. doi: 10.1523/JNEUROSCI.18-15-05652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi G, Wang J, Wei X, Deng B. Dendritic properties control energy efficiency of action potentials in cortical pyramidal cells. Front Cell Neurosci. 2017;11:265. doi: 10.3389/fncel.2017.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21(3):326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 6.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 7.Nimmo GA, Cohen P. The regulation of glycogen metabolism Purification and characterisation of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1978;87(2):341–351. doi: 10.1111/j.1432-1033.1978.tb12383.x. [DOI] [PubMed] [Google Scholar]
- 8.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262(5142):2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 9.Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, Okawa M, Yamada N, Hatten ME, Snyder SH, Ross CA, Sawa A. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100(1):289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murtomaki S, Risteli J, Risteli L, Koivisto UM, Johansson S, Liesi P. Laminin and its neurite outgrowth-promoting domain in the brain in Alzheimer’s disease and Down’s syndrome patients. J Neurosci Res. 1992;32(2):261–273. doi: 10.1002/jnr.490320216. [DOI] [PubMed] [Google Scholar]
- 11.Bakos J, Bacova Z, Grant SG, Castejon AM, Ostatnikova D. Are molecules involved in neuritogenesis and axon guidance related to autism pathogenesis? Neuromolecular Med. 2015;17(3):297–304. doi: 10.1007/s12017-015-8357-7. [DOI] [PubMed] [Google Scholar]
- 12.Lepagnol-Bestel AM, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide AL, Moalic JM, Muller D, Dean B, Yoshikawa T, Gorwood P, Buxbaum JD, Ramoz N, Simonneau M. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13(4):385–397. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- 13.Cornell B, Wachi T, Zhukarev V, Toyo-oka K. Regulation of neuronal morphogenesis by 14-3-3epsilon (Ywhae) via the microtubule binding protein, doublecortin. Hum Mol Genet. 2016;25(20):4405–4418. doi: 10.1093/hmg/ddw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Maldergem L, Hou Q, Kalscheuer VM, Rio M, Doco-Fenzy M, Medeira A, de Brouwer APM, Cabrol C, Haas SA, Cacciagli P, Moutton S, Landais E, Motte J, Colleaux L, Bonnet C, Villard L, Dupont J, Man H-Y. Loss of function of KIAA2022 causes mild to severe intellectual disability with an autism spectrum disorder and impairs neurite outgrowth. Hum Mol Genet. 2013;22(16):3306–3314. doi: 10.1093/hmg/ddt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y-C, Frei JA, Kilander MBC, Shen W, Blatt GJ. A subset of autism-associated genes regulate the structural stability of neurons. Front Cell Neurosci. 2016;10:263. doi: 10.3389/fncel.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs C, Trazzi S, Torricella R, Viggiano R, De Franceschi M, Amendola E, Gross C, Calza L, Bartesaghi R, Ciani E. Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3beta signaling. Neurobiol Dis. 2014;70:53–68. doi: 10.1016/j.nbd.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amendola E, Zhan Y, Mattucci C, Castroflorio E, Calcagno E, Fuchs C, Lonetti G, Silingardi D, Vyssotski AL, Farley D, Ciani E, Pizzorusso T, Giustetto M, Gross CT. Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS One. 2014;9(5):e91613. doi: 10.1371/journal.pone.0091613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roskoski R., Jr Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. doi: 10.1016/j.phrs.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burstein DE, Blumberg PM, Greene LA. Nerve growth factor-induced neuronal differentiation of PC12 pheochromocytoma cells: lack of inhibition by a tumor promoter. Brain Res. 1982;247(1):115–119. doi: 10.1016/0006-8993(82)91033-2. [DOI] [PubMed] [Google Scholar]
- 21.Burstein DE, Greene LA. Evidence for RNA synthesis-dependent and -independent pathways in stimulation of neurite outgrowth by nerve growth factor. Proc Natl Acad Sci USA. 1978;75(12):6059–6063. doi: 10.1073/pnas.75.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao J, Liu Y. Differential roles of ERK and JNK in early and late stages of neuritogenesis: a study in a novel PC12 model system. J Neurochem. 2003;86(6):1516–1523. doi: 10.1046/j.1471-4159.2003.01961.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang CM, Shui HA, Wu YT, Chu PW, Lin KG, Kao LS, Chen ST. Proteomic analysis of proteins in PC12 cells before and after treatment with nerve growth factor: increased levels of a 43-kDa chromogranin B-derived fragment during neuronal differentiation. Brain Res Mol Brain Res. 2001;92(1–2):181–192. doi: 10.1016/s0169-328x(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 24.Greene LA, Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- 25.Xiao J, Zhou Q, Liu Y. Variant PC12 cell line that spontaneously differentiates and extends neuritic processes. J Neurosci Res. 2002;69(1):104–109. doi: 10.1002/jnr.10260. [DOI] [PubMed] [Google Scholar]
- 26.Westerink RHS, Ewing AG. The PC12 cell as model for neurosecretion. Acta physiologica (Oxford, England) 2008;192(2):273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, Chu D, Sun SH. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 2009;21(6):881–891. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 28.Mao AJ, Bechberger J, Lidington D, Galipeau J, Laird DW, Naus CC. Neuronal differentiation and growth control of neuro-2a cells after retroviral gene delivery of connexin43. J Biol Chem. 2000;275(44):34407–34414. doi: 10.1074/jbc.M003917200. [DOI] [PubMed] [Google Scholar]
- 29.Fan S, Ramirez SH, Garcia TM, Dewhurst S. Dishevelled promotes neurite outgrowth in neuronal differentiating neuroblastoma 2A cells, via a DIX-domain dependent pathway. Mol Brain Res. 2004;132(1):38–50. doi: 10.1016/j.molbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Cornell B, Wachi T, Zhukarev V, Toyo-Oka K. Regulation of neuronal morphogenesis by 14-3-3 epsilon (Ywhae) via the microtubule binding protein, doublecortin. Hum Mol Genet. 2016;25(20):4405–4418. doi: 10.1093/hmg/ddw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Algarni AS, Hargreaves AJ, Dickenson JM. Role of transglutaminase 2 in PAC1 receptor mediated protection against hypoxia-induced cell death and neurite outgrowth in differentiating N2a neuroblastoma cells. Biochem Pharmacol. 2017;128:55–73. doi: 10.1016/j.bcp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Tahirovic S, Bradke F. Neuronal polarity. Cold Spring Harbor Perspect Biol. 2009;1(3):a001644. doi: 10.1101/cshperspect.a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer AW, Schoonderwoert VTG, Ji L, Mederios N, Danuser G, Forscher P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15(1):146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sainath R, Gallo G. Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res. 2015;359(1):267–278. doi: 10.1007/s00441-014-1955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schelski M, Bradke F. Neuronal polarization: from spatiotemporal signaling to cytoskeletal dynamics. Mol Cell Neurosci. 2017;84:11–28. doi: 10.1016/j.mcn.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 36.da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3(9):694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z-Q, Franz EW, Leach MK, Winterroth F, White CM, Rastogi A, Gu Z-Z, Corey JM. Mechanical tension applied to substrate films specifies location of neuritogenesis and promotes major neurite growth at the expense of minor neurite development. J Biomed Mater Res Part A. 2015;104:966–974. doi: 10.1002/jbm.a.35627. [DOI] [PubMed] [Google Scholar]
- 38.Gärtner A, Fornasiero EF, Munck S, Vennekens KL, Seuntjens E, Huttner WB, Valtorta F, Dotti CG. N-cadherin specifies first asymmetry in developing neurons. EMBO J. 2012;31(8):1893–1903. doi: 10.1038/emboj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S-X, Duan L-H, Qian H, Yu X. Actin aggregations mark the sites of neurite initiation. Neurosci Bull. 2016;32(1):1–15. doi: 10.1007/s12264-016-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S-X, Duan L-H, He S-J, Zhuang G-F, Yu X. Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation. Cell Res. 2017;27(2):253–273. doi: 10.1038/cr.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vignjevic D, Peloquin J, Borisy GG. In vitro assembly of filopodia-like bundles. Methods Enzymol. 2006;406:727–739. doi: 10.1016/S0076-6879(06)06057-5. [DOI] [PubMed] [Google Scholar]
- 42.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160(3):409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller BK. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- 44.Dwane S, Durack E, O’Connor R, Kiely PA. RACK1 promotes neurite outgrowth by scaffolding AGAP2 to FAK. Cell Signal. 2014;26(1):9–18. doi: 10.1016/j.cellsig.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Anglister L, Farber IC, Shahar A, Grinvald A. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev Biol. 1982;94(2):351–365. doi: 10.1016/0012-1606(82)90353-0. [DOI] [PubMed] [Google Scholar]
- 46.Sogawa Y, Yoshimura Y, Otaka A, Yamauchi T. Ca(2+)-independent activity of Ca(2+)/calmodulin-dependent protein kinase II involved in stimulation of neurite outgrowth in neuroblastoma cells. Brain Res. 2000;881(2):165–175. doi: 10.1016/s0006-8993(00)02838-9. [DOI] [PubMed] [Google Scholar]
- 47.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 48.Kleinschmidt EG, Schlaepfer DD. Focal adhesion kinase signaling in unexpected places. Curr Opin Cell Biol. 2017;45:24–30. doi: 10.1016/j.ceb.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grant SG, Karl KA, Kiebler MA, Kandel ER. Focal adhesion kinase in the brain: novel subcellular localization and specific regulation by Fyn tyrosine kinase in mutant mice. Genes Dev. 1995;9(15):1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- 50.Burgaya F, Menegon A, Menegoz M, Valtorta F, Girault JA. Focal adhesion kinase in rat central nervous system. Eur J Neurosci. 1995;7(8):1810–1821. doi: 10.1111/j.1460-9568.1995.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 51.Lim ST. Nuclear FAK: a new mode of gene regulation from cellular adhesions. Mol Cells. 2013;36(1):1–6. doi: 10.1007/s10059-013-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 53.Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18(5):725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monje FJ, Kim EJ, Pollak DD, Cabatic M, Li L, Baston A, Lubec G. Focal adhesion kinase regulates neuronal growth, synaptic plasticity and hippocampus-dependent spatial learning and memory. Neurosignals. 2012;20(1):1–14. doi: 10.1159/000330193. [DOI] [PubMed] [Google Scholar]
- 55.Kim G, Choe Y, Park J, Cho S, Kim K. Activation of protein kinase A induces neuronal differentiation of HiB5 hippocampal progenitor cells. Brain Res Mol Brain Res. 2002;109(1–2):134–145. doi: 10.1016/s0169-328x(02)00550-8. [DOI] [PubMed] [Google Scholar]
- 56.Shea TB, Beermann ML, Leli U, Nixon RA. Opposing influences of protein kinase activities on neurite outgrowth in human neuroblastoma cells: initiation by kinase A and restriction by kinase C. J Neurosci Res. 1992;33(3):398–407. doi: 10.1002/jnr.490330306. [DOI] [PubMed] [Google Scholar]
- 57.Vogt Weisenhorn DM, Roback LJ, Kwon JH, Wainer BH. Coupling of cAMP/PKA and MAPK signaling in neuronal cells is dependent on developmental stage. Exp Neurol. 2001;169(1):44–55. doi: 10.1006/exnr.2001.7651. [DOI] [PubMed] [Google Scholar]
- 58.Shea TB, Cressman CM, Spencer MJ, Beermann ML, Nixon RA. Enhancement of neurite outgrowth following calpain inhibition is mediated by protein kinase C. J Neurochem. 1995;65(2):517–527. doi: 10.1046/j.1471-4159.1995.65020517.x. [DOI] [PubMed] [Google Scholar]
- 59.Lazarovici P, Jiang H, Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol Pharmacol. 1998;54(3):547–558. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272(30):18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 61.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392(6676):622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 62.Ryu H, Chung M, Dobrzyński M, Fey D, Blum Y, Lee SS, Peter M, Kholodenko BN, Jeon NL, Pertz O. Frequency modulation of ERK activation dynamics rewires cell fate. Mol Syst Biol. 2015;11(11):838. doi: 10.15252/msb.20156458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao KK, Wu MJ, Chen PY, Huang SW, Chiu SJ, Ho CT, Yen JH. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK- and PKC-dependent pathways. J Agric Food Chem. 2012;60(1):433–443. doi: 10.1021/jf203290r. [DOI] [PubMed] [Google Scholar]
- 64.Shinomiya M, Kawamura K, Tanida E, Nagoshi M, Motoda H, Kasanami Y, Hiragami F, Kano Y. Neurite outgrowth of PC12 mutant cells induced by orange oil and d-limonene via the p38 MAPK pathway. Acta Med Okayama. 2012;66(2):111–118. doi: 10.18926/AMO/48261. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Wang Z, Yao Y, Li J, Zhang X, Li C, Cheng Y, Ding G, Liu L, Ding Z. Essential role of ERK activation in neurite outgrowth induced by alpha-lipoic acid. Biochim Biophys Acta. 2011;1813(5):827–838. doi: 10.1016/j.bbamcr.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Martin JH, Mohit AA, Miller CA. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Mol Brain Res. 1996;35(1):47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- 67.Davies C, Tournier C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem Soc Trans. 2012;40(1):85–89. doi: 10.1042/BST20110641. [DOI] [PubMed] [Google Scholar]
- 68.Mullen LM, Pak KK, Chavez E, Kondo K, Brand Y, Ryan AF. Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res. 2012;1430:25–34. doi: 10.1016/j.brainres.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci. 2010;30(23):7804–7816. doi: 10.1523/JNEUROSCI.0372-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyne LJ, Martin K, Hockfield S, Fischer I. Expression and distribution of phosphorylated MAP1B in growing axons of cultured hippocampal neurons. J Neurosci Res. 1995;40(4):439–450. doi: 10.1002/jnr.490400403. [DOI] [PubMed] [Google Scholar]
- 71.Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclic AMP-dependent protein kinase, and Ca(2+)-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. Int J Dev Neurosci. 1993;11(3):357–368. doi: 10.1016/0736-5748(93)90007-z. [DOI] [PubMed] [Google Scholar]
- 72.Goshima Y, Ohsako S, Yamauchi T. Overexpression of Ca2+/calmodulin-dependent protein kinase II in Neuro2a and NG108-15 neuroblastoma cell lines promotes neurite outgrowth and growth cone motility. J Neurosci. 1993;13(2):559–567. doi: 10.1523/JNEUROSCI.13-02-00559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase–development of the energy sensor concept. J Physiol. 2006;574(Pt 1):7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amato S, Man H-Y. Bioenergy sensing in the brain. Cell Cycle. 2011;10(20):3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man H-Y. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science (New York, N.Y.) 2011;332(6026):247–251. doi: 10.1126/science.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramamurthy S, Chang E, Cao Y, Zhu J, Ronnett GV. AMPK activation regulates neuronal structure in developing hippocampal neurons. Neuroscience. 2014;259:13–24. doi: 10.1016/j.neuroscience.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 77.Wakita S, Izumi Y, Nakai T, Adachi K, Takada-Takatori Y, Kume T, Akaike A. Staurosporine induces dopaminergic neurite outgrowth through AMP-activated protein kinase/mammalian target of rapamycin signaling pathway. Neuropharmacology. 2014;77:39–48. doi: 10.1016/j.neuropharm.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15(8):702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 80.Lu J, Cao Y, Cheng K, Xu B, Wang T, Yang Q, Yang Q, Feng X, Xia Q. Berberine regulates neurite outgrowth through AMPK-dependent pathways by lowering energy status. Exp Cell Res. 2015;334(2):194–206. doi: 10.1016/j.yexcr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Zhu K, Chen X, Liu J, Ye H, Zhu L, Wu JY. AMPK interacts with DSCAM and plays an important role in netrin-1 induced neurite outgrowth. Protein Cell. 2013;4(2):155–161. doi: 10.1007/s13238-012-2126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13(10):2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4(5):385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 84.Wymann M, Arcaro A. Platelet-derived growth factor-induced phosphatidylinositol 3-kinase activation mediates actin rearrangements in fibroblasts. Biochem J. 1994;298(Pt 3):517–520. doi: 10.1042/bj2980517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson TR, Blader IJ, Hammonds-Odie LP, Burga CR, Cooke F, Hawkins PT, Wolf AG, Heldman KA, Theibert AB. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109(Pt 2):289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartnick CJ, Staecker H, Malgrange B, Lefebvre PP, Liu W, Moonen G, Van De Water TR. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J Neurobiol. 1996;30(2):246–254. doi: 10.1002/(SICI)1097-4695(199606)30:2<246::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 87.Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17(6):1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aoki K, Nakamura T, Matsuda M. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J Biol Chem. 2004;279(1):713–719. doi: 10.1074/jbc.M306382200. [DOI] [PubMed] [Google Scholar]
- 89.Nusser N, Gosmanova E, Zheng Y, Tigyi G. Nerve growth factor signals through TrkA, phosphatidylinositol 3-kinase, and Rac1 to inactivate RhoA during the initiation of neuronal differentiation of PC12 cells. J Biol Chem. 2002;277(39):35840–35846. doi: 10.1074/jbc.M203617200. [DOI] [PubMed] [Google Scholar]
- 90.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays. 2010;32(2):119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 91.Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71(3):201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- 92.Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30(36):12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sebok A, Nusser N, Debreceni B, Guo Z, Santos MF, Szeberenyi J, Tigyi G. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J Neurochem. 1999;73(3):949–960. doi: 10.1046/j.1471-4159.1999.0730949.x. [DOI] [PubMed] [Google Scholar]
- 94.Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66(18):2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SR, Park JH, Park EK, Chung CH, Kang SS, Bang OS. Akt-induced promotion of cell-cycle progression at G2/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol. 2005;205(2):270–277. doi: 10.1002/jcp.20395. [DOI] [PubMed] [Google Scholar]
- 96.Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, Mo X. Brain development and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61(3):379–384. doi: 10.1007/s12031-016-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takitoh T, Kumamoto K, Wang CC, Sato M, Toba S, Wynshaw-Boris A, Hirotsune S. Activation of Aurora-A is essential for neuronal migration via modulation of microtubule organization. J Neurosci. 2012;32(32):11050–11066. doi: 10.1523/JNEUROSCI.5664-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, Saya H, Wynshaw-Boris A, Hirotsune S. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11(9):1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 99.Zhu J, Li W, Mao Z. Cdk5: mediator of neuronal development, death and the response to DNA damage. Mech Ageing Dev. 2011;132(8–9):389–394. doi: 10.1016/j.mad.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duhr F, Deleris P, Raynaud F, Seveno M, Morisset-Lopez S, Mannoury la Cour C, Millan MJ, Bockaert J, Marin P, Chaumont-Dubel S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat Chem Biol. 2014;10(7):590–597. doi: 10.1038/nchembio.1547. [DOI] [PubMed] [Google Scholar]
- 101.Speranza L, Chambery A, Di Domenico M, Crispino M, Severino V, Volpicelli F, Leopoldo M, Bellenchi GC, di Porzio U, Perrone-Capano C. The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology. 2013;67:155–167. doi: 10.1016/j.neuropharm.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 102.Lu XC, Zheng JY, Tang LJ, Huang BS, Li K, Tao Y, Yu W, Zhu RL, Li S, Li LX. MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem. 2015;35(1):246–258. doi: 10.1159/000369692. [DOI] [PubMed] [Google Scholar]
- 103.Haas MA, Vickers JC, Dickson TC. Rho kinase activates ezrin-radixin-moesin (ERM) proteins and mediates their function in cortical neuron growth, morphology and motility in vitro. J Neurosci Res. 2007;85(1):34–46. doi: 10.1002/jnr.21102. [DOI] [PubMed] [Google Scholar]
- 104.Roloff F, Scheiblich H, Dewitz C, Dempewolf S, Stern M, Bicker G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS One. 2015;10(2):e0118536. doi: 10.1371/journal.pone.0118536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lavalley NJ, Slone SR, Ding H, West AB, Yacoubian TA. 14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening. Hum Mol Genet. 2016;25(1):109–122. doi: 10.1093/hmg/ddv453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi Y, Ayaki T, Li F, Tsujimura A, Kamada M, Ito H, Maki T, Sawamoto N, Urushitani M, Takahashi R. Phosphorylated NF-kappaB subunit p65 aggregates in granulovacuolar degeneration and neurites in neurodegenerative diseases with tauopathy. Neurosci Lett. 2019;704:229–235. doi: 10.1016/j.neulet.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 107.Cuberos H, Vallee B, Vourc’h P, Tastet J, Andres CR, Benedetti H. Roles of LIM kinases in central nervous system function and dysfunction. FEBS Lett. 2015;589(24 Pt B):3795–3806. doi: 10.1016/j.febslet.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 108.Tastet J, Cuberos H, Vallee B, Toutain A, Raynaud M, Marouillat S, Thepault RA, Laumonnier F, Bonnet-Brilhault F, Vourc’h P, Andres CR, Benedetti H. LIMK2-1 is a Hominidae-Specific Isoform of LIMK2 Expressed in Central Nervous System and Associated with Intellectual Disability. Neuroscience. 2019;399:199–210. doi: 10.1016/j.neuroscience.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 109.Kim W, Won SY, Yoon BJ. CRMP2 mediates GSK3beta actions in the striatum on regulating neuronal structure and mania-like behavior. J Affect Disord. 2019;245:1079–1088. doi: 10.1016/j.jad.2018.10.371. [DOI] [PubMed] [Google Scholar]
- 110.Pandey GN, Rizavi HS, Tripathi M, Ren X. Region-specific dysregulation of glycogen synthase kinase-3beta and beta-catenin in the postmortem brains of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2015;17(2):160–171. doi: 10.1111/bdi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wan G, Zhou L, Lim Q, Wong YH, Too HP. Cyclic AMP signalling through PKA but not Epac is essential for neurturin-induced biphasic ERK1/2 activation and neurite outgrowths through GFRalpha2 isoforms. Cell Signal. 2011;23(11):1727–1737. doi: 10.1016/j.cellsig.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Xu N, Engbers J, Khaja S, Xu L, Clark JJ, Hansen MR. Influence of cAMP and protein kinase A on neurite length from spiral ganglion neurons. Hear Res. 2012;283(1–2):33–44. doi: 10.1016/j.heares.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng C, Webber CA, Wang J, Xu Y, Martinez JA, Liu WQ, McDonald D, Guo GF, Nguyen MD, Zochodne DW. Activated RHOA and peripheral axon regeneration. Exp Neurol. 2008;212(2):358–369. doi: 10.1016/j.expneurol.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 114.Okada K, Tanaka H, Temporin K, Okamoto M, Kuroda Y, Moritomo H, Murase T, Yoshikawa H. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol. 2010;222(2):191–203. doi: 10.1016/j.expneurol.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 115.Jia L, Ji S, Maillet JC, Zhang X. PTEN suppression promotes neurite development exclusively in differentiating PC12 cells via PI3-kinase and MAP kinase signaling. J Cell Biochem. 2010;111(6):1390–1400. doi: 10.1002/jcb.22867. [DOI] [PubMed] [Google Scholar]
- 116.Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39(2):283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 117.Tashima K, Yamamoto H, Setoyama C, Ono T, Miyamoto E. Overexpression of Ca2+/calmodulin-dependent protein kinase II inhibits neurite outgrowth of PC12 cells. J Neurochem. 1996;66(1):57–64. doi: 10.1046/j.1471-4159.1996.66010057.x. [DOI] [PubMed] [Google Scholar]
- 118.Massé T, Kelly PT. Overexpression of Ca2+/calmodulin-dependent protein kinase II in pc12 cells alters cell growth, morphology, and nerve growth factor-induced differentiation. J Neurosci. 1997;17(3):924. doi: 10.1523/JNEUROSCI.17-03-00924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11(4):891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu C, Zhang CW, Zhou Y, Wong WQ, Lee LC, Ong WY, Yoon SO, Hong W, Fu XY, Soong TW, Koo EH, Stanton LW, Lim KL, Xiao ZC, Dawe GS. APP upregulation contributes to retinal ganglion cell degeneration via JNK3. Cell Death Differ. 2018;25(4):661–676. doi: 10.1038/s41418-017-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W-H, Cheng S-J, Tzen JTC, Cheng C-M, Lin Y-W. Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness. PLoS One. 2014;8(12):e83394. doi: 10.1371/journal.pone.0083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;20(11):4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 124.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 125.Namekata K, Watanabe H, Guo X, Kittaka D, Kawamura K, Kimura A, Harada C, Harada T. Dock3 regulates BDNF-TrkB signaling for neurite outgrowth by forming a ternary complex with Elmo and RhoG. Genes Cells. 2012;17(8):688–697. doi: 10.1111/j.1365-2443.2012.01616.x. [DOI] [PubMed] [Google Scholar]