Abstract
Calcitriol, the active metabolite of vitamin D, has been widely studied for its preventive and therapeutic activity against several cancers including oral squamous cell carcinoma (OSCC). However, the impact of dietary vitamin D supplementation on initiation and progression of OSCC is unclear. To address this gap in knowledge, we conducted preclinical trials using the 4-nitroquinoline-1-oxide (4NQO) carcinogen model of oral carcinogenesis. Female C57BL/6 mice were maintained on one of three vitamin D diets [ 25 IU, 100 IU, 10,000 IU] and exposed to 4NQO in drinking water for 16 weeks followed by regular water for 10 weeks. Body weight measurements obtained through the study duration did not reveal any differences between the three diets. Animals on 100 IU diet showed lower incidence of high-grade dysplasia/OSCC and higher CD3+ T cells compared to animals on 25 IU and 10000 IU diets. Serum 25(OH)D3 levels were highest in animals on 10000 IU diet at week 0 (prior to carcinogen exposure) but showed ~50% reduction at week 26. Histologic evaluation revealed highest incidence of OSCC in animals maintained on 10000 IU diet. Animals on 100 IU and 10000 IU diets showed higher vitamin D receptor (VDR) and CYP24A1 immunostaining in high-grade dysplastic lesions and OSCC compared to normal tongue. Validation studies performed in a 4NQO-derived OSCC model showed that short-term treatment of animals on a 25 IU diet with calcitriol significantly inhibited tumor growth compared to controls but did not affect tumor growth in animals on reference diet (1000 IU). Collectively, our results highlight the complex dynamics between vitamin D status and oral carcinogenesis. Our observations also suggest that therapeutic benefits of short-term calcitriol treatment may be more pronounced in vitamin D deficient hosts.
Keywords: vitamin D, oral carcinogenesis, diet, VDR, T cells, squamous cell carcinoma
1. INTRODUCTION
Oral squamous cell carcinomas (OSCC) are highly aggressive cancers that are a major cause of cancer related mortality worldwide [1, 2]. The development and progression of OSCC is a multi-step process that involves a complex series of molecular and genetic alterations induced by exposure to carcinogens such as tobacco, cigarette smoke and alcohol [3–5]. The exposure of the upper aero-digestive tract to these carcinogens results in multifocal disease (‘field cancerization’), which in turn, contributes to the high recurrence rates and risk of developing second primary tumors in this patient population [6, 7]. Strategies that prevent malignant transformation of oral premalignant lesions or slow OSCC disease progression could improve patient outcomes. In this regard, there has been considerable interest in examining the chemopreventive and therapeutic potential of vitamin D and its analogues against OSCC [8–11].
Vitamin D is fat soluble, pro-hormone that regulates calcium and phosphorous metabolism. In humans, vitamin D can either be derived from exposure to sunlight or dietary intake of fortified foods [12]. It is initially hydroxylated in the liver to 25-hydroxycholecalciferol [25(OH)D3] and further hydrolyzed in the kidney to calcitriol [1,25(OH)2D3], the active form of vitamin D. 1,25(OH)2D3 is catabolically inactivated by the enzyme 24-hydroxylase (encoded by the CYP24A1 gene) in the kidney [13]. Epidemiologic studies have suggested that low vitamin D levels are associated with increased risk of OSCC [14, 15]. Preclinical studies by others and us have shown that calcitriol can inhibit OSCC growth in vivo [8,16,17]. However, the risk of hypercalcemia associated with chronic administration of calcitriol complicates its clinical translation [18]. In this regard, dietary vitamin D supplementation is an alternative strategy that can be used to safely increase 25(OH)D3 and local production of 1,25(OH)2D3. In preclinical studies, dietary vitamin D supplementation has been shown to prevent breast and prostate cancers [19, 20]. However, the effects of vitamin D3 supplementation on OSCC have not been previously reported. Although clinical studies have reported a high prevalence of vitamin D3 insufficiency and deficiency in OSCC patients [21, 22], the manner and the extent to which vitamin D status affects oral carcinogenesis remains unclear. The consequences of treating vitamin D deficiency on OSCC progression are also not known.
The overall goal of the present study was to address these gaps in knowledge by conducting a preclinical investigation into the impact of dietary vitamin D3 on initiation and progression of oral cancer. To this end, experimental studies were conducted using the 4-nitroquinoline-1-oxide (4NQO) carcinogen model of oral carcinogenesis. We examined the effects of three different dietary vitamin D3 regimens on initiation and progression of oral cancer and the therapeutic impact of correcting vitamin D3 deficiency using calcitriol. Mechanistic assessment included examination of the effects of dietary supplementation on vitamin D metabolism, local vitamin D signaling and immune microenvironment in OSCC. The therapeutic impact of correcting vitamin D deficiency was also examined in an orthotopic murine model of OSCC.
2. MATERIALS AND METHODS
2.1. Animals and diets
Experiments were conducted using nine week-old female C57BL/6NCrmice (Charles River). Animals were housed in sterile micro isolator cages in an air-conditioned room with 12 hour light/dark cycles. Animals (n = 6 per group) were maintained on 25 IU vitamin D/kg, 100 IU vitamin D/kg or 10,000 IU vitamin D/kg throughout the study (Research Diets Inc, New Brunswick, NJ) [20] . All other diet components were identical.
2.2. 4NQO model
The carcinogen 4NQO (Sigma Aldrich, St. Louis, MO, USA) was dissolved in propylene glycol at 4 mg/ml. Autoclaved water was added to dilute the 4NQO to a final concentration of 100 μg/ml. Animals received a fresh batch of 4NQO dissolved in drinking water each week. Regular autoclaved water was administered to the animals for 10 weeks after 16 weeks of carcinogen exposure. Body weights were measured every three days. Animals were euthanized when weight loss exceeded >20% of the highest body weight or exhibited signs of morbidity (ruffled fur, lethargy). All experimental procedures were performed in accordance with institutional animal care and use committee (IACUC) approved protocols at Roswell Park Comprehensive Cancer Center.
2.3. Determination of vitamin D metabolites and calcium in serum
Serum samples were prepared from blood collected at week 0 and week 26. Serum obtained from mice at each diet level was pooled together for analysis. Pools were created by mixing equivalent volumes of serum from 5 to 6 mice per diet level. Serum levels of 1,25(OH)2D3 and 25(OH)D3 were determined by liquid chromatography mass spectrometry (Heartland Assays, IA, USA). To quantify 25(OH)D3 levels, serum samples along with standard curve and controls were protein precipitated with 0.2M Zinc sulfate solution, vortexed, followed by addition of methanol. The D3-25(OH)D2/D3-25(OH)D3 internal standard was added to samples and controls. Hexane was added to the samples and controls then capped and vortexed, followed by centrifugation. Organic layer was then transferred followed by drying. All standards, controls and samples were reconstituted with LCMS grade Methanol and water with 0.1% formic acid, then loaded onto the auto-sampler for analysis. The LC/MS/MS system used was an Agilent 1290 infinity HPLC coupled to an Agilent 6460 MS/MS with ESI source. Assay accuracy was determined to be >95% based on NIST certified standard assessment. For 1,25(OH)2D3 quantitation, serum samples, standard curve and QC were pipetted into borosilicate test tubes and then spiked with d6-1,25(OH)2D3 (Sigma-Aldrich, MA, USA) followed by protein precipitation with equal parts acetonitrile to samples. After acetonitrile was added samples were vortexed and centrifuged. The samples were extracted via solid phase extraction (SPE) using Diasorin C18-OH 0.5gram columns on a VAC-ELUT apparatus. After isolation via SPE, the elution was dried down and derivatized using 0.75 mg/mL 4-Phenyl-1,2,4-triazole-3,5-dione (PTAD, Sigma-Aldrich, MA , USA) in acetonitrile and allowed to react for 2 hours at room temperature . The samples were quenched with water, vortexed and dried down and reconstituted with LCMS grade methanol and water with 0.6% formic acid. The samples were re-constituted into LCMS grade methanol and water both with 0.6% formic acid, and then injected onto an Agilent 1290 HPLC coupled to an Agilent 6460 Triple-quad mass spec with electrospray ionization source (ESI) in positive mode. Extraction efficiencies was >90% based on certified standard recovery. Serum calcium levels were measured by colorimetry.
2.4. Histopathologic evaluation of oral lesions
Whole tongue tissues from mice were fixed in 10% neutral-buffered formalin (Sigma-Aldrich, St. Louis, MO, USA). Tongues were longitudinally dissected, and three sections 50μm apart were stained with hematoxylin and eosin (H&E). H&E stained tongue sections were evaluated for hyperplasia, hyperkeratosis, low grade (mild, moderate) dysplasia, severe dysplasia, and invasive squamous cell carcinoma as previously described by us [17] . Cellular abnormalities such as increased nuclear:cytoplasmic ratio, irregular nuclei, abnormal mitotic figures and architectural patterns such as pleomorphic parakeratosis and loss of polarity in the epithelial cells were identified. If these atypical features were present in the lower or middle third parabasal layers, lesions were classified as mild or moderate dysplasia, respectively. Lesions with the appearance of atypical features over two-thirds of the epithelial thickness were classified as severe dysplasia. Squamous cell carcinoma (SCC) was defined by the presence of atypical epithelial cells invading into the connective tissue stroma whereas superficially invasive SCC was defined by the presence of atypical cells that minimally invade the lamina propria [23]. Individual lesions on each tongue section were counted and reported as the total number of lesions per mouse and the area occupied by these lesions. Three contiguous sections (50 μm apart) were obtained for each of the six mice and the worst histologic diagnosis of the three sections was used to calculate incidence of dysplasia and OSCC.
2.5. Immunohistochemistry
Immunostaining of tongue sections for VDR, 24-hydroxylase (CYP24A1) and CD3 was performed using the envision technique, Dako real envision System and Peroxidase/DAB+ (Agilent Technologies, Santa Clara, CA, USA), as described previously [17]. Formalin fixed paraffin embedded (FFPE) tongue sections were stained with antibodies specific for VDR (Thermo Fisher Scientific; MA1-710; 1:500 for 30 minutes at room temperature), CYP24A1 (MyBioSource, Inc.; MBS178241; 1:1000; 1 hour at 37°C) and CD3 (Dako; A0452; 1:200; 30 mins at room temperature). The FFPE slides were digitized using ScanScope XT system (Aperio Technologies). Two-three fields per tongue were captured for each mouse for quantification of VDR, CYP24A1 staining intensity and CD3+ T counts. Quantification of nuclear VDR, cytoplasmic CYP24A1 and CD3 T cells was performed on captured digital images (20X magnification) using the Image J IHC profiler Macro (NIH Image J, Version 1.51j8). A binarized image was created in NIH Image J and used to quantify the number of stained cells in a given field (CD3+ count/field). Positive and negative controls were used to validate the absence of non-specific binding of all antibodies used.
2.6. Magnetic resonance imaging of murine OSCC response to calcitriol
Murine OSCC cells were generated by enzymatic dissociation of a tongue lesion in a 4NQO-exposed mouse that was maintained on 1000 IU rodent house chow diet. Following dissociation, flow cytometry based sorting of epithelial cell adhesion molecule (EpCAM; CD326; clone G8.8, BD Biosciences, San Jose, CA, USA) and CD44 (clone IM7, BD Biosciences) cells was performed to generate these cells [24, 25]. 1 million cells suspended in 30μl Dulbecco’s modified eagle media were injected into the floor of the mouth of C57Bl/6 mice maintained on a 25 IU vitamin D deficient diet or a 1000 IU reference diet for 6 weeks. Calcitriol was prepared in 100% ethanol at a stock concentration of 200 μg/ml, stored at −80°C, and diluted in phosphate buffered saline to a concentration of 1 μg/ml for treatment. Non-invasive magnetic resonance imaging (MRI) was performed using a 4.7T/33-cm horizontal bore magnet (GE NMR Instruments, CA) within the Translational Imaging Shared Resource at Roswell Park. Beginning at 10 days post implantation, multi-slice T2 weighted MR images were acquired on the axial plane to monitor tumor growth over a 28 day period. Starting at day 15 post implantation, calcitriol was administered at a dose of 0.1 μg by intraperitoneal (i.p) three times a week (Mon, Wed, Fri) for two weeks. Tumor volumes were calculated by manually tracing the extent of the tumor on MR images as previously described by us [26].
2.7. Statistical considerations
The Chi-square test was used to analyze the difference in incidence of disease phenotypes on histology among the three dietary regimens. Comparison of multiplicity of disease phenotypes, T cell levels, VDR and 24-OHase expression among the diet cohorts were performed using one-way ANOVA with Tukey adjusted post-hoc comparisons. Unpaired parametric t-test was used to compare the tumor growth in calcitriol treated and control mice maintained on either deficient or reference diet. GraphPad Prism (GraphPad Software, Version 7.0; San Diego, CA) was used to perform all the statistical analysis. P- Value <0.05 was considered statistically significant.
3. RESULTS
3.1. Dietary vitamin D3 supplementation in a carcinogen-induced mouse model of oral cancer
The overall study design, cohorts and end points for dietary vitamin D3 supplementation in the 4NQO model is shown in Fig. 1A. All three dietary vitamin D3 regimens were well tolerated with no significant reduction in body weight or clinical signs of toxicity observed during the study period. Body weights of mice measured once every three days throughout the duration of the study did not reveal any significant difference between the three dietary regimens (Fig. 1B).
Figure 1. Effect of dietary supplementation on body weight and vitamin D metabolites.

(A) Study design, cohorts and end points. C57BL/6 mice were randomized to receive one of 3 vitamin D3 diets: 25 IU, 100 IU, or 10,000 IU. Six weeks after initiation of diets, mice were exposed to carcinogen 4NQO in drinking water for 16 weeks. Mice were fed regular drinking water for an additional 10 weeks. Mice were maintained on the assigned diets for the entire study. Blood was collected at week 0 (6 weeks post initiation of diet prior to 4NQO exposure) and at week 26 for determination of vitamin D3 metabolites by LC-MS/MS. At study termination (week 26), tongue tissues were collected for histology and immunohistochemistry. (B) Body weights of mice measured once every three days throughout the duration of the study did not reveal any significant difference between the three dietary regimens. Absolute levels of 25(OH)D3 (C) at week 26 and change in 25(OH)D3 levels of mice from week 0 to 26 (D) for three dietary vitamin D3 cohorts. Levels of 1,25(OH)2D3 (E) and calcium (F) at week 26 in animals maintained on the three diets.
To investigate the effects of dietary supplementation on vitamin D homeostasis in our model system, we measured serum 25(OH)D3, 1,25(OH)2D3 and calcium levels in mice maintained on the three diets at week 26. Serum levels of 25(OH)D3 (Fig. 1C) was markedly higher in animals maintained on the 10,000 IU diet (21 ng/ml) compared to those on the 25 IU (1.5 ng/ml) and 100 IU (3.9 ng/ml) diets. However, measurement of relative change (Δ) in serum 25(OH)D3 levels from week 0 (prior to carcinogen exposure) showed ~50% decrease at week 26 in mice maintained on the 10,000 IU diet (Fig. 1D). Minimal change in serum levels of 25(OH)D3 was seen in animals on the 25 IU or 100 IU diets. Serum 1,25(OH)2D3 levels in mice on the 25 IU or 100 IU diets were 49.3 pg/ml and 97.3 pg/ml, respectively. Mice on the 10,000 IU diet had very high serum 1,25(OH)2D3 levels (381 pg/ml; Fig. 1E). Serum calcium levels were within the normal range of 8-11 mg/dl in mice across the three diets (Fig. 1F).
3.2. Impact of dietary vitamin D3 on oral premalignant lesions and OSCC
Next, we examined the impact of long-term dietary vitamin D3 supplementation on the incidence of 4NQO-induced oral premalignant lesions and invasive OSCC. Photomicrographs of hematoxylin and eosin (H&E) stained sections (10X, scale bar - 200μm) of normal tongue epithelium, dysplasia and OSCC of a representative mouse from each diet group are shown in Fig. 2A. No difference was observed in the histologic appearance of dysplastic lesions across the three diet groups. Donut charts shown in Fig. 2B represent the incidence of moderate dysplasia (grey) and severe dysplasia + OSCC (black) based on worst histologic diagnosis of three tongue sections. Mice on the 100 IU diet (middle) had the lowest incidence of severe dysplasia (SD) + OSCC (33%, 2 out of 6) compared to mice maintained on 25 IU (50%, 3 out of 6) or 10000 IU (83%, 5 out of 6) diets. Quantification of the total number of lesions did not reveal any significant difference in the number of moderate dysplasia (MD) (Fig. 2C) or SD + OSCC (Fig. 2D) between animals on the three diets. However, animals on 100 IU diet exhibited superficial OSCC while mice on the 25 IU and 10000 IU diets exhibited invasive SCC. Tongue sections from animals maintained on the 25 IU diet showed higher OSCC lesion area compared to mice on 100 IU or 10000 IU diets (Supplementary Fig S1).
Figure 2. Impact of dietary vitamin D3 intake on incidence and multiplicity of oral premalignant lesions and OSCC.
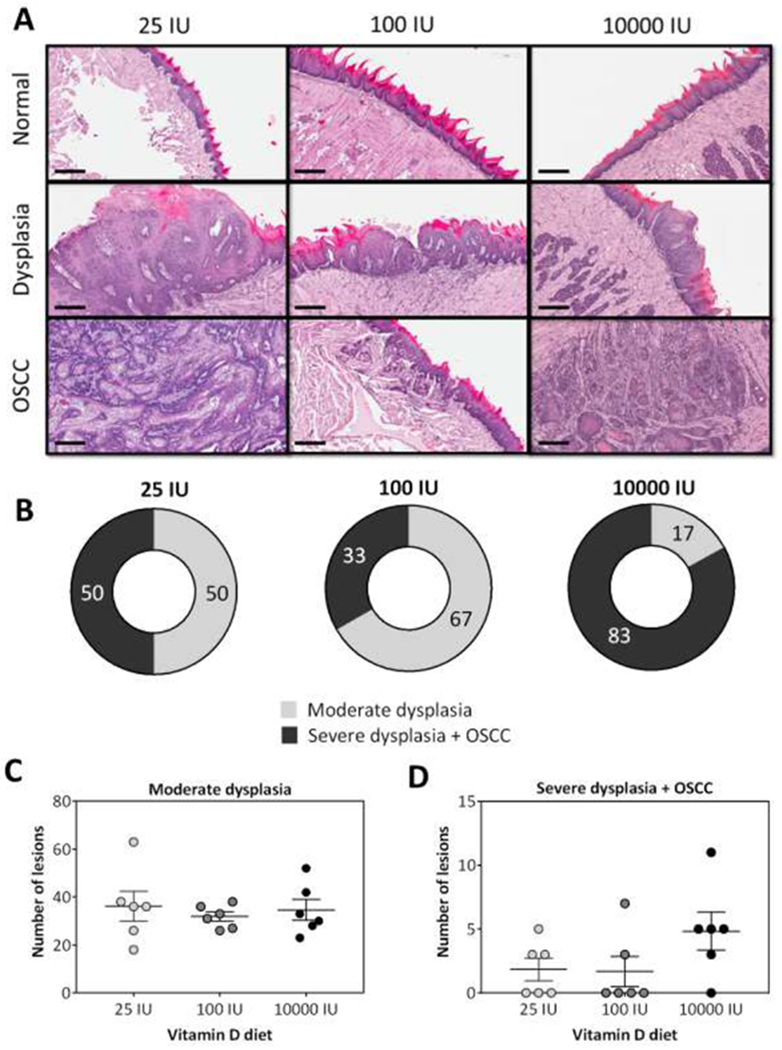
(A) Photomicrographs of hematoxylin and eosin (H&E) stained sections (10X, scale bar - 200μm) of normal tongue epithelium, dysplasia and OSCC of a representative mouse from each diet group. (B) Donut charts illustrate the incidence (%) of oral premalignant and malignant lesions in mice maintained on the three diets. The number of oral lesions (Moderate dysplasia; C) (Severe dysplasia + OSCC; D) across the three diet groups are also shown (p>0.05). Histologic evaluation revealed lowest incidence of severe dysplasia + OSCC in mice maintained on the adequate diet while mice maintained on 10000 IU showed the highest incidence of OSCC. Reported values represent mean ± standard error.
3.3. Effect of dietary vitamin D3 supplementation on VDR and CYP24A1 expression in tongue tissues
The CYP24A1 gene that encodes 24-OHase is a direct VDR target and is responsible for catabolic inactivation of 1,25(OH)2D3. We therefore measured the effects of dietary vitamin D3 intake on VDR and CYP24A1 in the tongue tissues of mice as a measure of local vitamin D3 signaling. The panel of images shown in Figure 3 represent photomicrographs of (A) VDR and (C) CYP24A1 (10X magnification, scale bar: 200μm) immunostained sections of normal tongue epithelium, dysplasia and OSCC in mice maintained on 25, 100 or 10000 IU diets. Quantitative estimates of VDR (B) and CYP24A1 (D) staining intensity are also shown. As expected, subcellular localization of VDR was observed in the nucleus, whereas CYP24A1 staining was restricted to the cytoplasm. VDR expression was elevated in SD+OSCC tissue compared to the normal epithelium in mice, although this increase was not statistically significant. CYP24A1 expression was comparable across all histologies in mice on the 25 IU diet. In contrast, a significant increase in CYP24A1 was seen in moderate dysplasia (p<0.01) and SD+ OSCC (p<0.001) compared to the normal epithelium in mice on 100 IU and 10000 IU (Fig. 3D) diets. In mice maintained on the 100 IU diet, a significant increase (p<0.05) in CYP24A1 was detected in SD+OSCC compared to MD (Supplementary Fig. S2).
Figure 3. Dietary vitamin D3 supplementation and expression of VDR and CYP24A1 in tongue tissues.
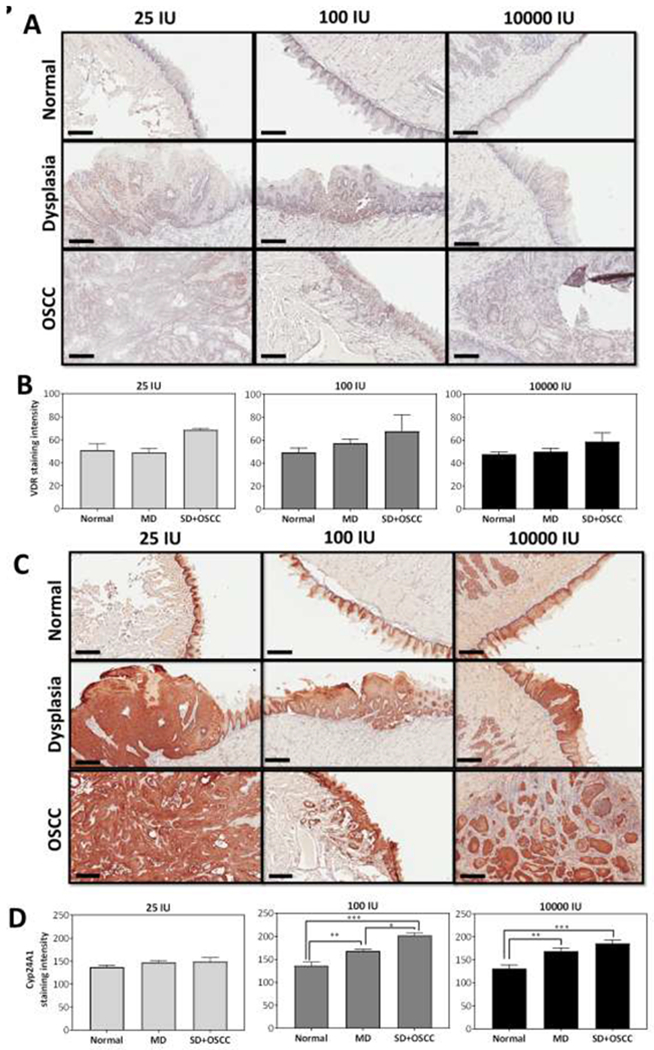
Photomicrographs of (A) VDR and (C) CYP24A1 (10X magnification, scale bar: 200μm) immunostained sections of normal tongue epithelium, dysplasia and OSCC in mice maintained on 25 IU, 100 IU or 10000 IU. Quantitative estimates of VDR (B) and CYP24A1 (D) staining intensity are also shown. Animals on 100 IU and 10000 IU demonstrated significantly higher CYP24A1 staining in MD and SD+OSCC compared to normal tongue. Values represent the mean ± standard error. *p < 0.05; ** p <0.01; ***p <0.001.
3.4. Dietary vitamin D3 alters T cell levels in tongue tissue
To gain further insight into the observed impact of dietary vitamin D on oral carcinogenesis, we examined the effect of the three diets on T cell levels in normal, dysplasia and OSCC. Tongue sections from individual mice were stained for the pan T cell marker, CD3. The panel of images in Fig. 4A represents photomicrographs of CD3 stained sections (20X, scale bar - 100μm) of normal tongue epithelium, dysplasia and OSCC of a representative mouse from each diet group. Normal tongue epithelium showed comparable CD3 staining across the three diet groups. Mice maintained on 25 IU diet showed comparable levels of CD3+ T cells across different histologies (A, B). Compared to normal epithelium, CD3+ T cell levels were significantly (p<0.05) higher in dysplastic regions (MD) of the tongue in animals on 100 IU (C) and 10000 IU (D). Mice that were fed 100 IU diet had significantly higher (p<0.05 or greater) CD3+T cell levels in the SD+OSCC lesions compared to normal epithelium and dysplasia (C). Comparative assessment of SD + OSCC tissue across the three diet groups revealed higher CD3+ T cell counts in animals on 100 IU diet compared to 25 IU or 10000 IU diets (p<0.05) (E).
Figure 4. Dietary vitamin D3 alters T cell levels in tongue tissues.
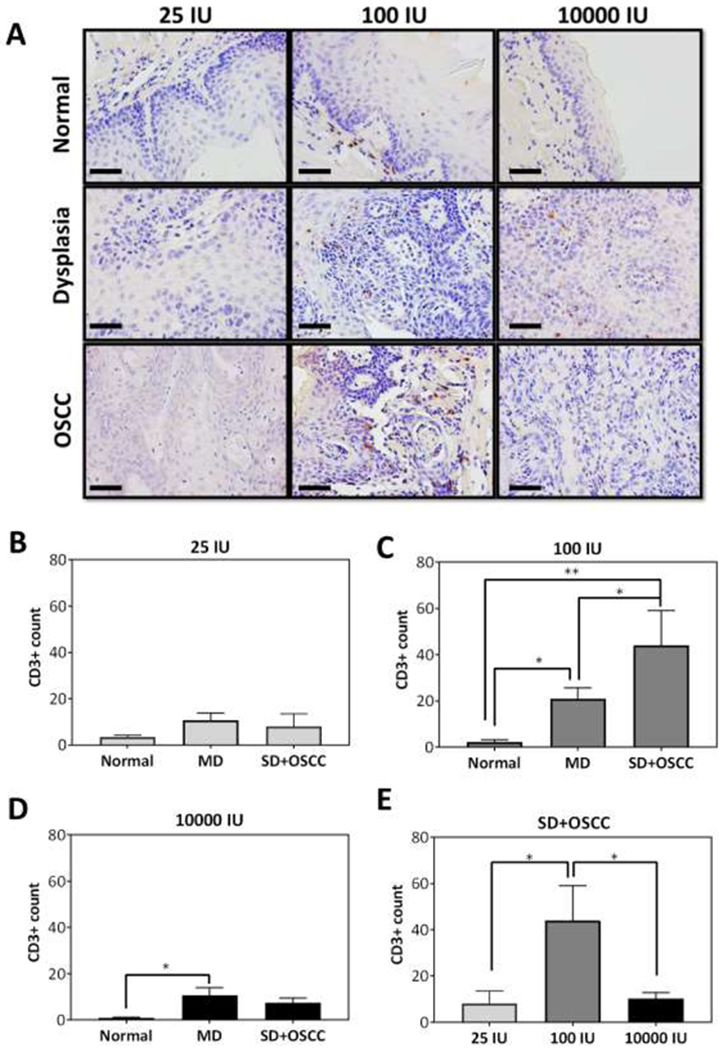
(A) Photomicrographs of CD3 stained sections (40X, scale bar - 50μm) of normal tongue epithelium, dysplasia and OSCC of a representative mouse from each diet group. (B-D) Counts of CD3+ T cells in normal, moderate dysplasia and severe dysplasia + OSCC tissues in mice across the three diets. (E) CD3+ T cell counts in SD+OSCC lesions in the three vitamin D3 diet cohorts. Mice on 100 IU showed significant increase in CD3+ T cell counts in SD+ OSCC compared to mice on 25 IU or 10000 IU. Reported values represent mean ± standard error. *p < 0.05, ** p <0.01
3.5. Short-term Calcitriol treatment to vitamin D3 deficient hosts inhibits growth of orthotopic murine OSCC
And finally, to validate our observations in the 4NQO model, we examined the therapeutic impact of correcting vitamin D deficiency using short-term calcitriol treatment in a 4NQO-derived murine model of OSCC. Orthotopic OSCC tumors were established in C57Bl/6 mice maintained on 25 IU vitamin D3 deficient diet or 1000 IU vitamin D3 reference diet for 6 weeks. Mice with established tumors were assigned to control or calcitriol (0.1 μg, i.p. MWF) arms (n = 4-6 animals per group). Non-invasive MRI was employed to monitor tumor growth over a 28 day period. The panel of images in Fig. 5 represent axial T2-weighted MR images of mice on the 25 IU (A) and the 1000 IU (B) diet. The extent of the tumor is outlined in white on the images in Fig. 5A. No statistically significant difference in tumor growth kinetics was observed between untreated control animals on the two diets. In animals maintained on the 1000 IU diet, calcitriol had no growth inhibitory effects in vivo (Fig. 5A, C). In contrast, longitudinal MRI revealed tumor growth inhibition in vitamin D deficient animals (Fig. 5B). A significant inhibition of tumor growth was observed following calcitriol treatment compared to untreated controls (D). Quantification of total CD3+ T cell levels in the tumors revealed comparable CD3+ T cell counts in the tumors of mice maintained on either 25 IU vs 1000 IU (Supplementary Figure S3). Serum 25(OH)D3 levels were significantly lower (p =0.023) in mice maintained on 25 IU (4.2 ng/ml) compared to 1000 IU diet (13.5 ng/ml). Calcitriol treated mice maintained on 1000 IU showed three fold (21.3 pg/ml) reduction in 1,25(OH)2D3 serum levels compared to controls (68.6 pg/ml) . Mice maintained on 25 IU had higher 1,25(OH)2D3 levels in the calcitriol treated group (26.3 pg/ml) than the controls (20.9 pg/ml) (Supplementary Fig. S4).
Figure 5. Short-term Calcitriol administration to vitamin D3 deficient hosts inhibits growth of orthotopic murine OSCC.

Axial T2-weighted MR images showing growth of orthotopic murine OSCC (white arrows) in control animals (top row) maintained on 1000 IU vitamin D3 (A) or 25 IU vitamin D3 (B), respectively. Corresponding MR images of animals on the respective diets treated with calcitriol (0.1 μg, i.p. MWF for 2 weeks) treated animals are shown in the bottom row. (C) Tumor volumes calculated from longitudinal multi-slice MR images over a 28 period revealed significant tumor growth inhibition following calcitriol treatment (D) compared to controls in mice on the 25 IU diet. No evidence of tumor growth inhibition (C) was observed in mice on 1000 IU diet treated with calcitriol. Reported values represent mean ± standard error. *p < 0.05.
DISCUSSION
The development of clinically translatable strategies to prevent or treat OSCC progression can have a significant impact on the survival and quality of life in this patient population. In this regard, we have been investigating the potential utility of vitamin D in oral cancer. We have recently shown that the chemopreventive efficacy of calcitriol is critically influenced by the stage of intervention and duration of exposure [17]. Given the limitations associated with chronic calcitriol use, in the present study, we examined the interaction between dietary vitamin D supplementation and oral carcinogenesis. Using the 4NQO mouse model of OSCC, we evaluated the effect of three different vitamin D diets (deficient, adequate or pharmacologic) on the development of oral premalignant lesions and invasive OSCC. Histopathologic evaluation revealed lower incidence of high-grade dysplasia and OSCC in animals maintained on 100 IU vitamin D diet (AD) while animals maintained on 10,000 IU diet showed highest incidence of OSCC. The diets were well tolerated and no statistically significant difference in body weights was seen between animals on the three diets over the duration of the study. However, the mean body weight of animals in the 25 and 10,000 IU diets showed a decreasing trend following 16 weeks of carcinogen exposure. This could be potentially reflective of increase in disease burden seen in animals on the 25 IU and 10, 000 IU diets.
The 4NQO model is a clinically-relevant model for the evaluation of preventive strategies as it mimics all the stages of OSCC progression [16,17,27]. 4NQO is a compound found in tobacco, one of the risk factors for OSCC. 4NQO administration in drinking water induces histologic and molecular modifications such as oxidative stress and DNA adduct formation in the oral mucosa of mice, similar to those observed in human OSCC [28, 29]. Given that local CYP24A1 levels are a critical determinant of the anticancer activity of vitamin D3, we examined the levels of VDR and CYP24A1 in the normal tongue epithelium, premalignant and malignant lesions in this model system.. Examination of tongue lesions also revealed higher expression of CYP24A1 in dysplasia and SD+OSCC compared to normal epithelium in mice receiving 100 IU or 10000 IU diet, whereas no difference in CYP24A1 expression was observed in mice receiving the 25 IU diet. This finding is in agreement with previous reports in colon, lung and breast cancer [30–32] and may potentially explain the increased incidence of OSCC with 10000 IU diet. Fleet et al., have previously demonstrated downregulation of CYP27B1 in mice receiving pharmacologic levels of vitamin D which in turn represses local production of 1,25(OH)2D3 [33]. However, a limitation of our study is that we did not measure tissue levels of the vitamin D metabolites. It can therefore only be speculated that in animals maintained on the 10000 IU diet, local 1,25(OH)2D3 levels may be lower rendering them more susceptible to malignant transformation. Consistent with accelerated catabolism of vitamin D metabolites in mice fed 10000 IU, we observe a reduction in 25(OH)D3 serum level by 25 ng/ml between week 0 and week 26 of study. The other two diet cohorts did not show a time dependent decline in 25(OH)D3 levels. We have previously reported on a similar phenomenon associated with chronic administration of calcitriol that enhances carcinogenesis by sustained induction of CYP24A1 and suppression of endogenous 1,25(OH)2D3 levels [17]. Although we did not measure renal CYP24A1 and CYP27B1 levels in the present study, our findings suggest that high level of dietary vitamin D3 leads to downregulation of 25(OH)D3 which ultimately leads to the increased incidence of SD+OSCC.
It is well recognized that immune evasion is one of the hallmarks of carcinogenesis [34]. A consistent body of evidence has documented the immune modulatory effects of vitamin D in preclinical models of cancer including OSCC [35–38]. In this context, it has been suggested that presence of CD3-expressing T-cells may play a role in preventing malignant transformation of oral premalignant lesions [39]. We therefore quantified CD3+ T cells in normal, dysplastic and invasive SCC lesions on the tongues of animals maintained on the three diets and exposed to 4NQO. Immunostaining of tongue sections revealed increased T cell levels in severe dysplasia/OSCC lesions on tongue sections obtained from animals on the 100 IU diet. Conversely, animals maintained on a 25 IU or 10,000 IU diet showed reduced T cell levels. These findings are consistent with previous clinical observations. Walsh et al. have also shown that treatment with 1,25(OH)2D3 can stimulate immune cell infiltration in OSCC patients [40]. A recent report by Bochen et al. have recently shown that head and neck cancer patients with low vitamin D3 (25(OH)D3<15 ng/ml) had significantly low CD3+ intratumoral T cell levels compared to vitamin D3 high patients (25(OH)D3>15 ng/ml) [41]. Whether this increase in T cell levels is due to enhanced recruitment/infiltration or increased proliferation of resident T cells remains to be investigated. In contrast to the 4NQO study in which the animals were maintained on their respective diets for 26 weeks, we did not observe any differences in intratumoral CD3+ T cells in orthotopic murine tumors established in animals maintained on 25 IU and 1000 IU diets for 6 weeks. This suggests the immune-modulatory effects of dietary vitamin D3 in oral cancer may be influenced by duration of exposure.
And finally, a significant number (45-90%) of OSCC patients exhibit severe vitamin D3 deficiency, which is strongly correlated with disease progression [21, 22]. However, the impact of correcting deficiency on OSCC progression has not been previously reported. In our study, we observed that short-term supplementation with calcitriol was much more effective in controlling tumor growth in vitamin D3 deficient animals compared to those on a reference diet (1000 IU). As expected, serum 25(OH)D3 levels were significantly lower in mice maintained on 25 IU compared to 1000 IU diet. Short-term calcitriol treatment increased 1,25(OH)2D3 levels in mice on the 25 IU diet compared to controls and inhibited tumor growth. In contrast, calcitriol treatment of mice maintained on a 1000 IU diet decreased 1,25(OH)2D3 levels and failed to inhibit tumor growth in vivo. This is likely due to increased renal CYP24A1 expression that actively catabolized 1,25 (OH)2D3 [20].
In summary, our results highlight the complex dynamics between vitamin D status and oral carcinogenesis. To the best of our knowledge, this is the first report on the impact of dietary vitamin D on initiation and progression of oral cancer in vivo. Our findings have important clinical implications. Anand et al. have reported that OSCC patients who received vitamin D3 supplementation (1,000 IU VD3/kg) showed reduced therapy-related debilitating effects and had improved quality of life compared to patients who did not receive vitamin D3 [42]. However, extrapolation of our observations to the clinical setting should be done with caution. As such, there is considerable skepticism on the utility of vitamin D3 supplementation in cancer due to failed clinical trials of vitamin D compounds. Recent results from a large (25,000 participants) randomized, placebo-controlled trial of vitamin D3 supplementation (2000 IU per day) and omega-3 fatty acids did not show any reduction in the incidence of breast, prostate or colorectal cancers [43]. It should be noted that the VITAL study did not evaluate the incidence of oral cancers. Similarly, published negative trials of vitamin D or related compounds have been conducted in unselected patient cohorts [44, 45]. Dietary supplementation of vitamin D3 is safe and cost-effective and is therefore attractive for implementation in the clinical setting. Whether correcting vitamin D deficiency through dietary supplementation or calcitriol could have beneficial effects in preventing malignant transformation and slowing oral cancer progression remains to be investigated. In this regard, our findings suggest the need for a ‘precision medicine’ approach to fully realize the benefit of vitamin D through development of tailored dietary supplementation regimens based on individual vitamin D status.
Supplementary Material
HIGHLIGHTS.
Malignant transformation and disease progression of oral lesions is critically impacted by host vitamin D status.
Dietary supplementation of 100 IU vitamin D lowers incidence of oral cancer while pharmacologic levels of dietary vitamin D (10000 IU) potentiate malignant progression.
Short-term calcitriol treatment inhibited growth of oral cancer in vitamin D deficient but not replete hosts.
ACKNOWLEDGEMENTS
This work was supported by grants R01DE024595, R01CA204636, Roswell Park Alliance Foundation and utilized shared resources supported by Roswell Park Comprehensive Cancer Center Support Grant from the National Cancer Institute P30CA06156. The authors would like to thank staff members of the following shared resources at Roswell Park for their technical support in conducting the experimental studies presented in this manuscript - Translational Imaging Resource, Laboratory Animal Resource, and the Pathology Resource Network.
ABBREVIATIONS
- OSCC
Oral squamous cell carcinoma
- VDR
Vitamin D receptor
- 4NQO
4-Nitroquinoline-1-Oxide
- MD
Moderate dysplasia
- SD
Severe dysplasia
- H&E
Hematoxylin and eosin
- MRI
Magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors do not have any conflicts to disclose. The funding sponsors had no role in the design of the study, collection, analyses or interpretation of the data, writing of the manuscript and the decision to publish the results.
REFERENCES
- 1.Warnakulasuriya S Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45(4-5) (2009) 309–316. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 65 (2) (2015) 87–108. [DOI] [PubMed] [Google Scholar]
- 3.Driemel O, Hertel K, Reichert TE, Kosmehl H. Current classification of precursor lesions of oral squamous cell carcinoma principles of the WHO classification 2005. Mund Kiefer Gesichtschir 10 (2) (2006) 89–93. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Ishigamori R. Understanding carcinogenesis for fighting oral cancer. J Oncol 2011 (2011) 603740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zygogianni AG, Kyrgias G, Karakitsos P, Psyrri A, Kouvaris J, Kelekis N, et al. Oral squamous cell cancer: early detection and the role of alcohol and smoking. Head Neck Oncol 3 (2011) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer 70 (1) (1992) 14–19. [DOI] [PubMed] [Google Scholar]
- 7.Mayne ST, Cartmel B, Kirsh V, Goodwin WJ Jr., Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev 18 (12) (2009) 3368–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier JD, Enepekides DJ, Poirier B, Bradley CA, Albala JS, Farwell DG. Treatment with 1-alpha,25-dihydroxyvitamin D3 (vitamin D3) to inhibit carcinogenesis in the hamster buccal pouch model. Arch Otolaryngol Head Neck Surg 133 (11) (2007) 1149–1152. [DOI] [PubMed] [Google Scholar]
- 9.Osafi J, Hejazi A, Stutz DD, Keiserman MA, Bergman CJ, Kingsley K. Differential effects of 1,25-dihydroxyvitamin D3 on oral squamous cell carcinomas in vitro. J Dietary Suppl 11 (2014) 145–154. [DOI] [PubMed] [Google Scholar]
- 10.Prudencio J, Akutsu N, Benlimame N, Wang T, Bastien Y, Lin R, Black MJ, Alaoui-Jamali MA, White JH. Action of low calcemic 1alpha,25-dihydroxyvitamin D3 analogue EB1089 in head and neck squamous cell carcinoma. J Natl Cancer Inst 93 (10) (2001) 745–753. [DOI] [PubMed] [Google Scholar]
- 11.Yang SW, Tsai CY, Pan YC, Yeh CN, Pang JH, Takano M, Kittaka A, Juang HH, Chen TC, Chiang KC. MART-10, a newly synthesized vitamin D analog, represses metastatic potential of head and neck squamous carcinoma cells. Drug Des Devel Ther 10 (2016) 1995–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int 92 (2) (2013) 163–176. [DOI] [PubMed] [Google Scholar]
- 13.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007. September;7(9):684–700. [DOI] [PubMed] [Google Scholar]
- 14.Fanidi A, Muller DC, Midttun O, Ueland PM, Vollset SE, Relton C, et al. Circulating vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the EPIC cohort. Sci Rep 6 (2016) 36017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta D, Vashi PG, Trukova K, Lis CG, Lammersfeld CA. Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Exp Ther Med 2(2) (2011) 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bothwell KD, Shaurova T, Merzianu M, Suresh A, Kuriakose MA, Johnson CS, et al. Impact of Short-term 1,25-Dihydroxyvitamin D3 on the Chemopreventive Efficacy of Erlotinib against Oral Cancer. Cancer Prev Res (Phila) 8(9) (2015) 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent-Chong VK, DeJong H, Attwood K, Hershberger PA, Seshadri M. Preclinical Prevention Trial of Calcitriol: Impact of Stage of Intervention and Duration of Treatment on Oral Carcinogenesis. Neoplasia 21 (4) (2019) 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith EA, Frankenburg EP, Goldstein SA, Koshizuka K, Elstner E, Said J, et al. Effects of long-term administration of vitamin D3 analogs to mice. J Endocrinol 165 (1) (2000) 163–172. [DOI] [PubMed] [Google Scholar]
- 19.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 153 (6) (2012) 2576–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleet JC, Kovalenko PL, Li Y, Smolinski J, Spees C, Yu JG, Thomas-Ahner JM, Cui M, Neme A, Carlberg C, Clinton SK. Vitamin D Signaling Suppresses Early Prostate Carcinogenesis in TgAPT121 Mice. Cancer Prev Res (Phila) 12 (6) (2019) 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orell-Kotikangas H, Schwab U, Osterlund P, Saarilahti K, Makitie O, Makitie AA. High prevalence of vitamin D insufficiency in patients with head and neck cancer at diagnosis. Head Neck 34 (10) (2012) 1450–1455. [DOI] [PubMed] [Google Scholar]
- 22.Grimm M, Cetindis M, Biegner T, Lehman M, Munz A, Teriete P, et al. Serum vitamin D levels of patients with oral squamous cell carcinoma (OSCC) and expression of vitamin D receptor in oral precancerous lesions and OSCC. Med Oral Patol Oral Cir Bucal 20 (2) (2015) e188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol 45(4-5) (2009) 361–85. [DOI] [PubMed] [Google Scholar]
- 24.Biddle A, Gammon L, Liang X, Costea DE, Mackenzie IC. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. EBioMedicine 4 (2016) 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen JH, Graham MP, Chinn SB, Darr OF, Chepeha DB, Wolf GT, et al. Novel method of cell line establishment utilizing fluorescence-activated cell sorting resulting in 6 new head and neck squamous cell carcinoma lines. Head Neck 38 (2016) Suppl 1:E459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A, Rich LJ, Vincent-Chong VK, Seshadri M. Visualizing the effects of metformin on tumor growth, vascularity, and metabolism in head and neck cancer. J Oral Pathol Med 47(5) (2018):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou G, Hasina R, Wroblewski K, Mankame TP, Doci CL, Lingen MW. Dual inhibition of vascular endothelial growth factor receptor and epidermal growth factor receptor is an effective chemopreventive strategy in the mouse 4-NQO model of oral carcinogenesis. Cancer Prev Res (Phila) 3(11) (2010) 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 42 (7) (2006) 655–667. [DOI] [PubMed] [Google Scholar]
- 29.Ikenaga M, Ishii Y, Tada M, Kakunaga T, Takebe H. Excision-repair of 4-nitroquinolin-1-oxide damage responsible for killing, mutation, and cancer. Basic Life Sci 5B (1975) 763–771. [DOI] [PubMed] [Google Scholar]
- 30.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol 57(2) (2006) 234–240. [DOI] [PubMed] [Google Scholar]
- 31.Zhalehjoo N, Shakiba Y, Panjehpour M. Gene expression profiles of CYP24A1 and CYP27B1 in malignant and normal breast tissues. Mol Med Rep 15 (1) (2017) 467–473. [DOI] [PubMed] [Google Scholar]
- 32.Mazzilli SA, Hershberger PA, Reid ME, Bogner PN, Atwood K, Trump DL, et al. Vitamin D Repletion Reduces the Progression of Premalignant Squamous Lesions in the NTCU Lung Squamous Cell Carcinoma Mouse Model. Cancer Prev Res (Phila). 2015;8(10):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr 138(6) (2008) 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144(5) (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- 35.Karkeni E, Morin SO, Bou Tayeh B, Goubard A, Josselin E, Castellano R, et al. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front Immunol 10 (2019) 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY, Chen WC, et al. 1alpha,25-Dihydroxyvitamin D3 Inhibits Esophageal Squamous Cell Carcinoma Progression by Reducing IL6 Signaling. Mol Cancer Ther. 2015;14(6):1365–75. Epub 2015/04/01. doi: 10.1158/1535-7163.MCT-14-0952. . [DOI] [PubMed] [Google Scholar]
- 37.Young MR, Day TA. Immune regulatory activity of vitamin d3 in head and neck cancer. Cancers (Basel) 5(3) (2013) 1072–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker DD, Reeves TD, de Costa AM, Schuyler C, Young MR. Immunological modulation by 1α,25-dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine 58(3) (2012) 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohman J, Mowjood R, Larsson L, Kovacs A, Magnusson B, Kjeller G, et al. Presence of CD3-positive T-cells in oral premalignant leukoplakia indicates prevention of cancer transformation. Anticancer Res. 35 (1) (2015) 311–317. [PubMed] [Google Scholar]
- 40.Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 71 (7) (2010) 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bochen F, Balensiefer B, Korner S, Bittenbring JT, Neumann F, Koch A, et al. Vitamin D deficiency in head and neck cancer patients - prevalence, prognostic value and impact on immune function. Oncoimmunology. 7 (9) (2018) e1476817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand A, Singh S, Sonkar AA, Husain N, Singh KR, Singh S, et al. Expression of vitamin D receptor and vitamin D status in patients with oral neoplasms and effect of vitamin D supplementation on quality of life in advanced cancer treatment. Contemp Oncol (Pozn) 21 (2) (2017) 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 380 (1) (2019) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antunac Golubic Z, Barsic I, Librenjak N, Plestina S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer. Nutr Cancer 70 (3) (2018) 413–417. [DOI] [PubMed] [Google Scholar]
- 45.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354(7) (2006) 684–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


