Abstract
Human alcohol laboratory studies use two routes of alcohol administration: ingestion and infusion. The goal of this paper is to compare and contrast these alcohol administration methods. The work summarized in this report was the basis of a 2019 Research Society on Alcoholism Roundtable, “To Ingest or Infuse: A Comparison of Oral and Intravenous Alcohol Administration Methods for Human Alcohol Laboratory Designs.” We review the methodological approaches of each and highlight strengths and weaknesses pertaining to different research questions. We summarize methodological considerations to aid researchers in choosing the most appropriate method for their inquiry, considering exposure variability, alcohol expectancy effects, safety, bandwidth, technical skills, documentation of alcohol exposure, experimental variety, ecological validity, and cost. Ingestion of alcohol remains a common, and often a preferable, methodological practice in alcohol research. Nonetheless, the main problem with ingestion is that even the most careful calculation of dose and control of dosing procedures yields substantial and uncontrollable variability in the participants’ brain exposures to alcohol. Infusion methodologies provide precise exposure control but are technically complex and may be limited in ecological validity. We suggest that alcohol ingestion research may not be the same thing as alcohol exposure research; investigators should be aware of the advantages and disadvantages that the choice between ingestion and infusion of alcohol invokes.
Keywords: oral alcohol ingestion, intravenous alcohol infusion, human laboratory research, alcohol experiments
Human alcohol laboratory studies use two routes of alcohol administration: ingestion and infusion. This paper summarizes contributions to a 2019 Research Society on Alcoholism Roundtable “To Ingest or Infuse: A Comparison of Oral and Intravenous Alcohol Administration Methods for Human Alcohol Laboratory Designs.” We review the methodological procedures of each and highlight strengths and weaknesses pertaining to various research questions. We summarize methodological considerations to aid researchers in choosing the most appropriate administration method for their inquiry, considering exposure variability, alcohol expectancy effects, safety, bandwidth, technical skills, documentation of alcohol exposure, experimental variety, ecological validity, and cost.
For both alcohol ingestion and infusion, we review two laboratory paradigms: ‘alcohol challenge’ and ‘alcohol self-administration.’ In an alcohol challenge, researchers prescribe a target alcohol exposure, usually with the goal of holding the exposure constant across participants in order to examine the effects of that exposure on dependent measures of interest. In alcohol self-administration paradigms, researchers usually perform a manipulation and measure its effects on the amount of alcohol self-administered; thus, variability in alcohol administration (e.g., rate, total volume, obtained Breath Alcohol Concentration (BrAC), etc.) is desired, as the focus is on determinants on alcohol intake. Although inhalation and transdermal perfusion routes are employed productively in animal studies, their application to human alcohol studies is not yet established; we do not address them.
Comparing and Contrasting Paradigms
Alcohol ingestion paradigms.
Oral alcohol challenge.
Here, the alcohol input is the dose of alcohol (gm), usually scaled to the individual’s weight (kg) and sex, or to total body water, then diluted according to a formula in a vehicle (usually some form of distilled spirits or grain alcohol mixed with sweetened drinks or juices, although beer is sometimes used when target BrAC values are comparatively low). A publicly available program provides an efficient approach for such calculations (Curtin & Fairchild, 2003). Doses are based on safe prescription of a target average peak BrAC ranging from 0 mg/dl (i.e., a placebo dose) to usually 80 mg/dl (see reviews by Quinn and Fromme, 2011; Zimmermann, O’Connor and Ramchandani, 2011); although some implement “high dose” or similar challenges producing mean BrACs of above 80 mg/dl (Amlung, McCarty, Morris, Tsai, & McCarthy, 2015; Bradford, Shapiro, & Curtin, 2013; Donohue, Curtin, Patrick, & Lang, 2007; King, De Wit, McNamara, & Cao, 2011; Moberg, Weber, & Curtin, 2011) and one recent study was able to target 120 mg/dl without adverse effects (Vena & King, 2019). Experimenters usually rely on the trajectory of BrACs as good approximations of the current brain alcohol concentration (Gomez et al., 2012) as they are closer to momentary arterial alcohol concentration than to venous alcohol concentration (Lindberg et al., 2007; Martin, Moll, Schmid, & Dettli, 1984). The goals include achieving a rapid climb to the target BrAC, a similar brain exposure across participants, and reducing confounds related to drink preference or experience.
The resultant beverage must be ingested by each participant over a relatively short period of time (e.g., <30 minutes). This constraint attempts to reduce alcohol exposure variability across participants, so that dependent measures (e.g., driving performance, subjective response, cognition) can be measured at the same BrAC level across participants. Measurements are planned during the ascending and/or descending limbs and/or at BrAC peak. Due to the substantial inter-individual variation in alcohol absorption, distribution, and elimination, standardizing BrAC across participants in an oral alcohol challenge is an unsolved challenge.
Oral alcohol self-administration.
In oral alcohol self-administration paradigms, participants are allowed to drink as much or as little of a predefined range of provided alcoholic beverages and placebo drinks (if provided) over a period of time (e.g., 1-2 hours of ad libitum drinking) or choose between alcoholic beverage self-administration and another reinforcer (e.g., money). For safety, participants are prevented from drinking more than a pre-determined amount in order to stay below a BrAC safety limit (e.g., 100 mg/dl or 120 mg/dl) (Corbin, Gearhardt, & Fromme, 2008; Leeman et al., 2013). The outcome of interest is often the amount of alcohol ingested (in ml or grams). Alcoholic beverages can be matched to participants’ preferences, either by offering the participant’s most frequently consumed beverage or by targeted recruitment of participants who consume the beverage provided in the study. These procedures maximize the likelihood that participants will engage in their usual drinking behavior in the laboratory. Priming doses (where the participant consumes a drink usually targeting a BrAC of 20-40 mg/dl prior to the self-administration period) are often used (e.g., to increase the participants’ desire to drink during the ensuing self-administration period, to induce craving, etc.). The goal of employing alternative reinforcers (e.g., money, water, non-alcoholic drinks, food) or concomitant auditory or visual cues, is to determine factors that escalate or suppress drinking. Outside factors potentially influence consumption (e.g., drinking in the lab with the hopes of leaving while still intoxicated or avoiding drinking to leave early). Investigators attempt to address this confound by reminding participants of the BrAC they need to achieve (e.g., usually 20-40 mg/dl) and/or setting a minimum time in lab before they are dismissed.
Alcohol Infusion paradigms.
Several alcohol infusion techniques have been reported over time (e.g., Chapman and Williams, 1951; Korsten et al., 1975; Gibbens and Chard, 1976; Nishimura, Hasumura and Takeuchi, 1980; Hansbrough et al., 1984; Jones, Norberg and Hahn, 1997; Ray and Hutchison, 2004; Ray et al., 2007; Aalto et al., 2015; Westman, Bujarski and Ray, 2017). The most commonly used technique utilizes a program called the Computer-assisted Alcohol Infusion System (CAIS) (Zimmermann et al., 2008). Prior to infusion, the participant’s age, height, weight, and sex are entered into CAIS, which transforms those measurements into parameters of an individualized, physiologically-based pharmacokinetic (PBPK) model of alcohol distribution and elimination (Plawecki et al., 2007; Plawecki et al., 2008). The program continuously calculates the infusion rate profile required to achieve the desired BrAC trajectory or level. CAIS supports a wide range of paradigms and the wealth of published studies employing it is the basis for limiting our discussion to its capacities and limitations.
Intravenous alcohol challenge.
In the intravenous alcohol challenge, researchers do not prescribe a dose of alcohol; rather they target a BrAC trajectory. The methodological goal is to achieve a precise, prescribed BrAC time course and, thus, brain alcohol exposure trajectory, in all participants in the experiment, removing confounds related to variations within and across participants. The most common intravenous alcohol challenge experiment is the alcohol clamp (O’Connor, Morzorati, Christian, & Li, 1998; Ramchandani, Bolane, Li, & O’Connor, 1999), where a target BrAC is achieved at a specified time and maintained for as long as is desired to complete outcome assessments (e.g., between 30 minutes and 3 hours). Participants are infused with an individualized, outcome-adapted time course of a 6.0% (v/V) ethanol solution that achieves the prescribed trajectory. During the session, BrAC measurements are used by the system to ensure fidelity to the desired exposure trajectory and to adjust the infusion rate profile, if necessary. Published clamping studies, using CAIS as well as other approaches, bracketing a wide variety of scientific inquiry, have used target levels ranging from 20 mg/dl to 100 mg/dl (Gilman, Ramchandani, Crouss, & Hommer, 2012; Gilman, Ramchandani, Davis, Bjork, & Hommer, 2008; Gilman, Smith, Ramchandani, Momenan, & Hommer, 2012; Kosobud et al., 2015; Marshall et al., 2014a; Ramchandani et al., 2011; Yoder et al., 2016) and some have used multiple targets within a session (Subramanian et al., 2002; Westman, Bujarski and Ray, 2017; Plawecki, Koskie, et al., 2018).
Intravenous alcohol self-administration.
Intravenous alcohol self-administration commonly uses one of three techniques, all of which can include priming exposures or alternative reinforcers. The three techniques described here are not exclusive, as other methodological strategies for intravenous alcohol exposure have been successfully employed but this review will focus on three methods commonly tied to simultaneous assessment of subjective responses and other outcomes to generate extensive within-person data and allow for high resolution modeling of temporal processes. The first technique is free access, where participants receive prescribed incremental BrAC exposures by pressing a button. This paradigm serves as an analog to the oral ad libitum paradigm described above and is commonly employed to examine the rewarding aspects of alcohol consumption, for example reward satiation. The incremental alcohol exposure, identical across participants when using CAIS, is then delivered intravenously to the participant over a prescribed interval (usually ~2.5 minutes, although this can be modified). Following the increment, the BrAC descends at a user-prescribed rate until the participant requests another alcohol reward. Free access to alcohol rewards is available for a prescribed period of time (usually 2-3 hours), with maximum BrAC levels allowed up to a pre-determined safety limit. Because there is good accuracy of achieving the target BrAC, maximum allowable BrAC levels range up to 200mg/dl, although most infusion self-administration studies set the limit between 100 mg/dl and 180mg/dl (e.g. VanderVeen et al., 2016; Stangl et al., 2017; Plawecki, White, et al., 2018).
The second technique utilizes a progressive ratio work paradigm, where participants earn incremental BrAC exposure rewards through completion of a task, with successive rewards requiring more work. The increasing work schedule makes it difficult to continuously raise or maintain one’s BrAC throughout the experiment. This paradigm is commonly employed to probe aspects of motivated behavior and effort. Within the CAIS system, currently available work is one of two types: simple button presses (Farokhnia et al., 2018) or the Constant Attention Task (CAT) (Plawecki et al., 2013). The CAT is an operant task requiring participants to attend and respond to a stimulus in a specified time period. The difficulty of the task adjusts so that fatigue or intoxication do not affect the success rate across the experiment.
The third, recently introduced, technique uses rate control (Plawecki et al., 2016). In this paradigm, participants are still free to administer alcohol ad libitum, but they choose the rate of change (i.e., slope) of the next, 3-minute segment of the BrAC trajectory. This paradigm aims to examine the response to reward, provides participants increased freedom to define their reward trajectory, and aims to discriminate high-risk drinking patterns, such as binge drinking. Participants express their preferences for the steepness of the ascending and descending limbs and the level of peak exposure. In contrast to other infusion paradigms, where participants experience identical incremental rewards, each incremental exposure is under the participant’s control.
Issues to Consider in Choosing Route of Administration
There are a variety of issues to consider when choosing the route of administration for a laboratory alcohol study. These include participant responses (i.e., exposure variability, alcohol expectancy effects, safety, and bandwidth) and practical/methodological considerations (technical skills required, documentation of alcohol exposure, ecological validity, experimental validity, and cost). For each issue, we compare and contrast the performance of ingestion and infusion methodologies. We point out, when possible, which route of alcohol administration overcomes challenges in future work.
Exposure Variability.
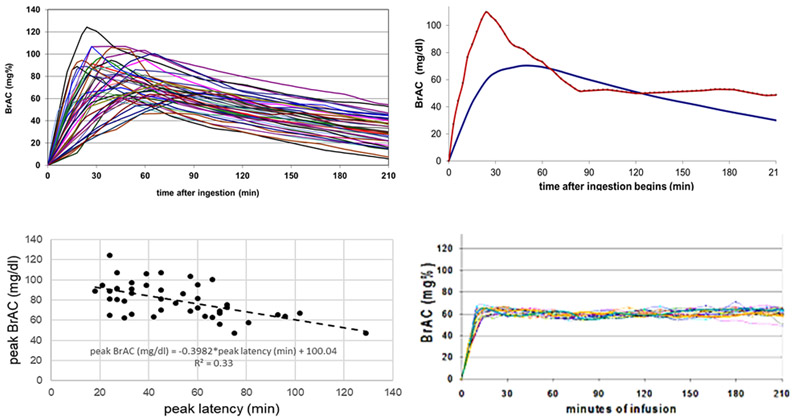
Most manuscripts reporting the results of alcohol ingestion report that the mean trajectory of BrAC did not differ between 2 groups of interest. Such a statement can be misleading on two accounts. The first neglects the within-group variation in alcohol exposure. The kinetics of absorption, distribution and metabolism after an identical dose of ethanol all vary across participants. The variation in absorption kinetics, in particular, is substantial, and – to a disconcerting degree – uncontrollable. Figure 1 presents the BrAC trajectories of 44 young adult participants who ingested individualized doses of alcohol (adjusted for total body water, minimizing the influence of age, and sex), targeting a 80mg/dl peak BrAC, under experimental conditions that were crafted to be as similar as possible across the sample population (Ramchandani et al., 2009).
Figure 1A-1D. Comparison of Experienced BrAC trajectories across ingestion and infusion paradigms. 1A top left. 1B top right. 1C bottom left. 1D bottom right.
A. Individual BrAC trajectories in ingestion. Alcohol ingestion yields variable trajectories of BrAC even under carefully controlled circumstances. Here, individual BrAC trajectories after ingestion are plotted for 44 participants. The doses were calibrated according to total body water, based on the participant’s age, height, weight, and gender, to produce a peak BrAC of 80 mg/dl, then administered to minimize further variability in BrAC due to time of day, food, consumed, technician and testing environment.
B. Mean and range of BrAC over time in ingestion. Lower smooth line: The average BrAC as a function of time after ingestion of 1.0 gm alcohol per liter total body water, calculated from data in Figure 1A. BrAC was measured approximately every ~10 min, then interpolated precisely to every 3 min over the 4-hour experiment. Upper jagged line: The range of BrAC between the maximum and minimum BrACs noted in the sample across time are plotted; expressing the uncertainty in brain exposure to alcohol across time after ingestion, even when control of dosing was the only goal.
1C. Peak BrAC and latency in ingestion. The relationship between an individual’s peak BrAC and the latency to that peak BrAC (min) in the response to careful oral dosing, calculated from Figure 1A. Peak BrAC ranges from 47 to 124; mean = 80 mg/dl. Latency to peak ranged from 18 to 129; mean = 51 min. In general, the longer it took to reach peak BrAC, the lower the peak concentration, reflecting the interplay between variable absorption and first-pass metabolism in alcohol pharmacokinetics.
D. BrAC trajectory in infusion clamping. A trajectory of brain exposure to alcohol can be prescribed and achieved by real-time computation of an individual’s required infusion rate profile using CAIS-based PBPK modeling. The example shown here is from a BrAC clamping experiment conducted in 50 young adults with the goal of raising BrAC to 60 mg/dl in10 minutes, then maintaining it for 3 hours. The result minimizes the variability in BrAC trajectories across participants. Nearly any prescribed, physiologic trajectory, including the biphasic oral alcohol exposure curve, can be achieved.
Following ingestion of the same total body water-referenced dose of alcohol, there is a 2 to 3-fold variation in the peak amplitude of BrAC (Figure 1A), the mean and range of BrAC (Figure 1B), and the time that the peak is reached (Figure 1C). Peak BrAC ranged from 47mg/dl to 124mg/dl (although the mean was at the targeted 80mg/dl level) and a range of time to peak BrAC between 18 to 129 minutes (M= 51 minutes). Some research teams try to overcome this variation by covarying for BrAC in statistical models; although this procedure reduces error variance to some extent, it makes it difficult to directly compare outcome measures at the same BrAC level. Some researchers address the problem by collecting measures at a standardized BrAC level, which can reduce variability in BrAC at the point of measurement, but adds variability in the time between assessments and the duration of exposure prior to assessment. Unfortunately, such approaches do not control for the rate of ascent or descent of BrAC (which produce qualitatively different intoxication experiences; Fillmore & Vogel-Sprott, 1998; Martin & Earleywine, 1990; Morris, Amlung, Tsai, & McCarthy, 2017; Wetherill et al., 2012). Ascending trajectories varied quite markedly and peak BrAC values remained stable within an individual for only a short period of time (Figure 1A). Alcohol infusion achieves a substantial reduction in BrAC variability across participants compared to ingestion (see Figure 1D).
In summary, alcohol ingestion results in large variability of BrAC and brain exposure, as well as timing of dependent measures, even within the same participant in repeated sessions. If the research goal is to examine and/or include the natural variability in alcohol kinetics from standard dosing, then the ingestion route may be preferred. However, if the research goal necessitates consistent exposure across and within individuals, infusion may be advantageous.
Alcohol expectancy effects.
A recent study (Conrad, McNamara, & King, 2012) identified baseline expectation of alcohol’s subjective effects as a dominant determinant of subjective perceptions after alcoholic beverage ingestion. There is a vast literature linking alcohol expectancies with drinking behavior and alcohol-related consequences (Goldman, Del Boca, & Darkes, 1999; Jones, Corbin, & Fromme, 2001), expectancies developed prior to alcohol exposure with later drinking outcomes (Christiansen, Smith, Roehiing, & Goldman, 1989), and alcohol expectancies with alcohol self-administration and subjective effects (Stangl et al., 2017). Thus, it is important to consider expectancy effects in alcohol administration studies and the extent to which ingestion and infusion approaches can appropriately account for them.
It is not clear if expectations vary as a function of experience with and preference for the specific beverage consumed, but for infused alcohol, participants typically have no previous experience. Whether that fact is an advantage or disadvantage depends on the investigator’s interest in the influence of experience and expectations vs. the pharmacologic effect of alcohol, per se. However, because drinking in the real-world always involves ingesting alcohol, it is possible that expected and experienced effects differ when alcohol is administered in a manner that is unfamiliar to the individual. Prior studies have shown that physical and social context impact alcohol expectancies and reported alcohol effects (Corbin, Scott, Boyd, Menary, & Enders, 2015; Sayette et al., 2012; Wall, McKee, & Hinson, 2000; Wall, McKee, Hinson, & Goldstein, 2001). Technician blinding is possible with the use of BrAC meters displaying zero, storing time stamped measurements for later review and analysis. However, with infusion, at least one technician cannot be blinded to the presence of alcohol in the infusate.
It may also be more difficult to facilitate an effective placebo response in infusion studies. Ingestion paradigms typically capitalize on visual (placebo poured from an alcohol bottle), olfactory and taste (rimming glasses with alcohol, floating alcohol on top of the drink), and behavioral (e.g., drinking from a glass) cues for this purpose. A common alternative is to minimize expectancy effects; some investigators using both oral and intravenous alcohol challenge techniques tell participants that they may receive one of several substances, including placebo. This approach may be even more effective using infusion, as the cues associated with alcohol ingestion (especially taste) can only be masked to a degree, especially at high doses. Although the approach of minimizing expectancies (regardless of route of administration) may help isolate alcohol’s pharmacologic effects, it may reduce generalizability, as real-world drinking experiences are always a result of the combination of expectancies and pharmacology. Multiple intravenous alcohol clamping studies have used saline as a control condition and report analyses on these effects (Kerfoot et al., 2013; Kosobud et al., 2015; Plawecki, Windisch, et al., 2018; Ramchandani et al., 2002), but the efficacy of such as a placebo technique is not established, as manipulation checks have not been reported. Thus, some expectancy effects may remain in alcohol infusion studies.
One realm of similarity between ingestion and infusion is in studying subjective responses to alcohol. One published experiment examined the fidelity with which infused alcohol could mimic the idiosyncratic BrAC trajectories of individuals (Figure 1) following ingestion of a standardized dose in the same laboratory setting (Ramchandani et al., 2009). In both experimental sessions, participants provided a battery of subjective perceptions about current alcohol effects on a regular, frequent basis. Adopting placebo response techniques from oral alcohol challenges, participants were blinded to the route of administration, receiving both an infusion (alcohol versus saline) and an orally consumed drink (normalized alcohol dosage or placebo with alcohol floated on top). In this study, BrAC curves for participants were nearly identical for ingestion and infusion routes of alcohol administration and, likewise, their subjective responses to alcohol were also nearly identical regardless of route of administration. This finding suggests a negligible impact of the route of alcohol administration on the experience of intoxication (Plawecki et al., 2019).
Overall, if the researcher would like to increase generalizability to real-world drinking contexts, ingestion may be the better choice. However, if the study design necessitates a tightly controlled pharmacokinetic variable without the confound of prior alcohol use cues, then infusion may be a better choice. Regardless of the route of administration, ingestion and infusion paradigms can control or provide alcohol cues as desired and the subjective experience of intoxication is similar across both routes of administration. Intravenous alcohol administration may be a prime method to isolate alcohol expectancy effects.
Safety.
Most alcohol beverages prepared for ingestion research do not exceed 20% alcohol (16,000 mg/dl), which is safe (unless the participant has a peptic ulcer). The alcohol concentration that the stomach and liver encounter in infusion paradigms is the peak experimental BrAC, two orders of magnitude less than ingestion of a 20% alcohol beverage.
First pass metabolism of alcohol is inevitable when ingesting alcohol. After ingestion of an initial alcohol dose, an uncertain percentage (~10-20%) is metabolized by gut mucosa and liver before it reaches the bloodstream. This portion accounts for some of the variability in the BrAC trajectory after standardized oral dosing. After the liver’s alcohol elimination rate is saturated, subsequent dosing is subject to any variance in gut metabolism, but differing ascending limb trajectories are primarily a function of variance in absorption.
Even when oral dosing is kept within the bounds of an accurate recent drinking history and participant characteristics, alcohol ingestion can still pose some potential problems. Alcohol absorption can be slowed by gastric retention and the delay leads to the “reservoir effect” (i.e., BrAC rises after consumption ceases). Should the experiment be discontinued in the first 45 min after ingestion (e.g., due to nausea), it may be difficult or impossible to prevent further rise in the BrAC because absorption of the gastric reserve continues. In contrast, infused alcohol bypasses the gut; there is no reservoir effect. The instant the infusion pump is turned off, the BrAC starts to decrease. In this regard, infusion is safer than ingestion. It is true, however, that careful screening of participants and matching exposures to normal drinking levels minimizes the likelihood of adverse events in both ingestion and infusion; such events are generally rare.
In other regards, alcohol ingestion is safer than infusion. Before infusion can occur, an indwelling catheter is inserted into a vein, usually in the antecubital fossa of the non-dominant arm. Insertion involves minor discomfort, can cause local bruising, and there is a minor risk of infection unless sterile technique is employed. If the catheter penetrates both sides of the vein, subsequent infusion infiltrates perivascular interstitial space. Vaso-vagal reaction to catheter insertion causing dizziness is rare (1/500); catheter insertion with the participant in a supine position and performed by a skilled phlebotomist is the best precaution. Participants with extreme fear of medical procedures involving placement of intravenous catheters may need to be excluded from infusion paradigms.
Pain due to endothelial irritation limits the concentration of alcohol that can be infused. The irritation is due to the venous concentration of ethanol just proximal to the catheter insertion site. That concentration combines the typical 6% v/V ethanol at the current infusion rate, diluted by the aggregate flow of blood from veins distal to the insertion site. The higher infusion rates at the beginning of an experiment yield the least dilution and the highest rates of complaints of minor, transient pain (in ~5% of participants) using 6% ethanol. Catheter placement in a hand or forearm vein yields less dilution, accommodating a lower infusate concentration limit. Participants complain of pain before harm is done and ending the infusion alleviates the discomfort. Use of two catheters, one in each arm, each carrying half the total infusion rate also mitigates this concern.
The infusate is usually prepared by a compounding pharmacy from sterile pharmaceutical grade ethanol diluted to 60ml ethanol per liter of infusate, with the vehicle comprising half-normal saline, normal saline, or Ringer’s lactate. Unlike ingestion, whatever is infused must be sterile and pyrogen free, thus the infusate must be prepared appropriately. Any concentration of salt in the vehicle less than half the normal value in plasma risks intravascular lysis of red blood cells. In the thousands of CAIS sessions performed at Indiana University School of Medicine, only three types of infusion-specific adverse events have been identified; none serious and all rare to very rare. The first involved alcohol infiltration into the ante-cubital space due to improper catheter placement. The other events occurred before the infusion of alcohol began: occasional vaso-vagal reaction resulting in transient dizziness, fainting, and one grand mal seizure in a participant who had not reported this known prior history.
Overall, both ingestion and infusion procedures are relatively safe in terms of gastrointestinal tract alcohol exposure and are well-tolerated in the human laboratory. Infused alcohol is safer in terms of limiting the reservoir effect. Intravenous alcohol poses some risk related to catheter placement and alcohol infusate concentration.
Bandwidth.
The BrAC bandwidth involves the amplitude, range, peak exposure, and rate of change of BrAC, and the duration of time over which these kinetics may be safely manipulated by the investigator. A greater range of controlled BrAC and rates provides more research possibilities. Both ingestion and infusion can adequately dose alcohol to yield low BrAC levels where safety concerns are minimal since only persons who have experience with alcohol are studied. However, at higher dosing with ingested alcohol, the investigator must choose a dose that will keep the peak BrAC of the fastest absorber below a safety limit. One does not know which participant might be a fast absorber, so this issue constrains the highest dose given to all participants, which is an important limitation when attempting to achieve ecologically valid BrACs in heavier drinkers. For example, if oral dosing targets a peak BrAC of 80 mg/dl, it is quite likely that some participants will exceed 120 mg/dl ( Ramchandani et al., 2009).
For self-administration paradigms, ingestion-based techniques often limit the frequency of small doses available, give a smaller total available dose of alcohol, or establish a cut-off beyond which participants are not allowed to consume another drink. This procedure controls the peak BrAC and prevents the accumulation of too much alcohol in the gut before absorption. Such limits constrain the range of alcohol choice outcomes and often do not represent ecologically valid drinking behaviors. In these paradigms, each dose-based reward uniquely influences the brain exposure, yielding a lack of precision in bandwidth and inconsistent reward values across the experiment. Although the total number of consumed drinks or grams of alcohol are the most common outcome variables, BrAC during or at the conclusion of the free drinking period is also used. Repeatedly measuring BrAC interrupts the drinking period and may impact drinking behavior. In summary, the substantial overall variability in peak BrAC across participants (Figure 1), uncontrollable variability in incremental BrAC, and difficulties in repeatedly assessing BrAC during ad-libitum consumption pose a limitation to the BrAC bandwidth in ingestion approaches.
In contrast, BrAC bandwidth is one of the main advantages to alcohol infusion. Within CAIS, PBPK modeling allows for calculation of each individual participant’s alcohol infusion profile to achieve a specified overall or incremental BrAC exposure trajectory. Within an alcohol challenge, the software accommodates BrAC measurement feedback and the infusion profile is optimized to attain and maintain the investigator-defined exposure trajectory, commonly achieving precision within 5 mg/dl of the target BrAC. For intravenous self-administration designs, future BrAC, given the past and any future alcohol reward-associated alteration of the infusion profile, is calculated frequently; alcohol requests that would increase BrAC above a preset safety limit are blocked. This safety feature permits infusion experiments to employ larger BrAC bandwidths than ingestion experiments. Routine infusion BrAC safety limits are set at 120 mg/dl for social drinkers, 150mg/dl for heavy drinkers and 180 mg/dl for dependent drinkers. Some participants still hit those limits (~20% according to Kosobud et al., in preparation).
Overall, if the experimenter desires a broader upper range of BrAC exposure, then infusion would be preferred. This expanded range more closely mimics the BrAC levels individuals reach in real-life drinking scenarios without compromising participant safety.
Technical skills.
Laboratory experiments employing alcohol ingestion require beverage preparation, participant instruction, performance of priming procedures, if any, administration monitoring, dependent measures collection, BrAC measurement, and detoxification monitoring. The majority of these tasks are performed by study staff with skill sets in standardized human research, good clinical practice, participant interaction, and some degree of basic computer skills. In comparison, experiments employing infusion require these same skills and constraints, but may also require additional institutional and staff requirements for intravenous catheter insertion, alcohol infusate preparation and administration, computer skills, and safety. Either route requires that the investigators carefully control and report the time of day, environment, recent diet, last alcohol ingestion, dismissal expectations, manner/degree of sobering up before dismissal, and the influence of payment amounts/timing. Both approaches generally require access to onsite medical support and an on-call study physician and/or principal investigator. Overall, ingestion has the advantage over infusion, as it requires fewer technical demands and can be more easily implemented in non-medical settings.
Documentation of alcohol exposure.
With alcohol ingestion, the best approximation to the trajectory of the brain’s exposure to alcohol is the serial BrAC readings obtained throughout the experiment. Good research protocols reduce the confound of residual oral alcohol that may adversely affect accurate BrAC measurement, as well as emission of stomach gas (belching), especially when multiple doses are administered. These challenges are mitigated with a 10-minute non-drinking period before BrAC assessment, having the participant rinse their mouth with water, and/or avoiding taking measures during moments of belching or hiccupping.
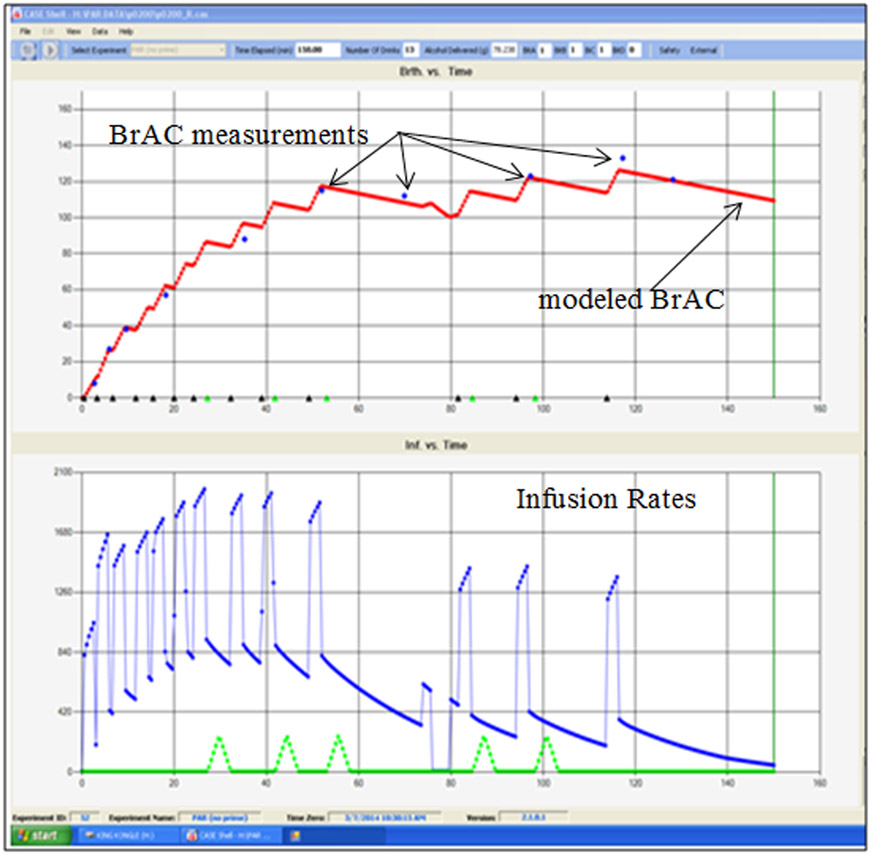
There are several advantages of alcohol infusion in terms of estimation of brain alcohol exposure. First, since no alcohol is consumed orally, there are no delays or artifacts in BrAC measurements due to residual mouth alcohol. Second, no gut reservoir exists, such that the gut is spared exposure to any more than vascular concentrations of alcohol, eliminating the impact of gas emission and making BrAC readings more reliable. Readings logged in CAIS appear in the technician’s real-time display of experimental progress (Figure 2, top panel). Third, CAIS uses BrAC readings to improve subsequent predictions and infusion rates. Finally, since the model’s differential equations can be solved in milliseconds, the model can be adjusted in real time (see Figure 2) and the latest estimate of the entire BrAC time course is always displayed. Infusion has the advantage in expensive research environments where exposure precision is key. Infusion affords a substantial reduction in the incremental or overall variance in BrAC exposure, which can be used to reduce the size of the samples required for alcohol challenge and self-administration research. Many alcohol infusion projects have been IRB-approved in a wide range of expensive environments (e.g. PET, MRI, fMRI, electrophysiological and hybrid) where such precision is important. Similarly, the consistency of incremental BrAC exposures within and across subjects allows for consistent rewards and simplifies the interpretation of self-administration experiments. However, given that real-life drinking might not show such consistency in brain exposure across drinking sessions, such variation may be seen either as an experimental confound or a prime ecological experience of the typical drinker. Infusion also affords access to some research that cannot be performed with ingestion of alcohol: infusion can mimic BrAC trajectories achieved after ingestion (Ramchandani et al., 2009), but not vice versa. In alcohol ingestion, BrAC levels follow usual limb trajectories (ascending, descending levels) and clamping at a prescribed level has not been demonstrated. Alcohol infusion, on the other hand, can produce clamps that hold BrAC steady for hours, thus allowing for the disentangling of BrAC level and limb effects and for unambiguous assessment of acute within-session tolerance, though ecological validity may be limited as typical drinking occasions are likely to show limb effects. Overall, if there is a need to accurately and precisely record the alcohol exposure time course across the experiment, infusion is the preferred technique.
Figure 2:
Portion of the CAIS technician’s video monitor screen displays the BrAC trajectory (top) and the infusion rate profile employed (bottom) for the entire experimental session. This example is from a hazardous drinker’s self-administered BrAC trajectory in a progressive work paradigm using infused water as an alternative reward. Triangles along the bottom of the upper screen indicate where rewards were administered. BrAC measurements obtained occasionally during the experiment are shown in dots along the upper trajectory of the modeled BrAC, validating the trajectory of modeled BrAC, and used by CAIS to adjust a parameter of the PBPK model in order to overcome any modeling errors. The bottom portion notes the infusion rate for alcohol (upper lines) and water (smaller triangles along the bottom) rewards. The increment in BrAC is a ‘slopelet’; a steady increase in BrAC for a specified interval, followed by a steady decline until the next increment commences. The slopelet used in this experiment raised the BrAC by 12 mg/dl in 3 min (4.0 mg/dl/min) before descending at −0.75 mg/dl/min and is one such example. Parameters of the slopelet are specified by the investigator in the setup file linked to the particular experiment. Other incremental shapes and exposure parameters are possible.
Experimental variety.
Drinking occurs in as many different situations and environments as there are people who drink. Ingestion research is more flexible than infusion in this regard. For example, prior ingestion studies have demonstrated that alcohol increases social bonding in groups and that effects of social drinking differ based on the physical context in which alcohol is consumed. Other studies have demonstrated alcohol-related aggression in dyads, and modeling of alcohol consumption in groups (Collins, Parks, & Marlatt, 1985; Corbin et al., 2015; Leonard, 1984; Sayette et al., 2012). So far, an infusion paradigm has not been developed that effectively incorporates social interaction, limiting the current use of this approach to address critical questions about the social context of alcohol use. Current field studies using mobile devices allow for the testing of similar hypotheses about alcohol and have expanded the range of settings and situations where drinking behavior can be assessed. Work is also underway to unscramble recordings from transdermal alcohol concentration devices so that the brain exposure to momentary assessments can be computed retrospectively. Infusion paradigms would clearly be difficult to perform in many of these real-world settings. Overall, alcohol ingestion can be more flexibly applied to a wider range of laboratory questions, using rough estimates of the consequent BrAC.
Ecological validity.
The most common form of alcohol use is ingestion; thus, in some ways alcohol ingestion paradigms appear to be more ecologically valid than infusion paradigms. However, there is a tradeoff between the ability to generalize findings (i.e., matching the research methods closely to what is experienced in real life) and experimental control. For example, using naturalistic settings allows examining behavior that approximates real life; however, the numerous confounds and inability to control for them might compromise the validity of the findings. Ingestion provides greater external validity and infusion provides greater internal validity.
Despite the potentially greater ecological validity of ingestion paradigms, it is important to recognize that all laboratory-based alcohol administration paradigms, regardless of route of administration, have limited ecological validity. Most alcohol administration studies occur in the laboratory or other controlled settings where most ecological details are limited, not mimicking real-life drinking. Settings range from a traditional laboratory setting to hospital settings, simulated bar labs, and simulated living room environments. While some investigators go to great lengths to simulate the bar experience, the size, lighting, color, and other ambient characteristics of these facilities cannot fully reproduce a genuine bar setting, and not all drinkers consume alcohol in bar settings in their real life. Importantly, many studies have individuals consuming alcohol in isolation, or with a confederate who treats every participant in a standardized way, which may reduce ecological validity. Regardless of route of administration, studying alcohol in a laboratory will always, to some extent, have limited ecological validity.
It is sometimes assumed that the use of infusion methods constitutes serious limitations in ecological validity. In some ways, once one makes the decision to use infusion methods, maximizing ecological validity may not need to be the primary consideration. While the mismatch between infusion and the usual route of administration reduces ecological validity, the increased BrAC bandwidth (discussed above) may result in the experience of more ecologically valid levels of alcohol exposure, especially for heavy drinkers. Recall that ingestion experiments limit alcohol exposure to avoid dangerous levels of BrAC due to pharmacokinetic variability, on average limiting BrAC to 80mg/dl or below in challenge studies and 60 mg/dl in self-administration studies. Infusion have safely allowed BrACs of 180mg/dl, although they can approach 100 to 120 mg/dl in ad libitum studies. Infusion has safely allowed BrACs of 180mg/dl. To understand high-risk drinking patterns, such as binge or extreme binge drinking, participants should be allowed to produce brain alcohol exposures similar to those experienced in “real life” (within safety limits). Therefore, providing individuals smaller ingested dosages to prevent high alcohol exposures, although matching the route of administration, does not match the level of intoxication they experience in real-world drinking. The more ecologically valid brain exposure levels available with infusion could benefit many research questions that require a better match between real world intoxication levels and what is modeled in the laboratory.
Further supporting this idea, data suggest that alcohol infusion behaviors correspond with “real world” behavioral and clinical phenomena of interest, similar to how alcohol ingestion behaviors correspond with these outcomes. Response to the alcohol clamp has demonstrated sensitivity to family history of alcoholism (Blekher et al., 2002; Kareken et al., 2010; Morzorati, Ramchandani, Flury, Li, & O’Connor, 2002; Ramchandani, O’Connor, et al., 1999) (albeit, like the oral literature, inconsistently), drinking history (Kerfoot et al., 2013), neurophysiology (Gilman, Ramchandani, et al., 2012; Kareken et al., 2012; Kareken, Liang, et al., 2010; Oberlin et al., 2015; Strang et al., 2015; Yoder et al., 2016, 2009), pharmacologic targets or interventions (Gowin et al., 2016; Leggio, Schwandt, Oot, Dias, & Ramchandani, 2013; Ralevski et al., 2017; Spagnolo et al., 2014), metabolism (Marshall et al., 2014b; Neumark et al., 2004; Ramchandani, Kwo, & Li, 2001), and interactions with known risk genotypes (Kosobud et al., 2015; Roh et al., 2011; Sloan et al., 2018) and personality traits (Hendershot et al., 2015; Leeman et al., 2014; Plawecki, Windisch, et al., 2018). Other alcohol infusion challenge designs have also corresponded with neurophysiology (Oberlin et al., 2018) and drinking history (Wetherill et al., 2012). The literature associating intravenous alcohol self-administration paradigms to behavioral and clinical phenotypes is emerging. Intravenous alcohol self-administration has been associated with family history of alcoholism (Zimmermann et al., 2009), drinking history (Bujarski et al., 2018; Stangl et al., 2017), including binge drinking (Sloan et al., 2019), AUD risk (Gowin, Sloan, Stangl, Vatsalya, & Ramchandani, 2017), craving (Green et al., 2019; Wardell, Ramchandani, & Hendershot, 2015), personality traits (Stangl et al., 2017; VanderVeen et al., 2016), sex differences (Cyders et al., 2016; Plawecki, White, et al., 2018), pharmacologic targets or interventions (Suchankova et al., 2017), and risk genotypes (Hendershot, Claus, & Ramchandani, 2016; Hendershot, Wardell, McPhee, & Ramchandani, 2017; Plawecki et al., 2013; Sloan et al., 2018; Suchankova et al., 2017).
Overall, both ingestion and infusion routes employ many methodological details that reduce ecological validity to some extent in order to increase the internal validity of the study and the power to address the questions being addressed. Alcohol ingestion has the advantage that it matches the route of administration with that which is typically experienced in real-world alcohol use. Infusion allows for more ecologically valid brain exposures, however, but with a route of administration that does not map onto real-world alcohol use.
Cost.
Even after the one-time cost of infusion apparatus, most infusion experiments incur more costs per session than similar experiments using ingestion attributable to the cost of alcohol infusate versus beverage preparation (up to $10 versus $150-300 depending on the experimental design). Project technician costs vary per institutional requirements, but at many locations are the same in research performed with either route of alcohol administration although for iv alcohol experiments the technical expertise of a phlebotomist and compounding pharmacy are required in the preparatory phase. Further, should blinding of data collection be required, infusion-based approaches would require an additional technician. The cost difference in route of administration, however, may be mitigated by the experimental design and need for exposure precision, as discussed previously. Increased control over the primary independent variable (i.e., alcohol exposure) reduces noise and thus increases the power to detect the outcome of interest for a given sample size. Overall, ingestion paradigms are less costly per session.
Human Laboratory Alcohol Paradigm Methodological Considerations (Table 1)
Table 1.
Comparison of Ingestion and Infusion Paradigms
| Aspect of Method | Ingestion | Intravenous Infusion |
|---|---|---|
| Control: controlled variable for alcohol administration | Dose of alcohol ingested | BrAC Trajectory |
| Control: main barrier to precision | Uncontrollable absorption | BrAC meter precision |
| Control: individual peak BrAC | SD ~ 20-30% of intended mean | SD ~ 3% of intended mean |
| Control: latency to peak BrAC | SD ~ 25 min | SD ~ 2 min |
| Control of overall BrAC trajectory | Poor: see Figure 1A-1C | Precise: see Figure 1D |
| Control: first pass metabolism | Uncertain and uncontrollable | None |
| Power: Influence on size of sample population required for adequate hypothesis testing | Determined by concern for dealing with within-group variability in brain exposure to alcohol during analysis | Maximized by elimination of absorption and individualized modeling of alcohol distribution and elimination kinetics |
| Safety: reservoir effect | Unavoidable | Does not exist |
| Safety: peak BrAC | Mean value limited by uncertainty: absorption kinetics vs. toxicity | Limited by toxicity only: investigator specifies a preset value that cannot be exceeded by participants. |
| Ability to control beverage characteristics, including concentration, amount, taste, aroma, texture, and temperature | Excellent, but need to control them all, and to know relationship of each to subject’s preference | No beverage, but important to know infusate alcohol concentration, and to control infusate temperature |
| Ability to blind the first data-collection technician regarding alcohol vs. control beverage | Good | Currently unavailable |
| Ability to control for expectancy effects with an effective placebo control | Good to excellent depending on aspects of study design | Good to excellent depending on aspects of study design |
| Use of preferred alcoholic beverages | Feasible but uncommon | Not applicable |
| Presence of sensory cues associated with alcohol consumption | Excellent; olfactory and taste cues and behavioral responses (e.g., drinking from a glass). | Limited on purpose, but can be added depending on experimental question |
| Documentation of BrAC achieved | Stored, BrAC meter readings; unchecked if technician blinded | Real-time, monitored BrAC meter readings and continuous estimate of BrAC |
| Documentation of events, comments | Manual, changeable record | Time-stamped, permanent record |
| Documentation of dosing schedule in each session | Intermittent, by technician | Continuous, by CAIS |
| Laboratory settings where research can be conducted | Includes simulated bar, lab, simulated living room, fMRI, CT, etc. | Includes simulated bar, lab, CT, SPECT, PET, and fMRI |
| Inclusion of social interaction as variable of interest | Possible, limited, published | currently limited, unpublished |
| Repeated within-session administrations | Easy, but unreliable result | Easy and reliable |
| Ability to study acute within-session tolerance | Possible but typically confounded by limb effects | Easy and reliable |
| Main domain of validity | Ecological route of administration | Ecological alcohol exposures |
| Cost; # technicians required for per-session data collection | Minimum of 1; 2 if blinding required | Minimum of 1; 2 if blinding required |
| Cost: preparation of beverage/alcohol administered | Variable; usually by lab technician from readily available components | Infusate: $150 – $300 per session; usually performed by a research pharmacy |
| Cost: optimization of alcohol delivery paradigm | Variable depending on investigator experience | 20 hours including free consultation using CAIS simulation mode |
| Cost: training of technicians | Weeks on protocol | Weeks on protocol + Days on CAIS |
| Cost: CAIS hardware | $0 | $2,500 - $4,000 |
| Cost: CAIS software | $0 | $0 if paradigm used anywhere previously |
| Cost: CAIS bench testing | $0 | Free, but shipping at cost |
| Cost: CAIS training | $0 | ~ $500 a day + travel |
Note. BrAC= Breath Alcohol Concentration; CAIS= Computer Assisted Infusion Software
Overall, we assert that both ingestion and infusion laboratory paradigms are useful for alcohol research. Both paradigms can be safely conducted in the human laboratory and used to address a wide variety of research questions. However, there are important differences in these methodologies, making each differentially suited for particular research questions and parameters.
Alcohol ingestion paradigms are more appropriate when studying alcohol expectancy effects, when using an ecological route of administration is valued, when there is a specific interest in contextual factors (e.g., social interaction), and when costs need to be limited or key resources are not available (e.g., when conducting research outside of a medical setting or when infusion expertise is not available). Ingestion paradigms are better designed to study determinants of alcohol drinking, rather than the effects of a precise, well-controlled brain alcohol exposures.
On the other hand, infusion paradigms are best suited for studies in which brain alcohol is a key experimental consideration, when precise measurement and documentation of BrAC is necessary, and when higher exposures to alcohol are crucial. Infusion offers increased internal validity and experimental control over ingestion designs. Infusion paradigms have a safety advantage at higher BrAC bandwidth due to eliminating the reservoir effect and more precise control to avoid exceeding the safety limit. Although the cost per session is higher for infusion paradigms, the increased experimental control may reduce the sample size necessary for adequate power depending on the design (for expensive neuroimaging). Infusion paradigms are more appropriate when the research question pertains to the effects of well-controlled trajectory of brain exposure on an outcome of interest. For example, infusion paradigms are particularly well suited to brain imaging contexts and to studying acute tolerance, where the ability to maintain control over alcohol exposure overly lengthy sessions is critical. Infusion paradigms also allow for the study of rapid BAC changes, providing a unique context for studying within-person, exposure-dependent phenomena at a level of resolution not possible with ingestion methods. Such control offers advantages for generating rich within-person data and modeling temporal processes with high resolution, and likely advantages for statistical power.
In conclusion, oral dosing with alcohol remains a common, portable, and often preferable methodological practice in alcohol administration research. However, a major limitation with the method is that even the most careful dose calculation, there remains substantial variability in BrAC trajectories that serve as a proxy of participants’ brain exposures to alcohol. These inherent differences across participants are important facets to study in alcohol research, as ingestion challenge paradigms demonstrate this variability by the route of choice (ingestion) in drinkers’ natural environments. However, such exposure variability brings challenges, some of which are difficult or impossible to overcome. In these situations, infusion methods may be superior due to the precision in alcohol exposure control. Interestingly, given their distinct strengths and weaknesses, the combination of ingestion and infusion paradigms could provide key complementary information concerning both behavioral determinants and consequences of alcohol administration. There is no one preferred method to use in human alcohol research; we do not advocate using IV methods to replace oral administration in either alcohol challenge or self-administration studies, per se or vice versa. Rather, investigators need to be aware of the scientific, pharmacologic, pharmacokinetic, safety, validity, financial, and technical trade-offs in the choice between ingestion and infusion to best address their area of scientific inquiry.
Acknowledgments
Development of the CAIS system was supported by the Indiana Alcohol Research Center (P60 AA007611). The most current version of CAIS is available to interested investigators at cost; contact mplaweck@iupui.edu for information. Preparation of this article was supported by P60 AA07611 (MC, MP, SO), R01 AA027236 (MC, MP), R01 AA019546 (DM), K01 AA024519 (JW), and R01 AA013746 (AK). The authors have no conflicts of interest to report. The authors would like to acknowledge Ms. Katie Shircliff for her help in preparation of this manuscript.
Footnotes
This manuscript was based in part on a roundtable presented at the 2019 Research Society on Alcoholism Annual Meeting in Minneapolis, MN.
References
- Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Någren K, Rinne JO, & Scheinin H (2015). Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [11 C]raclopride. Journal of Perinatology, 35(3), 424–431. 10.1038/jcbfm.2014.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, McCarty KN, Morris DH, Tsai CL, & McCarthy DM (2015). Increased behavioral economic demand and craving for alcohol following a laboratory alcohol challenge. Addiction, 110(9), 1421–1428. 10.1111/add.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekher T, Ramchandani V. a, Flury L, Foroud T, Kareken D, Yee RD, Li TK, & O’Connor S (2002). Saccadic eye movements are associated with a family history of alcoholism at baseline and after exposure to alcohol. Alcoholism, Clinical and Experimental Research, 26(10), 1568–1573. 10.1097/01.ALC.0000033121.05006.EF [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, & Curtin JJ (2013). How Bad Could It Be? Alcohol Dampens Stress Responses to Threat of Uncertain Intensity. Psychological Science, 24(12), 2541–2549. 10.1177/0956797613499923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, David Jentsch J, Roche DJO, Ramchandani VA, Miotto K, & Ray LA (2018). Differences in the subjective and motivational properties of alcohol across alcohol use severity: Application of a novel translational human laboratory paradigm. Neuropsychopharmacology, 43(9), 1891–1899. 10.1038/s41386-018-0086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, & Williams PT (1951). Intravenous alcohol as an obstetrical analgesia. American Journal of Obstetrics and Gynecology, 61(3), 676–679. 10.1016/0002-9378(51)91422-6 [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Smith GT, Roehiing PV, & Goldman MS (1989). Using Alcohol Expectancies to Predict Adolescent Drinking Behavior After One Year. Journal of Consulting and Clinical Psychology, 57(1), 93–99. 10.1037/0022-006X.57.1.93 [DOI] [PubMed] [Google Scholar]
- Collins RL, Parks GA, & Marlatt GA (1985). Social Determinants of Alcohol Consumption. The Effects of Social Interaction and Model Status on the Self-Administration of Alcohol. Journal of Consulting and Clinical Psychology, 53(2), 189–200. 10.1037/0022-006X.53.2.189 [DOI] [PubMed] [Google Scholar]
- Conrad M, McNamara P, & King A (2012). Alternative substance paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Experimental and Clinical Psychopharmacology, 20(5), 382–389. 10.1037/a0029261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, & Fromme K (2008). Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology, 197(2), 327–337. 10.1007/s00213-007-1039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, Menary KR, & Enders CK (2015). Contextual influences on subjective and behavioral responses to alcohol. Experimental and Clinical Psychopharmacology, 23(1), 59–70. 10.1037/a0038760 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, & Fairchild BA (2003). Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology, 112(3), 424–436. 10.1037/0021-843X.112.3.424 [DOI] [PubMed] [Google Scholar]
- Cyders MA, VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken DA, & O’Connor S (2016). Gender-specific effects of mood on alcohol-seeking behaviors: Preliminary findings using intravenous alcohol self-administration. Alcoholism: Clinical and Experimental Research, 40(2), 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, & Lang AR (2007). Intoxication level and emotional response. Emotion, 7(1), 103–112. 10.1037/1528-3542.7.1.103 [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, Stangl BL, Schwandt ML, Farinelli LA, Momenan R, Ramchandani VA, & Leggio L (2018). Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Molecular Psychiatry, 23, 2029–2038. 10.1038/mp.2017.226 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M (1998). Behavioral Impairment Under Alcohol: Cognitive and Pharmacokinetic Factors. Alcoholism: Clinical and Experimental Research, 22(7), 1476–1482. 10.1111/j.1530-0277.1998.tb03938.x [DOI] [PubMed] [Google Scholar]
- Gibbens GLD, & Chard T (1976). Observations on maternal oxytocin release during human labor and the effect of intravenous alcohol administration. American Journal of Obstetrics and Gynecology, 126(2), 243–246. 10.1016/0002-9378(76)90283-0 [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, & Hommer DW (2012). Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology, 37(2), 467–477. 10.1038/npp.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, & Hommer DW (2008). Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. Journal of Neuroscience, 28(8), 4583–4591. 10.1523/JNEUROSCI.0086-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, Momenan R, & Hommer DW (2012). The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction Biology, 17(2), 465–478. 10.1111/j.1369-1600.2011.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Del Boca FK, & Darkes J (1999). Alcohol expectancy theory: The application of cognitive neuroscience. In Psychological Theories of Drinking and Alcoholism, 2, 203–246. [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, & Mason GF (2012). Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biological Psychiatry, 71(3), 239–246. 10.1016/j.biopsych.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, & Ramchandani VA (2017). Vulnerability for alcohol use disorder and rate of alcohol consumption. American Journal of Psychiatry, 174(11), 1094–1101. 10.1176/appi.ajp.2017.16101180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Vatsalya V, Westman JG, Schwandt ML, Bartlett S, Heilig M, Momenan R, & Ramchandani VA (2016). The Effect of Varenicline on the Neural Processing of Fearful Faces and the Subjective Effects of Alcohol in Heavy Drinkers. Alcoholism: Clinical and Experimental Research, 40(5), 979–987. 10.1111/acer.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RJ, Grodin E, Lim AC, Venegas A, Bujarski S, Krull J, & Ray LA (2019). The Interplay Between Subjective Response to Alcohol, Craving, and Alcohol Self-Administration in the Human Laboratory. Alcoholism: Clinical and Experimental Research, 43(5), 907–915. 10.1111/acer.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbrough JF, Zapata-Sirvent RL, Carroll WJ, Johnson R, Saunders CE, & Barton CA (1984). Administration of intravenous alcohol for prevention of withdrawal in alcoholic burn patients. The American Journal of Surgery, 148(2), 266–269. 10.1016/0002-9610(84)90235-6 [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, & Ramchandani VA (2016). Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addiction Biology, 21(1), 125–135. 10.1111/adb.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, McPhee MD, & Ramchandani VA (2017). A prospective study of genetic factors, human laboratory phenotypes, and heavy drinking in late adolescence. Addiction Biology, 22(5), 1343–1354. 10.1111/adb.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Strang NM, Markovich MSD, Claus ED, & Ramchandani VA (2015). Application of an alcohol clamp paradigm to examine inhibitory control, subjective responses, and acute tolerance in late adolescence. Experimental and Clinical Psychopharmacology, 23(3), 147–158. 10.1037/pha0000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Norberg Å, & Hahn RG (1997). Concentration-Time Profiles of Ethanol in Arterial and Venous Blood and End-Expired Breath During and After Intravenous Infusion. Journal of Forensic Sciences, 42(6),1088–1094. 10.1520/jfs14265j [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, & Fromme K (2001). A review of expectancy theory and alcohol consumption. Addiction, 96(1), 57–72. 10.1046/j.1360-0443.2001.961575.x [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, & O’Connor SJ (2010). Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. NeuroImage, 50(1), 267–276. 10.1016/j.neuroimage.2009.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Grahame N, Dzemidzic M, Walker MJ, Lehigh CA, & O’Connor SJ (2012). fMRI of the brain’s response to stimuli experimentally paired with alcohol intoxication. Psychopharmacology, 220(4), 787–797. 10.1007/s00213-011-2526-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O’Connor SJ, & Foroud T (2010). A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcoholism: Clinical and Experimental Research, 34(12), 2169–2178. 10.1111/j.1530-0277.2010.01293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, & Petrakis IL (2013). Effects of Family History of Alcohol Dependence on the Subjective Response to Alcohol Using the Intravenous Alcohol Clamp. Alcoholism: Clinical and Experimental Research, 37(12), 2011–2018. 10.1111/acer.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, De Wit H, McNamara PJ, & Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68(4), 389–399. 10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsten MA, Matsuzaki S, Feinman L, & Lieber CS (1975). High Blood Acetaldehyde Levels after Ethanol Administration. New England Journal of Medicine, 292(8), 386–389. 10.1056/nejm197502202920802 [DOI] [PubMed] [Google Scholar]
- Kosobud AEK, Wetherill L, Plawecki MH, Kareken DA, Liang T, Nurnberger JL, Windisch K, Xuei X, Edenberg HJ, Foroud TM, & O’Connor SJ (2015). Adaptation of Subjective Responses to Alcohol is Affected by an Interaction of GABRA2 Genotype and Recent Drinking. Alcoholism: Clinical and Experimental Research, 39(7), 1148–1157. 10.1111/acer.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Corbin WR, Nogueira C, Krishnan-Sarin S, Potenza MN, & O’Malley SS (2013). A human alcohol self-administration paradigm to model individual differences in impaired control over alcohol use. Experimental and Clinical Psychopharmacology, 21(4), 303–314. 10.1037/a0033438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O’Malley SS, & Petrakis IL (2014). Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology, 231(14), 2867–2876. 10.1007/s00213-014-3458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Schwandt ML, Oot EN, Dias AA, & Ramchandani VA (2013). Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: A within-subject placebo-controlled study. Psychoneuroendocrinology, 38(12), 3085–3091. 10.1016/j.psyneuen.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE (1984). Alcohol consumption and escalatory aggression in intoxicated and sober dyads. Journal of Studies on Alcohol, 45(1), 75–80. 10.15288/jsa.1984.45.75 [DOI] [PubMed] [Google Scholar]
- Lindberg L, Brauer S, Wollmer P, Goldberg L, Jones AW, & Olsson SG (2007). Breath alcohol concentration determined with a new analyzer using free exhalation predicts almost precisely the arterial blood alcohol concentration. Forensic Science International, 168(2-3), 200–207. 10.1016/j.forsciint.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Marshall VJ, Ramchandani VA, Kalu N, Kwagyan J, Scott DM, Ferguson CL, & Taylor RE (2014). Evaluation of the Influence of Alcohol Dehydrogenase Polymorphisms on Alcohol Elimination Rates in African Americans. Alcoholism: Clinical and Experimental Research, 38(1), 51–59. 10.1111/acer.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, & Earleywine M (1990). Ascending and descending rates of change in blood alcohol concentrations and subjective intoxication ratings. Journal of Substance Abuse, 2(3), 345–352. 10.1016/S0899-3289(10)80006-9 [DOI] [PubMed] [Google Scholar]
- Martin E, Moll W, Schmid P, & Dettli L (1984). The pharmacokinetics of alcohol in human breath, venous and arterial blood after oral ingestion. European Journal of Clinical Pharmacology, 26(5), 619–626. 10.1007/BF00543496 [DOI] [PubMed] [Google Scholar]
- Moberg CA, Weber SM, & Curtin JJ (2011). Alcohol dose effects on stress response to cued threat vary by threat intensity. Psychopharmacology, 218(1), 217–227. 10.1007/s00213-011-2304-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DH, Amlung MT, Tsai CL, & McCarthy DM (2017). Association between overall rate of change in rising breath alcohol concentration and the magnitude of acute tolerance of subjective intoxication via the Mellanby method. Human Psychopharmacology, 32(1). 10.1002/hup.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani V. a, Flury L, Li T-K, & O’Connor S (2002). Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcoholism, Clinical and Experimental Research, 26(8), 1299–1306. 10.1097/01.ALC.0000025886.41927.83 [DOI] [PubMed] [Google Scholar]
- Neumark YD, Friedlander Y, Durst R, Leitersdorf E, Jaffe D, Ramchandani VA, O’Connor S, Carr LG, & Li TK (2004). Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res, 28(1), 10–14. 10.1097/01.ALC.0000108667.79219.4D [DOI] [PubMed] [Google Scholar]
- Nishimura M, Hasumura Y, & Takeuchi J (1980). Effect of an intravenous infusion of ethanol on serum enzymes and lipids in patients with alcoholic liver disease. Gastroenterology, 78(4), 691–695. 10.1016/0016-5085(80)90669-1 [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, & Li TK (1998). Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism, Clinical and Experimental Research, 22(1), 202–210. 10.1111/j.1530-0277.1998.tb03639.x [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Eiler WJA, Carron CR, Soeurt CM, Plawecki MH, Grahame NJ, O’Connor SJ, &. Kareken DA (2018). Pairing neutral cues with alcohol intoxication: new findings in executive and attention networks. Psychopharmacology, 235(9), 2725–2737. 10.1007/s00213-018-4968-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O’Connor SJ, Yoder KK, & Kareken DA (2015). Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology, 232(5), 861–870. 10.1007/s00213-014-3720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin H., DeCarlo RA, Ramchandani VA, & O’Connor S (2007). Improved transformation of morphometric measurements for a priori parameter estimation in a physiologically-based pharmacokinetic model of ethanol. Biomedical Signal Processing and Control, 2(2), 97–110. 10.1016/j.bspc.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin H., Han JJ, Doerschuk PC, Ramchandani VA, & O’Connor SJ (2008). Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Transactions on Biomedical Engineering, 55(12), 2691–2700. 10.1109/TBME.2008.919132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin H., Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, & O’Connor S (2013). Voluntary Intravenous Self-Administration of Alcohol Detects an Interaction Between GABAergic Manipulation and GABRG1 Polymorphism Genotype: A Pilot Study. Alcoholism: Clinical and Experimental Research, 37(SUPPL.1). 10.1111/j.1530-0277.2012.01885.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin H., Windisch KA, Wetherill L, Kosobud AEK, Dzemidzic M, Kareken DA, & O’Connor SJ (2018). Alcohol affects the P3 component of an adaptive stop signal task ERP. Alcohol, 70, 1–10. 10.1016/j.alcohol.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin Henry, Durrani AM, Boes J, Wetherill L, Kosobud A, O’Connor S, & Ramchandani VA (2019). Comparison of Subjective Responses to Oral and Intravenous Alcohol Administration Under Similar Systemic Exposures. Alcoholism: Clinical and Experimental Research, 43(4), 597–606. 10.1111/acer.13970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki Martin Henry, Koskie S, Kosobud A, Justiss MD, & O’Connor S (2018). Alcohol intoxication progressively impairs drivers’ capacity to detect important environmental stimuli. Pharmacology Biochemistry and Behavior, 175, 62–68. 10.1016/j.pbb.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Plawecki Martin Henry, Vitvitskiy V, Millward JB, Haines J, Hays J, Shehkar S, Kosobud AEK, Wetherill L, & O’Connor S (2016). Alcohol Exposure Rate Control – A Laboratory Model of Heavy Episodic Drinking? In Alcoholism, Clinical and Experimental Research, 40 (S1), 97A. [Google Scholar]
- Plawecki Martin Henry, White K, Kosobud AEK, Grahame N, Zimmermann US, Crabb D, & O’Connor S (2018). Sex Differences in Motivation to Self-Administer Alcohol After 2 Weeks of Abstinence in Young-Adult Heavy Drinkers. Alcoholism: Clinical and Experimental Research, 42(10), 1897–1908. 10.1111/acer.13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, & Fromme K (2011). Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical and Experimental Research, 35(10), 1759–1770. 10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, & Petrakis I (2017). Ghrelin is supressed by intravenous alcohol and is related to stimulant and sedative effects of alcohol. Alcohol and Alcoholism, 52(4), 431–438. 10.1093/alcalc/agx022 [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, & Heilig M (2011). A genetic determinant of the striatal dopamine response to alcohol in men. Molecular Psychiatry, 16(8), 809–817. 10.1038/mp.2010.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani V. a, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li TK, & O’Connor S (2002). Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. Journal of Studies on Alcohol, 63(6), 734–744. [DOI] [PubMed] [Google Scholar]
- Ramchandani Vijay A., Bolane J, Li TK, & O’Connor S (1999). A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical and Experimental Research, 23(4), 617–623. 10.1097/00000374-199904001-00008 [DOI] [PubMed] [Google Scholar]
- Ramchandani Vijay A., Kwo PY, & Li T-K (2001). Effect of Food and Food Composition on Alcohol Elimination Rates in Healthy Men and Women. The Journal of Clinical Pharmacology, 41(12), 1345–1350. 10.1177/00912700122012814 [DOI] [PubMed] [Google Scholar]
- Ramchandani Vijay A., O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, & Li T-K (1999). A Preliminary Study of Acute Responses to Clamped Alcohol Concentration and Family History of Alcoholism. Alcoholism: Clinical and Experimental Research, 23(8), 1320–1330. 10.1111/j.1530-0277.1999.tb04353.x [DOI] [PubMed] [Google Scholar]
- Ramchandani Vijay A., Plawecki M, Li TK, & O’Connor S (2009). Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcoholism: Clinical and Experimental Research, 33(5), 938–944. 10.1111/j.1530-0277.2009.00906.x [DOI] [PubMed] [Google Scholar]
- Ray LA, & Hutchison KE (2004). A polymorphism of the μ,-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism: Clinical and Experimental Research, 28(12), 1789–1795. 10.1097/01.ALC.0000148114.34000.B9 [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Kahler CW, Leventhal AM, Monti PM, Swift R, & Hutchison KE (2007). Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: A pilot study. Psychopharmacology, 193(4), 449–456. 10.1007/s00213-007-0794-z [DOI] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, & Higuchi S (2011). Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alcoholism: Clinical and Experimental Research, 35(3), 400–407. 10.1111/j.1530-0277.2010.01357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Kirchner TR, Levine JM, & Moreland RL (2012). Alcohol and Group Formation: A Multimodal Investigation of the Effects of Alcohol on Emotion and Social Bonding. Psychological Science, 23(8), 869–878. 10.1177/0956797611435134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Klepp TD, Gowin JL, Swan JE, Sun H, Stangl BL, & Ramchandani VA (2018). The OPRM1 A118G polymorphism: Converging evidence against associations with alcohol sensitivity and consumption. Neuropsychopharmacology, 43(7), 1530–1538. 10.1038/s41386-017-0002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan Matthew E., Gowin JL, Janakiraman R, Ester CD, Stoddard J, Stangl B, & Ramchandani VA (2019). High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addiction Biology. 10.1111/adb.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, & Heilig M (2014). Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcoholism: Clinical and Experimental Research, 38(12), 3024–3032. 10.1111/acer.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, & Ramchandani VA (2017). Exposure-response relationships during free-access intravenous alcohol self-administration in nondependent drinkers: Influence of alcohol expectancies and impulsivity. International Journal of Neuropsychopharmacology, 20(1), 31–39. 10.1093/ijnp/pyw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, & Hendershot CS (2015). Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology, 232(4), 733–744. 10.1007/s00213-014-3706-z [DOI] [PubMed] [Google Scholar]
- Subramanian MG, Heil SH, Kruger ML, Collins KL, Buck PO, Zawacki T, Abbey A, Sokol RJ, & Diamond MP (2002). A three-stage alcohol clamp procedure in human subjects. Alcoholism: Clinical and Experimental Research, 26(10), 1479–1483. 10.1111/j.1530-0277.2002.tb02446.x [DOI] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Jerlhag E, Engel JA, Hodgkinson CA, Ramchandani VA, & Leggio L (2017). The Leu72Met polymorphism of the prepro-ghrelin gene is associated with alcohol consumption and subjective responses to alcohol: Preliminary findings. Alcohol and Alcoholism, 52(4), 425–430. 10.1093/alcalc/agx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken DA, O’Connor S, & Cyders MA (2016). Negative urgency, mood induction, and alcohol seeking behaviors. Drug and Alcohol Dependence, 165, 151–158. 10.1016/j.drugalcdep.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vena A, & King AC (2019). Testing the Allostasis theory in light, heavy, and AUD drinkers. In Alcoholism: Clinical & Experimental Research. 10.1111/acer.1405 [DOI] [Google Scholar]
- Wall AM, McKee SA, & Hinson RE (2000). Assessing variation in alcohol outcome expectancies across environmental context: An examination of the situational-specificity hypothesis. Psychology of Addictive Behaviors, 14(4), 367–375. 10.1037/0893-164X.14.4.367 [DOI] [PubMed] [Google Scholar]
- Wall AM, McKee SA, Hinson RE, & Goldstein A (2001). Examining alcohol outcome expectancies in laboratory and naturalistic bar settings: A within-subject experimental analysis. Psychology of Addictive Behaviors, 15(3), 219–226. 10.1037/0893-164X.15.3.219 [DOI] [PubMed] [Google Scholar]
- Wardell JD, Ramchandani VA, & Hendershot CS (2015). A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. Journal of Abnormal Psychology, 124(4), 1050–1063. 10.1037/abn0000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman JG, Bujarski S, & Ray LA (2017). Impulsivity moderates subjective responses to alcohol in alcohol-dependent individuals. Alcohol and Alcoholism, 52(2), 249–255. 10.1093/alcalc/agw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T, Windisch K, Darlington T, Zimmerman US, Plawecki MH, & O’Connor SJ (2012). Subjective Perceptions Associated with the Ascending and Descending Slopes of Breath Alcohol Exposure Vary with Recent Drinking History. Alcoholism: Clinical and Experimental Research, 36(6), 1050–1057. 10.1111/j.1530-0277.2011.01642.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, Herring CM, Hile KL, Walters JW, Liang T, Plawecki MH, O’Connor S, & Kareken DA (2016). Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug and Alcohol Dependence, 160, 163–169. 10.1016/j.drugalcdep.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, & Kareken DA (2009). When what you see isn’t what you get: Alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcoholism: Clinical and Experimental Research, 33(1), 139–149. 10.1111/j.1530-0277.2008.00821.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, & O’Connor S (2009). Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE). Psychopharmacology, 202(4), 689–697. 10.1007/s00213-008-1349-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, & Connor SO (2008). Development and Pilot Validation of Computer-Assisted Self-Infusion of Ethanol (CASE): A New Method to Study Alcohol Self-Administration in Humans. Alcoholism: Clinical and Experimental Research, 32(7), 1321–1328. 10.1111/j.1530-0277.2008.00700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, & Ramchandani VA (2013). Modeling alcohol self-administration in the human laboratory. Current Topics in Behavioral Neurosciences, 13, 315–353. 10.1007/7854_2011_149 [DOI] [PubMed] [Google Scholar]