Abstract
Background:
Prenatal exposure to ethanol has lasting effects on neuropeptide and neuroimmune systems in the brain alongside detrimental alcohol-related behaviors. At low-to-moderate doses, prenatal ethanol stimulates neurogenesis in lateral hypothalamus (LH) and increases neurons that express the orexigenic peptides, hypocretin/orexin (Hcrt/OX) and melanin-concentrating hormone (MCH), and the proinflammatory chemokine CCL2 which through its receptor CCR2 stimulates cell differentiation and movement. Our recent studies demonstrated that CCL2 and CCR2 colocalize with MCH neurons and are involved in ethanol’s stimulatory effect on their development but show no relation to Hcrt/OX. Here we investigated another chemokine CXCL12 and its receptor CXCR4 which promote neurogenesis and neuroprogenitor cell proliferation, to determine if they also exhibit peptide specificity in their response to ethanol exposure.
Methods:
Pregnant rats were intraorally administered a moderate dose of ethanol (2 g/kg/d) from embryonic day 10 (E10) to E15. Their embryos and postnatal offspring were examined using real-time quantitative PCR and immunofluorescence histochemistry, to determine if ethanol affects CXCL12 and CXCR4 and the colocalization of CXCR4 with Hcrt/OX and MCH neurons in the LH and with radial glia neuroprogenitor cells in the hypothalamic neuroepithelium (NEP).
Results:
Prenatal ethanol strongly stimulated CXCL12 and CXCR4 in LH neurons of embryos and postnatal offspring. This stimulation was significantly stronger in Hcrt/OX than MCH neurons in LH and also occurred in radial glia neuroprogenitor cells dense in the NEP. These effects were sexually dimorphic, consistently stronger in females than males.
Conclusions:
While showing prenatal ethanol exposure to have a sexually dimorphic, stimulatory effect on CXCL12 and CXCR4 in LH similar to CCL2 and its receptor, these results reveal their distinct relationship to the peptide neurons, with the former closely related to Hcrt/OX and the latter to MCH, and they link ethanol’s actions in LH to a stimulatory effect on neuroprogenitor cells in the NEP.
Keywords: Prenatal ethanol exposure, Hypocretin/Orexin, Melanin-concentrating hormone, CXCL12/CXCR, radial glia neuroprogenitors
Introduction
Maternal consumption of ethanol during pregnancy has varying teratogenic effects on neurological development and later behaviors of the offspring, including an increased risk for alcohol abuse and anxiety-related behaviors (Malone et al., 2010, Caputo et al., 2016, Gupta et al., 2016). Animal models in studies from this lab (Chang et al., 2015, 2018) and others (Fabio et al., 2015, Wille-Bille et al., 2018) reproduce such behavioral disturbances after prenatal ethanol exposure and provide evidence that they result from changes in neurochemical systems in the brain. These include the neuropeptides, hypocretin/orexin (Hcrt/OX) and melanin-concentrating hormone (MCH), which are expressed in neurons of the lateral hypothalamus (LH) with broad projections throughout the brain (Sakurai et al., 1998, de Lecea et al., 1998) and have a role in the development of alcohol abuse and addictive-like behaviors (DiLeone et al., 2003, Li et al., 2014, Karlsson et al., 2016). While chronic high doses of prenatal ethanol cause morphological and functional defects in neuronal development (Guizzetti et al., 2014, Komada et al., 2017), prenatal ethanol at low-to-moderate doses (1–3 g/kg/d) from embryonic day 10 (E10) to E15, which raise blood ethanol to 85–115 mg/dL during peak hypothalamic neurogenesis, markedly stimulates neural differentiation (Chang et al., 2012), consistent with in vitro evidence showing lower ethanol doses to increase neurogenesis and neuroprogenitor cell proliferation (Santillano et al., 2005, Vangipuram and Lyman, 2010). As shown in adolescent rats, ethanol also increases the density of Hcrt/OX and MCH neurons while causing greater alcohol drinking behavior and anxiety (Chang et al., 2012, 2015, 2018). Interestingly, these neural and behavioral changes observed in rats are confirmed in another vertebrate species, zebrafish, which after low ethanol doses in the water exhibit a stimulation of Hcrt/OX neurons along with increased voluntary alcohol consumption (Collier et al., 2019, Sterling et al., 2016).
Neuroimmune factors in the brain are also profoundly affected by ethanol exposure (Drew and Kane, 2014, Crews et al., 2015). One such factor is the C-C motif chemokine 2 (CCL2), which is a pro-inflammatory agent that functions through its main receptor, CCR2, to stimulate cell differentiation, motility and migration (Watson et al., 2019). In rats, embryonic ethanol exposure at low-to-moderate levels stimulates both CCL2 and CCR2 in the LH, predominantly in neurons, and it increases their colocalization in up to 90% of the MCH neurons (Chang et al., 2015, 2018, Terasaki and Schwarz, 2016), a neuropeptide known itself to have inflammatory properties (Ziogas et al., 2014). These effects, which are observed in embryos as well as postnatal and adolescent offspring, are sexually dimorphic, consistently stronger in females than males (Chang et al., 2018, 2019, Terasaki et al., 2016,), in agreement with clinical evidence that alcohol drinking and rates of alcohol use disorder are greater in women (Foster et al., 2015, Peltier et al., 2019). Furthermore, the endogenous CCL2/CCR2 axis is found to have a role in mediating ethanol’s stimulatory actions on the development of MCH neurons and alcohol-related behaviors, as shown with pharmacological manipulations of CCR2 and administration of CCL2 itself during pregnancy (Chang et al., 2018, 2019).
While closely linked to MCH neurons, the CCL2/CCR2 axis stimulated by ethanol shows little relation to Hcrt/OX neurons (Chang et al., 2018, Le Thuc et al., 2016), leading us to investigate here a different neuroimmune system possibly involved in mediating ethanol’s stimulatory effect on this neuropeptide. This is the C-X-C motif chemokine 12, CXCL12, which while similarly inducible along with its receptor CXCR4 under inflammatory conditions is classified into a separate category of homeostatic chemokines that are constitutively expressed (Devarapu et al., 2016, Trettel et al., 2019, Watson et al., 2019). While there are no studies in the brain, CXCL12 in the periphery is shown to be stimulated by ethanol (Gil-Bernabe et al., 2011, Karim et al., 2013), and there is evidence in the spinal cord that CXCL12 exists in the neuroepithelium (NEP) (Zhu et al., 2015), where radial glia neuroprogenitor cells are dense and a primary source of neurons (Bedont et al., 2015, Florio and Huttner, 2014). Whereas both the CXCL12/CXCR4 and CCL2/CXCR2 chemokine networks have a role in embryonic development and cell migration, they have specific functions that differ, with the former involved in stimulating neurogenesis and neuroprogenitor cell proliferation and the latter more active in the differentiation and motility/movement of cells (Cheng et al., 2017, Watson et al., 2019, Janssens et al., 2018).
Here we investigated in the embryo and postnatal offspring whether CXCL12 and its receptor CXCR4 are stimulated by prenatal ethanol exposure and how CXCR4 relates not only to the peptide neurons in the LH but also to the radial glia neuroprogenitor cells in the NEP. We also examined both females and males to determine if ethanol’s effects are sexually dimorphic. In addition to revealing a role of the CXCL12/CXCR4 axis in mediating ethanol’s stimulatory effects on the development of peptide neurons and also on neuroprogenitor cells, our results provide evidence for how the brain’s various neuroimmune networks after ethanol stimulation can function differentially in relation to distinct populations of neurons, such as Hcrt/OX and MCH, that promote alcohol-related behaviors.
Materials and Methods
Animals
All procedures were conducted in a fully accredited AAALAC facility (22°C, 12:12-h light-dark cycle with lights off at 7 am), in accordance with protocols approved by The Rockefeller University Animal Care and Use Committee and consistent with the NIH Guide to the Care and Use of Laboratory Animals.
Time-pregnant, Sprague-Dawley rats (220–240g) (Charles River Breeding Laboratories, Hartford, CT) arrived at the facility on embryonic day 5 (E5) and were acclimated to the laboratory conditions until E10, at which time experiments began as described in detail below. In all experiments, rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO) and filtered water were available ad libitum. Two brain areas were examined in the female and male offspring, namely, the NEP in the embryos sacrificed at E19, when the peptide neurons first exhibit an adult-like pattern (Brischoux et al., 2001) and are found to be stimulated by ethanol at this age (Chang et al., 2019), and also the LH of postnatal offspring at P10, when the peptide neurons are further developed and more readily seen. One female and 1 male rat were taken from each litter, with the number of rats/sex/group (n = 6–8) equal to the number of litters/group.
Maternal administration of ethanol
Pregnant rats (n = 7–8/experiment) were intraorally administered, from E10-E15 when the peptide neurons are developing in the hypothalamus, either a 2 g/kg/day ethanol solution (30% v/v) (“Ethanol”) or a control solution of maltose-dextrin made isocaloric to the ethanol solution (“Control”) (Chang et al., 2012) with an additional group of pregnant rats that were untreated controls (“Untreated”). The daily dose of ethanol was split in half with all rats gavaged twice daily, with the first gavage of 1 g/kg occurring 2 h after start of the dark cycle and the second gavage of this dose occurring 7 h later. In blood collected from the tail vein at 2 h after the morning ethanol gavage on E11, blood ethanol concentration (BEC) was measured using Analox GM7 Alcohol Analyzer (Lunenburg, MA, USA) and was elevated to ~80 mg/dL, consistent with previous reports (Qiang et al., 2002, Chang et al., 2012). There were no differences between the Ethanol, Control and Untreated groups in terms of the dam’s body weight (224–251 g), chow intake (60–80 kcal/day), and litter size (7–14), with no spontaneous abortions.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to measure the gene expression of CXCL12, CXCR4, Hcrt/OX and MCH in the LH. The rats were sacrificed at P10, and the LH was dissected as described (Chang et al., 2018). Total RNA was then extracted from each microdissected sample, cDNA was synthesized, and qRT-PCR was performed, as previously described in our publications (Chang et al., 2012, 2018, Barson et al., 2013) and others (West et al., 2017, Adachi et al., 2018). The primers for CXCL12, CXCR4 and the neuropeptides were designed with ABI Primer Express Version 3.0 software from published sequences, and their sequences and concentrations are presented in Table 1. The mRNA levels of the target gene in each rat were normalized within subject relative to mRNA levels of the internal housekeeping gene, cyclophilin, in the same sample, and this ratio of target gene expression to housekeeping gene expression was calculated in each rat using the standard delta-delta Ct method. An ANOVA was then run on this ratio calculated for each subject, with the effect of ethanol on target gene expression determined by comparing the average ratio in the experimental groups to the control groups, as well as the control groups to each other. The mRNA gene expression data are presented in the figures as a fold change in the Ethanol group relative to Control groups.
Table 1:
Primers for measurements of mRNA levels using qRT-PCR
| Gene | GenBank Accession | Forward 5’−3’ | Reverse 5’−3’ | Concentration (nM) |
|---|---|---|---|---|
| Cyclophilin | NM_001004279 | TGTGCTGAATATTGGTGCTTGTAA | TGTGCTGAATATTGGTGCTTGTAA | 200 |
| CXCL12 | NM_001033883 | AGCCAACGTCAAACATCTGAAA | CGGGTCAATGCACACTTGTC | 200 |
| CXCR4 | NM_022205 | CGAGCATTGCCATGGAAATA | CGGAAGCAGGGTTCCTTGT | 200 |
| Hcrt/OX | AF019565 | AGATACCATCTCTCCGGATTGC | CCAGGGAACCTTTGTAGA AGGA | 50 |
| MCH | M_29712 | CAAACAGGATGGCGAAGATGA | AGGCTTTCCCCATCCTGAAT | 50 |
Abbreviations: CXCL12, C-X-C motif chemokine 12; CXCR4, C-X-C receptor type 4; Hcrt/OX, hypocretin/orexin; MCH, melanin-concentrating hormone.
Single- and double-label immunofluorescence histochemistry
Single-label immunofluorescence histochemistry (IF) was used, as previously described (Chang et al., 2013, 2015, 2018), to characterize the distribution pattern and quantify the density of CXCL12-immunoreactive (CXCL12+), CXCR4-immunoreactive (CXCR4+), Hcrt/OX-immunoreactive (Hcrt/OX+), and MCH-immunoreactive (MCH+) cells in the LH of postnatal offspring at P10 and the density of CXCR4+ and brain lipid-binding protein (BLBP+) cells, a marker of radial glia neuroprogenitors, in the hypothalamic NEP of E19 embryos. The precise areas of LH and NEP are outlined in Fig. 1A and 1B, which illustrate CXCR4+ cells, respectively, in the LH at P10 and in NEP at E19, respectively. The CXCR4 antibody was verified by preabsorption with CXCR4 protein which totally eliminated the staining, while CXCL12+ cells were undetectable at this young age using currently available antibodies. To obtain the brains at P10 and E19, the postnatal rats and pregnant dams, respectively, were perfused intracardially with 0.9% normal saline followed by 4% paraformaldehyde in phosphate buffer (PB), the tails of the offspring and embryos were collected for genotyping to determine the sex, and their brains were removed and post-fixed for 4 h in the same fixative at 4° C, cryo-protected in 25% sucrose at 4° C for 72–96 h, and then frozen and stored at −80° C. Brains were cut at 30 μm with a cryostat, and free-floating coronal sections were incubated in the primary antibody at 4° C for 72 h and then in the secondary antibody at room temperature in the dark for 2 h, with 30 minutes wash in PBS between the two incubations. The primary and secondary antibodies are listed in Table 2. Fluorescence images in entire LH from P10 offspring were captured using a Zeiss fluorescence microscopy with 10x objective, and fluorescence images in NEP from E19 embryos were acquired using a Zeiss LSM 880 confocal microscope with 20x objective. For each brain, 8–12 images were collected at the anterior-posterior level of A 3.2 – A 2.3 mm in P10 offspring (Sherwood and Timiras, 1970) and at E20 coronal plates 12 to 13 for E19 embryo brains (Altman and Bayer, 1995). The density of single-labeled immunofluorescent cells was quantified using Image-Pro Plus software (Version 4.5; Media Cybernetics) as previously described (Chang et al., 2013, 2015, 2018). Only intact cells with an area of 50–100 μm2 were counted, and the population density of these cells is reported as cells/μm2.
Figure 1:
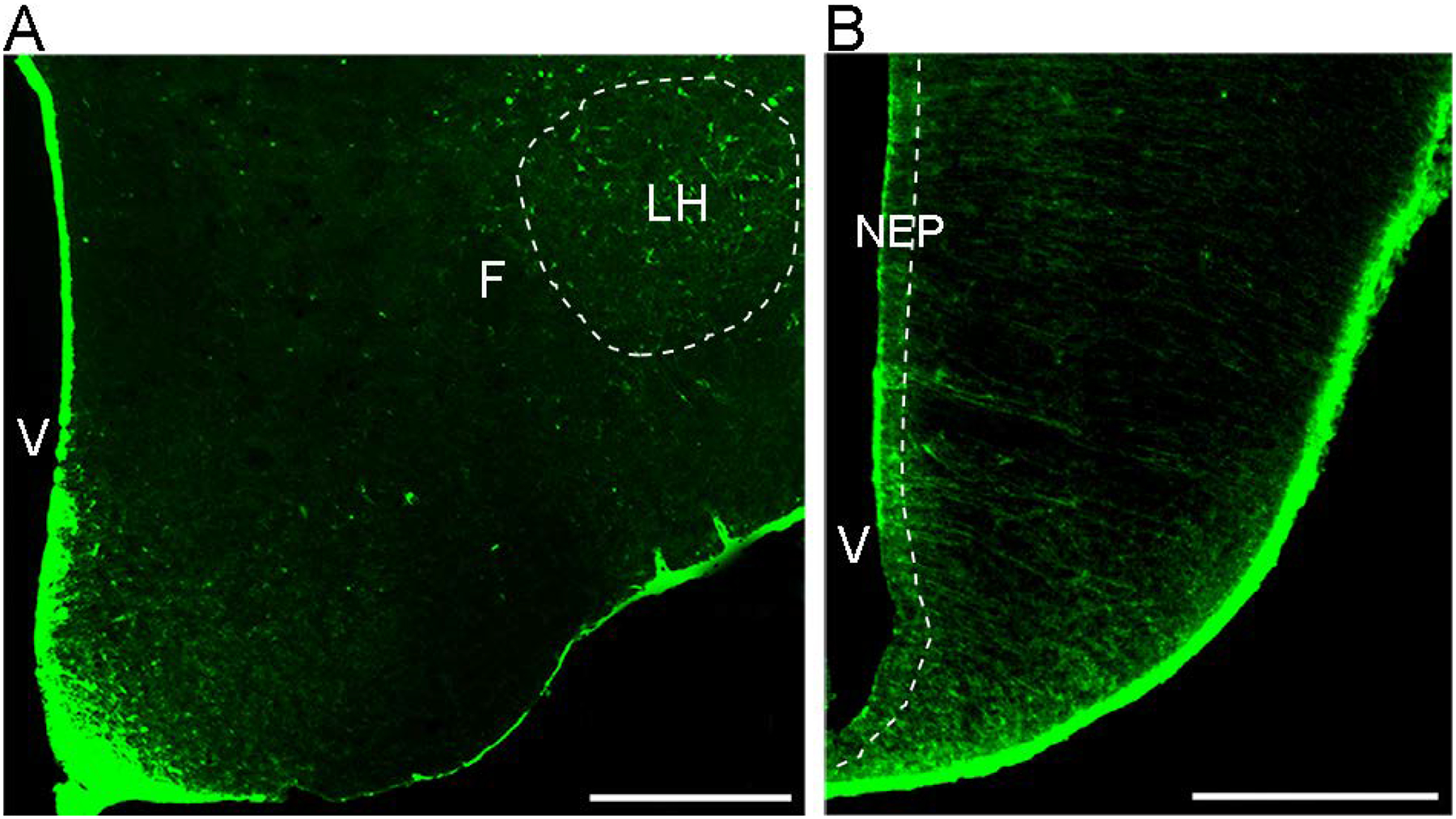
Photomicrographs illustrate CXCR4-immunoreactive cells in the two areas, lateral hypothalamus (LH) in postnatal offspring and hypothalamic neuroepithelium (NEP) in the embryo, that were examined in this manuscript. Abbreviations: V, ventricle; F, fornix, LH, lateral hypothalamus; NEP, neuroepithelium; Scale bar, 500 μm.
Table 2:
Antibodies used for single-labeling immunofluorescence histochemistry
| Primary antibody | Dilution | Vendor | Secondary antibody | Dilution | Vendor |
|---|---|---|---|---|---|
| Goat anti-CXCR4 | 1:100 | Abcam, MA | Bio-Horse anti-Goat IgG + TSA | 1:100 | Vector, CA |
| Rabbit anti-BLBP | 1:200 | Abcam, MA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch |
| Laboratories Inc, PA | |||||
| Rabbit anti-MCH | 1:1000 | Phoenix | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch |
| Pharmaceuticals, CA | Laboratories. Inc, PA | ||||
| Mouse anti-NeuN | 1:100 | EMD Millipore, MA | Cy3-Donkey anti-Mouse IgG | 1:100 | Jackson ImmunoResearch |
| Laboratories. Inc, PA | |||||
| Rabbit anti- | 1:500 | Phoenix | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch |
| Hcrt/OX | Pharmaceuticals, CA | Laboratories. Inc, PA |
Abbreviations: CXCR4, C-X-C receptor type 4; BLBP, brain lipid-binding protein; MCH, melanin-concentrating hormone; NeuN, neuronal nuclei; Hcrt/OX, hypocretin/orexin.
Double-labeled IF was used to determine if CXCR4 colocalizes with the neuronal marker NeuN, Hcrt/OX and MCH peptide neurons in LH and with the radial glia marker BLBP in NEP. While CXCL12/CXCR4 signaling in glia has been extensively studied in adult animals and in vitro (Banisadr et al., 2005, Sowa et al., 2017, Slusarczyk et al., 2015), we did not examine the glia marker, GFAP, here since our studies in embryos and postnatal rats have revealed very few labeled cells in the hypothalamus, no effect of low-to-moderate ethanol doses on their density, and no co-labeling with CCL2 and CCR2 at this early age (Chang et al., 2015, 2018, 2019). The double-labeling with NeuN, Hcrt/OX, MCH and BLBP was performed using antibodies listed in Table 3, based on our procedures described previously (Chang et al., 2018, 2019). After incubation in the primary antibody as above, sections were rinsed in PBS for 6 × 10 min and then incubated for 90 min in a combination of biotin-conjugated horse anti-goat IgG for CXCR4, with Cy3-conjugated donkey anti-mouse IgG for NeuN or with Cy3-conjugated donkey anti-rabbit IgG for BLBP, Hcrt/OX and MCH. This was followed by 30 min incubation in streptavidin-HRP (1:100, Perkin Elmer) and 5 min incubation in fluorescein tyramide (1:50, Perkin Elmer) to amplify CXCR4 immunolabeling, with all incubations conducted in the dark at room temperature. Double-labeling images were captured using a Zeiss LSM 880 confocal microscope, with double-labeled cells counted in Z-Stacks (30 μm thick with a step size of 0.44–0.50 μm) using a 20x lens and further confirmed using a 40x oil-immersion lens. In all analyses, the cells were counted in each section, and only the cells of a designated size (area of 50–100 μm) were counted. The double-labeled cells in each rat in each group were averaged and reported as the percentage of total single-labeled cells. To confirm that the BLBP+ and CXCR4+ cells in the NEP are epithelial cells, we used DAPI to stain the nucleus. Confocal Z-Stack sectioning (30μm thick and step size of 1.02 μm) of double-staining of DAPI with BLBP or CXCR4 in the NEP showed with a 40x objective that almost all of the radial glia cells and CXCR4 cells contained a nucleus, confirming that they are epithelial in nature.
Table 3:
Antibodies used for double-labeling immunofluorescence histochemistry
| Combination | Primary antibody | Dilution | Vendor | Secondary antibody | Dilution | Vendor |
|---|---|---|---|---|---|---|
| CXCR4+BLBP | Goat anti-CXCR4 | 1:200 | Abcam, MA | Bio-Horse anti-Goat IgG + TSA | 1:100 | Vector, CA |
| Rabbit anti-BLBP | 1:200 | Abcam, MA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch | |
| Laboratories Inc, PA | ||||||
| CXCR4+NeuN | Goat anti-CXCR4 | 1:200 | Abcam, MA | Bio-Horse anti-Goat IgG + TSA | 1:100 | Vector, CA |
| Mouse anti-NeuN | 1:100 | EMD Millipore, MA | Cy3-Donkey anti-Mouse IgG | 1:100 | Jackson ImmunoResearch | |
| Laboratories Inc, PA | ||||||
| CXCR4+Hcrt/OX | Goat anti-CXCR4 | 1:200 | Abcam, MA | Bio-Horse anti-Goat IgG + TSA | 1:100 | Vector, CA |
| Rabbit anti-Hcrt/OX | 1:500 | Phoenix Pharmaceuticals, CA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch Laboratories Inc, PA |
|
| CXCR4+MCH | Goat anti-CXCR4 | 1:200 | Abcam, MA | Bio-Horse anti-Goat IgG + TSA | 1:100 | Vector, CA |
| Rabbit anti-MCH | 1:1000 | Phoenix Pharmaceuticals, CA | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch Laboratories Inc, PA |
Abbreviations: CXCR4, C-X-C receptor type 4; BLBP, brain lipid-binding protein; NeuN, neuronal nuclei; Hcrt/OX, hypocretin/orexin; MCH, melanin-concentrating hormone.
Statistical Analysis
All data were presented as mean ± SEM and were analyzed using SPSS (Version 23). All graphs were prepared using the GraphPad Prism software (Version 6). Data were analyzed using a two-way ANOVA when both females and males were examined and a one-way ANOVA when only females were examined. The two-way ANOVA tested within-subject main effects of maternal ethanol administration and their respective controls, between-subject main effects of sex, and the interactions between sex and maternal treatment. A significant interaction was interpreted using simple main effect analyses to test the differences between sexes as well as the differences within each sex. Paired t-tests were performed to directly compare the effects of maternal treatment vs control groups within each sex and the effects of maternal ethanol to isocaloric control when only two groups in one sex were examined.
Results
Prenatal ethanol increases expression of CXCL12, CXCR4, and neuropeptides in LH of postnatal offspring
Here we tested using qRT-PCR the effects of maternal intraoral ethanol administration (2 g/kg/d, E10-E15) compared to the Control (isocaloric maltose-dextrin solution) and Untreated control groups on gene expression in LH of both CXCL12 and CXCR4 and the peptides Hcrt/OX and MCH in female and male postnatal offspring at P10 (n = 8/sex/group). Analysis via a two-way ANOVA revealed a significant main effect of prenatal ethanol on mRNA levels of CXCL12 (F(2,42) = 17.79, p = 0.00) and CXCR4 (F(2,42) = 9.32, p = 0.00) and also of Hcrt/OX (F(2,42) = 48.17, p = 0.000) and MCH (F(2,42) = 55.25, p = 0.000) (Fig. 2). In addition to a significant main effect of sex on CXCL12 (F(1,42) = 37.26, p = 0.00) and CXCR4 (F(1,42) = 35.00, p = 0.00) but not Hcrt/OX (F(1,42) = 1.37, p = 0.714) and MCH (F(1,42) = 12.36, p = 0.010), it revealed a significant interaction between sex and ethanol treatment for mRNA expression of CXCL12 (F(2,42) = 13.29, p = 0.00) and CXCR4 (F(2,42) = 13.46, p = 0.00) and also of Hcrt/OX (F(2,42) = 4.36, p = 0.019) and MCH (F(2,42) = 17.75, p = 0.000).
Figure 2:

Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared to Control and Untreated groups (n = 8/sex/group) altered mRNA levels (using qRT-PCR) of CXCL12, CXCR4, Hcrt/OX and MCH in the LH of postnatal offspring at P10, with the data presented here as mRNA fold change compared to control groups. Ethanol significantly increased mRNA levels of CXCL12 and CXCR4 in female but not male offspring, and it increased Hcrt/OX and MCH mRNA in females more than male offspring. There were no sex differences in the control groups and no differences between the two control groups. Abbreviations: CXCL12, C-X-C motif ligand 12; CXCR4, C-X-C receptor 4; Hcrt/OX, hypocretin/orexin; MCH, melanin-concentrating hormone; P10, postnatal day 10; LH, lateral hypothalamus. Data are mean ± SEM. *p < 0.05 versus control group. #p < 0.05 versus males.
Simple main effect analyses of the two control groups, Control and Untreated, showed no differences between the females and males. This is in their expression of CXCL12 (p = 0.069 for Control and p = 0.324 for Untreated), CXCR4 (p = 0.381 for Control and p = 0.089 for Untreated), Hcrt/OX (p = 0.834 for Control and p = 0.110 for Untreated), and MCH (p = 0.453 for Control and p = 0.973 for Untreated). There were also no differences between the Control and Untreated groups, indicating that the intraoral infusion itself had little impact on gene expression in postnatal offspring as previously shown in adolescent offspring (Chang et al., 2018). This was evident for females, with measurements of CXCL12 (p = 0.142), CXCR4 (p = 0.298), Hcrt/OX (p = 0.544) and MCH (p = 0.278), and for males, with measurements of CXCL12 (p = 0.535), CXCR4 (p = 0.844), Hcrt/OX (p = 0.423), and MCH (p = 0.712).
After ethanol exposure, however, the female and male offspring were clearly different (Fig. 2). The ethanol-exposed females compared to males had significantly higher mRNA levels of CXCL12 (p = 0.000) and CXCR4 (p = 0.000) and also of Hcrt/OX (p = 0.017) and MCH (p = 0.000). Interestingly, whereas ethanol strongly increased in female offspring expression of CXCL12 compared to their Control (p = 0.000) and Untreated (p = 0.000) groups and of CXCR4 compared to their Control (p = 0.000) and Untreated (p = 0.000) groups, it had no impact in male offspring on both CXCL12 compared to their Control (p = 0.931) or Untreated (p = 0.480) groups and CXCR4 compared to their Control (p = 0.642) or Untreated (p = 0.508) groups. Further, while ethanol increased Hcrt/OX and MCH mRNA in both females (p = 0.000 and p = 0.000, respectively) and males (p = 0.000 and p = 0.005, respectively) compared to their Control group and both females (p = 0.000 and p = 0.000 respectively) and males (p = 0.000 and p = 0.013, respectively) compared to their Untreated group, direct comparisons between the sexes (via paired t-test) revealed significantly stronger effects on these peptides in the ethanol-exposed females. The ethanol-induced increase in Hcrt/OX mRNA was significantly greater in females than males when compared to the Control (t(14) = 2.794, p = 0.014) and Untreated (t(14) = 2.874, p = 0.012) groups, as was the ethanol-induced increase in MCH mRNA when compared to the Control (t(14) = 4.804, p = 0.000) and Untreated (t(14) = 5.824, p = 0.000) groups. Thus, prenatal ethanol stimulates gene expression of both CXCL12 and CXCR4 along with the peptide neurons in the LH of female more than male postnatal offspring.
Prenatal ethanol increases density of CXCR4 and peptide neurons in LH of postnatal offspring
This experiment used single-label IF to examine the effects of prenatal ethanol exposure compared to Control and Untreated groups on the single-labeled CXCR4+, Hcrt/OX+ and MCH+ cells in the LH of female and male postnatal offspring at P10 (n = 7/sex/group). As with the peptides, the CXCR4+ cells were found to be particularly dense in the LH, although still evident throughout the hypothalamus. Analysis via a two-way ANOVA of these single-labeled cells revealed a significant main effect of ethanol compared to the Control group on the density of CXCR4+ cells (F(2,36) = 45.31, p = 0.000), along with the Hcrt/OX+ (F(2,36) = 18.88, p = 0.000) and MCH+ (F(2,36) = 35.96, p = 0.00) cells (Fig. 3A). It also showed a significant main effect of sex on the density of CXCR4+ (F(1,36) = 18.90, p = 0.000), Hcrt/OX+ (F(1,36) = 7.39, p = 0.010) and MCH+ (F(1,36) = 9.23, p = 0.004) cells and a significant interaction between sex and ethanol treatment for CXCR4+ (F(2,36) = 10.33, p = 0.00), Hcrt/OX+ (F(2,36) = 3.32, p = 0.048) and MCH+ (F(2,36) = 5.79, p = 0.007) cells.
Figure 3:

Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared with Control and Untreated groups (n = 7/sex/group) affected CXCR4+, Hcrt/OX+, and MCH+ cells in the LH of postnatal offspring at P10. A, Ethanol compared with control groups significantly increased the density of single-labeled CXCR4+ (top), Hcrt/OX+ (middle) and MCH+ (bottom) cells in both female and male offspring, with the effects in females significantly greater. B, This effect in female offspring is illustrated in representative immunostaining confocal images of single-labeled CXCR4+, Hcrt/OX+ and MCH+ cells. Abbreviations: F, fornix; CXCR4, C-X-C Receptor 4; Hcrt/OX, hypocretin/orexin; MCH, melanin-concentrating hormone; P10, postnatal day 10; LH, lateral hypothalamus. Scale bar, 100 μm. Data are mean ± SEM. *p < 0.05 versus control group. #p < 0.05 versus males.
Simple main effect analyses revealed no difference between female and male control groups in their density of CXCR4+ (p = 0.588 for Control and p = 0.450 for Untreated), Hcrt/OX+ (p = 0.682 for Control and p = 0.536 for Untreated), and MCH+ (p = 0.776 for Control and p = 0.659 for Untreated) cells in the LH. In contrast, ethanol-exposed females compared to ethanol-exposed males had a significantly higher density of the CXCR4+ (p = 0.000), Hcrt/OX+ (p = 0.001) and MCH+ (p = 0.000) cells (Fig. 3A). Also, ethanol compared to control groups significantly increased in females the density of CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated), Hcrt/OX+ (p = 0.000 for Control and p = 0.000 for Untreated), and MCH+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, as illustrated in the photomicrographs (Fig. 3B). Whereas ethanol compared to control groups also significantly increased in males the density of CXCR4+ (p = 0.002 for Control and p = 0.008 for Untreated), Hcrt/OX+ (p = 0.028 for Control and p = 0.047 for Untreated), and MCH+ (p = 0.001 for Control and p = 0.023 for Untreated) cells, direct comparisons between the sexes (via paired t-test) showed ethanol’s stimulatory effects to be significantly greater in females. This was found for the CXCR4+ (t(12) = 9.144, p = 0.000 compared to Control and t(12) = 8.828, p = 0.000 compared to Untreated), Hcrt/OX+ (t(12) = 6.339, p = 0.000 compared to Control and t(12) = 6.000, p = 0.000 compared to Untreated), and MCH+ (t(12) = 3.716, p = 0.000 compared to Control and t(12) = 3.581, p = 0.000 compared to Untreated) cells. Thus, the stimulatory effects of ethanol on the chemokine receptor and peptide neurons in the LH of postnatal offspring are consistent with the ethanol-induced changes in gene expression, with both found to be sexually dimorphic.
Prenatal ethanol stimulates CXCR4 in neurons of LH in postnatal offspring
Using double-labeling IF, we further characterized the CXCR4+ cells in the LH of female offspring at P10 (n = 6/group), to determine if they are neurons colocalizing the neuronal marker NeuN and, if so, whether ethanol alters the percentage of CXCR4+ cells that are neurons. While increasing the density of single-labeled CXCR4+ cells (5.12 × 10−5 ± 1.87 × 10−7 vs 4.71 × 10−5 ± 1.89 × 10−6 cells/μm2, t(10) = −1.07, p = 0.0404) as shown above and also single-labeled NeuN+ cells (6.23 × 10−5 ± 2.45 × 10−6 vs 4.3247 × 10−5 ± 2.374 × 10−6 cells/μm2, t(10) = −2.215, p = 0.032) as described previously (Chang et al., 2012), ethanol compared to Control significantly increased the density of double-labeled CXCR4+/NeuN+ neurons in the LH. This is relative to the single-labeled CXCR4+ cells (t(10) = −10.65, p = 0.00) and NeuN+ cells (t(10) = −15.23, p = 0.00) (Fig. 4A), as illustrated in the photomicrographs (Fig. 4B). The percentage of CXCR4+ cells that were neurons colabeling NeuN was increased from 30% under control conditions to almost 80% in response to ethanol.
Figure 4:

Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared with Control group (n = 6/group) altered the percentage of double-labeled CXCR4+/NeuN+ cells in the LH of P10 offspring. A, Ethanol compared to control group significantly increased the percentage of double-labeled CXCR4+/NeuN+ cells in female P10 offspring. B, This effect in female offspring on double-labeled CXCR4+/NeuN+ cells is illustrated in the 20x photomicrographs showing (white arrows) the double-labeled CXCR4+/NeuN+ cells, with those in the box showing at higher magnification the single-labeled CXCR4+ (green) and NeuN+ (red) and the double-labeled CXCR4+/NeuN+ cells (yellow). Abbreviations: CXCR4, C-X-C Receptor 4; NeuN, neuronal nuclei; P10 postnatal day 10; LH, lateral hypothalamus. Scale bars, 100 μm. Data are mean ± SEM. *p < 0.05 versus control group.
Prenatal ethanol stimulates co-labeling of CXCR4 with peptide neurons in LH of postnatal offspring
This experiment used double-label IF to test whether prenatal ethanol exposure compared to Control and Untreated groups affects the colocalization of CXCR4 specifically with peptide neurons in the LH of female and male offspring at P10 (n = 7/sex/group). Analysis via a two-way ANOVA revealed a significant main effect of prenatal ethanol on the density of double-labeled CXCR4+/Hcrt/OX cells, relative to single-labeled Hcrt/OX+ (F(2,36) = 119.77, p = 0.000) and CXCR4+ (F(2,36) = 69.241, p = 0.000) cells, and also of double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (F(2,36) = 121.45, p = 0.000) and CXCR4+ (F(2,36) = 168.63, p = 0.000) cells (Fig. 5A). It also showed a significant main effect of sex on the density of double-labeled CXCR4+/Hcrt/OX cells, relative to single-labeled Hcrt/OX+ (F(1,36) = 58.103, p = 0.000) and CXCR4+ (F(1,36) = 28.559, p = 0.000) cells, and of double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (F(1,36) = 50.03, p = 0.000) and CXCR4+ (F(1,36) = 110.86, p = 0.000) cells. Further, there was a significant interaction between sex and ethanol treatment for double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (F(2,36) = 50.41, p = 0.000) and CXCR4+ (F(2,36) = 25.65, p = 0.000) cells, and for double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (F(2,36) = 48.22, p = 0.000) and CXCR4+ (F(2,36) = 103.30, p = 0.000) cells.
Figure 5:

Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared to Control and Untreated groups (n = 7/sex/group) altered the percentage of double-labeled CXCR4+/Hcrt/OX+ cells and CXCR4+/MCH+ cells in the LH of P10 offspring. A, Ethanol compared to control groups significantly increased the percentage of double-labeled CXCR4+/Hcrt/OX+ (top) and CXCR4+/MCH+ (bottom) cells relative to single-labeled CXCR4+ and Hcrt/OX+ or MCH+ cells, in both female and male offspring, with the effects in females significantly greater. B, This effect of ethanol in female postnatal offspring on double-labeled CXCR4+/Hcrt/OX+ and CXCR4+/MCH+ cells is illustrated in the 20x images showing (white arrows) the double-labeled CXCR4+/peptide+ cells, with those in the box enlarged to the right at higher magnification showing the single-labeled CXCR4+ (green) and Hcrt/OX+ or MCH+ (red) cells and the double-labeled CXCR4+/peptide+ cells (yellow). Abbreviations: CXCR4, C-X-C Receptor 4; Hcrt/OX, hypocretin/orexin; MCH, melanin-concentrating hormone; P10, postnatal day 10; LH, lateral hypothalamus. Scale bars, 100 μm. *p < 0.05 versus control group. #p < 0.05 versus male group.
Simple main effect analyses again revealed no differences between female and male control groups, in their density of double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (p = 0.837 for Control and p = 0.694 for Untreated) and CXCR4+ (p = 0.889 for Control and p = 0.856 for Untreated) cells, and of double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (p = 0.952 for Control and p = 0.930 for Untreated) and CXCR4+ (p = 0.795 for Control and p = 0.874 for Untreated) cells. This is in contrast to the ethanol-exposed females compared to ethanol-exposed males, which had a significantly higher density of both double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (p = 0.000) and CXCR4+ (p = 0.000) cells, and double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (p = 0.000) and CXCR4+ (p = 0.000) cells (Fig. 5A). Further, ethanol exposure compared to Control groups in females significantly increased the density of both double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (p = 0.000 for Control and p = 0.000 for Untreated) and CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, and double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (p = 0.000 for Control and p = 0.000 for Untreated) and CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, as illustrated in the photomicrographs (Fig. 5B). In males, ethanol also increased the density of double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (p = 0.001 for Control and p = 0.003 for Untreated) and CXCR4+ (p = 0.009 for Control and p = 0.007 for Untreated) cells, and of double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (p = 0.001 for Control and p = 0.001 for Untreated) and CXCR4+ (p = 0.025 for Control and p = 0.015 for Untreated) cells. However, direct comparisons between sexes (via paired t-test) showed this stimulatory effect on double-labeled cells to be significantly greater in females than males. This was the case for the double-labeled CXCR4+/Hcrt/OX+ cells, relative to single-labeled Hcrt/OX+ (t(12) = 7.78, p = 0.000 compared to Control and t(12) = 7.665, p = 0.000 compared to Untreated) and CXCR4+ (t(12) = 5.196, p = 0.000 compared to Control and t(12) = 5.172, p = 0.000 compared to Untreated) cells, and double-labeled CXCR4+/MCH+ cells, relative to single-labeled MCH+ (t(12) = 7.072, p = 0.000 compared to Control and t(12) = 7.040, p = 0.000 compared to Untreated) and CXCR4+ (t(12) = 10.465, p = 0.000 compared to Control and t(12) = 10.518, p = 0.000 compared to Untreated) cells. The stimulatory effect of ethanol on the percentage of peptide neurons that colocalize CXCR4 was significantly greater (t(12) = −3.362, p = 0.006) for Hcrt/OX neurons (58%) than it was for MCH neurons (18%).
Prenatal ethanol stimulates CXCR4 and BLBP cells in NEP of the embryo
Using single-label IF, we examined the effects of prenatal ethanol compared to Control and Untreated groups on CXCR4+ and BLBP+ radial glia cells in the NEP of female and male embryos at E19 (n = 7/sex/group). Analysis via a two-way ANOVA revealed a significant main effect of prenatal ethanol exposure compared to Control group on the density of CXCR4+ (F(2,36) = 26.44, p = 0.000) and BLBP+ (F(2,36) = 56.697, p = 0.000) cells in the NEP (Fig. 6A). It also showed a significant main effect of sex on the density of CXCR4+ (F(1,36) = 4.58, p = 0.039) and BLBP+ (F(1,36) = 7.87, p = 0.008) cells, along with a significant interaction between sex and ethanol treatment for CXCR4+ (F(2,36) = 7.93, p = 0.001) and BLBP+ (F(2,36) = 13.34, p = 0.000) cells.
Figure 6:
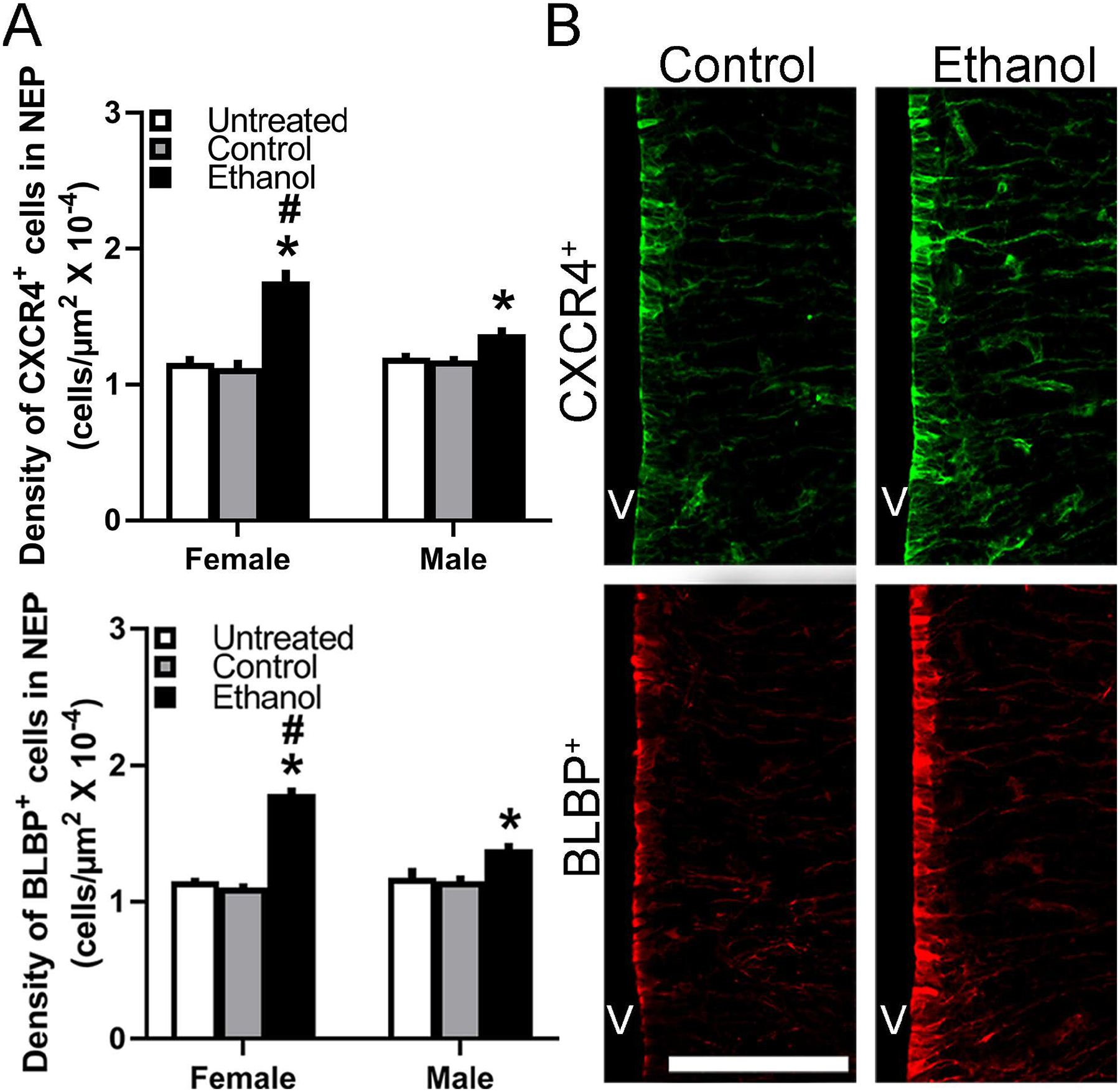
Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared with Control and Untreated groups affected the single-labeled CXCR4+ and BLBP+ cells in the NEP of E19 embryos (n = 7/sex/group). A, Ethanol compared with control groups significantly increased the density of CXCR4+ (top) and BLBP+ (bottom) cells in both female and male embryos, with the effects in females significantly greater. B, This effect in E19 embryos is illustrated in representative immunostaining confocal images of single-labeled CXCR4+ (green) and BLBP+ (red) single-labeled neurons. Abbreviations: CXCR4, C-X-C Receptor 4; BLBP, brain lipid binding protein; E19, embryonic day 19; NEP, neuroepithelium; V, ventricle. Scale bar, 100 μm. Data are mean ± SEM. *p < 0.05 versus control group. #p < 0.05 versus male group.
Simple main effect analyses revealed no differences between female and male control groups, in their density of single-labeled CXCR4+ (p = 0.538 for Control and p = 0.884 for Untreated) and BLBP+ (p = 0.531 for Control and p = 0.734 for Untreated) cells. The ethanol-exposed female embryos, however, compared to ethanol-exposed males had a significantly higher density of both CXCR4+ (p = 0.000) and BLBP+ (p = 0.000) cells in the NEP (Fig. 6A). Ethanol compared to the control groups increased in female embryos the density of CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated) and BLBP+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, as illustrated in the photomicrographs (Fig. 6B). Whereas ethanol compared to the control groups also increased in male embryos the density of CXCR4+ (p = 0.048 for Control and p = 0.031 for Untreated) and BLBP+ (p = 0.001 for Control and p = 0.003 for Untreated) cells, direct comparisons between sexes (via paired t-test) showed this stimulatory effect of ethanol to be significantly greater in females than males. This was found for both the CXCR4+ (t(12) = 4.478, p = 0.001 compared to Control and t(12) = 4.302, p = 0.001 compared to Untreated) and BLBP+ (t(12) = 6.823, p = 0.000 compared to Control and t(12) = 6.506, p = 0.000 compared to Untreated) cells, showing clear sex differences in ethanol’s stimulatory effects on chemokine receptor and radial glia cells in the embryonic NEP.
Prenatal ethanol stimulates co-labeling of CXCR4 with radial glia cells in NEP of the embryo
Using double-label IF, we tested the effects of maternal intraoral ethanol administration compared to Control and Untreated groups on the colocalization of CXCR4 with BLBP+ radial glia cells in the NEP of female and male embryos at E19 (n = 7/sex/group). Analysis via a two-way ANOVA revealed a significant main effect of prenatal ethanol on the density of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (F(2,36) = 695.4, p =0.000) and CXCR4+ (F(2,36) = 568.7, p = 0.000) cells (Fig. 7A). It also showed a significant main effect of sex on the density of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (F(1,36) = 58.58, p = 0.000) and CXCR4+ (F(1,36) = 66.45, p = 0.000) cells, and a significant interaction between sex and ethanol treatment for double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (F(2,36) = 46.75, p = 0.000) and CXCR4+ (F(2,36) = 15.039, p = 0.000) cells.
Figure 7:

Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared to Control and Untreated groups (n = 7/sex/group) altered the percentage of double-labeled CXCR4+/BLBP+ cells in the NEP of E19 embryos. A, Ethanol compared to control groups significantly increased in the NEP the percentage of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled CXCR4+ and BLBP+ cells, in both female and male offspring, with the effects in females significantly greater. B, This effect of ethanol on double-labeled CXCR4+/BLBP+ cells is illustrated in the 20x images showing (white arrowheads) the double-labeled CXCR4+/BLBP+ cells, with those in the box enlarged to the right (40x) showing the single-labeled CXCR4+ (green) and BLBP+ (red) cells and the double-labeled CXCR4+/BLBP+ cells (yellow). Abbreviations: CXCR4, C-X-C Receptor 4; BLBP, brain lipid binding protein; E19, embryonic day 19; NEP, neuroepithelium; V, ventricle. Scale bars, 100 μm. *p < 0.05 versus control group. #p < 0.05 versus male group.
Simple main effect analyses revealed no differences between female and male control groups, in their density of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (p = 0.668 for Control and p = 0.613 for Untreated) and CXCR4+ (p = 0.809 for Control and p = 0.443 for Untreated) cells. However, they showed ethanol-exposed females compared to ethanol-exposed males to have a higher density of the double-labeled CXCR4+/BLBLP+ cells, relative to single-labeled BLBP+ (p = 0.000) and CXCR4+ (p = 0.000) cells in the NEP (Fig. 7A). In females, ethanol compared to control groups increased the density of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (p = 0.000 for Control and p = 0.000 for Untreated) and CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, as illustrated in the photomicrographs (Fig. 7B). Whereas ethanol compared to control groups also increased in males the density of double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ (p = 0.000 for Control and p = 0.000 for Untreated) and CXCR4+ (p = 0.000 for Control and p = 0.000 for Untreated) cells, direct comparisons between the sexes (via paired t-test) showed this stimulatory effect to be significantly greater in females than males. This was shown for double-labeled CXCR4+/BLBP+ cells, relative to single-labeled BLBP+ cells (t(12) = 7.290, p = 0.000 compared to Control and t(12) = 7.242, p = 0.000 compared to Untreated) and single-labeled CXCR4+ cells (t(12) = 4.168, p = 0.001 compared to Control and t(12) = 3.850, p = 0.002 compared to Untreated). These analyses provide clear evidence for ethanol’s stimulatory effect on CXCR4 in radial glia cells of the embryonic NEP.
Discussion
While there is strong evidence that ethanol stimulates different inflammatory factors in the brain and periphery of adult animals (Drew and Kane, 2014, Vetreno and Crews, 2014), there are only a few studies of the CXCL12/CXCR4 chemokine axis, with ethanol shown to increase expression of CXCR4 in liver endothelial cells (Karim et al., 2013) and plasma levels of CXCL12 (Gil-Bernabe et al., 2011). Here we provide strong evidence that prenatal exposure to moderate levels of ethanol, during a 5-day period (E10-E15) of peak neurogenesis, stimulates this chemokine network in the LH as shown in postnatal rats. While CXCL12 and CXCR4 in the brain exist in different cell types (Banisadr et al., 2003, Sowa et al., 2017), our results here in the LH show that a majority of the CXCR4 cells stimulated by ethanol have a neuronal phenotype and colabel the neuronal marker, NeuN, consistent with published studies (Banisadr et al., 2005). This evidence, together with the established neuromodulatory functions attributed to the CXCL12/CXCR4 axis (Guyon, 2014, Luo et al., 2016), suggest its involvment in the stimulatory effects of prenatal ethanol on neuronal development.
This is supported by our additional finding that prenatal ethanol exposure, while increasing the density of Hcrt/OX and MCH neurons in postnatal rats as previously shown in adolescent rats (Chang et al., 2012, 2015), produces this effect in the same LH area where the CXCR4 cells are particularly dense and this chemokine receptor colocalizes with the peptide neurons. Notably, these peptide neurons differ in their relationship to CXCR4, with their low percentage of colocalization (<10%) under control conditions increased to almost 60% in Hcrt/OX neurons but only 18% in MCH neurons. This differential co-labeling may reflect a variety of differences between these distinct populations of peptide neurons. These include differences in their anatomical distribution, with Hcrt/OX neurons denser in the medial area and MCH neurons denser in the lateral area of the LH (Sakurai et al., 1998, de Lecea et al., 1998), and also in their responses to the inflammatory agent liposaccharide (Palomba et al., 2014)https://www.ncbi.nlm.nih.gov/pubmed/24708924 and their co-expression of the peptide cocaine‐ and amphetamine‐regulated transcript (Jancsik et al., 2018). These Hcrt/OX and MCH neurons also differ in their functional activity, signaling through distinct electrical pathways to promote addictive-like behaviors (DiLeone et al., 2003, Moorman, 2018, Diniz and Bittencourt, 2017). With the CXCL12/CCR4 axis shown to modulate electrical excitability of peptide-expressing neurons (Guyon et al., 2005), it may have differential effects on the signaling of Hcrt/OX and MCH neurons, with stronger effects on Hcrt/OX system due to its greater colocalization.
In addition to modulating neuronal excitability and function, the CXCL12/CXCR4 axis also plays an obligate role in the processes of neurogenesis and proliferation of neuroprogenitor cells (Trettel et al., 2019) as well as neuronal migration (Watson et al., 2019). This chemokine axis is believed to function in the embryonic NEP (Zhu et al., 2015), a primary site of neurogenesis in the brain (Bedont et al., 2015, Florio and Huttner, 2014), where radial glia cells, the main neuroprogenitors of the brain (Taverna et al., 2014), are dense and distinct in their elongated bipolar morphology (Barry et al., 2014). With no prior studies of ethanol’s effects in the NEP, our results here in the embryo are the first to reveal a dense concentration of radial glia cells along with CXCR4 cells in the hypothalamic NEP and a strong stimulatory effect of prenatal ethanol exposure on their density and co-labeling, with ethanol increasing to almost 60% the percentage of BLBP cells that coexpress CXCR4. With the known functions of CXCL12/CXCR4 signaling in promoting neurogenesis, neuroprogenitor cell proliferation and migration (Trettel et al., 2019, Cheng et al., 2017, Zhu et al., 2015), these findings offer new insight into the effects of moderate ethanol levels on neuronal development in the embryonic brain. They identify the hypothalamic NEP as an important site of action where ethanol increases CXCR4 signaling within radial glia cells, similar to its effect on peptide neurons in the LH. Analyses of another CXCL12 receptor, CXCR7, which has overlapping but different roles in modulating neuroprogenitor cells (Cheng et al., 2017), should further elucidate the role of this chemokine signaling in ethanol’s stimulatory actions on neuronal development.
Notably, these effects on the fetal neuroimmune system and neuronal development are sexually dimorphic, with female embryos and postnatal offspring exhibiting a greater sensitivity to ethanol than males, consistent with evidence obtained in adolescent offspring (Chang et al., 2018). While effects of estrogen on inflammatory agents have been studied mostly in glia in vitro (Cerciat et al., 2010, Astiz et al., 2014), there is a general consensus that females are more vulnerable to the effects of ethanol and exhibit a greater increase in inflammatory mediators along with more neuroinflammatory damage, neurodevelopmental dysfunction, and behavioral disturbances (Alfonso-Loeches et al., 2013, Pascual et al., 2017). Although the precise mechanisms underlying these sex differences in ethanol’s actions have yet to be characterized, they likely reflect the effects of sex hormones, receptors and enzymes known to stimulate neurogenesis (Brocca and Garcia-Segura, 2019, Pellegrini et al., 2016). With higher estrogen levels detected in the male embryonic brain (Bakker et al., 2006), the neuroprotective effects attributed to this steroid (Chakrabarti et al., 2016, McEwen and Milner, 2017) may serve to diminish in males the ethanol-induced increase in chemokine signaling.
Our results here with analyses of the CXCL12/CXCR4 network, building on our published studies of the CCL2/CCR2 axis (Chang et al., 2015, 2018), give us a broader perspective and understanding of how prenatal ethanol affects neuroimmune functions in the embryonic brain as they relate to the development of neurons such as Hcrt/OX and MCH. Whereas both chemokine systems serve important roles in embryonic development and cell migration, they differ in several ways, with CXCL12 having primary functions in neurogenesis and neural progenitor cell proliferation and CCL2 affecting the differentiation and motility/movement of cells (Watson et al., 2019, Trettel et al., 2019). While both systems are similarly stimulated by ethanol in a sexually dimorphic manner, they clearly differ in their relationship to the peptide neurons, with CXCR4 colocalizing with Hcrt/OX neurons far more than MCH neurons as shown here and CCR2 colocalizing mostly with MCH but not Hcrt/OX neurons as shown previously (Chang et al., 2018, 2019). This minimal or no overlap of CXCR4 with MCH and CCR2 with Hcrt/OX indicates that, when stimulated by ethanol, these neuroimmune systems have differential effects on distinct populations of neurons. Whereas factors such as dose, age and duration of ethanol exposure may determine the nature of these different chemokine/neuropeptide functional pathways, other factors are likely involved. These could include differences in birth dates of the chemokine systems (Tissir et al., 2004, Mizutani et al., 2012) and specific peptide neurons to which they are related (Ogawa et al., 2017, Brischoux et al., 2001) and also in their anatomical distribution, with the CXCR4 cells found here to be dense in the LH although still evident throughout the hypothalamus and the CCR2 cells reported to be tightly clustered in the LH while sparse elsewhere in the hypothalamus (Chang et al., 2018, 2019). A possible relationship of CXCR4 to other neuropeptide systems is suggested by a study showing this chemokine receptor to colocalize with the peptide, gonadotropin-releasing hormone, and affect the migration of its neurons from the basal forebrain to the hypothalamus (Schwarting et al., 2006).
In conclusion, this study provides further insight into the functional pathways and mechanisms that may be responsible for the changes in the neurobiology and ultimately behavior after moderate prenatal ethanol exposure. Until now, the CXCL12/CXCR4 axis has mainly been implicated in influencing neurogenesis and progenitor cell proliferation as well as migration while receiving little attention in the alcohol field, despite its likely role in the development and patterning of neurons in the brain. Our findings here, demonstrating for the first time a close link between CXCL12/CXCR4 signaling and Hcrt/OX neurons that is distinct from the CCL2/CCR2 axis linked to MCH neurons, reveal the complexity of the neuroimmune systems as they relate to neurochemical circuits in the larger functional network to control behavior. They focus attention on the radial glia neuroprogenitors in the NEP where neuroimmune systems may initially respond to ethanol and act in a sexually dimorphic manner to generate peptide neurons in the LH that promote the overconsumption of alcohol.
Acknowledgements
This research was supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA024798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We extend gratitude to The Rockefeller University’s Bio-Imaging Resource Center and Translational Technology Core Laboratory for the use of their equipment.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Adachi N, Suzuki S, Matsuoka H, Fushimi S, Ono J, Ohta KI, Hirai Y, Miki T, Koshimizu H (2018) Corticotropin-releasing hormone-binding protein is up-regulated by brain-derived neurotrophic factor and is secreted in an activity-dependent manner in rat cerebral cortical neurons. Journal of neurochemistry. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C (2013) Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311:27–34. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1995) Atlas of Prenatal Rat Brain Development, CRC Press, Boca Raton. [Google Scholar]
- Astiz M, Acaz-Fonseca E, Garcia-Segura LM (2014) Sex differences and effects of estrogenic compounds on the expression of inflammatory molecules by astrocytes exposed to the insecticide dimethoate. Neurotoxicity research 25:271–285. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C (2006) Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nature neuroscience 9:220–226. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM (2005) Chemokines and brain functions. Curr Drug Targets Inflamm Allergy 4:387–399. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM (2003) Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. The European journal of neuroscience 18:1593–1606. [DOI] [PubMed] [Google Scholar]
- Barry DS, Pakan JM, McDermott KW (2014) Radial glial cells: key organisers in CNS development. The international journal of biochemistry & cell biology 46:76–79. [DOI] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF (2013) Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol Clin Exp Res 37 Suppl 1:E141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, Newman EA, Blackshaw S (2015) Patterning, specification, and differentiation in the developing hypothalamus. Wiley interdisciplinary reviews. Developmental biology 4:445–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Fellmann D, Risold PY (2001) Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. The European journal of neuroscience 13:1733–1744. [DOI] [PubMed] [Google Scholar]
- Brocca ME, Garcia-Segura LM (2019) Non-reproductive Functions of Aromatase in the Central Nervous System Under Physiological and Pathological Conditions. Cellular and molecular neurobiology 39:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Wood E, Jabbour L (2016) Impact of fetal alcohol exposure on body systems: A systematic review. Birth defects research. Part C, Embryo today : reviews 108:174–180. [DOI] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA (2010) Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 58:93–102. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M, Das A, Samantaray S, Smith JA, Banik NL, Haque A, Ray SK (2016) Molecular mechanisms of estrogen for neuroprotection in spinal cord injury and traumatic brain injury. Reviews in the neurosciences 27:271–281. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Devi Sai Sri Kavya B, Leibowitz SF(2019) CCL2/CCR2 Chemokine System in Embryonic Hypothalamus: Involvement in Sexually Dimorphic Stimulatory Effects of Prenatal Ethanol Exposure on Peptide-Expressing Neurons. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Halkina V, Edelstien J, Ramirez E, Leibowitz SF (2018) Hypothalamic CCL2/CCR2 Chemokine System: Role in Sexually Dimorphic Effects of Maternal Ethanol Exposure on Melanin-Concentrating Hormone and Behavior in Adolescent Offspring. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:9072–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2013) Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:13600–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2015) Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience 310:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF (2012) Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience 222:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Wang H, Zhang X, Zhao S, Zhou Z, Mu X, Zhao C, Teng W (2017) The Role of SDF-1/CXCR4/CXCR7 in Neuronal Regeneration after Cerebral Ischemia. Frontiers in neuroscience 11:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AD, Halkina V, Min SS, Roberts MY, Campbell SD, Camidge K, Leibowitz SF (2019) Embryonic ethanol exposure affects the early development, migration and location of hypocretin/orexin neurons in zebrafish. Alcoholism: Clinical and Experimental Research 43:1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP (2015) Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol research : current reviews 37:331–341, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG(1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity Proceedings of the National Academy of Sciences of the United States of America 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarapu SK, Kumar Vr S, Rupanagudi KV, Kulkarni OP, Eulberg D, Klussmann S, Anders HJ (2016) Dual blockade of the pro-inflammatory chemokine CCL2 and the homeostatic chemokine CXCL12 is as effective as high dose cyclophosphamide in murine proliferative lupus nephritis Clinical immunology (Orlando, Fla.) 169:139–147. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768. [DOI] [PubMed] [Google Scholar]
- Diniz GB, Bittencourt JC (2017) The Melanin-Concentrating Hormone as an Integrative Peptide Driving Motivated Behaviors. Frontiers in systems neuroscience 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJ (2014) Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol 118:41–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, Macchione AF, Nizhnikov ME, Pautassi RM (2015) Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. The European journal of neuroscience 41:1569–1579. [DOI] [PubMed] [Google Scholar]
- Florio M, Huttner WB (2014) Neural progenitors, neurogenesis and the evolution of the neocortex Development (Cambridge, England: ) 141:2182–2194. [DOI] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M (2015) Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med 45:3047–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bernabe P, Boveda-Ruiz D, D’Alessandro-Gabazza C, Toda M, Miyake Y, Mifuji-Moroka R, Iwasa M, Morser J, Gabazza EC, Takei Y (2011) Atherosclerosis amelioration by moderate alcohol consumption is associated with increased circulating levels of stromal cell-derived factor-1. Circulation journal : official journal of the Japanese Circulation Society 75:2269–2279. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Zhang X, Goeke C, Gavin DP (2014) Glia and neurodevelopment: focus on fetal alcohol spectrum disorders. Frontiers in pediatrics 2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KK, Gupta VK, Shirasaka T (2016) An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin Exp Res 40:1594–1602. [DOI] [PubMed] [Google Scholar]
- Guyon A (2014) CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Frontiers in cellular neuroscience 8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Banisadr G, Rovere C, Cervantes A, Kitabgi P, Melik-Parsadaniantz S, Nahon JL (2005) Complex effects of stromal cell-derived factor-1 alpha on melanin-concentrating hormone neuron excitability. The European journal of neuroscience 21:701–710. [DOI] [PubMed] [Google Scholar]
- Jancsik V, Bene R, Sotonyi P, Zachar G (2018) Sub-cellular organization of the melanin-concentrating hormone neurons in the hypothalamus. Peptides 99:56–60. [DOI] [PubMed] [Google Scholar]
- Janssens R, Struyf S, Proost P (2018) Pathological roles of the homeostatic chemokine CXCL12. Cytokine & growth factor reviews 44:51–68. [DOI] [PubMed] [Google Scholar]
- Karim S, Liaskou E, Hadley S, Youster J, Faint J, Adams DH, Lalor PF (2013) An in vitro model of human acute ethanol exposure that incorporates CXCR3- and CXCR4-dependent recruitment of immune cells. Toxicological sciences : an official journal of the Society of Toxicology 132:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Rehman F, Damadzic R, Atkins AL, Schank JR, Gehlert DR, Steensland P, Thorsell A, Heilig M (2016) The melanin-concentrating hormone-1 receptor modulates alcohol-induced reward and DARPP-32 phosphorylation. Psychopharmacology 233:2355–2363. [DOI] [PubMed] [Google Scholar]
- Komada M, Hara N, Kawachi S, Kawachi K, Kagawa N, Nagao T, Ikeda Y (2017) Mechanisms underlying neuro-inflammation and neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to ethanol. Sci Rep 7:4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thuc O, Cansell C, Bourourou M, Denis RG, Stobbe K, Devaux N, Guyon A, Cazareth J, Heurteaux C, Rostene W, Luquet S, Blondeau N, Nahon JL, Rovere C (2016) Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep 17:1738–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L (2014) The hypocretins/orexins: integrators of multiple physiological functions. British journal of pharmacology 171:332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Wang X, Xia Z, Chung SK, Cheung CW (2016) CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Reviews in the neurosciences 27:83–92. [DOI] [PubMed] [Google Scholar]
- Malone SM, McGue M, Iacono WG (2010) Mothers’ maximum drinks ever consumed in 24 hours predicts mental health problems in adolescent offspring. Journal of child psychology and psychiatry 51:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE (2012) The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. Journal of immunology 188:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE (2018) The hypocretin/orexin system as a target for excessive motivation in alcohol use disorders. Psychopharmacology (Berl) 235:1663–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kanda T, Vogt K, Yanagisawa M (2017) Anatomical and electrophysiological development of the hypothalamic orexin neurons from embryos to neonates. J Comp Neurol 525:3809–3820. [DOI] [PubMed] [Google Scholar]
- Palomba M, Seke Etet PF, Veronesi C (2014) Effect of inflammatory challenge on hypothalamic neurons expressing orexinergic and melanin-concentrating hormone. Neuroscience letters 570:47–52. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ, Guerri C (2017) Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol 22:1829–1841. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Diotel N, Vaillant-Capitaine C, Perez Maria R, Gueguen MM, Nasri A, Cano Nicolau J, Kah O (2016) Steroid modulation of neurogenesis: Focus on radial glial cells in zebrafish. The Journal of steroid biochemistry and molecular biology 160:27–36. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiology of stress 10:100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Wang MW, Elberger AJ (2002) Second trimester prenatal alcohol exposure alters development of rat corpus callosum. Neurotoxicol Teratol 24:719–732. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC (2005) Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC neuroscience 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S (2006) Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:6834–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N, Timiras P (1970) A Stereotaxic Atlas of the Developing Ear Brain, University of California Press. [Google Scholar]
- Slusarczyk J, Trojan E, Glombik K, Budziszewska B, Kubera M, Lason W, Popiolek-Barczyk K, Mika J, Wedzony K, Basta-Kaim A (2015) Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Frontiers in cellular neuroscience 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa JE, Slusarczyk J, Trojan E, Chamera K, Leskiewicz M, Regulska M, Kotarska K, Basta-Kaim A (2017) Prenatal stress affects viability, activation, and chemokine signaling in astroglial cultures. Journal of neuroimmunology 311:79–87. [DOI] [PubMed] [Google Scholar]
- Sterling M, Chang G-Q, Karatayev O, Chang S, Leibowitz S (2016) Effects of embryonic ethanol exposure at low doses on neuronal development, voluntary ethanol consumption and related behaviors in larval and adult zebrafish: role of hypothalamic orexigenic peptides. Behavioural brain research 304:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB (2014) The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annual review of cell and developmental biology 30:465–502. [DOI] [PubMed] [Google Scholar]
- Terasaki LS, Gomez J, Schwarz JM (2016) An examination of sex differences in the effects of early-life opiate and alcohol exposure. Phil. Trans. R. Soc. B 371:20150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki LS, Schwarz JM (2016) Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. Journal of Neuroimmune Pharmacology 11:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM (2004) Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res 149:63–71. [DOI] [PubMed] [Google Scholar]
- Trettel F, Di Castro MA, Limatola C (2019) Chemokines: Key Molecules that Orchestrate Communication among Neurons, Microglia and Astrocytes to Preserve Brain Function. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD (2010) Ethanol alters cell fate of fetal human brain-derived stem and progenitor cells. Alcohol Clin Exp Res 34:1574–1583. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT (2014) Current hypotheses on the mechanisms of alcoholism. Handbook of clinical neurology 125:477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AES, Goodkey K, Footz T, Voronova A (2019) Regulation of CNS precursor function by neuronal chemokines. Neuroscience letters:134533. [DOI] [PubMed] [Google Scholar]
- West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Gortz D, This S, Stockenhuber K, Pott J, Friedrich M, Ryzhakov G, Baribaud F, Brodmerkel C, Cieluch C, Rahman N, Muller-Newen G, Owens RJ, Kuhl AA, Maloy KJ, Plevy SE, Oxford IBDCI, Keshav S, Travis SPL, Powrie F (2017) Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med 23:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Bille A, Miranda-Morales RS, Pucci M, Bellia F, D’Addario C, Pautassi RM (2018) Prenatal ethanol induces an anxiety phenotype and alters expression of dynorphin & nociceptin/orphanin FQ genes. Progress in neuro-psychopharmacology & biological psychiatry 85:77–88. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Matsumoto T, Nagasawa T, Mackay F, Murakami F (2015) Chemokine Signaling Controls Integrity of Radial Glial Scaffold in Developing Spinal Cord and Consequential Proper Position of Boundary Cap Cells. J Neurosci 35:9211–9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziogas DC, Karagiannis AK, Geiger BM, Gras-Miralles B, Najarian R, Reizes O, Fitzpatrick LR, Kokkotou E (2014) Inflammation-induced functional connectivity of melanin-concentrating hormone and IL-10. Peptides 55:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


