Abstract
Background:
Prenatal alcohol exposure (PAE) has been linked to poorer performance on the Morris Water Maze (MWM), a test of spatial navigation in rodents that is dependent on hippocampal functioning. We recently confirmed these findings in children with PAE on a human analog of the MWM, the virtual water maze (VWM). Previous studies have shown that the hippocampus is particularly sensitive to PAE. Our aim was to determine whether hippocampal volume mediates the relation between PAE and virtual navigation.
Methods:
VWM and MRI hippocampal data were collected from 50 right-handed 10-yr-old children in a heavily exposed Cape Town, South African sample. PAE data had been collected from their mothers during pregnancy, and the children were examined by expert fetal alcohol spectrum disorder (FASD) dysmorphologists. In the VWM the participant attempts to learn the location of a hidden platform in a virtual pool of water across a series of learning trials using only distal room cues. Hippocampal volumes were derived using FreeSurfer from MRI scans administered within 1 week of completing the VWM task.
Results:
Both the fetal alcohol syndrome (FAS)/partial FAS and non-syndromal heavy exposed (HE) groups had smaller hippocampal volumes than controls. PAE was associated with reduced right hippocampal volumes even after control for total intracranial volume (ICV). Hippocampal volume was also positively associated with VWM performance. The relation between PAE and VWM performance was partially mediated by right hippocampal volume but not by total ICV.
Conclusions:
These data confirm previous reports linking PAE to poorer spatial navigation on the VWM and are the first to provide direct evidence that volume reductions in this region partially mediate the relation of FASD diagnosis to place learning, suggesting that PAE specifically impairs the ability to encode the spatial information necessary for successful location of the hidden platform on a navigation task.
Keywords: Spatial navigation, virtual environment, place learning, hippocampal volume, fetal alcohol spectrum disorders, fetal alcohol syndrome, prenatal alcohol exposure
INTRODUCTION
Fetal alcohol spectrum disorders (FASD), the umbrella term used to describe the broad range of adverse outcomes associated with prenatal alcohol exposure (PAE) (Hoyme et al., 2005), is among the most prevalent and preventable forms of mental retardation in the U.S. and worldwide (Lange et al., 2017). Endemic areas, such as the Western Cape province of South Africa, have the highest rates of FASD and fetal alcohol syndrome (FAS), the most severe form of FASD, in the world (May et al., 2013; 2018). Identifying the specific brain regions affected in the performance of tasks that are adversely affected by PAE is a major challenge to the field.
Laboratory animal studies have shown that the hippocampus is particularly susceptible to insult from PAE that occurs in the equivalent of the human 2nd and 3rd trimesters. In rodents, reductions in hippocampal cell count (Bonthius and West, 1990), cell density, and total hippocampal volume (Livy et al., 2003) have been observed following PAE. These effects are more pronounced when animals are exposed to binge-like episodes of ethanol than when lower levels are administered more gradually (Bonthius and West, 1990; Greene et al., 1992; Livy et al., 2003). Consistent with the rodent data, hippocampal volume reductions have also been detected in eight MRI studies of humans with FASD (Lebel et al., 2011). Given that reductions in overall brain volume are a well-documented feature of FASD, these studies have considered whether the hippocampus is disproportionally smaller in relation to total brain volume. Three of these studies found reductions in hippocampal volume after adjusting statistically for total intracranial volume (ICV) (Archibald et al., 2001; Nardelli et al., 2011; Willoughby et al., 2008); five did not (Astley et al., 2009; Coles et al., 2011; Joseph et al., 2014; Riikonen et al., 2005; Roussotte et al., 2011).
Place learning has been shown to be highly dependent upon specific neurocircuitry in the hippocampus in rodents (Morris et al., 1982) and humans (e.g., Bohbot et al., 2007; Hsu et al. 2000; Iaria et al., 2003; Maguire et al., 1998). Lesions to the hippocampus impair performance on the widely-used Morris Water Maze task (MWM; Morris et al., 1982, 1984), and the volume of the lesion has been shown to be directly related to degree of MWM impairment (Moser et al., 1993; 1995). In the MWM, a test of hippocampus-dependent spatial learning (e.g., Berman and Hannigan, 2000), rodents are required to swim to the location of a platform to escape from a pool of opaque water across a series of acquisition trials. In the place learning variant of the MWM (see Dodge et al., 2019, Fig. 1), the platform is submerged beneath the surface of the water, and there are no proximal cues marking its location. During an acquisition phase the rodents attempt to learn the location of the platform based on the arrangement of distal environmental cues on the walls in the room. Performance during a cued navigation phase at the end of the task is a control condition which requires no learning and checks that acquisition is not due to motor impairment by generating data on navigating to a visible platform. The hippocampus is critical for both the encoding and retrieval of the spatial associations formed between the hidden escape platform and the distal room cues (Riedel et al., 1999).
Figure 1.
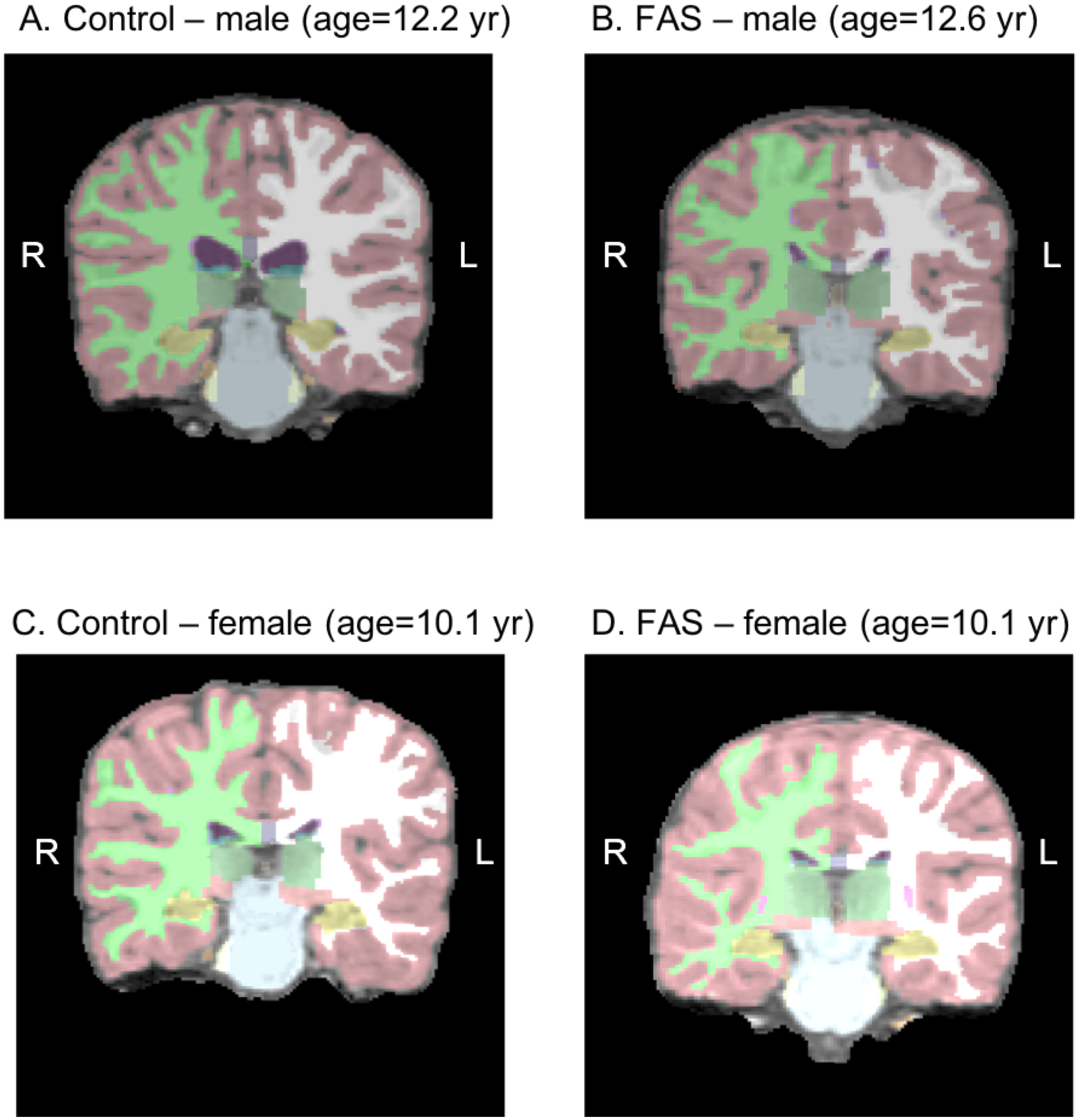
Control (A,C) and FAS (B,D) brains generated by FreeSurfer matched by age and sex. The hippocampus is colored in yellow.
Hippocampal involvement in place learning/navigation has been demonstrated in humans using a human analog of the MWM, the virtual water maze (VWM) task, which uses 3-dimensional computer-generated environments. Patients with epilepsy who have undergone hippocampal resection showed severe impairment in the ability to learn the location of the hidden platform in the VWM (Astur et al., 2002). These effects were evident regardless of the side of the hippocampal resection. By contrast, no differences were found in cued navigation, in which the platform is visible. Amnesic patients with hippocampal damage also showed selective impairment in place learning on the VWM (Goodrich-Hunsaker et al., 2010). Damage to the hippocampus has been linked to impaired spatial navigation in other tasks that require navigation through virtual space (Bohbot et al., 1998; Maguire et al., 1998; Spiers et al., 2001). Gray matter volume in the hippocampus has also been shown to relate to the ability to navigate mazes (Bohbot et al., 2007; Brown et al., 2014), and age-related declines in hippocampal volume correlate with reduced hippocampal activation during navigation and performance on spatial learning tasks (Moffat et al., 2006). Two prior studies using voxel-based morphometry found that hippocampal volume was associated with the use of mapping strategies employed during navigation even after correcting for brain size (Bohbot et al., 2007; Brown et al., 2014).
Rodent models examining the effects of ethanol on the MWM have found specific effects of PAE on place learning and not on cued navigation (e.g., Goodlett et al., 1987; Kelly et al., 1988). In a seminal paper, Hamilton et al. (2003) reported impaired place learning in a small sample of male adolescents with FAS compared to normal male controls on the VWM, whereas cued navigation, when the platform was visible, was spared. This behavioral dissociation between place learning and cued navigation is consistent with the findings from laboratory animals with hippocampal damage and rodent models with alcohol exposure.
We have recently reported that children with FAS or partial FAS (PFAS) also show longer latency and path lengths to locate the hidden platform in the VWM (Dodge et al., 2019). Among boys, both the FAS/PFAS and nonsyndromal heavily exposed (HE) groups performed more poorly than controls during acquisition, and both boys and girls born to women who binge drank during pregnancy performed more poorly than those born to abstainers and light drinkers. In addition, both amount and frequency of PAE were related to poorer performance during a probe trial and not during cued navigation, indicating that the deficits were related specifically to encoding and retrieval. These alcohol-related deficits in spatial navigation persisted, even after control for IQ, indicating that they were specific to this domain of cognitive function and not attributable to overall poorer cognitive function.
Although place learning in both rodents and humans has been shown to be dependent on the hippocampus (Morris et al., 1982; Moser et al., 1993; 1995; Pearce et al., 1998; Astur et al., 2002; Goodrich-Hunsaker et al., 2010) and the hippocampus has been found to be altered by PAE, no previous human study has examined directly whether differences in hippocampal volume play a role in mediating the impact of PAE on spatial navigation. We used MRI to examine hippocampal volumes in a sample of heavily exposed and control children who had recently been assessed on the VWM (Dodge et al., 2019). The aims of this study were to determine (1) whether hippocampal volume is reduced in relation to both FASD diagnosis and continuous measures of PAE; (2) whether hippocampal volume is reduced in children with PAE both before and after adjustment for intracranial volume, that is, whether it is disproportionately smaller relative to other brain regions; (3) whether reduced hippocampal volume is associated with poorer VWM performance; and (4) whether hippocampal volume plays a role in (partially mediates) the effects of FASD diagnostic group and PAE on VWM performance.
METHODS
Participants
The participants were recruited from the Cape Coloured (mixed ancestry) community in Cape Town, where prevalence of heavy drinking during pregnancy continues to be among the highest in the world (May et al., 2013; 2018). As described in Dodge et al. (2019), the sample consisted of 62 right-handed, school-aged children (M = 10.4 years; SD = 1.2), recruited between 2004–2006, who were administered the VWM. 36 were the older siblings of participants in our original prospective Cape Town Longitudinal Cohort (Jacobson et al., 2008), and 26 were recruited by screening all 8- to 12-year-olds from an elementary school in a nearby rural section of Cape Town with an even higher incidence of PAE than that seen in urban Cape Town (Jacobson et al., 2011). Of the 62 children, MRI scans were successfully obtained from 50 children at 10.2 years (SD = 0.6). MRIs from the remaining 12 children (2 FAS/PFAS, 4 nonsyndromal heavily exposed (HE), 6 controls) were excluded because of poor image quality due primarily to excessive movement.
Approval from human subjects ethics committees were obtained from the Wayne State University and University of Cape Town (UCT) Faculty of Health Sciences, and informed consent was obtained from the mothers and assent from the children. All children received a small gift and the mothers received a photo of the child and remuneration consistent with guidelines from the UCT ethics committee. A staff driver and research nurse transported the mother and child to our UCT laboratory for the behavioral VWM and IQ assessments and to the Cape Universities Brain Imaging Center (CUBIC) for the MRIs. The mean number of days between the VWM assessment and the neuroimaging scan was 1.5 days (SD = 1.4); all were conducted within 8 days.
Procedure
Assessment of prenatal alcohol and drug use and demographic background
Each mother in the cohort was interviewed in English or Afrikaans by a research nurse using a timeline follow-back approach, adapted for use with women in this community (Jacobson et al., 2008, 2011). In the timeline follow-back interview, the mother is asked about her drinking during a typical 2-week period during her pregnancy. The interview included information about sharing (size and type of container shared by how many women) for use in the calculation of standard drinks. Volume was recorded for each type of alcoholic beverage consumed and converted to absolute alcohol (AA), using multipliers developed by Bowman et al. (1975): liquor—0.4, beer—0.05, wine—0.12. Frequency of alcohol consumption was also recorded. These values were averaged across the prenatal visits to obtain three measures of pregnancy drinking: oz AA/day, oz AA/occasion, and frequency of drinking days/week across pregnancy. A dichotomous measure of binge drinking for women (≥4 drinks/occasion; yes/no) was also constructed based on the NIAAA criteria (https://www.niaaa.nih.gov › alcohol-health › moderate-binge-drinking). Information on whether the mothers had used cocaine, marijuana, opiates, and methaqualone (“mandrax”), and average number of cigarettes/day during the pregnancy was also obtained. Demographic information including socioeconomic status (Hollingshead, 2011), maternal age, education, marital status, and parity, and child sex and age at testing were collected during the visit to our UCT laboratory.
FASD diagnoses
FAS is characterized by microcephaly, pre- and or postnatal growth retardation, and a distinctive pattern of craniofacial dysmorphology, including short palpebral fissures, thin upper lip, and a smooth philtrum; PFAS, as the presence of at least two of these features and microcephaly, growth retardation, or cognitive or behavioral dysfunction (Hoyme et al., 2005). In 2005 and 2009, we organized clinics at which each child was independently examined by two expert FAS dysmorphologists (H.E. Hoyme, M.D., and L.K. Robinson, M.D.) for growth and FAS anomalies using a standard protocol (Hoyme et al., 2005) based on the Revised Institute of Medicine criteria (see Jacobson et al., 2008, 2011). The two dysmorphologists were blind regarding maternal alcohol history. FAS and PFAS diagnoses were subsequently determined at daily case conferences attended by HEH, LKR, SWJ, JLJ, and CDM. Children who did not meet criteria for FAS or PFAS were designated as either non-syndromal, heavily exposed (HE) or controls, depending on the maternal alcohol history. The diagnoses were confirmed in follow-up clinics led by HEH in 2013 and 2016.
Virtual Water Maze and IQ assessment
The VWM procedure used was developed by Hamilton et al. (2003) and described in detail in Dodge et al. (2019). The virtual environment was comprised of a circular pool centered in a square room with four different rectangular objects of equal size placed on each of the distal room walls. The participant navigated from the starting point to the hidden platform during the acquisition phase. Starting locations varied between 5 blocks with participants informed that the platform would always be in the same location relative to the distal cues but that starting positions would differ. Latency (total time to reach the hidden platform) and path length were recorded for each trial. The probe phase consisted of a single 45-s trial in which the platform was removed from the environment. The starting location was selected randomly from one of two points that were farthest from the location of the platform. Percentage of time spent in the platform quadrant was recorded as the dependent variable. The final cued navigation phase consisted of 2 four-trial blocks in which the platform was visible and all other parameters were the same as in the acquisition phase. Latency and path length to reach the platform during the final two blocks of acquisition were used as the measures of performance on the VWM.
Seven of 10 subtests from the WISC-III—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and Matrix Reasoning from the WISC-IV were administered in Afrikaans or English at the children’s first laboratory visit (M = 10.2 yr, SD = 1.2). IQ was estimated using Sattler’s (1992) formula for computing Short Form IQ, and validity coefficients for Sattler Short Form IQ based on ≥5 subtests consistently exceed r = 0.90. Handedness was assessed on the Annett (1970) Behavioral Handedness Inventory.
MRI acquisition and image analysis
Upon arrival at CUBIC, a research-dedicated, child-friendly neuroimaging facility, the children were familiarized with the scanning procedures by first practicing in a mock scanner. The children were imaged using a 1.5T Magnetom Symphony MRI scanner (Siemens Medical Systems, Erlangen, Germany). High-resolution anatomical images were acquired in the sagittal plane using a three-dimensional inversion recovery gradient echo sequence (72 slices, TR=1900 ms, TE=3.93 ms, TI=1100 ms, slice thickness 2mm), 250 × 250 mm field of view, resolution 1.4 × 1.0 × 2 mm. Intracranial volume and hippocampal volumes were extracted by NCD using FreeSurfer V5.1.0 automated segmentation.
Demographic background variables
The following demographic background variables were collected: maternal age at delivery, socioeconomic status (SES; Hollingshead (2011) Index), education (yr), marital status (married/unmarried), parity, smoking and marijuana during pregnancy, and child sex and age at testing.
Data Analysis
To assess the effects of FASD group (FAS/PFAS vs. HE vs. controls) on hippocampal volume, analyses of variance (ANOVAs) were performed separately for right and left hippocampal volumes. All significant ANOVAs were followed with least-significant differences post hoc tests. These analyses were also repeated with ICV added as a covariate. Correlation analyses were used to assess whether the amount of PAE (oz AA/day, oz AA/occasion, frequency of drinking days) was associated with reduced hippocampal volumes. These correlations were then re-run as partial correlations to adjust for ICV. Correlations were used to test whether hippocampal volumes and ICV were related to acquisition and probe trial performance on the VWM, and multiple regression analysis was performed to examine potential interaction of sex and hippocampal volume on VWM performance.
To determine if hippocampal volume mediated the association of FASD diagnosis or PAE with VWM performance, estimates of the indirect effect were determined using the MEDIATE macro for SPSS (Hayes and Preacher, 2014), a multivariate extension of the Sobel (1982) method. This macro allows the use of multi-categorical independent variables and multiple mediators. Mediation is determined based on the size of the indirect effects, which are the product of the regression coefficients for the direct effects of the independent variable (IV) on the mediating variable (MV), and the MV on the DV. Statistical significance of the indirect effects is determined based on 95% bootstrap percentile confidence intervals. Indirect effects are deemed statistically significant when the confidence intervals do not overlap with 0. Separate analyses were run using two different predictors—FASD diagnosis and binge group—using latency in blocks 4–5 as the dependent variable.
RESULTS
Table 1 presents the sample characteristics for the 50 children for whom both VWM and neuroimaging data were available. All of the children came from the same socioeconomically disadvantaged, predominantly Cape Coloured community. There were no between-group differences for the FAS/PFAS, HE, and controls based on maternal age, SES, marital status, or parity, but mothers of the FAS/PFAS and HE children were less educated than controls (both ps < 0.05). There were also no differences between the FAS/PFAS and HE groups on the three measures of alcohol exposure. Mothers in the FAS/PFAS and HE groups reported drinking about ≈ 10.6–12.4 standard drinks/occasion on 2–3 days/week. Seventeen (89.4%) of the 19 controls abstained from drinking during pregnancy; the remaining 2 controls reported consuming only 2 drinks on 1–2 occasions during the pregnancy. The number of cigarettes smoked/day during pregnancy was greater in the HE group compared to controls (p < 0.05). None of the women reported using cocaine or methaqualone, and only one HE mother (4.5%) reported using marijuana during pregnancy. The FAS/PFAS, HE, and control groups did not differ in terms of child sex or age at testing. As expected, the children’s IQ scores were lower in the FAS/PFAS group compared to those in the HE and control groups (both p’s < 0.05), and the HE group had lower IQ scores than the controls (p < 0.05). Nor were there any sex differences in age at testing, p > 0.20.
Table 1.
Sample characteristics (N = 50)
| FAS/PFAS (n= 9) | HE (n = 22) | Controls (n = 19) | F or χ2 | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at delivery (years) | 27.6 (5.8) | 24.1 (5.4) | 24.0 (6.2) | 1.36 |
| Socioeconomic statusa | 18.2 (6.8) | 15.6 (5.7) | 20.3 (8.1) | 2.35 |
| Education (years)b | 7.3 (3.3) | 7.0 (2.1) | 8.6 (2.0) | 2.44† |
| Marital status (% married) | 33.3 | 50.0 | 68.4 | 3.28 |
| Parity | 2.3 (1.2) | 2.4 (1.3) | 2.2 (1.6) | 1.01 |
| Prenatal exposure | ||||
| Alcohol | ||||
| oz AA/dayb | 2.8 (2.6) | 2.1 (1.7) | 0.003 (0.01) | 13.23*** |
| oz AA/occasionb | 6.2 (2.5) | 5.3 (3.7) | 0.1 (0.3) | 24.04*** |
| Frequency (days/week)b | 2.9 (1.6) | 2.8 (1.7) | 0.02 (0.1) | 26.67*** |
| Cigarettes/dayc | 8.1 (6.1) | 12.1 (8.3) | 6.7 (7.6) | 2.62† |
| Child characteristics | ||||
| Age at testing (years) | 9.7 (1.4) | 10.7 (1.2) | 10.2 (1.1) | 2.36 |
| Sex (% male) | 33.3 | 45.5 | 47.4 | 0.52 |
| WISC-III IQb | 64.5 (10.6) | 67.1 (9.9) | 76.9 (11.5) | 6.00** |
AA = absolute alcohol. FAS = fetal alcohol syndrome. PFAS = partial FAS.
HE = heavily exposed nonsyndromal. WISC-III = Wechsler Intelligence Scale for Children, 3rd edition.
Values are mean (SD) or %. Post hoc comparisons are indicated below.
Based on Hollingshead Scale (2011).
FAS/PFAS, HE vs. Controls, all p’s < 0.05.
Smokers only. HE vs. Controls, p < 0.05.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Relation of sex and IQ to hippocampal volume
Hippocampal volumes were larger for boys than girls (left hippocampus, t(48) = 2.02, p = 0.049; right hippocampus, t(48) = 2.24, p = 0.030, as were ICVs, t(48) = 2.27, p = 0.028). In addition, WISC-III IQ was positively related to both left and right hippocampal volumes (r’s = 0.34 and 0.29, respectively, both p’s < 0.05) but only marginally related to ICV (r = 0.24, p = 0.097).
Relation of FASD and PAE to hippocampal volume before and after adjustment for ICV
Figure 1 displays FAS and control brains (generated by the FreeSurfer program), matched for sex and age, with the hippocampus shown in yellow. There was a significant effect of FASD diagnostic group on both the left and right hippocampus and on total ICV (Table 2). Hippocampal volumes and ICV were smaller in the FAS/PFAS, compared with the HE and control groups, and the HE group had smaller right hippocampal volumes than the controls. After controlling for ICV, the effect on the right hippocampus remained significant, and the effect on the left hippocampus fell just short of conventional levels of significance.
Table 2.
Effects of FASD diagnosis on hippocampal volumes and intracranial volume
| FAS/PFAS (n= 9) | HE (n = 22) | Controls (n = 19) | F or χ2 | |
|---|---|---|---|---|
| Hippocampus volume (mm3) | ||||
| Lefta | 3906.2 (514.3) | 4133.1 (360.4) | 4372.1 (517.5) | 4.53* |
| Rightb | 3844.0 (391.5) | 4089.7 (418.8) | 4367.1 (500.7) | 3.46* |
| Intracranial volume (cm3)b | 1231.0 (193.4) | 1424.0 (127.8) | 1427.7 (147.1) | 6.37** |
| Hippocampal volume (mm3) adjusted for intracranial volume | ||||
| Leftc | 4257.3 (514.3) | 4059.8 (360.4) | 4290.6 (517.5) | 2.92† |
| Rightc | 4183.3 (391.5) | 4018.9 (418.8) | 4288.3 (500.7) | 3.63* |
Values are mean (SD). Post hoc comparisons are indicated below.
FAS/PFAS vs. Controls, p < 0.05
FAS/PFAS, HE vs. Controls, p’s < 0.05
FAS/PFAS vs. HE, Controls, p’s < 0.05
HE vs. Controls, p < 0.05
p = 0.064
p < 0.05
p < 0.01
All three continuous measures of PAE were associated with smaller left and right hippocampal volumes (Table 3). Although the ICV was smaller in the FAS/PFAS group (see Table 2), PAE was not related to smaller ICV for the sample as a whole. Moreover, PAE continued to be related to right hippocampal volume, after control for ICV. There were no significant PAE by sex interactions (e.g., t’s = 0.13 and 0.56, p’s = 0.896 and 0.577, for right and left hippocampi, respectively).
Table 3.
Effects of prenatal alcohol exposure on hippocampal and intracranial volumes (N = 50)
| oz AA/day | oz AA/occasion | Frequency | ||||
|---|---|---|---|---|---|---|
| r | rpartial | r | rpartial | r | rpartial | |
| L Hippocampus | −0.29* | −0.22 | −0.30* | −0.23 | −0.25† | −0.23 |
| R Hippocampus | −0.35* | −0.31* | −0.33* | −0.27† | −0.30* | −0.31* |
| Intracranial volume | −0.19 | -- | −0.20 | -- | −0.13 | -- |
AA = absolute alcohol.
Values are Pearson r and partial r controlling for intracranial volume.
p < 0.10
p < 0.05.
Relation of hippocampal volume to VWM performance
Smaller left and right hippocampal volumes were significantly associated with longer mean trial times during blocks 2–3 and 4–5 of the VWM (see Table 4 and Fig. 2). The correlations between hippocampal volumes and latency to reach the hidden platform were strongest during Blocks 2–3 for the girls and during Blocks 4–5 for the boys. None of the hippocampus by sex interactions on performance were significant.
Table 4.
Relation of hippocampal volume to virtual water maze performance
| Whole sample (n = 50) | Boys (n = 22) | Girls (n = 28) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hippocampal volume | Hippocampal volume | Hippocampal volume | |||||||
| Left | Right | Intracranial volume | Left | Right | Intracranial volume | Left | Right | Intracranial volume | |
| Acquisition (mean trial time) | |||||||||
| Block 1a | −0.25† | −0.27† | −0.37** | −0.24 | −0.33 | −0.52* | −0.22 | −0.20 | −0.26 |
| Blocks 2–3b | −0.55*** | −0.48*** | −0.64*** | −0.40† | −0.33 | −0.57** | −0.63*** | −0.58** | −0.67*** |
| Blocks 4–5c | −0.35* | −0.40** | −0.40** | −0.57** | −0.48* | −0.35 | −0.12 | −0.28 | −0.39* |
| Probe trial (% time in platform quadrant)d | 0.08 | 0.09 | 0.16 | 0.16 | 0.13 | 0.05 | 0.01 | 0.05 | 0.22 |
Values are Pearson r.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Left hippocampus by sex interaction, t = −0.31, p = 0.759; Right hippocampus by sex interaction, t = −0.09, p = 0.932.
Left hippocampus by sex interaction, t = −1.42, p = 0.163; Right hippocampus by sex interaction, t = −1.63, p = 0.110.
Left hippocampus by sex interaction, t = 1.49, p = 0.144; Right hippocampus by sex interaction, t = −0.09, p = 0.932.
Left hippocampus by sex interaction, t = −0.46, p = 0.649; Right hippocampus by sex interaction, t = −0.19; p = 0.851.
Figure 2.
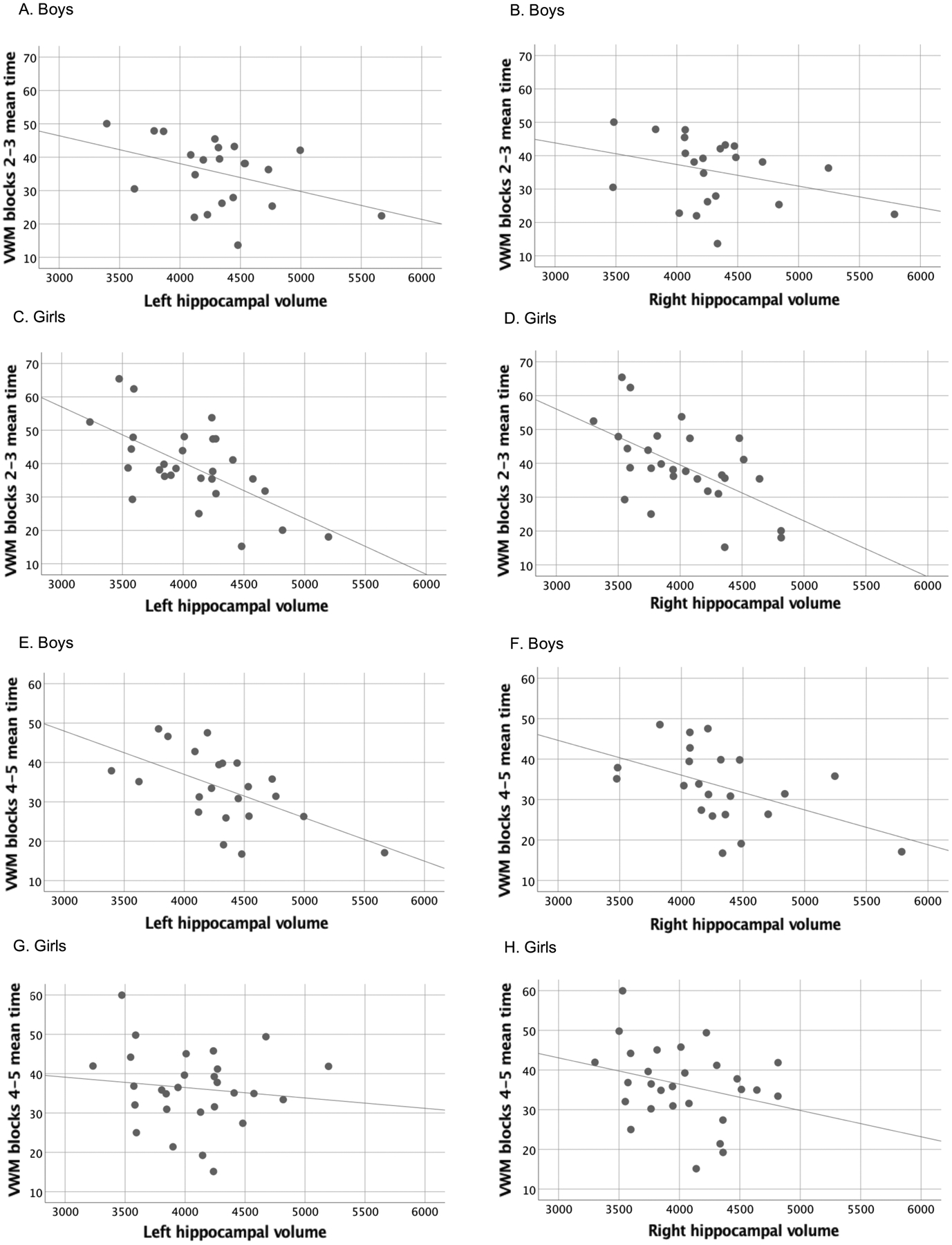
Scatterplots showing the relation between left and right hippocampal volume and performance during acquisition during blocks 2–3 (A-D) and blocks 4–5 (E-H) for boys and girls separately.
Mediation of the effect of FASD diagnostic group on VWM performance by hippocampal volume
Mean trial time in blocks 4–5 during acquisition was used as the dependent variable in the mediation analyses because this measure reflects place learning performance at the end of the acquisition phase. Figure 3A is a path model showing the mediation by right hippocampal volume of the relation between FASD diagnostic group and VWM performance controlling for sex. The FAS/PFAS and HE independent variables were indicator coded using the controls as the reference group. The model accounted for 20.5% of the variance in the dependent variable (F(4,45) = 4.15, p < 0.01). Both of the indirect effects, i.e., the paths from FAS/PFAS and HE through right hippocampal volume to VWM, were significant: FAS/PFAS indirect pathway effect = 2.66 (95% confidence interval = 0.34, 6.69), HE indirect pathway effect = 1.49 (95% confidence interval = 0.04, 4.85). The same model was re-run for the left hippocampal volume. This time, however, the indirect effects fell just short of statistical significance; FAS/PFAS indirect pathway effect = 1.87 (95% CI = −0.27, 6.38), HE indirect pathway effect = 1.01 (95% CI = −0.14, 3.87).
Figure 3. A.
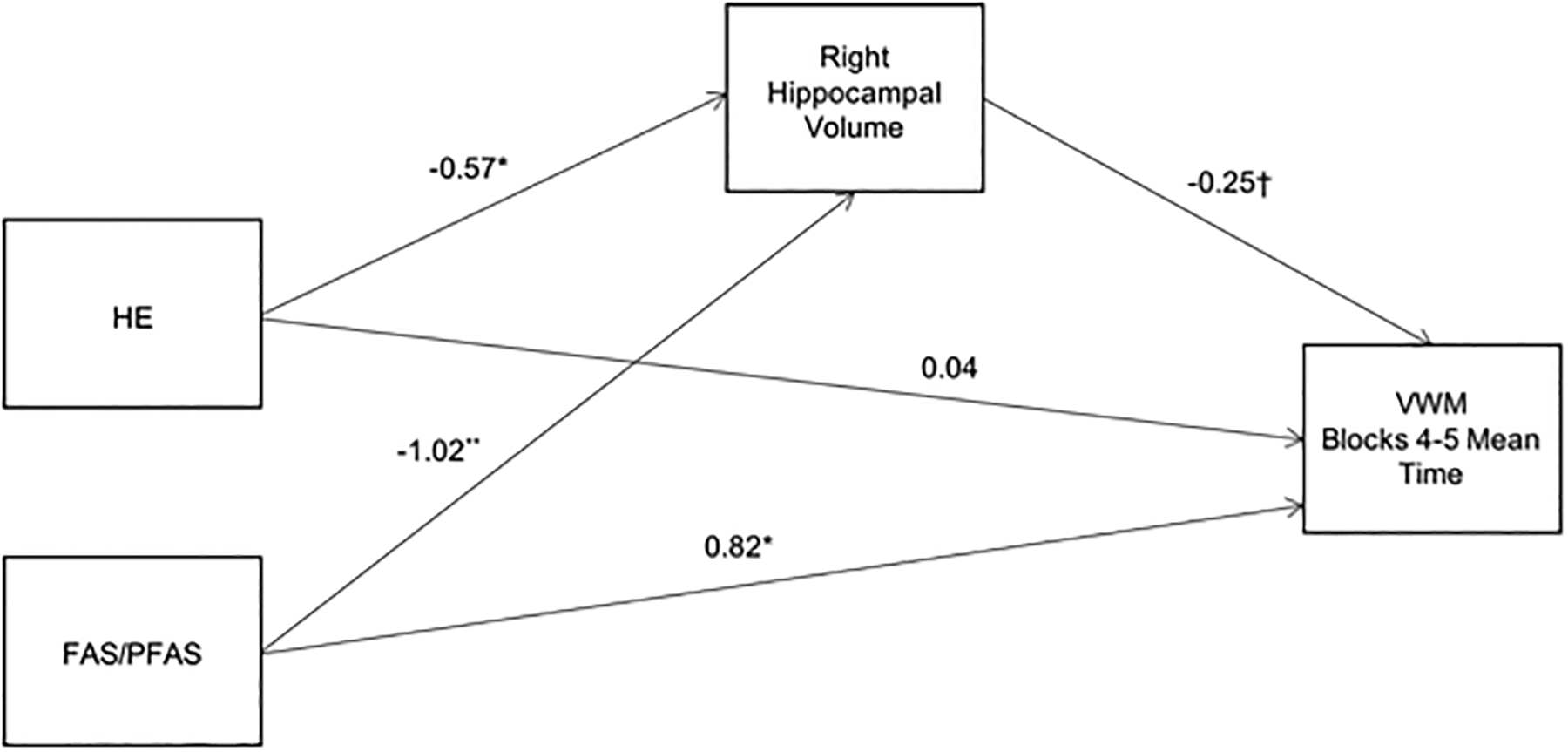
Path model showing the mediation by right hippocampal volume of the relation between diagnostic group and mean trial time during blocks 4 and 5 of the VWM. Values are standardized regression coefficients, adjusted for sex, that indicate by how many standard deviations the outcome changes for a unit change in the predictor. The control group was used as the reference group for the FAS/PFAS and HE coefficients.
†p < 0.10 *p < 0.05 **p < 0.01
To test whether the mediation of the effect of FASD diagnostic group on VWM performance by right hippocampal volume is primarily attributable to reductions in ICV, a second model testing ICV as a mediator was examined (Fig. 3B). The direct effect of ICV on VWM performance was not significant, nor were the indirect effects through ICV: FAS/PFAS = 2.49 (95% CI = −0.64, 8.43) and HE = 0.03 (95% CI = −1.25, 1.67), indicating that ICV did not mediate and, therefore, did not account for the relation between FASD diagnostic group and VWM performance.
Figure 3. B.
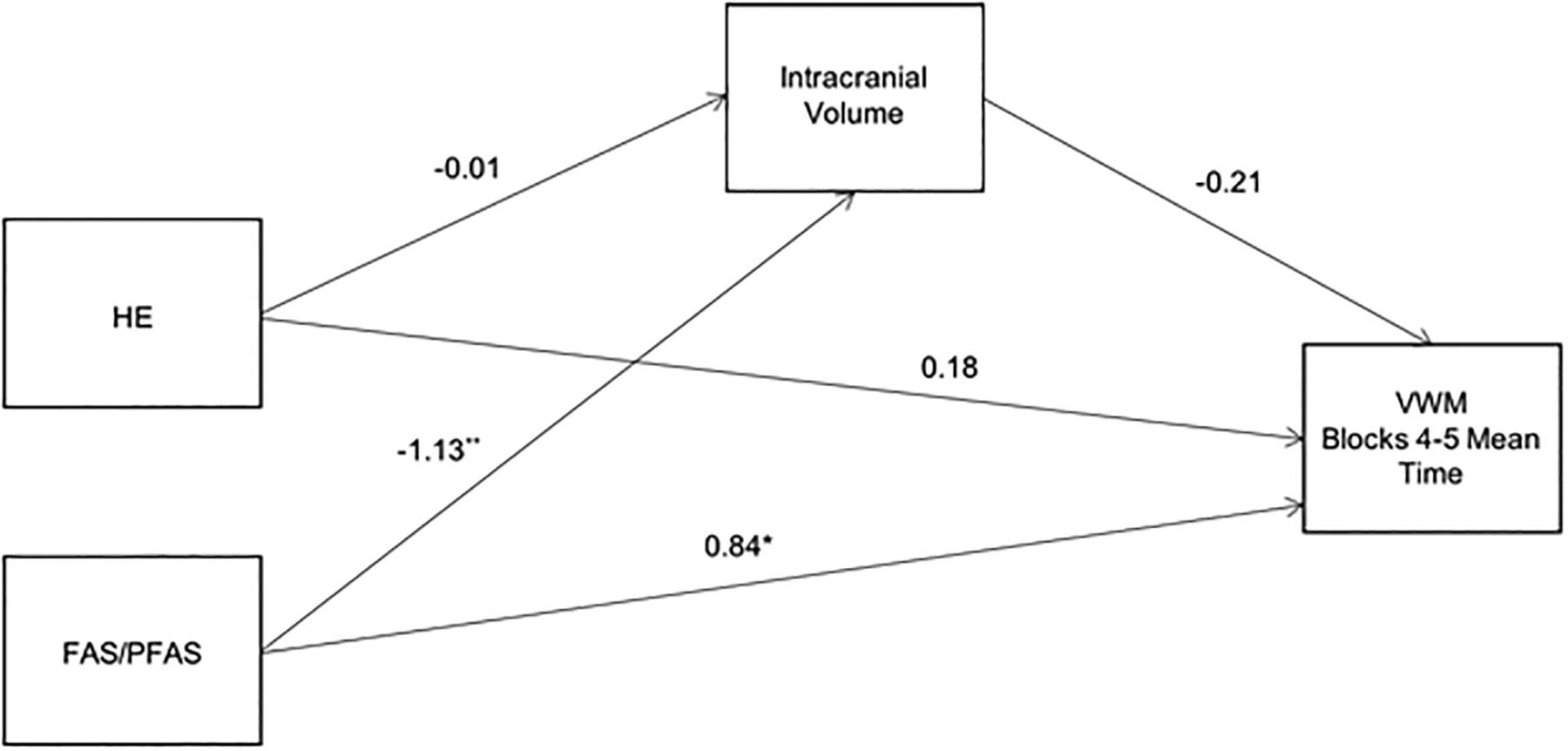
Path model showing mediation by intracranial volume (ICV) of the relation between diagnostic group and mean trial time during blocks 4 and 5 of the VWM. Values are standardized regression coefficients, adjusted for sex, that indicate by how many standard deviations the outcome changes by a unit change in the predictor. The control group was used as the reference group for FAS/PFAS and HE coefficients.
†p < 0.10 *p < 0.05 **p < 0.01
Figure 4 presents mediation by the right hippocampal volume of the relation between the PAE binge exposure measure (described in the Methods section) and VWM performance controlling for sex. The model accounted for 15.2% of the variance in the dependent variable (F(3,46) = 4.15, p < 0.05). The indirect path was significant, effect = 1.78 (95% CI = 0.21, 4.73). The same model was re-run using left hippocampal volume, but the indirect path was not significant, effect = 1.31 (95% CI = −0.02, 4.40). A second model testing ICV as a mediator was performed. The indirect effect through ICV was not significant, effect = 1.32 (95% CI = −0.05, 4.62), indicating that ICV did not mediate the relation between prenatal alcohol binge exposure and VWM performance.
Figure 4.
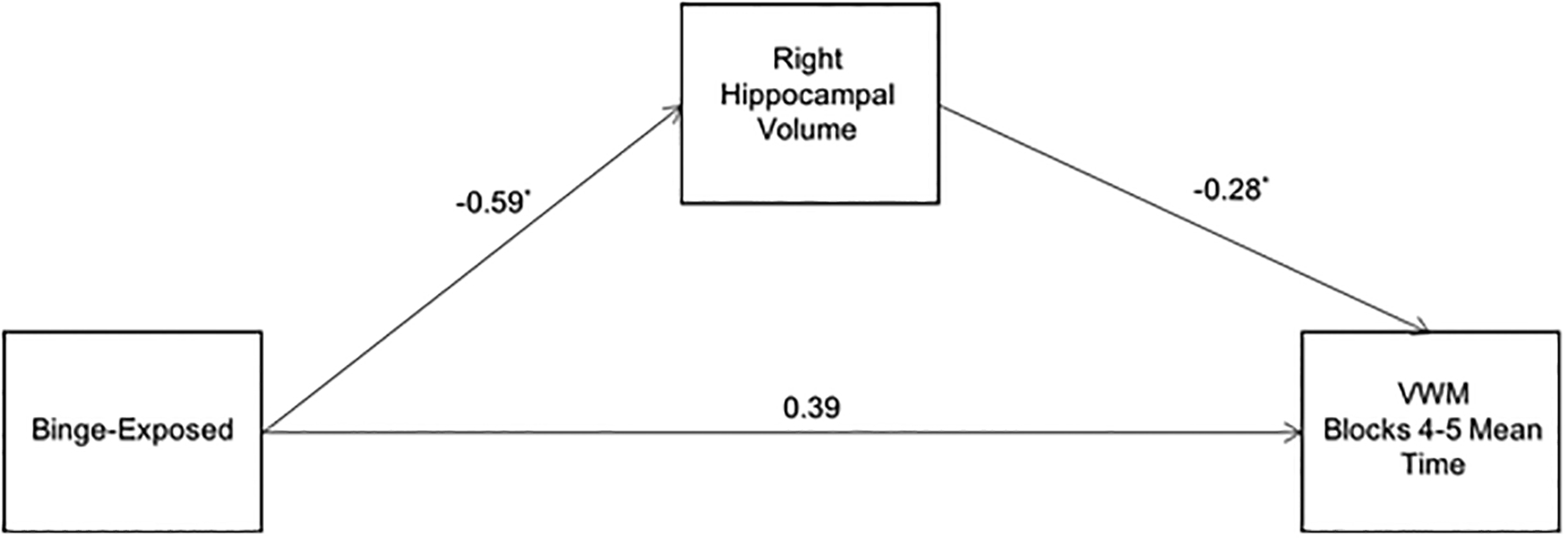
Path model showing the mediation by right hippocampal volume of the relation between prenatal alcohol binge exposure and mean trial time during blocks 4 and 5 of the VWM. Values are standardized regression coefficients, adjusted for sex, that indicate by how many standard deviations the outcome changes by a unit change in the predictor.
*p < 0.05
DISCUSSION
Although eight previous MRI studies have linked FASD diagnosis (Archibald et al., 2001; Astley et al., 2009; Coles et al., 2011) or the presence of PAE (Joseph et al., 2014; Nardelli et al., 2011; Riikonen et al., 2005; Roussotte et al., 2011; Willoughby et al., 2008) to reductions in hippocampal volume (Table 5), our study is the first to report inverse associations of hippocampal volume with both FASD diagnosis and continuous measures of PAE. While the exact mechanism of these fetal alcohol-associated hippocampal volume reductions are still unknown, laboratory animal studies have shown PAE-related reductions in both hippocampal cell count and cell density, which would be expected to contribute to reductions in hippocampal volume (Bonthius and West, 1990; Greene et al., 1992; Livy et al., 2003).
Table 5.
Summary of studies examining fetal alcohol spectrum disorders and hippocampal volume
| Persisted after ICV | N | Sex | Ethnic group | Age | Groups | MRI methods |
|---|---|---|---|---|---|---|
| Archibald et al. 2001 | 67 | Both | Not reported | 7–24 years | FAS vs. PAE vs. Control | Manual tracing |
| Nardelli et al. 2011 | 28 | Both | Mostly Caucasian, some aboriginal | 6–17 years | FASD vs. Controls | FreeSurfer |
| Willoughby et al. 2008 | 37 | Both | Not reported | 9–15 years | FASD vs. Controls | Manual tracing |
| Did not persist after ICV | ||||||
| Astley et al. 2009 | 81 | Both | Mostly Caucasian, some African American and Native American | M = 12, SD = 2 | FAS/PFAS vs. SE/AE vs. ND/AE vs. Controls | Manual tracing |
| Coles et al. 2011 | 92 | Both | Mostly African American | M = 21, SD = 2 | Dysmorphic vs. ARND vs. Controls | FreeSurfer |
| Joseph et al., 2014 | 21 | Both | Cape Coloured | M = 11.5, SD = 1.2 | FAS/PFAS vs. Controls | Manual tracing |
| Riikonen et al., 2005 | 22 | Both | Not reported | 5–16 years | FAS/FAE vs. Controls with indication for MRI | EasyMeasure |
| Roussotte et al. 2012 | 99 | Both | Multi-site study--Los Angeles and San Diego, mostly non-Hispanic Caucasian; South Africa site was Cape Coloured | M = 13; SD = 1.8 | FASD vs. Controls | FreeSurfer |
Three of the previous human studies have also provided evidence that the hippocampus is disproportionately smaller relative to total brain volume; that is after statistical adjustment for ICV (Archibald et al., 2001; Nardelli et al., 2011; Willoughby et al., 2008), but five others have not (Astley et al., 2009; Coles et al., 2011; Joseph et al., 2014; Riikonen et al., 2005; Roussotte et al., 2011). No apparent differences among these studies, including age, sex, ethnic group, or use of manual tracing vs. semi-automated or automated segmentation tools account for this discrepancy (Table 5). Our data are generally consistent with the studies showing disproportionate reductions in hippocampal volume, which we found in relation to both FASD diagnosis and continuous PAE measures, although this disproportionate reduction was statistically significant only for the right hippocampus after adjustment for ICV, falling short of conventional levels of significance for the left. Thus, although the link between PAE and reductions in overall brain volume has been demonstrated extensively in the FASD literature, the finding of a disproportionate reduction in hippocampal volume is somewhat less robust.
Previous studies in rodents (Morris et al., 1982; Moser et al., 1993; 1995; Riedel et al., 1999) and humans (Astur et al., 2002; Bohbot et al., 2007; Brown et al., 2014; Goodrich-Hunsaker et al., 2010; Hsu et al., 2000; Moffat et al., 2006; Parslow et al., 2004) have demonstrated that the hippocampus is critical for the encoding and retrieval of spatial relations needed for successful navigation based on distal cues. Our study adds to the literature in humans linking hippocampal volume to poorer performance during VWM acquisition trials but not during cued performance, when the platform is visible. Our findings of impaired place learning are consistent with the behavioral dissociation reported in rodents with hippocampal damage (Sutherland et al., 2001) and in animals exposed to alcohol during early brain development (Johnson and Goodlett, 2002). Given that much of the literature on the MWM and VWM has focused primarily on males, it is of interest that the effects of hippocampal volume on VWM performance were seen in both boys and girls and that the effects on the girls were seen more strongly earlier during acquisition, that is, during blocks 2–3 (Fig. 2).
The original Hamilton et al. (2003) study examined VWM performance in adolescents with FAS compared to controls. The finding of hippocampal volume reductions in the non-syndromal HE group indicates effects in children with heavy PAE who lack the overt dysmorphic features of FASD. Two prior studies also detected hippocampal volume reductions compared to non-exposed controls in individuals with FASD who lacked the physical dysmorphia of FAS or PFAS (Astley et al., 2009; Coles et al., 2011). However, two other studies did not detect hippocampal volume differences among the non-dysmorphic exposed group (Archibald et al., 2001; Nardelli et al., 2011). One possibility is that the lack of volumetric differences could be due to lower levels of exposure; however, exposure levels were not reported in those studies. The current study is also the first to report inverse associations between continuous measures of PAE and hippocampal volume.
Laboratory animal studies have reported that ethanol has adverse effects on the hippocampus and on the MWM assessment of hippocampal-sensitive spatial learning. To date, human studies have shown PAE effects on the hippocampus and on performance on the VWM. Our study is the first to demonstrate that effects of fetal alcohol-related volume reductions to the right hippocampus partially mediate the relation between FASD and spatial navigation on the VWM test. In addition, continuous measures of PAE were associated with smaller hippocampal volume, which in turn, mediated the PAE effects on place learning.
Whereas right hippocampal volume was a mediator when either FASD diagnostic group or binge exposure group was used as the independent variable, ICV was not a significant mediator of either effect on VWM performance, indicating that the observed mediation is not attributable to smaller overall brain size. These findings show that hippocampal volume partially mediates the effect of PAE on spatial navigation, although other brain regions that have also been found to be active during spatial navigation, such as the parahippocampal gyrus (Woods et al., 2018; Parslow et al., 2004) and frontal cortex (Hsu et al., 2000), likely also contribute to this effect.
Both left and right hippocampal volume predicted place learning, and volume reductions were detected in both hippocampi in relation to PAE. fMRI studies of navigation have consistently found bilateral activation of the hippocampus (Hsu et al., 2000; Maguire et al., 2008; Parslow et al., 2004). A more recent fMRI study demonstrated that activation in the right hippocampus predicted the use of allocentric navigation, that is, navigation based on the spatial configuration of environmental cues, whereas activation in the left hippocampus predicted the use of sequential egocentric representations, such as learning the sequence of direction changes needed to navigate to a goal (Igloi et al., 2010). Our finding that right hippocampal volume was more important than left in mediating the effect of PAE on the VWM is, therefore, not surprising, given that an allocentric navigation strategy is most efficient for learning the location of the hidden platform. Similar to the findings reported here, Coles et al. (2011) found that the right hippocampus volume primarily mediated the relation between the number of alcohol-related dysmorphic features and verbal and nonverbal recall. When our findings are considered together with those of Coles et al. (2011), prenatal alcohol-related insult to the hippocampus appears to impair not only place learning but also verbal and non-verbal learning, suggesting a general deficit in the encoding of information mediated by the hippocampus.
The limitations of this study are similar to those in other longitudinal studies of PAE. Error in the estimates of maternal alcohol consumption may obscure some associations. However, the validity of our alcohol use ascertainment techniques has been demonstrated in relation to fatty acid ethyl ester metabolites in meconium (Bearer et al., 2003). Additionally, the predictive validity of the timeline follow-back has been shown in relation to infant and child cognition (Jacobson et al., 2002, 2008; Lewis et al., 2016), somatic growth (Carter et al., 2016), and brain structure (De Guio et al., 2014; Fan et al., 2016; Jacobson et al., 2017; Meintjes et al., 2014) and function (Woods et al., 2015; 2018). Nutritional differences might have contributed to these effects, but alcohol consumption during pregnancy was not associated with differences in maternal diet or anthropometric measures in this population (Carter et al., 2017), indicating that poor nutrition among the drinkers is unlikely to play an important role in the extensively reported effects of PAE on growth and neurobehavior, including performance on the VWM. Regional brain volume can be considered a relatively crude measure of teratological insult in that it does not indicate whether the deficit in function is localized to the region in question, the neurocognitive functional networks it participates in, or both.
In summary, this study demonstrated that both FASD diagnosis and severity of alcohol exposure are related to reduced hippocampal volumes. In terms of FASD diagnosis, our data extend previous findings by showing that reduced hippocampal volumes are also seen in nonsyndromal HE boys. Hippocampal volume was also related to our continuous measures of PAE. These data confirm previous reports linking PAE to poorer spatial navigation on the VWM and are the first to provide direct evidence that volume reductions in this region partially mediate the relation of FASD diagnosis and PAE to place learning, suggesting that PAE specifically impairs the ability to encode the spatial information necessary for successful location of the hidden platform on a navigation task. These findings add to the growing body of evidence suggesting that hippocampal damage plays an important role in the teratogenic effects of PAE.
Acknowledgments:
First of all, we thank Derek Hamilton, who generously provided his computer-simulated virtual water maze (VWM) task to us for use in our FASD research. We thank the Cape Universities Brain Imaging Centre (CUBIC) radiographers Marie-Louise de Villiers and Nailah Maroof; and research staff, Maggie September, Mariska Pienaar, and Renee Sun, for their contributions to recruitment and data collection. We thank H. Eugene Hoyme, M.D., and Luther K. Robinson, M.D., who conducted the Cape Town FASD diagnostic dysmorphology examinations. We appreciate the consultation by Andre van der Kouwe, Ph.D., regarding preparation of the brain images included in this paper. The VWM study was conducted as part of the doctoral dissertation of Neil Dodge. Thanks to Ty Partidge, Ph.D., and John Hannigan, Ph.D., for their input as members of Dr. Dodge’s dissertation committee. We also thank the mothers and children for their participation. Portions of this research were presented at the 2016 meetings of the Research Society on Alcoholism. Funded by the National Institute on Alcohol Abuse and Alcoholism (R01AA016781, U01AA014790, U01AA014809, U24AA014815); a Fogarty International Research Collaboration Award (R03TW007030); a Wayne State University Children’s Bridge Program Research Enhancement Grant; a Focus Area grant (FA2005040800024) from the National Research Foundation of South Africa, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, the Medical Research Council of South Africa; and the Lycaki-Young Fund from the State of Michigan.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Annett M (1970) A classification of hand preference by association analysis. Br J Psychol 61:303–321. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL (2001) Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol 43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T (2009) Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 33:1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ (2002) Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res 132:77–84. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CD, Viljoen DL, Marais AS, Chiodo LM, Cwik AS (2003) Validation of a new biomarker of fetal exposure to alcohol. J Pediatr 143:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH (2000) Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 10:94–110. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Riley EP, Hannigan JH (1987) Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicol Teratol 9:253–258. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel LY (1998) Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36:1217–1238. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP (2007) Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci 27:10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR (1990) Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res 14:107–118. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR (1975) Measurement and interpretation of drinking behavior. J Stud Alcohol Drug 36:1154. [DOI] [PubMed] [Google Scholar]
- Brown TI, Whiteman AS, Aselcioglu I, Stern CE (2014) Structural differences in hippocampal and prefrontal gray matter volume support flexible context-dependent navigation ability. J Neurosci 34:2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW (2016) Fetal alcohol growth restriction and cognitive impairment. Pediatr 138:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RC, Senekal M, Dodge NC, Bechard L, Meintjes EM, Molteno CD, Duggan C, Jacobson JL, Jacobson SW (2017) Maternal alcohol use and nutrition during pregnancy: Diet and anthropometry. Alcohol Clin Exp Res 41:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X (2011) Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn 75:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guio F, Mangin JF, Riviere D, Perrot M, Molteno CD, Jacobson SW, Meintjes EM, Jacobson JL (2014) A study of cortical morphology in children with fetal alcohol spectrum disorders. Human Brain Mapp 35:2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Thomas KGF, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW (2019) Spatial navigation in children and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 43:2536–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Jacobson SW, Taylor PA, Molteno CD, Dodge NC, Stanton ME, Jacobson JL, Meintjes EM (2016) White matter deficits mediate effects of prenatal alcohol exposure on cognitive development in childhood. Human Brain Mapp 37:2943–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, West JR (1987) Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiol 15:64–74. [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO (2010) Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus 20:481–491. [DOI] [PubMed] [Google Scholar]
- Greene PL, Diaz-Granados JL, Amsel A (1992) Blood ethanol concentration from early postnatal exposure: Effects on memory-based learning and hippocampal neuroanatomy in infant and adult rats. Behav Neurosci 106:51. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD (2003) Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res 143:85–94. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Preacher KJ (2014) Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol 67:451–470. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (2011) Four factor index of social status. Yale J Sociol 8:21–51. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK (2005) A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatr 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Ryan L, Nadel L, Thomas K, Jacobs WJ (2000) Functional neuroimaging of place learning in a computer-generated space. Neuroimage 11:S441. [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD (2003) Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci 23:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N (2010) Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Nat Acad Sci 107:14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL (2002) Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr 109:815–825. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2008) Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res 32:365–372. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL (2011) Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res 35:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Molteno CD, Warton C, Wintermark P, Hoyme HE, De Jong G, Taylor P, Warton F, Lindinger NM, Carter RC (2017) Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res 41:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR (2002) Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposre in male rats. Alcohol Clin Exp Res 26:83–93. [PubMed] [Google Scholar]
- Joseph J, Warton C, Jacobson SW, Jacobson JL, Molteno CD, Eicher A, Marais P, Phillips OR, Narr KL, Meintjes EM (2014) Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders. Human Brain Mapp 35:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR (1988) Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res 27:247–257. [DOI] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S (2017) Global prevalence of fetal alcohol spectrum disorder among children and youth: a systematic review and meta-analysis. JAMA Pediatr 171:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER (2011) Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev 21:102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KGF, Molteno CD, Kliegel M, Meintjes EM, Jacobson JL, Jacobson SW (2016) Prospective memory impairment in children with prenatal alcohol exposure. Alcohol Clin Exp Res 40:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR (2003) Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol 25:447–458. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J (1998) Knowing where and getting there: a human navigation network. Science 280:921–924. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S (2013) Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res 37:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H (2018) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Pirnia T, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW (2014) A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure. Neuroimage Clin 5:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM (2006) Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging 27:965–972. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683. [DOI] [PubMed] [Google Scholar]
- Morris RGM (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth 11:47–60. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995) Spatial learning with a minislab in the dorsal hippocampus. Proc Nat Acad Sci 92:9697–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C (2011) Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35:1404–1417. [DOI] [PubMed] [Google Scholar]
- Parslow DM, Rose D, Brooks B, Fleminger S, Gray JA, Giampietro V, Brammer MJ, Williams S, Gasston D, Andrew C, Vythelingum GN, Ioannou G, Simmons A, Morris RG (2004) Allocentric spatial memory activation of the hippocampal formation measured with fMRI. Neuropsychol 18:450. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Roberts AD, Good M (1998) Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature 396:75–77. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AGM, Roloff EVL, Martin SJ, Bridge H, Morris RGM (1999) Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature Neurosci 2:898–905. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, Valkonen K, Kolehmainen AI, Kumpulainen KI, Könönen M, Vanninen RS, Kuikka JT (2005) Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: a study with nor-β-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatr 57:1565–1572. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O’Connor MJ, Bookheimer SY, Sowell ER (2011) Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage 54:3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM (1992) Assessment of Children, 3rd edition San Diego, CA: Jerome M. Sattler, Inc. [Google Scholar]
- Sobel ME (1982) Asymptotic confidence intervals for indirect effects in structural equation models In Leinhart S (ed.), Sociological Methodology, San Francisco: Jossey-Bass, pp. 290–312. [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O’Keefe J (2001) Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124:2476–2489. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS Hanlon FM, Koerner A (2001) Retrograde amnesia after hippocampal damage: recent versus remote memories in two tasks. Hippocampus 11:47–62. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J (2008) Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14:1022–1033. [DOI] [PubMed] [Google Scholar]
- Woods KJ, Meintjes EM, Molteno CD, Jacobson SW, Jacobson JL (2015) Parietal dysfunction during number processing in children with fetal alcohol spectrum disorders. Neuroimage Clin 8:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KJ, Thomas KGF, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM (2018) Sex differences in prenatal alcohol related alterations in brain function during place learning in a virtual environment. Brain Behav e01103. [DOI] [PMC free article] [PubMed] [Google Scholar]


