Abstract
Objective:
To document prevalence and traits of children with fetal alcohol spectrum disorders (FASD) and maternal risk factors in a Rocky Mountain City.
Methods:
Variations on active case ascertainment methods were used in two first grade cohorts in all city schools. The consent rate was 59.2%. Children were assessed for physical growth, dysmorphology, and neurobehavior and their mothers interviewed.
Results:
Thirty-eight children were diagnosed with FASD and compared with 278 typically-developing controls. Total dysmorphology scores summarized well the key physical indicators of FASD and defined specific diagnostic groups. On average, children with FASD performed significantly poorer than controls on intellectual, adaptive, learning, attention, and behavioral tasks. More mothers of children with FASD reported drinking prior to pregnancy, 1st and 2nd trimesters, and had partners with drinking problems than mothers of controls; however, reports of co-morbid alcohol and other drug use were similar for both maternal groups. Mothers of children with FASD were significantly younger at pregnancy, had lower average weight before pregnancy and less education, initiated prenatal clinic visits later, and reported more health problems (e.g., stomach ulcers, accidents). Children with FASD had significantly lower birth weight, more problems at birth, and were less likely to be living with biological mother and father. Controlling for other drug and tobacco use, a FASD diagnosis is 6.7 times (OR=6.720, 95% CI = 1.6-28.0) more likely among children of women reporting pre-pregnancy drinking of three drinks per drinking day (DDD) and 7.6 times (OR=7.590, 95% CI = 2.0-31.5) more likely at five DDD. Prevalence of FAS was 2.9–5.8 per 1,000 children, and total FASD was 34.9 – 82.5 per 1,000 children, or 3.5 - 8.3% at this site.
Conclusion:
This site had the second highest prevalence of FASD of the four CoFASP sites and clearly identifiable child and maternal risk traits.
Keywords: fetal alcohol spectrum disorders, alcohol use and abuse, maternal risk, prenatal alcohol use, prevalence, children with FASD
INTRODUCTION
Many individuals have wondered for years what the prevalence of fetal alcohol spectrum disorders (FASD) is in large populations across the globe, what the specific characteristics are of children within the continuum of FASD, and what the distribution of these traits is in general populations. Furthermore, it is vital that we understand the most influential maternal risk factors for actual cases of FASD if we are to lessen the severity of, reduce, or eliminate FASD among children in the United States (U.S.) and the world. Recent reviews indicated that there is a general lack of adequate empirical research on the prevalence of FASD throughout the world (Lange et al., 2017; Roozen et al., 2016), especially literature that links detailed alcohol exposure data and other maternal characteristics to specific FASD outcomes (Roozen et al., 2018).
The Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP)
In 2010, CoFASP was created by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) from two extramural applications submitted to a request for applications. The collaboration required the use of the best available methods to establish the prevalence of FASD in U.S. communities that were believed to be representative of their geo-political regions, if not the overall general U.S. population (NIAAA Strategic Plan 2017-2021, 2017). Over the eight years of collaboration, an advisory group composed of NIAAA program personnel and several FASD experts met regularly with the investigative teams to discuss and finalize: sampling methods; diagnostic, inclusion, and exclusion criteria; and other matters related to the study, publication of results, and the preparation of a public use dataset.
Final CoFASP data were used to estimate prevalence rates for eight cohort samples, two samples each in four regions of the U.S.: Midwest, Pacific Southwest, Rocky Mountain and Southeast. A prevalence summary of the eight CoFASP samples was published in 2018 (May et al., 2018a). The prevalence of FASD in these regional sites was found to be substantially higher than had been previously estimated for the overall U.S. population. FASD had been believed to be, and often quoted, as 9 to 10 per 1,000 (1%) in the U.S. (Sampson et al., 1997). However, even the most conservative prevalence estimates from CoFASP collaborative samples ranged from 11.3 to 50.0 per 1,000 children (1.1- 5.0%). Furthermore, the CoFASP collaboration produced less conservative, weighted prevalence estimates which ranged from 31.1 to 98.5 per 1,000 children (3.1 - 9.9%) across the eight samples.
Since the diagnosis of fetal alcohol syndrome (FAS) was first described (Jones and Smith, 1973), surveillance systems, prenatal clinic-based studies, and special referral clinics have generally proven inadequate for determining the prevalence of FAS or FASD and for describing the traits of children on the continuum of FASD in a general population (May et al., 2009). The CoFASP research group chose to use three variations on active case ascertainment (ACA) methods.
Active Case Ascertainment Studies of FASD
The first, large, active case ascertainment (ACA) studies of the prevalence and characteristics of FASD began in schools in South Africa in 1997. Every child consented into the study was screened for traits of FASD. This approach yielded results that were representative of the local population (May et al., 2000). Not only were South African studies continued for years thereafter, but later, similar studies were initiated in Italy (May et al., 2006). Both efforts in foreign countries were initiated and funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Warren et al., 2001).
Prior to 2000, many scholars thought that it might be impossible to undertake successful ACA studies in U.S. schools due to resistance within local communities. However, one ACA study on fetal alcohol syndrome (FAS) was initiated in two counties in Washington State (Clarren et al., 2001) in the late 1990’s. Success was obtained in only one county where passive consent was permitted. In the other county, where active written consent was required, no results could be reported due to lack of participation. The Washington study identified seven children with fetal alcohol syndrome (FAS), only one of whom had been diagnosed previously. The rate of FAS was 3.1 per 1,000 children. Another site where an ACA study was approved and carried out was a Head Start program in a northern Native American community. Burd and colleagues screened children over a nine-year period. The rate of FAS was 5.9 per 1,000 children with FAS in one sample (Burd et al., 1999), and 4.3 per 1,000 in another (Poitra et al., 2003).
The Rocky Mountain Region Site and Study Objectives
The study locale for this paper is a small city in the Rocky Mountain Region of the U.S. The Fetal Alcohol Syndrome Epidemiology Research (FASER) Team of the University of New Mexico received a contract in 1999 from a State Department of Public Health to provide FASD referral and screening clinics in this community. In 2006, a city/county health department official suggested that an in-school prevalence study would be meaningful in this city and worked to get it approved. After approval from the Board of Trustees of the city schools, pilot studies were carried out in three cohorts of first grade students from 2007 through 2010. Summary results were reported on the characteristics of children with FAS and partial fetal alcohol syndrome (PFAS) and a limited description of maternal risk. The combined prevalence of FAS and Partial FAS in this city was 10.9 to 25.2 per 1,000 (May et al., 2015).
The major objectives of this manuscript, prepared from CoFASP study data, are twofold: 1) to present a detailed analysis of the growth, dysmorphology, and developmental traits of children with FASD and compare them with randomly-selected, typically-developing controls from the same community; and 2) to detail significant maternal risk factors for FASD at this site.
The Research Site Described
The site is a city of 60,000 people in the Rocky Mountain Region of the U.S. (Table 1). Its economy is based primarily on ranching, agriculture, banking, general business, medical services, and a Federal government installation. The population of this city and county is not growing rapidly; its growth rate in 2015 was about 1/5 that of the United States (U.S.) overall. The racial and ethnic composition of the city is 87% non-Hispanic, White, 5% American Indian/Alaska Native, 3.4% Hispanic, and 1% Black, non-Hispanic. The median age is similar to the overall U.S. and mean household value is less than the U.S. average. More people have graduated from high school than in the general population, but fewer people are college graduates (“U.S. Census Bureau QuickFacts: United States,” 2015). Per capita income and median household income are lower than the general U.S. population, and a higher percentage of the population is classified in poverty. Seventy percent (70%) of the population of this state report that they are affiliated with Christian, Jewish, or Muslim institutions and 30% are unaffiliated (“nones”) with an organized religion (Pew Research Center, 2015). This state’s health rank falls between 20 and 25 of the 50 states, but alcohol use is higher in this state, city and county (America’s Health Rankings Annual Report, 2015). Annual state per capita alcohol consumption is 30% higher than U.S. averages and binging and excessive drinking are higher in this state and county than the US population average (LaVallee and Yi, 2011). This city, however, reports excessive drinking that is about 1/3 of the U.S. average at 5% (“CDC - BRFSS,” 2013).
Table 1.
Demographic Indicators for the Rocky Mountain Region City compared to the United States
| Demographic Indicator | Rocky Mountain City |
United States |
|---|---|---|
| Population (7/2015)1 | 59,638 | 321,418,820 |
| (percentage of US population) | (0.02%) | (100%) |
| Population change (%) since 20101 | 0.9% | 4.1% |
| Race/Hispanic Ethnicity (2010)1 | ||
| White, non-Hispanic | 86.7% | 63.7% |
| Black, non-Hispanic | 1.1% | 12.6% |
| American Indian and Alaskan Native | 5.0% | 0.9% |
| Asian | 0.9% | 4.8% |
| Two or more races | 3.8% | 2.9% |
| Hispanic or Latino | 3.4% | 16.3% |
| Foreign born persons1 | 2.2% | 13.1% |
| Age – years (median) | 38.9 | 37.2 |
| Housing1 | ||
| Median household value | $158,900 | $176,700 |
| Education1 | ||
| High School graduate or higher, % ages ≥25 years | 91.1% | 86.3% |
| Bachelor’s degree or higher, % ages ≥25 years | 25.5% | 29.3% |
| Economy1 | ||
| Per capita income in past 12 months (2014 dollars) | $24,733 | $28,555 |
| Median household income | $43,374 | $53,482 |
| Persons in poverty | 16.1% | 14.8% |
| Religion5 | ||
| Composition | ||
| Christian | 65% | 70.6% |
| Non-Christian | 5% | 5.9% |
| Unaffiliated (“nones”) | 30% | 22.8% |
| Importance of Religion | ||
| Very important | 44% | 58% |
| Somewhat important | 25% | 24% |
| Not too important/not at all | 30% | 16% |
| Health Behavior | Median 25 | |
| Overall state health Rank in US2 | 20-25 | (Range 1-50) |
| Alcohol Use | ||
| Binge drinking^ state %, (US rank)2 | 18.9% (41) | 16.% |
| Excessive drinking+, state % (US rank)2 | 20.8% (42) | Median = 17.4% |
| Excessive drinking, county3 | 20.0% | Mean = 16.8% |
| Heavy drinking#, city3 | 4.9% | |
| State per capita ethanol consumption (2009), | 2.99 gallons | 2.30 gallons |
| volume per person 14 years and older4 | 11.32 liters | 8.71 liters |
Sources:
US Census, 2015
United Health Foundation, America’s Health Rankings, 2015
Behavioral Risk Factor Surveillance System (BRFSS) 2013 data of the CDC. Reported in local city and county statistical reports
LaVallee and Yi, 2011.NIAAA Surveillance Report #92
Pew Research Center. America’s Changing Religion Landscape, 2015. Online. www.pewresearch.org.
Binge drinking defined as: during the past 30 days, the consumption of 5 or more drinks for men or 4 or more drinks for females on an occasion
Heavy drinking is defined as males having more than two drinks per day and females having more than one drink per day
Excessive drinking of alcohol is defined as both binge drinking (above) and chronic drinking also referred to as heavy drinking (above)
METHODS
Protocols and consent forms were approved by The University of New Mexico School of Medicine, HRRC #10-342, and the University of North Carolina, #11-0717. Active consent from parents was required for children to participate in the study, and maternal interviews required a separate consent.
Diagnostic Criteria
The Revised Institute of Medicine (IOM) diagnostic guidelines for FASD (Hoyme et al., 2005) were used along with revised cut-off values established by the CoFASP advisory group (Hoyme et al., 2016). Physical assessments were made by fellowship-trained pediatricians in medical genetics/dysmorphology. Licensed school psychologists performed all neurobehavioral testing. Grant-employed nurses and social workers administered face-to-face maternal risk interviews. All research team members were blinded from prior knowledge of children and mothers. The domains assessed for all study participants who completed the entire study were: (1) physical growth, (2) dysmorphology; (3) cognitive tests and behavioral assessments, and (4) maternal risk factors impacting the index pregnancy. In the diagnostic process, other recognizable malformations and syndromes were ruled out by the dysmorphologists. Dysmorphologists made the final diagnoses in formal, structured, data-driven case conferences after the examiners of the individual domains presented detailed findings and assessments.
The continuum of FASD has four specific diagnoses: fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD) (Hoyme et al., 2016). Each diagnostic category was utilized in this study, yet ARBD has been found to be rare in any population (May et al., 2016a, 2016b, 2015, 2014, 2011a). The diagnosis of FAS without a confirmed history of alcohol exposure can be made according to the original IOM criteria (Stratton et al., 1996), and revised criteria (Hoyme et al., 2005, 2016). Revised criteria also permit diagnosis of PFAS without directly-reported evidence of prenatal drinking. Some women underreport alcohol use during pregnancy, especially precise levels and frequencies (Alvik et al., 2006; Bakhireva et al., 2017; Wurst et al., 2008). Yet in some populations both alcohol use and levels of drinking are reported accurately (Fortin et al., 2017; May et al., 2018a). The diagnosis of FASD in epidemiology studies is rarely made without direct maternal reports of alcohol use prior to pregnancy recognition, during pregnancy, or collateral reports. An ARND diagnosis always requires direct confirmation of alcohol use in the index pregnancy.
Sampling in Two Cohorts
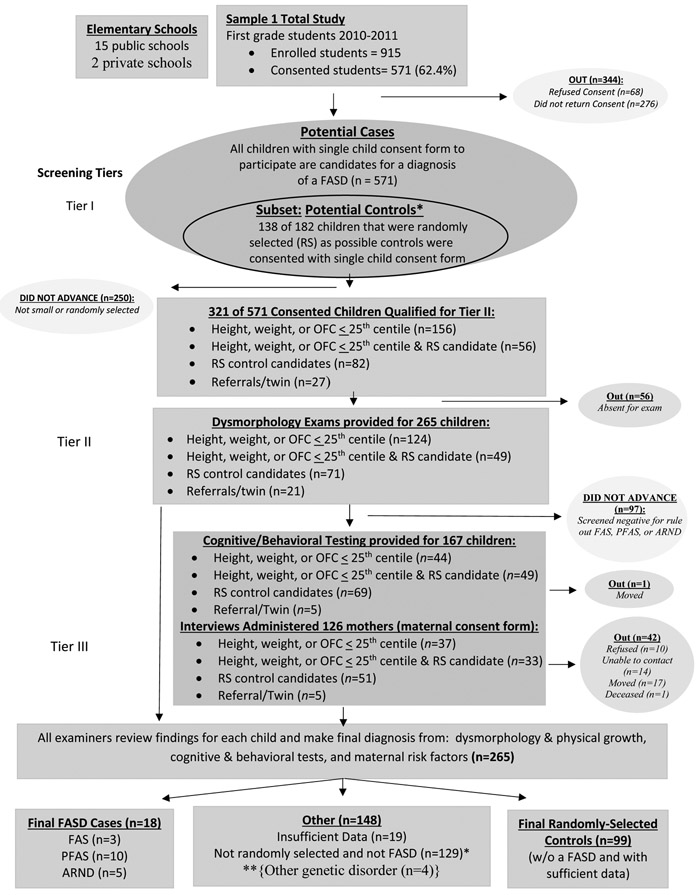
There were 15 public elementary schools in this city and two private, Christian schools; all participated in the study. In the first cohort sample, consent forms were sent to parents/guardians of all first grade students (n=915) enrolled in the city that year. Six-hundred thirty-nine (639) forms were returned (69.8%), 68 of which were refusals, 571 students or 62.4% had consent to participate. The sampling process and numbers of children and mothers in Cohort Sample 1 is described in Figure 1.
Figure 1. Sampling Methodology for Prevalence of FASD in Rocky Mountain City (SM1): Sample 1.
*If a child was randomly selected and found to have an FASD or another known genetic or teratogenic disorder, he/she was classified appropriately and removed from the control group. **4 were not FASD, with other genetic disorders
Consented children entered Sample 1 primarily via one or both of two criteria: 1) oversampling of all consented small children ≤ 25 centile on height, weight and/or head circumference (occipitofrontal circumference-OFC) and/or 2) random selection from class rolls. This census of all consented small children was to capture most of the children with FAS and PFAS. Random sample entry was utilized to: 1) capture a representative proportion of children with ARND, 2) provide a representative comparison (control) group of typically-developing not FASD children from this population, and 3) provide accurate proportions for the occurrence of each FASD diagnosis in this population. Children selected randomly and found not to have a diagnosis of a FASD or another anomaly constituted the final control group. Additionally, 21 children entered the study because of teacher or parent referrals or because a child picked for the study had a twin enrolled in the study. Children who entered via non-random selection routes, and found to be “not-FASD” or affected by another known birth defect, did not default to the control group. Only randomly-selected children who were verified to be developing normally by the examination and testing process, qualified for the control group. All children who participated through all tiers of the study received identical exams and testing.
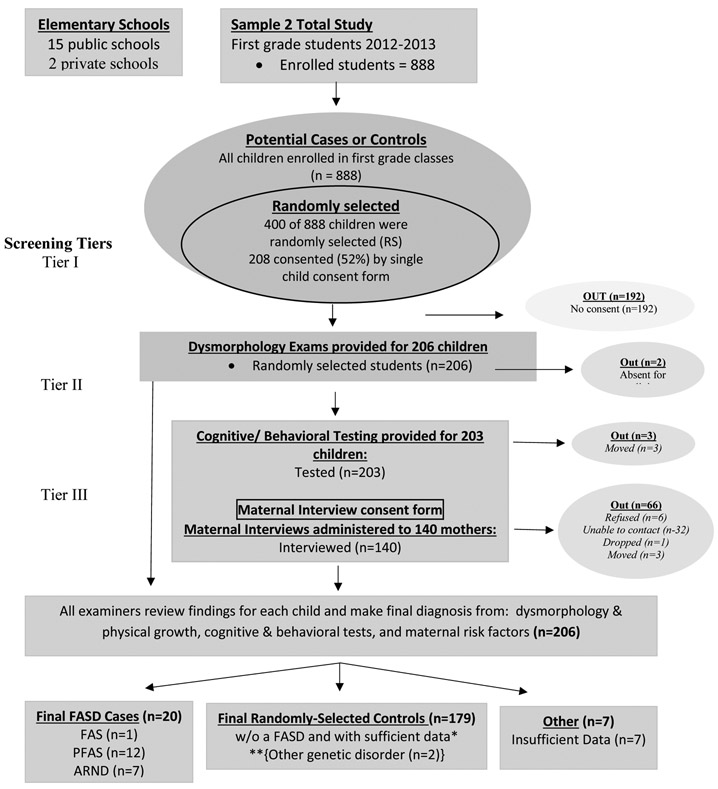
Cohort Sample 2 was collected exclusively via a simple random sample drawn two years after Cohort Sample 1 from the entire first grade population. There were 888 students in this cohort, and 400 unique numbers were chosen via a computer randomization program. As in the first sample, parents/guardians were contacted and provided consent forms through each school’s take-home folder communication. Two-hundred eight (208) consent forms were returned (52%). The combined-sample participation rate was 59.2%. In Sample 2, each child was assessed in the same three-tier process described below, but there was no pre-screening by size.
Diagnostic Process - Three Tiers of Assessment
In Tier I, all consented children in Cohort Sample 1 were measured first by the research team, and any consented child ≤ 25th centile on OFC or height or weight, those referred by teachers, and all randomly-selected children in either cohort were included in Tier II physical exams (Figure 1). All children selected randomly for Cohort Sample 2 went immediately to Tier II.
In Tier II, research teams took final growth measurements, frontal and profile facial photographs and dysmorphologists provided structured dysmorphology examinations. Exams assessed multiple facial measurements and minor anomalies of the craniofacies, limbs, skin, hair, hands, and hearts, utilizing a structured dysmorphology form. Later, completed forms for each child were reviewed by the examining dysmorphologist to summarized which cardinal FASD features and other minor anomalies were found and to assign a total dysmorphology score. A preliminary diagnosis was assigned: a) not-FASD, b) diagnosis deferred – rule out a specific FASD diagnosis or a related disorder, or c) probable FAS or PFAS. All randomly-selected children and children classified in categories b and c advanced to Tier III.
Although the total dysmorphology score is not used directly for assignment of a specific FASD diagnosis, the presence or absence of specific cardinal features, other minor anomalies, and degrees of growth deficiency provide the criteria for a final diagnosis (Hoyme et al., 2016). The dysmorphology score correlates well with maternal drinking and learning/behavior difficulties (Ervalahti et al., 2007). Inter-rater reliability using revised IOM criteria was assessed in previous studies (May et al., 2011b, 2000; Viljoen et al., 2005). Exam techniques balance sensitivity and specificity for capturing the complete range of FASD (Hoyme et al., 2016).
Tier III - Child Testing and Maternal Risk Questionnaires
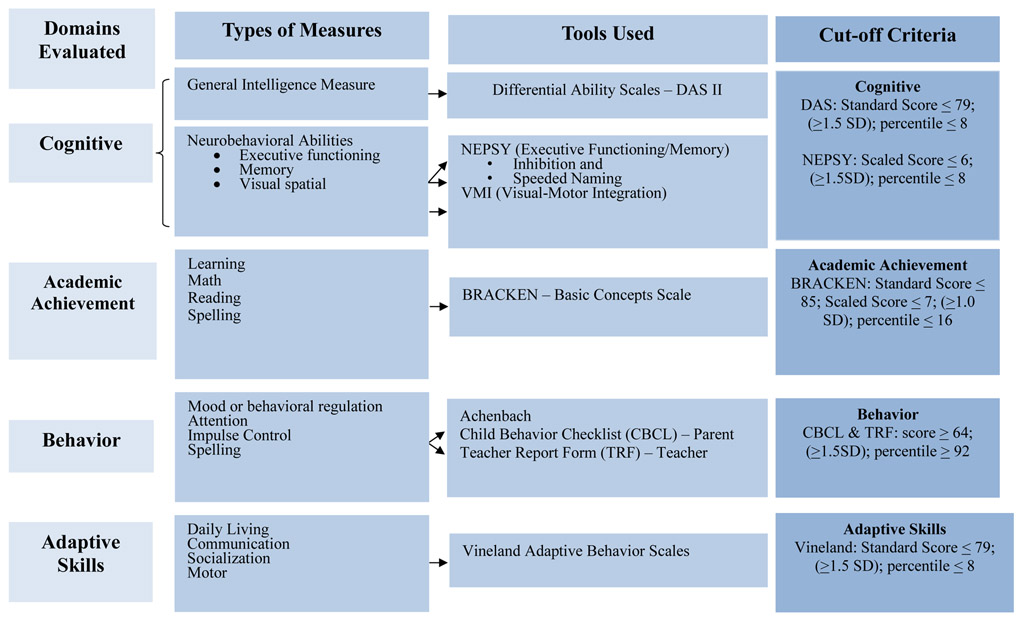
Child cognition, academic achievement, and behavior were assessed in Tier III with the CoFASP consensus battery and cut-off points (Figure 3). Each child was tested by school psychologists with the: Differential Ability Scales (DAS-II) (Elliott, 2007) for general intelligence; NEPSY-II (Korkman et al., 2007) to assess executive functioning, memory, and visual spatial integration; Developmental Test of Visual-Motor Integration (VMI) (Beery and Beery, 2004) for eye-hand coordination; Bracken Basic Concepts Scale (Bracken, 1998) for concept development in math, reading, and spelling; Child Behavior Checklist (CBCL) by both parents and teachers (TRF) (Achenbach and Rescorla, 2001); and the Vineland Adaptive Behavior Scales (Sparrow et al., 2005).
Figure 3.
CoFASP Cut Off Criteria Set for all Domains: Neurobehavioral Testing Battery
Also in Tier III, consenting mothers of children advanced to Tier III were provided in-person interviews. The sequenced questions were intended to maximize accurate reporting of: health and physical status, reproduction, nutrition, alcohol use, and socioeconomic status (SES). Drinking questions used a timeline, follow-back sequence (Sobell et al., 2001, 1988), and Vessels alcohol product methodology for accurate calibration of standard alcohol units (Kaskutas and Graves, 2001, 2000; Kaskutas and Kerr, 2008). Current alcohol consumption for the week preceding the interview was embedded into dietary intake questions (King, 1994) to aid accurate reporting and calibration of drinking quantity, frequency, and timing of alcohol use before, during, and after the index pregnancies (Alvik et al., 2006; May et al., 2013, 2008, 2005). Retrospective reports of alcohol use have been found to be accurate when designed and administered properly (Czarnecki et al., 1990; Fortin et al., 2017; Hannigan et al., 2010). The accuracy of data produced by this approach has been confirmed by biomarkers in at least one population (May et al., 2018b).
Maternal risk data were gathered for 126 and 140 of the children’s mothers in Samples 1 and 2, respectively (Figures 1 and 2). The American definition of a Standard Drink was used:14g of absolute alcohol, which is 12oz. (350mL of beer at 5% alcohol by volume); 5oz. (150mL) of wine (12% by volume); and 1.5oz. (44mL) of liquor (40% by volume) (“What Is A Standard Drink? ∣ National Institute on Alcohol Abuse and Alcoholism (NIAAA),” n.d.). Drinking during pregnancy was confirmed if at least one of the following criteria was met during the index pregnancy: a) six or more standard drinks per week for two or more weeks; b) a binge of three or more drinks per occasion on two or more occasions; or c) documentation of social or legal problems in proximity to the index pregnancy (e.g. treatment for alcohol abuse or driving under the influence). These CoFASP-approved criteria merely reflect cut-off levels believed to provide sufficient empirical proof of exposure in epidemiologic studies.
Figure 2. Sampling Methodology for Prevalence of FASD in Rocky Mountain City (SM2): Sample 2.
*If a child was randomly selected and found to have an FASD or another known genetic or teratogenic disorder, he/she was classified appropriately and removed from the control group. **2 were not FASD, with other genetic disorders
Multidisciplinary Case Conferences for Final Diagnoses and Assuring Accuracy
Following data collection and compilation, final diagnoses were made in structured, multidisciplinary case conferences. Findings for each child were discussed after results from the three domains were presented by research team members who participated in exams, testing, or interviews for the particular child and mother. During the presentations, two-dimensional, digital photo images of the child’s face (frontal and profile) were projected on a screen to contextualize the data. Whether the findings for each child met criteria for a FASD diagnosis (or another condition) was discussed. Final diagnoses were made by consensus, but in the rare cases of disagreement, the diagnosis was assigned by the examining dysmorphologist.
In the diagnostic process, consistency and quality assurance for the dataset were first enhanced by strict initial application of CoFASP criteria when preparing for case conferences. Second, all final diagnoses were double-checked for consistency and accuracy by the data management teams at UNC, UNM, and UCSD. Third, classifications were checked again by the CoFASP investigative teams via reciprocal exchange of all diagnostic data for all cases and a sample of controls. Each team was blinded to the other team’s classifications and determined whether criteria had been applied accurately.
Statistical Analysis
Data organization and analyses were performed with Excel (“Microsoft Excel,” 2016) and SPSS (IBM, 2017). All data were compared across diagnostic groups using chi square and one-way analysis of variance (Tabachnick and Fidell, 2013). Bonferroni adjustments of alpha values were used where appropriate. Alpha level for maternal risk comparisons was fixed at 0.05 due to the exploratory status of risk traits, but Bonferroni-adjusted values are also provided. With statistically significant ANOVAs, post-hoc analyses were performed using Dunnett’s correction (C) comparisons (α= .05).
Partial correlation and logistic regression were used to detect associations of child traits with alcohol use, and transformations were undertaken for most measures due to skewness. Logarithmic transforms were applied to usual number of drinks per drinking day before pregnancy (DDD), number of weeks before mother’s recognition of the index pregnancy, and teacher reports of rule-breaking and attention problems. Square root transformations were applied to the child’s total dysmorphology and general abilities scores. Although highly unbalanced, transformations could not be applied to “yes/no” items: maternal reports of drinking during pregnancy trimesters, and the covariate, whether mother had used drugs during the index pregnancy. Use of pairwise deletion ensured that all available data were included. A statistical criterion of p<.0017 was set for interpretation of partial correlations to control for Type I familywise error rate.
The site prevalence of FASD was calculated from the average of rates from the two, individual cohort samples published previously (May et al., 2018a). The lower rates for FASD represent the minimum (lower bound) prevalence possible given the number of children meeting CoFASP guidelines (numerator) in combined site samples divided by total children enrolled in both cohorts. Higher rates employed a conservative, weighted correction factor for each FASD diagnosis based on the proportion of diagnoses made within the subsamples of randomly-selected entrants. Weighted correction was applied to the unconsented students for each FASD diagnosis and also to small students who entered the study through growth qualification alone in order to estimate ARND cases in those small children who were not otherwise tested for neurobehavioral deficits. The calculation of rates is described fully in the e-appendix of the CoFASP prevalence summary paper (May et al., 2018a).
RESULTS
Diagnostic Numbers and Racial Distribution
There were 4 children diagnosed with FAS, 22 with PFAS, 12 ARND, and 276 typically-developing controls (Table 2). All controls had been selected randomly, were assessed fully and found to be developing within the normal range for age. If a randomly-selected child were given a diagnosis within the FASD continuum, he/she was included in the appropriate FASD category and not in the control group. There was no significant difference in the racial and ethnic distribution of children with FASD and the overall composition of the school population, whether measured by specific diagnosis or by FASD vs. not FASD.
Table 2.
Distribution of FASD Cases and Randomly-Selected (RS) Controls by Racial and Ethnic Categories: Rocky Mountain City
| All Children (n=314) |
FAS (n=4) |
PFAS (n=22) |
ARND (n=12) |
RS controls (n=276) |
X2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| White | 250 | 79.6 | 4 | 100.0 | 21 | 95.5 | 8 | 66.7 | 217 | 78.6 | ||
| Hispanic | 14 | 4.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 14 | 5.1 | ||
| African American | 10 | 3.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 10 | 3.6 | ||
| Other | 40 | 12.7 | 0 | 0.0 | 1 | 4.5 | 4 | 33.3 | 35 | 12.7 | 10.158 (df=9) | p = 0.338 |
| All Children | FASD | RS controls | X2 | p-value | ||||||||
| n | % | n | % | n | % | |||||||
| White | 250 | 79.6 | 33 | 86.8 | 217 | 78.6 | ||||||
| Hispanic | 14 | 4.5 | 0 | 0.0 | 14 | 5.1 | ||||||
| African American | 10 | 3.2 | 0 | 0.0 | 10 | 3.6 | ||||||
| Other | 40 | 12.7 | 5 | 13.2 | 35 | 12.7 | 3.594 (df=3) | p = 0.309 | ||||
Physical Growth, Physical Traits and Dysmorphology for Children with FASD
There was no significant difference in age or sex distribution among the diagnostic groups; however, there were significant differences from ANOVA tests at Bonferroni-adjusted significance level (α=.007) on all other child variables in Table 3 except three: child’s BMI percentage, inner pupillary distance, and maxillary arc measurements. Height and weight were significantly depressed for children with FAS and PFAS as per IOM criteria. Children with ARND were taller and heavier than controls. By definition, children with FAS had the smallest head circumferences (OFC) (50% were ≤3rd centile). Children with ARND had larger heads than controls (not statistically significant). For the three cardinal facial features of FAS: children with PFAS were most likely to have a smooth philtrum and narrow vermilion border (81.8% each), followed by FAS and mean PFL length was lowest for FAS and PFAS, but higher for children with ARND than controls. Twenty-five percent (25%) and 32% of the children with FAS and PFAS had a PFL ≤3rd centile, while more of the children with ARND had a PFL ≤10th centile. Additional minor anomalies differentiated the diagnostic groups from controls.
Table 3.
Physical Growth, Cardinal FAS Features, Other Minor Anomalies, and Total Dysmorphology Scores for Two Samples from a Rocky Mountain Region: 2012-2014
| Children with FAS (n=4) |
Children with PFAS (n=22) |
Children with ARND (n=12) |
Randomly- Selected Control Children (n=278) |
Test-score | p-value | |
|---|---|---|---|---|---|---|
| Growth and Cardinal Features | ||||||
| Sex (% Male) | 75.0 | 50.0 | 75.0 | 50.4 | χ2=3.702 | .296 |
| Current Age (in months) – Mean (SD) | 83.3 (6.6) | 84.5 (7.2) | 85.5 (6.2) | 82.8 (5.1) | F=1.630 | .182 |
| Height Percentile – Mean (SD) | 10.0 (12.3) | 33.1 (22.4) | 59.6 (34.3) | 52.5 (27.1) | F=6.986 | <.001B,C,E |
| Weight Percentile – Mean (SD) | 5.5 (4.2) | 40.4 (26.2) | 63.5 (33.2) | 58.4 (27.1) | F=8.046 | <.001A,B,C,E |
| Child's BMI Percentile – Mean (SD) | 24.0 (13.9) | 51.0 (30.2) | 65.3 (27.0) | 60.4 (27.2) | F=3.235 | .023B,C |
| Occipitofrontal Circumference (OFC) Percentile – Mean (SD) | 4.5 (3.9) | 32.7 (24.5) | 63.5 (27.3) | 59.8 (27.3) | F=12.186 | <.001A,B,C,D,E |
| OFC ≤3rd centile | 50.0 | 4.5 | 0.0 | 1.8 | χ2=37.805 | <.001 |
| OFC ≤10th centile | 100.0 | 31.8 | 0.0 | 5.4 | χ2=64.859 | <.001 |
| Palpebral Fissure Length (PFL) Percentile – Mean (SD) | 9.8 (13.6) | 14.2 (15.8) | 33.3 (15.8) | 30.6 (16.3) | F=9.119 | <.001D,E |
| PFL ≤3rd centile | 25.0 | 31.8 | 0.0 | 5.1 | χ2=25.373 | <.001 |
| PFL ≤10th centile | 75.0 | 54.5 | 0.0 | 9.0 | χ2=53.874 | <.001 |
| Smooth Philtrum (% Yes) | 50.0 | 81.8 | 8.3 | 15.5 | χ2=58.817 | <.001 |
| Narrow Vermilion (% Yes) | 75.0 | 81.8 | 16.7 | 21.2 | χ2=44.514 | <.001 |
| Other Minor Anomalies | ||||||
| Inner Pupillary Distance (IPD) Percentile – Mean (SD) | 21.5 (18.5) | 48.7 (25.5) | 56.7 (24.5) | 58.5 (24.6) | F=3.923 | .009 |
| Outer Canthal Distance (OCD) Percentile – Mean (SD) | 9.0 (7.6) | 22.0 (16.2) | 35.7 (21.5) | 36.8 (19.4) | F=6.609 | <.001B,C,E |
| Maxillary Arc (in cm) – Mean (SD) | 23.7 (0.4) | 24.4 (0.9) | 25.4 (1.4) | 24.7 (1.1) | F=3.520 | .015 B,C |
| Mandibular Arc (in cm) – Mean (SD) | 24.3 (0.4) | 25.6 (1.0) | 26.7 (1.7) | 25.8 (1.2) | F=4.441 | .005A,B,C |
| Strabismus (% Yes) | 25.0 | 0.0 | 0.0 | 1.4 | χ2=14.670 | .002 |
| Hypoplastic Nails (% Yes) | 0.0 | 4.5 | 0.0 | 0.0 | χ2=13.406 | .004 |
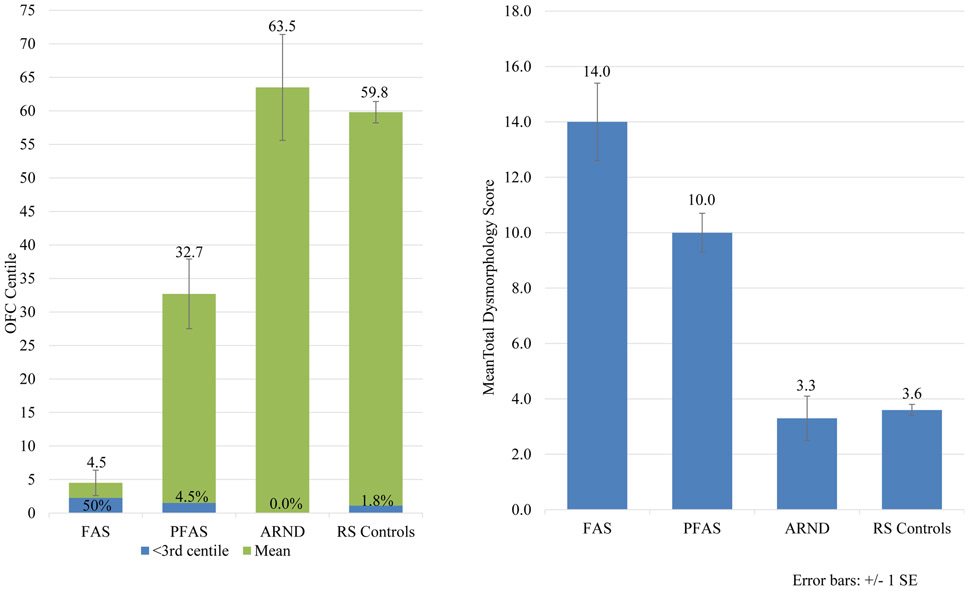
| Total Dysmorphology Score – Mean (SD) | 14.0 (2.7) | 10.0 (3.1) | 3.3 (2.9) | 3.6 (3.1) | F=42.880 | <.001B,C,D,E |
Post-hoc significant difference between:
FAS & PFAS
FAS & ARND
FAS & Controls
PFAS & ARND
PFAS & Controls
ARND & Controls.
Bonferroni adjusted significance level for Growth and Cardinal Features = 0.005; for other minor anomalies = .007
The total dysmorphology score summarized relevant anomalies. Diagnostic groups were on average significantly different from one another. All but two bivariate comparisons are statistically significant in average total dysmorphology: FAS vs. PFAS and ARND vs. controls (Table 3 and Figure 4).
Figure 4.
Occipitalfrontal Circumference (OFC) and Total Dysmorphology Score by FASD Diagnosis, Rocky Mountain City
Neurobehavioral Traits
On most tests of intellectual performance, executive function, and learning in Table 4, children with FASD scored significantly lower than controls. Although, when broken out by individual diagnoses, the children with FAS test in the normal range on many of the cognitive tests and children with ARND performed significantly poorer than all other groups on most tests (see Table E1). Visual spatial scores indicate impairment, as do mood regulation measures for the FASD group vs. controls. The categories of anxious/depressed, withdrawn/depressed, internalizing problems, and affective problems also stand out as issues for the children with FASD. Attention problems are consistently reported for the FASD group, as are impulse control, especially aggressive behavior. Finally, adaptive function scores are all significantly different between children with FASD and controls, with the exception of parent reports of daily living. All other reports of communication skills, daily living, and socialization indicate that children with FASD performed poorer than controls.
Table 4.
Neurobehavioral Findings Among Children with FASD and Randomly-Selected Controls from a Rocky Mountain Region City
| Children with FAS (n=38) |
Randomly- Selected Control Children (n=273) |
t-test | p-value | |
|---|---|---|---|---|
| Mean(SD) | Mean(SD) | |||
| Intellectual Domain | (n=38) | (n=273) | ||
| General Abilities Percentile | 44.7 (30.8) | 59.7 (24.5) | −2.887 | .006** |
| Verbal Cluster Percentile | 49.3 (33.1) | 58.6 (25.7) | −1.654 | .105 |
| Nonverbal Reasoning Cluster Percentile | 43.1 (28.1) | 56.0 (26.4) | −2.792 | .006** |
| Spatial Cluster Percentile | 41.8 (25.7) | 57.9 (23.8) | −3.871 | <.001** |
| Executive Function | (n=38) | (n=273) | ||
| INN (Naming) combined scaled score | 8.2 (4.2) | 10.2 (3.3) | −3.415 | .001** |
| INN vs. INI Contrast Scaled Score | 9.0 (3.4) | 9.7 (3.3) | −3.415 | .001** |
| INI (Inhibition) combined scaled score | 8.3 (3.7) | 9.8 (3.3) | −1.314 | .190 |
| INS (Switching) combined scaled score | 7.3 (4.5) | 7.9 (3.8) | −0.737 | .462 |
| Speeded Naming Combined scaled score | 8.2 (3.2) | 10.0 (3.0) | −3.272 | .003** |
| Learning1 | (n=35) | (n=268) | ||
| BBCS School Readiness Composite Scaled Score | 11.1 (3.3) | 12.4 (2.0) | 2.330 | .025** |
| BBCS Readiness Composite Standard Score | 105.4 (16.2) | 111.8 (9.8) | −2.294 | .027** |
| Visual Spatial | (n=38) | (n=273) | ||
| VMI Standard Score | 91.8 (13.2) | 97.9 (7.1) | −2.748 | .009** |
| Visuomotor Precision Combined scaled score | 8.6 (3.4) | 10.3 (3.7) | −2.610 | .009** |
| Mood Regulation2 | ||||
| CBCL Anxious/depressed t-score | 55.9 (7.6) | 52.5 (4.7) | 2.544 | .015* |
| TRF Anxious/depressed t-score | 56.5 (7.2) | 52.8 (5.4) | 3.759 | <.001** |
| CBCL Withdrawn/depressed t-score | 57.7 (9.3) | 52.6 (4.1) | 3.137 | .003** |
| TRF Withdrawn/depressed t-score | 55.8 (5.5) | 53.0 (5.7) | 2.921 | .005* |
| CBCL Internalizing Problems t-score | 53.1 (13.3) | 47.5 (9.3) | 2.391 | .022* |
| TRF Internalizing Problems t-score | 54.1 (10.3) | 46.7 (9.2) | 4.578 | <.001** |
| CBCL Externalizing Problems t-score | 53.1 (14.0) | 47.4 (8.9) | 2.318 | .026* |
| TRF Externalizing Problems t-score | 56.0 (11.9) | 50.2 (8.8) | 2.906 | .006* |
| CBCL Affective problems t-score | 57.8 (8.3) | 52.5 (4.3) | 3.661 | <.001** |
| TRF Affective problems t-score | 57.9 (8.3) | 52.6 (5.4) | 3.847 | <.001** |
| CBCL Anxiety problems t-score | 56.6 (8.1) | 52.5 (4.8) | 2.956 | .005* |
| TRF Anxiety problems t-score | 57.7 (7.7) | 53.2 (5.6) | 3.475 | .001** |
| Attention2 | ||||
| CBCL Attention problems t-score | 59.5 (10.5) | 53.8 (6.4) | 3.095 | .004** |
| TRF Attention problems t-score | 59.8 (9.6) | 54.0 (6.1) | 3.595 | .001** |
| CBCL Attention deficit/hyperactivity problems t-score | 57.7 (7.7) | 53.3 (5.5) | 3.214 | .003** |
| TRF Attention deficit/hyperactivity problems t-score | 59.9 (9.9) | 54.6 (6.6) | 3.224 | .002** |
| Impulse Control2 | ||||
| CBCL Rule-breaking behavior t-score | 55.9 (7.0) | 52.9 (4.5) | 2.458 | .018* |
| TRF Rule-breaking behavior t-score | 56.7 (7.5) | 53.3 (5.5) | 2.684 | .010* |
| CBCL Aggressive behavior t-score | 58.9 (10.6) | 52.7 (4.9) | 3.361 | .002** |
| TRF Aggressive behavior t-score | 59.1 (11.6) | 53.9 (6.5) | 2.655 | .011* |
| CBCL Oppositional defiant problems t-score | 58.1 (8.8) | 54.0 (5.3) | 2.662 | .011* |
| TRF Oppositional defiant problems t-score | 57.6 (9.3) | 53.8 (6.3) | 2.399 | .021* |
| CBCL Conduct problems t-score | 57.1 (8.8) | 52.9 (5.2) | 2.749 | .009* |
| TRF Conduct problems t-score | 57.3 (10.0) | 53.2 (5.8) | 2.503 | .016* |
| Adaptive Function3 | ||||
| Vineland (Parent) VABS Communication Standard Score | 99.8 (17.0) | 113.8 (25.6) | −3.112 | .002** |
| Vineland (Teacher) VABS Communication Standard Score | 94.4 (18.8) | 103.5 (14.1) | −3.536 | <.001** |
| Vineland (Parent) VABS Daily Living Skills Standard Score | 104.3 (16.4) | 108.5 (14.4) | −1.553 | .122 |
| Vineland (Teacher) VABS Daily Living Skills Standard Score | 95.2 (20.7) | 104.9 (16.6) | −3.219 | .001** |
| Vineland (Parent) VABS Socialization Standard Score | 99.6 (16.9) | 108.4 (14.6) | −3.202 | .002** |
| Vineland (Teacher) VABS Socialization Standard Score | 93.7 (13.6) | 103.6 (15.4) | −3.773 | <.001** |
Children less than 7 at time evaluation did not complete a BRACKEN.
For CBCL: n=35 for FASD; n=195 for Controls. For TRF: n=38 for FASD; n=269 for Controls.
For Vineland (Parent): n=35 for FASD; n=194 for Controls. For Vineland (Teacher): n=38 for FASD; n=262 for Controls.
Significant at 0.05
Significant at Bonferroni-adjusted level: Intellectual: 0.0125; Executive Function: 0.01; Learning: 0.025; Visual Spatial: 0.025; Mood Regulation: 0.004; Attention: 0.0125; Impulse Control: 0.006; Adaptive Function: 0.008
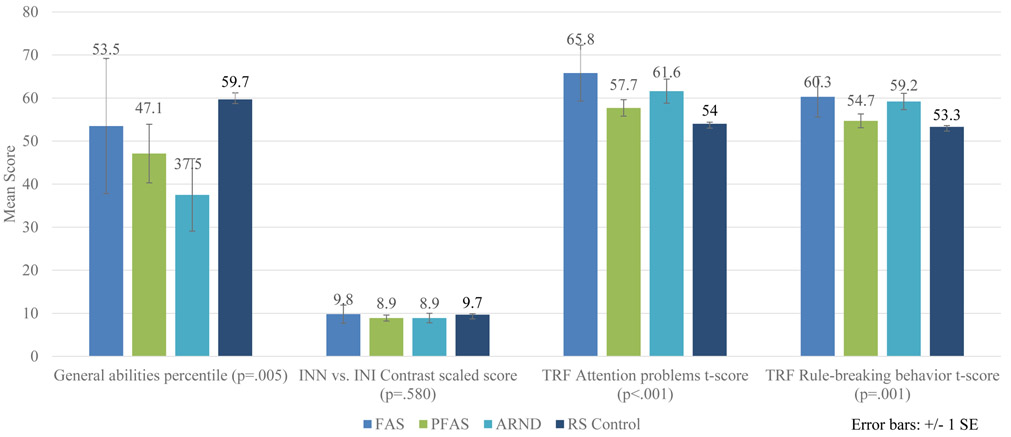
Figure 5 illustrates key cognitive, executive functioning and behavioral tests that might discriminate the four specific FASD diagnostic groups from one another. Children with ARND have the poorest general abilities percentile scores. There is not a significant difference between scores for the four groups on INN and INI (naming and inhibition) contrast scaled scores. The INN score is a challenging naming task where the child must name shapes or the direction of arrows as quickly as possible. The INI task is an inhibition task that requires a child to say the opposite shape name or arrow direction as quickly as possible. The INN vs. INI Contrast Scaled Score is a comparison of the Naming speed score and the Inhibition score. Teacher Report Forms (TRF) reflected norm and rule-breaking behavior measured by t-scores. Children with FAS have more attention problems and rule-breaking scores. More details on neurobehavioral traits by individual FASD diagnosis are found in Table E1.
Figure 5.
Selected Cognitive and Behavioral Measures by FASD Diagnoses, Rocky Mountain City
Maternal Risk Traits –Proximal Variables: Alcohol and Drug Use
Proximal maternal variables in Table 5 indicate that pre-pregnancy drinking variables are more useful and accurate assessments of usual drinking patterns that affect first trimester drinking than are reports made for pregnancy or prenatal time periods. Three months prior to pregnancy, 78.1% of mothers of children with FASD reported consuming alcohol compared to 60.3% of mothers of controls (p=.054). Mothers of children with FASD reported a mean of 5.3 (SD=3.1) drinks per drinking day (DDD), a median of 4.0 DDD, and 72% drink one to two days per week. Mothers of controls report drinking a mean of 3.2 (SD=2.6) DDD, a median of 2.0 DDD, and 46% drink one to two days per week. Prior to pregnancy, between-groups DDD is statistically significant, while percentage drinking and frequency of drinking merely approaches significance. For first and second trimester, only the binary measure of drinking, “yes/no” is significantly different between groups. Quantity (DDD) and frequency (drinking days) did not differ for those who said that they drank during these trimesters. But three to five times as many mothers of children with FASD reported drinking in the first and second trimester than controls. Only two other proximal variables were significantly different between maternal groups: mothers of children with FASD were six times more likely (9.7 vs. 1.6%) to report drinking due to anxiety than controls and 42% used marijuana in their lifetime. Approaching significance was use of any drug in lifetime (67.6 vs. 49.7%), with the mothers of children with FASD more prone to co-morbid alcohol and drug use. Also, approaching significance was that 6.3% of mothers of children with FASD used both marijuana and alcohol during the index pregnancy vs. 1.6% for controls. Overall, there are few significant differences in drinking and drug use patterns between the two groups except more binge drinking and the higher percentage of the FASD group who report drinking in first and second trimesters.
Table 5.
Proximal Maternal Risk Factors for FASD: Combined Sample Data for Physical, Demographic, Childbearing, Alcohol and Drug Use from a Rocky Mountain Region City
| Children with FASD (n=35) |
Randomly-Selected Control Children (n=197) |
χ2 or t-test |
p-value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Alcohol Use – Before and During Pregnancy | ||||
| Drank before pregnancy (% Yes) | 78.1 | 60.3 | 3.719 | .054 |
| # of drinks consumed on usual drinking day before pregnancy1 | 5.3 (3.1) | 3.2 (2.6) | 3.510 | .001** |
| Mdn = 4.0 | Mdn = 2.0 | - | - | |
| Usual frequency – before pregnancy1 | ||||
| Everyday or almost everyday | 8.0 | 4.3 | ||
| 3-4 times per week | 16.0 | 2.2 | ||
| 1-2 times per week | 48.0 | 39.2 | ||
| 2-3 times per month | 16.0 | 15.2 | ||
| 1 time per month or less | 12.0 | 39.1 | 7.928 | .094 |
| Drank in 1st trimester (% Yes) | 18.2 | 6.3 | 5.335 | .033A,* |
| # of drinks on usual drinking day1 −1st | 4.1 (2.0) | 2.5 (2.2) | 1.341 | .201 |
| Usual frequency1 – 1st | ||||
| Everyday or almost everyday | 0.0 | 9.1 | ||
| 3-4 times per week | 0.0 | 9.1 | ||
| 1-2 times per week | 0.0 | 18.2 | ||
| 2-3 times per month | 0.0 | 0.0 | ||
| 1 time per month or less | 100.0 | 63.6 | 3.955 | .683 |
| Drank in 2nd trimester (% Yes) | 15.2 | 3.7 | 7.262 | .019A,* |
| # of drinks on usual drinking day1 - 2nd | 5.3 (2.9) | 1.2 (0.6) | 2.451 | .130 |
| Usual frequency1 – 2nd | ||||
| Everyday or almost everyday | 0.0 | 14.3 | ||
| 3-4 times per week | 0.0 | 14.3 | ||
| 1-2 times per week | 0.0 | 0.0 | ||
| 2-3 times per month | 0.0 | 0.0 | ||
| 1 time per month or less | 100.0 | 71.4 | 5.102 | .531 |
| Drank in 3rd trimester (% Yes) | 6.1 | 3.2 | .685 | .337A |
| # of drinks on usual drinking day1 - 3rd | -- -- | 1.1 (0.5) | -- | -- |
| Usual frequency1 – 3rd | 0.0 | |||
| Everyday or almost everyday | 16.7 | |||
| 3-4 times per week | 0.0 | |||
| 1-2 times per week | 0.0 | |||
| 2-3 times per month | 0.0 | |||
| 1 time per month or less | -- | 0.0 | -- | -- |
| Alcohol Use - Current | ||||
| Drink in past 30 days (% Yes) | 64.5 | 69.0 | .249 | .618 |
| Binge 5+ in past month (% Yes) | 29.0 | 27.7 | .023 | .880 |
| Why usually drink: because others drink | 6.5 | 7.6 | .052 | 1.00A |
| Why usually drink: to feel less anxious | 9.7 | 1.6 | 6.333 | .040A,* |
| Current drinking problem (% Yes) | 6.3 | 1.1 | 4.059 | .104A |
| Recovering drinker (% Yes) | 15.8 | 4.8 | 3.242 | .104A |
| Drug Use | ||||
| Used tobacco – during pregnancy (% Yes) | 17.6 | 20.3 | .129 | .819A |
| Used any drugs in pregnancy (% Yes) | 8.8 | 6.2 | .337 | .472A |
| Abused prescription – during pregnancy | 2.9 | 2.6 | .015 | 1.00A |
| Used marijuana – during pregnancy (% Yes) | 8.8 | 5.7 | .499 | .455A |
| Used marijuana & alcohol – during pregnancy (% Yes) | 6.3 | 1.6 | 2.620 | .157A |
| Used club drugs – during pregnancy (% Yes) | 2.9 | 0.5 | 1.929 | .279A |
| Used cocaine - during pregnancy (% Yes) | 2.9 | 1.0 | .804 | .387A |
| Used methamphetamine – pregnancy (% Yes) | 2.9 | 2.6 | .015 | 1.00A |
| Used tobacco – in lifetime | ||||
| Yes, within last 30 days | 26.5 | 27.5 | ||
| Yes, in lifetime | 35.3 | 29.0 | ||
| Never | 38.2 | 43.5 | 1.282 | .527 |
| Used any drug in lifetime (% Yes) | 67.6 | 49.7 | 3.721 | .054 |
| Used marijuana – in lifetime (% Yes) | 67.6 | 47.6 | 4.620 | .032* |
| Used methamphetamine – in lifetime (% Yes) | 20.0 | 13.1 | 1.162 | .294A |
| Used heroin – in lifetime (% Yes) | 5.9 | 3.8 | .313 | .634A |
| Used club drugs – in lifetime (% Yes) | 5.9 | 4.3 | .160 | .656A |
| Used crack/cocaine – in lifetime (% Yes) | 14.7 | 10.8 | .446 | .555A |
| Abused pain killers – in lifetime (% Yes) | 5.9 | 5.3 | .016 | 1.00A |
Among women who drank in that specific time period.
A collateral interview confirmed alcohol consumption but with unknown quantity and/or frequency.
Fisher’s Exact Test
Significant at 0.05
Significant at Bonferroni-adjusted level: Bonferroni-adjusted significant levels: alcohol use – before and during pregnancy = 0.004; alcohol use – current = 0.008; other drug use = 0.003.
Maternal Risk Traits – Distal Variables
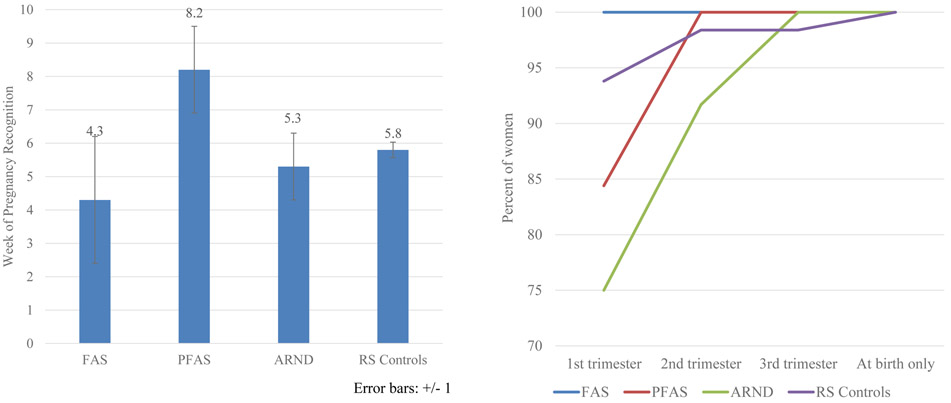
Only two distal maternal risk variables (Table 6) were significant at the adjusted alpha level: mothers of children with FASD reported more stomach ulcers in their lifetimes and reported lower birth weight for their babies (2905g vs. 3298g) than controls. Significantly different between groups at the 0.05 level were younger age at pregnancy, lower weight before pregnancy, more asthma in lifetime, later first visits for prenatal care, more injuries during pregnancy, more problems for the child at birth, and lower education for the mothers of children with FASD. Additionally, children with FASD were less likely to live with their biological mother and father than controls, and fathers of children with FASD were reported to be more likely to have had a drinking problem than controls. Figure 6 and Table E3 present more detail on distal maternal risk by specific FASD diagnosis. For example, week of pregnancy recognition was latest for mothers of children with PFAS at 8.2 weeks, and mothers of children with ARND were generally later in visiting a healthcare provider for prenatal care. Also, in Table E3, significantly lower average birth weights among the children with FASD form a continuum by diagnosis: those with FAS averaged 2447g at birth, 2906g for PFAS and 3028g for ARND, all lower than controls at 3297g.
Table 6.
Distal Maternal Risk Factors for FASD: Combined Sample Data for Physical, Childbearing, and Postnatal Variables from a Rocky Mountain Region City
| Children with FASD (n=35) |
Randomly- Selected Control Children (n=197) |
χ2 or t-test |
p-value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Physical | ||||
| Age at pregnancy (yrs) | 25.9 (5.6) | 28.1 (5.7) | −2.070 | .040* |
| Height at interview (cm) | 164.8 (5.1) | 165.3 (6.6) | −0.452 | .653 |
| Weight at interview (kg) | 73.7 (18.3) | 78.7 (21.8) | −1.259 | .209 |
| Body Mass Index | 27.3 (7.2) | 28.8 (7.4) | −1.074 | .284 |
| Head circumference | 55.9 (1.7) | 56.2 (1.8) | −0.788 | .432 |
| Weight before pregnancy (in kg) | 64.7 (11.6) | 69.7 (17.4) | −2.087 | .041* |
| Asthma – in lifetime (% Yes) | 26.5 | 8.9 | 8.805 | .007A,* |
| Stomach ulcers – in lifetime (% Yes) | 17.6 | 2.6 | 14.212 | .002A,** |
| Neurological conditions/ epilepsy - lifetime | 0.0 | 4.7 | 1.651 | .362A |
| Liver problems / hepatitis – in lifetime | 2.9 | 2.6 | .014 | 1.00A |
| Depression – in lifetime (% Yes) | 55.9 | 49.7 | .436 | .578A |
| Childbearing | ||||
| Gravidity | 3.4 (2.5) | 3.2 (1.4) | .517 | .608 |
| Parity | 2.6 (1.6) | 2.6 (1.2) | −0.004 | .997 |
| Miscarriages | 1.0 (1.3) | 0.7 (0.9) | 1.328 | .186 |
| Abortions | 0.2 (0.5) | 0.2 (0.4) | .041 | .967 |
| Stillbirths | 0.1 (0.3) | 0.0 (0.3) | 1.061 | .291 |
| Birth order of index child | 1.8 (1.5) | 1.9 (1.0) | −0.425 | .671 |
| Week of pregnancy recognition | 3.4 (2.5) | 5.8 (3.2) | 1.234 | .226 |
| Prenatal Care | ||||
| Once knew pregnant, take vitamins (% Yes) | 87.9 | 94.8 | 2.277 | .233A |
| # of times seen by healthcare provider | ||||
| Never | 0.0 | 0.5 | ||
| 1-5 times | 6.1 | 2.6 | ||
| More than 5 times | 93.9 | 96.9 | 1.306 | .656 |
| When first seen by healthcare provider | ||||
| 1st trimester | 82.4 | 93.8 | ||
| 2nd trimester | 14.7 | 4.6 | ||
| 3rd trimester | 2.9 | 0.0 | ||
| Delivery only | 0.0 | 1.5 | 8.830 | .021A,* |
| Other health problems - during pregnancy | 50.0 | 31.9 | 4.158 | .051A |
| Accidents/injury – during pregnancy (% Yes) | 27.3 | 11.4 | 6.000 | .025A,* |
| Postpartum depression (%Yes) | 41.9 | 25.3 | 3.707 | .081A |
| Postnatal Variables | ||||
| Birth weight (grams) | 2905 (691) | 3298 (619) | −3.318 | .001** |
| Estimated Gestation Age at birth (in weeks) | 37.8 (3.2) | 38.7 (2.4) | −1.704 | .097 |
| COI had problem(s) at birth (% Yes) | 76.5 | 51.8 | 7.136 | .008* |
| Breastfed (% Yes) | 73.3 | 75.5 | .068 | .821 |
| Consumed alcohol in breastfeeding period1 | 13.6 | 22.7 | .926 | .413 |
| Pump and dump (% Yes)2 all mothers who consumed alcohol and breastfed | 33.3 | 56.7 | .599 | .579A |
| Age of biological father (in years) | 27.7 (6.6) | 29.5 (6.6) | −1.5237 | .131 |
| Child lives with biological mother (% Yes) | 82.9 | 89.8 | 1.460 | .245A |
| Child lives with: | ||||
| Foster/Adopted/Relative | 5.7 | 7.1 | ||
| Biological mother | 40.0 | 24.5 | ||
| Biological father | 11.4 | 2.6 | ||
| Biological mother and father | 42.9 | 65.8 | 11.178 | .013* |
| Partner ever had a drinking problem | ||||
| Never | 63.3 | 74.3 | ||
| In the past, but not currently | 10.0 | 16.22 | ||
| Currently | 3.3 | 0.0 | ||
| Both past and currently | 23.3 | 9.6 | 10.739 | .013* |
| Years of Education completed | 13.6 (2.6) | 14.6 (2.5) | −2.174 | .031* |
| Household yearly income - during pregnancy | 48066 (43221) | 50251 (35712) | −0.366 | .715 |
| Marital Status - current | ||||
| Married | 58.8 | 78.1 | ||
| Divorced/Widowed/Separated/Single | 29.4 | 15.6 | ||
| Living with partner | 11.8 | 6.3 | 5.773 | .061 |
| Marital Status – during pregnancy | ||||
| Married | 60.0 | 70.1 | ||
| Divorced/Widowed/Separated/Single | 14.3 | 11.9 | ||
| Living with partner | 25.7 | 18.0 | 1.493 | .493 |
| Spirituality: none [0] to high [10] | 6.2 (2.4) | 6.6 (2.2) | −0.726 | .469 |
Among drinkers only
Pump and dump is the colloquial name for expressing breastmilk after drinking alcohol and disposing of it.
Fisher’s Exact Test
Significant at 0.05
Significant at Bonferroni-adjusted level: Bonferroni-adjusted significance level: physical = 0.0045; childbearing = 0.007; prenatal care = 0.008; postnatal = 0.004
Figure 6.
A) Week When Pregnancy Was First Recognized, Rocky Mountain City B) Timing of First Visit to Healthcare Provider by Trimester, Rocky Mountain City
There was one difference in socioeconomic status (SES) among the groups: mother’s level of education, but no significant difference by household income or marital status. Finally, there was no significant difference in self-ranked spirituality among the groups, nor a difference in religious service attendance or reported formal religious affiliation (not in Table 6).
Correlation Analysis
Correlation analysis measured associations between maternal and cognitive/behavioral variables, FASD diagnosis, and total dysmorphology scores after adjusting for whether mother had used other drugs during the index pregnancy (Table E4). Two measures of maternal alcohol use correlated significantly with greater probability of FASD diagnosis: the usual number of DDD consumed 3 months prior to pregnancy, and whether the mother drank during the first trimester. Statistically significant, absolute values of partial correlations adjusted for drug use were .28 and .48. Thus, each maternal variable accounted for no more than about 23% of the variance in the child’s diagnosis. Note, however, that correlations may be attenuated due to non-normality remaining, even after transformation, and to highly unbalanced frequencies in dichotomous “yes/no” categories. Thus, there was also a suggestion of a link between mother’s late recognition of pregnancy and reduced general abilities score. Drinking in the first trimester may have been linked with greater total dysmorphology score. There were suggested links between greater probability of total dysmorphology score and late recognition of pregnancy as well as drinking in the second trimester.
Binary logistic regression analysis further defined the relationship of alcohol consumption with a FASD diagnosis, and therefore child traits. Statistical adjustments were made for any illicit drug or tobacco use during pregnancy to control for these confounders , and 25 imputations were performed to adjust for missing data. Table 7 presents results for the relationship between a FASD diagnosis and reported DDD three months prior to pregnancy. Reported drinking of three DDD prior to pregnancy is statistically significant (p=.010), yielding an odds ratio of 6.7 (95%CI=1.6–28.0) over that of a non-drinking woman. Therefore, the likelihood of a FASD diagnosis in this community is 6 to 7 times greater for a woman who consumes three DDD prior to pregnancy than for women who abstain. The odds increase to 7.6 times greater (p=.002,95%CI=2.0–31.5) for women who reported drinking five or more DDD prior to the index pregnancy.
Table 7.
Adjusted Binary Logistic Regression Analysis of FASD Diagnosis as a Function of Usual Number of Drinks per Drinking Day 3 Months Prior to Pregnancy: Pooled Over 25 Imputations - Rocky Mountain Sample
| B | S.E. | Sig. | Odds Ratio Exp(B) |
95% CI | Fraction Missing Info. |
Relative Increase Variance |
Relative Efficiency |
||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| 1 drink per drinking day | 0.522 | 0.776 | 0.502 | 1.685 | 0.367 | 7.725 | 0.173 | 0.206 | 0.993 |
| 2 drinks per drinking day | 0.069 | 0.782 | 0.930 | 1.071 | 0.230 | 4.980 | 0.217 | 0.273 | 0.991 |
| 3 drinks per drinking day | 1.905 | 0.737 | 0.010 | 6.720 | 1.583 | 28.000 | 0.149 | 0.172 | 0.994 |
| 4 drinks per drinking day | 1.993 | 0.742 | 0.007 | 7.340 | 1.712 | 526.000 | 0.112 | 0.125 | 0.996 |
| 5+ drinks per drinking day | 2.027 | 0.664 | 0.002 | 7.590 | 2.006 | 31.463 | 0.163 | 0.192 | 0.994 |
| Covariates | 27.958 | ||||||||
| Used tobacco during pregnancy | 0.431 | 0.599 | 0.472 | 1.538 | 0.474 | 4.992 | 0.257 | 0.338 | 0.990 |
| Used any illicit drugs during pregnancy | 0.012 | 0.848 | 0.989 | 1.012 | 0.192 | 3.541 | 0.139 | 0.160 | 0.994 |
| Constant | −2.780 | 0.483 | 0.000 | 0.062 | 0.024 | 0.160 | 0.211 | 0.263 | 0.992 |
Prevalence of FASD Estimated
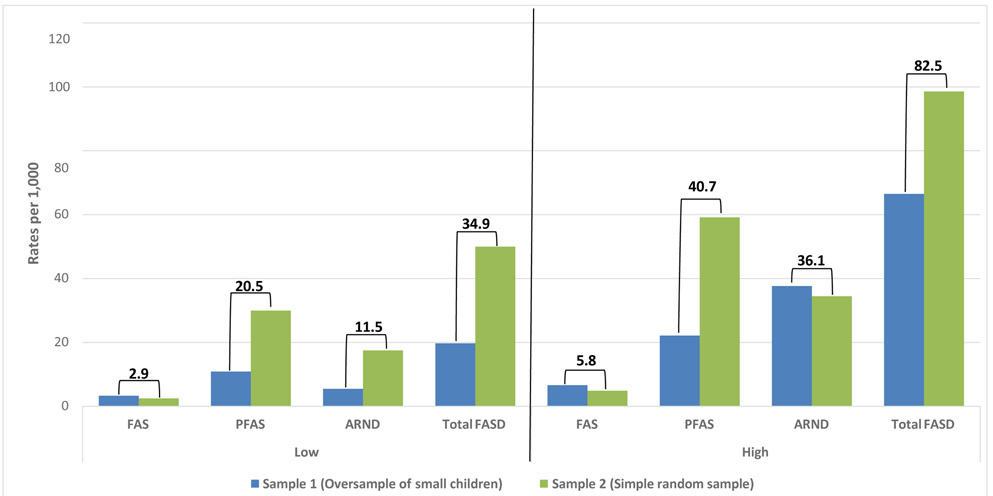
In Figure 7, the combined prevalence of FASD at this site was calculated from the average of rates from the two independent cohort samples published separately in the previous CoFASP prevalence summary (May et al., 2018a and E-appendix). Combined prevalence of FAS cannot be lower than 2.9 per 1,000 children, and weighted correction estimated prevalence at 5.8 per 1,000. PFAS was estimated at 20.5 to 40.7 per 1,000 children, and ARND prevalence was estimated at 11.5 to 36.1 per 1,000. Total FASD was at least 34.9 per 1,000 and likely to be 82.5 per 1,000. FASD affects 3.5% to 8.3% of children.
Figure 7.
Rocky Mountain Prevalence of FASD: Low and High Estimates for Sample 1 and 2 Combined
DISCUSSION
The rate of total FASD was found to be high at the Rocky Mountain city site; up to 8.3% percent of the first grade students are estimated to have qualified for a diagnosis on the continuum of FASD. This rate represents the second highest among the four CoFASP sites. The Pacific Southwest site had a similar combined sample rate of total FASD at 87.2 per 1,000 or 8.7% (May et al., 2018a). The cases of FASD at the Rocky Mountain site were equally distributed among the racial and (Hispanic) ethnic population in this community, for the distribution of FASD cases mirrored the overall racial and ethnic distribution of all students. This finding may have been partly influenced by the small population of the city and small number of children diagnosed with FASD (n=38).
The revised IOM diagnostic guidelines for FASD dysmorphology with CoFASP cut-off criteria discriminated the groups well; but unlike two other sites in CoFASP, children with ARND have a (non-significantly) lower average of minor anomalies and better growth than the randomly selected, typically-developing controls. Nevertheless, children with ARND perform the poorest of the groups on most neurobehavioral study indicators. Test results for children with FAS were quite variable or erratic on many neurobehavioral traits, and mothers of children with FAS surely under-reported alcohol use in pregnancy. Mothers of children with ARND reported the greatest number of DDD prior to pregnancy and more DDD and frequent drinking in the first trimester. Overall, significant maternal risk factors were: reporting of three or more DDD prior to pregnancy, younger age and weighing less immediately prior to pregnancy, later first visits to pre-natal care, drinking through at least part of first and second trimesters, co-morbid lifetime use of marijuana, other health problems, mothers who are less likely to be living with the child’s father and who have a male partner who has had a drinking problem. “Pump and Dump” is a common practice among mothers who breastfed and drank postpartum.
Reporting of alcohol correlated significantly, but weakly, with a child’s problems (when controlling for other drug exposure), and the odds ratio for a diagnosis of FASD was greatest for those who reported three or more drinks per drinking day prior to pregnancy. Co-morbid alcohol and other drug use at this site was reported by some mothers of both maternal groups. While alcohol is the most teratogenic of drugs commonly used in the U.S., simultaneous other drug exposure is a public health concern due to synergistic drug effects which may increase harm to the developing fetus. Since THC in marijuana has been demonstrated to be teratogenic, especially when used in combination with alcohol (Fish et al., 2019), co-morbid alcohol and marijuana use reported by mothers of both the FASD and control maternal groups (6.3 vs. 1.6%) is troubling. Also, reports that many fathers had alcohol use problems raised questions of both social influence on mothers and of an epigenetic effect on offspring.
Ecological explanations for the high rate of FASD in this community may be found in contemporary normative drinking practices influenced by loose social integration of western urban communities and low membership in local congregations of organized religion. Sixty-three percent of all women in this study reported pre-pregnancy drinking, which is higher than the 54% of women of childbearing age reported by the Centers for Disease Control (CDC) for a similar time period (Tan et al., 2015). Furthermore, many epidemiological studies of alcohol in the U.S. have reported significant increases in alcohol use, high risk drinking, and alcohol use disorders among women from 2001-2013 (Grant et al., 2017; Grucza et al., 2018; Keyes et al., 2011). Even with suspected under-reporting of alcohol quantity and frequency, measures of alcohol use before and during pregnancy indicated heavy and problematic use among the women at this site. The CDC also reported that 18.2% of women of childbearing age in the U.S. binge drink (four or more drinks per occasion), and logistic regression analysis in our study clearly links this level to a significantly increased odds ratio of a diagnoses on the FASD continuum that is six to eight times that of a non-drinker. Furthermore, recent increases in heavy drinking in the U.S. might be linked to the existence of fewer norms of moderation of, and abstinence from, alcohol use in primary social groups. The Rocky Mountain Region, specifically the urban areas of the region, have often been characterized as having loose social integration where men and women are allowed freedom for individualization of behavior, including drinking practices. Western social individualism, coupled with the current data on religious affiliation in this state, may allow much alcohol use and heavy drinking. Recent surveys report that 30% in this state have no affiliation (called “nones”) with an organized religion: Christian, Jewish or Muslim (Pew Research Center, 2015). Each of these religions emphasize moderation of, or abstinence from, alcohol use to varying degrees, which generally results in lower risk for alcohol use disorders among adherents of these religions (Isralowitz et al., 2018; Meyers et al., 2017). Of the other CoFASP sites, the sites with the lowest rates of FASD have the highest rates of formal religious affiliation and lowest rate of non-affiliation (“nones”) with these formal religious groups.
Strengths and Limitations
A strength of the research in this site was the research teams long term relationship in this community carrying out referral clinics for 15 years and completing three pilot in-school studies prior to CoFASP. School and community health personnel were supportive and efficient facilitators of the research. On the other hand, a limitation is the relatively small size of the community, which limited statistical power somewhat compared to other CoFASP sites; however, the large number of controls in this site facilitated the demonstration of significant differences between the child diagnostic groups and maternal risk comparisons utilizing bi-variate analysis. A second limitation was the likelihood of inaccurate reporting of alcohol use of the mothers of children with FASD, especially those with children with FAS. Mothers of children with ARND and controls may have been the most forthcoming with alcohol use information during pregnancy, and the information that they provided was rich in detail bolstering the FASD vs. control comparisons. Furthermore, data reported for drinking prior to pregnancy proved to be extremely valuable as the key maternal risk variable.
CONCLUSIONS
This small city had an estimated total FASD prevalence of 8.3%, which was the second highest of the CoFASP sites. There was no significant racial or ethnic difference in the distribution of cases. Children with PFAS accounted for 57.1% of the children with FASD, followed by 34.3% with ARND, and 8.6% with FAS. Children with ARND had the poorest scores and performance on cognitive and behavioral tests, and mothers of children with ARND also reported many risk factors, including the highest number of DDD prior to and during pregnancy and frequent, heavy drinking in the first trimester. Late reporting for prenatal care was a significant maternal risk variable, and the co-morbid use of alcohol, marijuana, tobacco and other drugs is a concern for both children with FASD and controls.
Supplementary Material
What’s Known on this Subject: There are few studies of the characteristics of children within the continuum FASD and their mothers in the general population of the United States. Additionally, most studies of FASD prevalence and maternal and child characteristics have been undertaken using passive methods of case ascertainment and underestimate the prevalence and range of traits that comprise the full continuum of FASD, and they are linked to a small number of maternal risk variables. Furthermore, most clinical and epidemiological studies of FASD do not provide a detailed overview of child physical and neurodevelopmental traits and maternal risk factors associated with fully diagnosed children with FASD compared to typically-developing children and their mothers in the same population.
What this Study Adds: Using active case ascertainment methods among children in a representative, middle class county in a Rocky Mountain Region in the United States, child characteristics and maternal risk traits for the continuum of FASD are described and compared to typically-developing children in the same community. The results of two studies in two independent cohorts of first grade students from the same city are presented here. The traits provide clear differentiation of the diagnostic groups and a continuum of effects. The prevalence of all diagnoses in the continuum of FASD was found to be substantially higher than previous estimates for the general U.S. population prior to this study.
ACKNOWLEDGEMENTS
This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894. Marcia Scott, Ph.D., Kenneth Warren, Ph.D., Faye Calhoun, D.P.A., and the late T-K Li, M.D. of NIAAA provided intellectual guidance, encouragement, and support for prevalence studies of FASD for many years. Our deepest thanks are extended to Carol Keaster, R.N., the Board of Trustees of the City Public School, the Superintendent of Schools, Boards of Education of the private schools, administrators, principals, psychologists, and teachers of the schools in the study community who have hosted and assisted in the research process over the years. Their professional support, guidance, and facilitation have been vital to the success of this study. We dedicate this paper to our deceased colleague and co-author of the early drafts of this manuscript. Joelene Goodover, M.A. passed away on February 6, 2019 after dedicating over three decades of hard work, boundless energy and expertise to school psychology in the Rocky Mountain Region City. Her efforts included overseeing all the testing for three pilot research projects on FASD at this site, which were the first studies of this kind in American schools. She also supervised all neurobehavioral testing, and performed some of the testing herself, for both cohort samples for the CoFASP study. Joelene’s generosity of spirit, caring, expertise and “can-do” attitude contributed greatly to the success of the project. We are also grateful for the advice and participation in the planning and implementation of the project by the CoFASP Advisory Committee members who were led by Marcia Scott, Ph.D., NIAAA Project Officer: Judith Arroyo, Ph.D., Michael Charness, M.D., William Dunty, Ph.D., Daniel Falk, Ph.D., Dale Herald, M.D., Ph.D., and Edward Riley, Ph.D.
Funding Source: This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894 as part of the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP) consortium. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ARBD
alcohol-related birth defects
- ARND
alcohol-related neurodevelopmental disorder
- BMI
Body Mass Index
- CDC
Centers for Disease Control and Prevention
- CoFASP
Collaboration on Fetal Alcohol Spectrum Disorders Prevalence
- CI
Confidence Intervals
- FASD
fetal alcohol spectrum disorders
- DDD
Drinks per Drinking Day
- FAS
fetal alcohol syndrome
- ICD
inner canthal distance
- IPD
inter pupillary distance
- IOM
Institute of Medicine
- OFC
occipitofrontal (head) circumference
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PFAS
partial fetal alcohol syndrome
- PFL
palpebral fissure length
- SES
socioeconomic status
- SD
Standard Deviations
Footnotes
Financial Disclosure: The authors have no financial relationship relevant to this article to disclose.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Achenbach T, Rescorla L (2001) Manual For The ASEBA School-Age Forms And Profiles. Burlington, VT, University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Alvik A, Haldorsen T, Lindemann R (2006) Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Paediatr 95:686–693. [DOI] [PubMed] [Google Scholar]
- America’s Changing Religious Landscape ∣ Pew Research Center (n.d.). Available at: https://www.pewforum.org/2015/05/12/americas-changing-religious-landscape/ Accessed November 15, 2019.
- America’s Health Rankings Annual Report (2015). Minnetonka, Minnesota. [Google Scholar]
- Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC (2017) Prevalence of Prenatal Alcohol Exposure in the State of Texas as Assessed by Phosphatidylethanol in Newborn Dried Blood Spot Specimens. Alcohol Clin Exp Res 41:1004–1011. [DOI] [PubMed] [Google Scholar]
- Beery KE, Beery NA (2004) The Beery-Buktenica Development Test of Visual-Motor Integration, Fifth Edit. ed. San Antonio, TX, Pearson Assessment. [Google Scholar]
- Bracken BA (1998) Braken Basic Concept Scale - Revised. San Antonio, Texas. [Google Scholar]
- Burd L, Cox C, Poitra B, Wentz T, Ebertowski M, Martsolf JT, Kerbeshian J, Klug MG (1999) The FAS Screen: a rapid screening tool for fetal alcohol syndrome. Addict Biol 4:329–336. [DOI] [PubMed] [Google Scholar]
- CDC - BRFSS (n.d.). Available at: https://www.cdc.gov/brfss/ Accessed November 15, 2019.
- Clarren SK, Randels SP, Sanderson M, Fineman RM (2001) Screening for fetal alcohol syndrome in primary schools: A feasibility study. Teratology 63:3–10. [DOI] [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D (1990) Five-year reliability of self-reported alcohol consumption. J Stud Alcohol 51:68–76. [DOI] [PubMed] [Google Scholar]
- Elliott CD (2007) Differential Ability Scales-II (DAS-II). San Antonio, Texas, Harcourt Assessment. [Google Scholar]
- Ervalahti N, Korkman M, Fagerlund Å, Autti-Rämö I, Loimu L, Hoyme HE (2007) Relationship between dysmorphic features and general cognitive function in children with fetal alcohol spectrum disorders. Am J Med Genet Part A 143A:2916–2923. [DOI] [PubMed] [Google Scholar]
- Fish EW, Murdaugh LB, Zhang C, Boschen KE, Boa-Amponsem O, Mendoza-Romero HN, Tarpley M, Chdid L, Mukhopadhyay S, Cole GJ, Williams KP, Parnell SE (2019) Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction. Sci Rep 9:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Muckle G, Jacobson SW, Jacobson JL, Belanger RE (2017) Alcohol use among Inuit pregnant women: Validity of alcohol ascertainment measures over time. Neurotoxicol Teratol 64:73–78. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013. JAMA Psychiatry 74:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, Hartz S, Virdi G, Bierut LJ (2018) Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcohol Clin Exp Res 42:1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V (2010) A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 44:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme H, May P, Kalberg W, Kodituwakku P, Gossage J, Trujillo P, Buckley D, Miller J, Aragon A, Khaole N, Viljoen D, Jones K, Robinson L (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM (2017) IBM SPSS Statistics for Windows. [Google Scholar]
- Isralowitz R, Reznik A, Sarid O, Dagan A, Grinstein-Cohen O, Wishkerman VY (2018) Religiosity as a Substance Use Protective Factor Among Female College Students. J Relig Health 57:1451–1457. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet (London, England) 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2001) Pre-pregnancy drinking: how drink size affects risk assessment. Addiction 96:1199–1209. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K (2000) An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse 12:67–78. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Kerr WC (2008) Accuracy of Photographs to Capture Respondent-Defined Drink Size. J Stud Alcohol Drugs 69:605–610. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS (2011) Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res 35:2101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC (1994) Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health 84:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007) NEPSY (NEPSY-II), Second ed. San Antonio, Texas, Pearson Assessment. [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee RA, Yi H (2011) Surveillance Report #92: Apparent per alcohol consumption: national, state, and regional trends, 1977-2009.
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and Characteristics of Fetal Alcohol Spectrum Disorders. Pediatrics 134:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D (2000) Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health 90:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Daniel F, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018a) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA - J Am Med Assoc 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, Jones KL, Hoyme D, Seedat S, Parry CDH, Hoyme HE (2016a) The continuum of fetal alcohol spectrum disorders in four rural communities in south africa: Prevalence and characteristics. Drug Alcohol Depend 159:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Hoyme HE, Aragon AS, Buckley D, Stellavato C, Gossage JP, Robinson LK, Jones KL, Manning M, Ceccanti M (2011a) Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: new estimated rates are higher than previous estimates. Int J Environ Res Public Health 8:2331–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Phillip Gossage J, Kalberg WO, Eugene Hoyme H, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M (2006) Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res 30:1562–1575. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL (2005) Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health 95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15:176–192. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL (2008) Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res 32:738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Hasken JM, De Vries MM, Marais AS, Stegall JM, Marsden D, Parry CDH, Seedat S, Tabachnick B (2018b) A utilitarian comparison of two alcohol use biomarkers with self-reported drinking history collected in antenatal clinics. Reprod Toxicol 77:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Gossage JP, Robinson LK, Manning M, Hoyme HE (2015) Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend 155:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, Adnams CM, Barnard R, Joubert B, Cloete M, Tabachnick B, Robinson LK, Manning MA, Jones KL, Bezuidenhout H, Seedat S, Parry CDH, Hoyme HE (2016b) The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend 168:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning M, Buckley D, Hoyme HE (2011b) Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend 119:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning MA, Blankenship J, Buckley D, Hoyme HE, Adnams CM (2013) Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr 34:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Brown Q, Grant BF, Hasin D (2017) Religiosity, race/ethnicity, and alcohol use behaviors in the United States. Psychol Med 47:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft Excel (2016).
- NIAAA Strategic Plan 2017-2021 (2017).
- Poitra BA, Marion S, Dionne M, Wilkie E, Dauphinais P, Wilkie-Pepion M, Martsolf JT, Klug MG, Burd L (2003) A school-based screening program for fetal alcohol syndrome. Neurotoxicol Teratol 25:725–729. [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Koek G, Curfs L (2018) Systematic literature review on which maternal alcohol behaviours are related to fetal alcohol spectrum disorders (FASD). BMJ Open 8:e022578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L (2016) Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res 40:18–32. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM (1997) Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology 56:317–326. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M (2001) Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse 36:313–331. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI (1988) The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol 49:225–232. [DOI] [PubMed] [Google Scholar]
- Sparrow SA, Cicchetti DV, Balla DA (2005) Vineland Adaptive Behavior Scales, Second ed. Bloomington, Minnesota. [Google Scholar]
- Stratton K, Howe C, Battaglia F (1996) Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington DC. [Google Scholar]
- Tabachnick BG, Fidell LS (2013) Using Multivariate Statistics, Sixth ed. Boston, Massachusetts, Pearson. [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D (2015) Alcohol use and binge drinking among women of childbearing age - United States, 2011-2013. MMWR Morb Mortal Wkly Rep 64:1042–1046. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau QuickFacts: United States (n.d.). Available at: https://www.census.gov/quickfacts/fact/table/US/PST045218 Accessed November 15, 2019.
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole NCO, Kodituwakku P, Asante KO, Findlay R, Quinton B, Marais A-S, Kalberg WO, May PA (2005) Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol 66:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KR, Calhoun FJ, May PA, Viljoen DL, Li TK, Tanaka H, Marinicheva GS, Robinson LK, Mundle G (2001) Fetal alcohol syndrome: an international perspective. Alcohol Clin Exp Res 25:202S–206S. [DOI] [PubMed] [Google Scholar]
- What Is A Standard Drink? ∣ National Institute on Alcohol Abuse and Alcoholism (NIAAA) (n.d.). Available at: https://www.niaaa.nih.gov/what-standard-drink Accessed November 18, 2019.
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I (2008) Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT-a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol 198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.