Abstract
Cervical cancer is the fourth most common type of cancer incidence in the world female population, and it has become a public health problem worldwide. Several factors are involved in this type of cancer, including intrinsic factors related to the inflammatory process, such as extracellular nucleotides and adenosine—components of the purinergic system. The present review focuses on the role of the purinergic system in cervical cancer, especially regarding the interaction of extracellular nucleotides with their respective receptors expressed in the tumor microenvironment of cervical cancer and their role in the host immune response. The high concentrations of extracellular nucleotides in the tumor microenvironment of cervical cancer interfere in the regulation, proliferation, differentiation, and apoptosis of cancer cells of the uterine cervix through different P1 and P2 receptor subtypes. Such diverse cellular processes that are mediated by adenosine triphosphate and adenosine across the tumor microenvironment and that also have effects on host immune defense will be reviewed here in detail.
Keywords: Purinergic receptors, Ectonucleotidases, Cervical cancer, Human papillomavirus
Introduction
Cervical cancer (CeCa) is the fourth most prevalent cancer in the female population worldwide, representing more than 300,000 deaths per year [1]. The development of CeCa occurs mainly due to the persistent infection of one of the carcinogenic types of human papillomavirus (HPV) [2, 3], which leads to the disruption of mechanisms that are involved in differentiation and programmed cell death. Such dysfunctional mechanisms are also related to tumor progression [4]. Intraepithelial lesions appear at first, throughout the cycle of viral replication and the process of cell differentiation. These lesions are called intraepithelial cervical neoplasms (CINs). These pre-cancer stages may later develop into an invasive tumoral process [5]. CINs classify into different cyto-histopathological grades based on the severity of the cellular anomalies, and their severity levels are subdivided into CIN I, CIN II, and CIN III [6].
Recently, the purinergic system has been associated with an essential signaling pathway related to tumor cell progression [7]—interfering with mechanisms such as disordered cell proliferation, promotion of angiogenesis, and failure of mechanisms controlling apoptosis [8]. These processes occur due to the nature of tumor environment that presents an unbalance in the concentrations of adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) nucleotides and adenosine (Ado) nucleoside, as well as overexpression or, in some cases, the low expression of P2 receptors and ectonucleotidases [9].
Tumor microenvironment (TME) of CeCa is rich in ATP and Ado [10]—suggesting that signaling and the purinergic pathway play an important role concerning the mechanisms that control cell growth and death in cancer [11]. These molecules—which typically exhibit opposite effects—are involved in purinergic signaling in several biological processes, such as proliferation, cell differentiation and growth, apoptosis, and mediation of immune responses [7, 12–14]. Nevertheless, regarding the apoptotic effect induced by these molecules, extracellular ATP has a minor effect on mechanisms that lead to programmed cell death on CeCa cancer cells when compared to Ado, formed by the degradation of ATP by ectonucleotidases. Ado acts as the main responsible for inducing apoptosis in these cells. If the inhibition of the conversion of Ado into AMP occurs, this will result in the inhibition of cytotoxic effects, indicating that Ado—originated from extracellular ATP—is the leading promoter of apoptosis in CeCa cells [12]. Ado is also a significant determinant of the immunosuppressive tumor milieu [15].
Indeed, purinergic signaling and its purinergic receptors have become extensively implicated in many mechanisms, including immune responses, inflammation, and platelet aggregation, as well as cell proliferation disorders, differentiation, and death—associated with tumor growth [16]. Therefore, this review will discuss the implication of purinergic signaling and specific subtypes of receptors in the pathophysiology of the CeCa. It will emphasize the most recent findings in this field and focus on the integration of purinergic signaling and its components with acknowledged oncogenic mechanisms.
Human papillomavirus and cervical cancer
Most HPV-associated infections are treated spontaneously by the immune system; however, if the cellular defense mechanism is ineffective, the infection may persist as an oncogenic process [17]. Currently, specific HPV genotypes are widely accepted as potential oncogenic factors and are associated with most cases of CeCa [18]. Besides, the endurance of infection due to HPV is the main factor in the development of CeCa [19]. High-risk HPV types—predominantly type 16, 18, 31, 33, and 35—have been associated with most CeCa and pre-cancer stages [20, 21] since they lead to genetic instability through integration with the cellular genome [22]. This instability is related to the action of E6 and E7 oncoproteins [5]. Such proteins participate in mechanisms that lead to damage to genetic material and mitotic process disorder [6, 22, 23] and have as their primary goal, during the HPV cycle of infection, the generation of a permissive microenvironment for viral replication [24]. During persistent high-risk HPV infection, the genetic material can suffer mutations not only under the effect of abnormal cellular action but also through other environmental factors [6, 20]. As viral DNA integrates with the genetic material of host cells, HPV manages to escape the cell and immune defense; at the same time, it promotes mechanisms that favor cell proliferation and inhibit apoptosis [3, 6, 22].
The exact mechanism the virus uses to alter the biology of infected cells is not yet precise to researchers. However, it is known that the inflammation-mediated DNA damage often precedes the genomic abnormalities caused by HPV infection, suggesting that the development of an inflammatory condition can be a potential mechanism that facilitates the integration of the viral genome by inducing breaks in both the viral and host genomes [25]. The HPV virus can also regulate the expression levels and activities of the surface proteins of the cells [26], which ultimately destroys the balance of the ectocervical epithelium [27]. Hence, CeCa cells express larger or smaller amounts of purinergic receptors that interact with the specific TME that forms along with HPV infection and early tumor development [27–30].
High-risk HPV infections also boost the production of immunosuppressive cytokines, such as interleukin 10 (IL-10) and transformer growth factor β (TGF-β) [31, 32]. These cytokines promote the downregulation of human class I leukocyte antigen system (HLAI), which serves as an immune evasion mechanism by damaged and infected cells. The downregulation of HLAI is, accordingly, significantly associated with the downregulation of several antigen processing machinery components. The decrease in HLAI and antigen processing machinery, promoted by the persistent infection of high-risk HPV types, ultimately leads to immune evasion by cervical carcinoma [33]—with leads to tumor proliferation [34–36] and interferes with the development of immunotherapies and cancer vaccines [33]. The high levels of TGF-β in TME also stabilize hypoxia-inducible factor 1α (HIF1α) [15]. HIF1α induces the expression of the enzymes diphosphoidrolase-1 triphosphate (CD39) and ecto-5′-nucleotidase (CD73), ectonucleotidases which are components of the purinergic system and are responsible for keeping TME rich in Ado [37–39].
Purinergic signaling and cancer
Only in 1972, nucleotides and nucleosides were understood to have distinct functions and roles depending on whether they were in the intracellular environment—where they have a role in cellular metabolism and as a source of cellular energy—or in the extracellular environment—where they act as molecular mediators of cellular signaling. However, until the early 1990s, this concept was not well accepted [40–42]. Currently, it is known that the components of the purinergic system act not only as neurotransmitters in the nervous system but are as well able to trigger several critical cellular processes, such as stimulation or inhibition of programmed cell death, proliferation, migration, and cell differentiation. They also act in the secretion of growth factors and inflammatory mediators [41, 43]. Hence, the purinergic system and its components are regularly associated with tumor progression and cancer-related immune responses [15, 44]. The physiological response of these molecular components depends on the number of purinergic receptors expressed in the plasma membrane in the cells that constitute the TME rich in ATP and Ado and the enzymatic activity performed by ectonucleotidases—enzymes responsible for the hydrolysis of extracellular nucleotides in nucleosides [45].
Purinergic receptors can be classified as P1 or P2 receptors. P1 receptors (coupled protein G), are purinergic receptors that mediate the biological actions of Ado. There are four distinct subgroups of P1 receptors—A1, A2A, A2B, and A3—and each subgroup has different affinities when interacting with Ado [10, 46, 47], whereas type P2 receptors, which recognize ATP as a principal agonist, are divided into metabotropic, P2YR, and ionotropic, P2XR, receptors. P2X receptors (P2XR) depend on the opening of ion channels and have seven different receptors subtypes (P2X1-7R) [9, 48]. P2Y receptors (P2YR) coupled to G protein, in turn, fall into eight types (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R) [41, 49]. P2Y receptors, besides ATP, can be activated by alternative agonist binders such as ADP, UTP, UDP, and UDP glucose [50]. Thus, high levels of nucleotides and nucleosides located outside the cell compartment can trigger several cellular mechanisms and events, while they also modulate the pathophysiological processes involved in the development and manifestation of CeCa [48, 51, 52].
Among the extracellular purines that are released in TME by the injured cells, extracellular ATP has different roles depending on its concentration on the tumor site and the P2 receptor subtypes expressed by the immune and cancer cells [10]. The high concentration of ATP in TME is mainly due to the regulated efflux of ATP—which can be stimulated by chronic inflammation, hypoxia, and ischemia—or by injury to the plasma membrane due to necrosis, apoptosis, or mechanical stress. Accumulated ATP can either trigger P2 purinergic receptors (P2XRs and P2YRs) or can be further degraded to adenosine by the sequential action of CD39 and CD73 ectonucleotidases [7, 53–55]. Extracellular ATP in TME behaves as a danger-associated molecular pattern (DAMP) and promotes both innate and adaptive immune responses. Alternatively, it also stimulates the development of both endothelial and tumor cells through interaction with P2 receptors expressed in these cells [15, 55–57].
Concurrently, as soon as extracellular ATP accumulates in TME, it is degraded quickly into AMP by CD39 ectonucleotidase activity. Then, CD73 ectonucleotidase allows for the dephosphorization of AMP, which leads to the formation of Ado. The catabolism of extracellular ATP is, therefore, the leading cause of the high levels of Ado in TME [15, 53, 57–59]. High levels of extracellular Ado potentially interact with P1 receptors, which can induce tumor angiogenesis, immune suppression, and metastasis. Ado’s accumulation at TME is, therefore, an essential mechanism for promoting tumor progression [54–56].
Purinergic signaling presents itself as an essential factor in the control of growth, survival, and tumor progression. Not only does it act directly on injured cells in TME, but it also serves as a modulator of the immune system through the mediation of interactions between cancerous cells and immune cells of the host [55]. Therefore, the purinergic constituents involved along the development and progression of tumors represent potential targets in the development of new antitumor therapies [48, 55, 60].
The physiological role of extracellular nucleotides in the cervix
The epithelium that covers the woman’s cervix serves primarily to regulate the lubrication of the genital canal and supply the necessary conditions for reproduction. The adequate functioning of the epithelium relies on the coordinated proliferation of a basal layer of cells, and the death of cells in the upper layer [27, 61, 62]. The cervical epithelium structures itself in confluent cell layers that limit the free flow of fluids and solutes from the blood into the lumen [62]. Moreover, healthy cervical cells usually exhibit a high level of permeability, which indicates that transcervical transport occurs mainly via the paracellular pathway [61].
The effects of ATP on the cervix occur through P2 receptors located on the surfaces of apical cells found in the lumen [61, 63–65]. ATP, present in nanomolar and micromolar concentrations in the extracellular environment of the cervix, actively regulates paracellular transport along the cervix epithelium when interacting with P2Y2 and P2X4 receptors [30, 66]. Cultures of human endocervical and ectocervical cells also express P2X7R. Such receptors, when activated, lead to the regulation of terminal differentiation and apoptosis of endocervical and ectocervical epithelial cells [27]. P2X7R also plays an essential role in protecting the cervix from infections, as was observed in a study conducted by Darville et al. [67], which showed that P2X7R activation induces the inhibition of chlamydia infection. Another study conducted by Stahl et al. [62] demonstrated that the P2X7 receptor, expressed in cervical cells, is upregulated under the inflammatory conditions found during microbial infection, thus helping to amplify inflammatory responses against cervix infection.
High concentrations of ATP in the tumor microenvironment of cervical cancer
P2X7 receptor and cancer
Among purinergic receptors, the P2X7R is continuously implicated in cytotoxicity situations [14]. P2X7R is an ionic channel encoded by the P2rx7 gene located on chromosome 12 (12q24.31) [68, 69]. These types of receptors are the most sizeable proteins of the P2X receptor family, as they present a protein structure composed of over 595 amino acids. Three identical (homotrimeric) subunits form P2X7R or, under certain conditions, can be found in the form of homohexamers [14].
Depending on the cell type and the level of interaction with agonists, P2X7R receptors have different physiological functions. Therefore, P2X7R may trigger cell death or stimulate proliferation, as well as immunosuppression or stimulation of the immune response in different situations [15]. P2X7R is expressed in both normal and cancerous cervical cells—although the expression of P2X7R in CeCa cells is considerably lower in cancer and pre-cancerous epithelial cells when compared to normal cells [70–72]. The activation of the P2X7R is associated with extreme cellular events such as increased plasma membrane permeability and membrane blebbing, phosphatidylserine exposure, cellular edema formation, and loss of mitochondrial potential [69, 73]. Thus, when activated, P2X7R promotes and stimulates purinergic signaling and inflammatory conditions [74]. The lower expression of P2X7R is associated with the development of CeCa; ergo the effect of substances that perform as agonists on these receptors could work as potential anti-metastatic agents and a therapeutic alternative for CeCa [16].
Extracellular ATP—abundant in TME and the endogenous agonist for the P2X7R [7, 75]—promotes the flow of Na+, K+, Ca+2, and other cations—by triggering P2X7R [76]. However, situations in which extracellular ATP interacts with P2X7R for long periods ultimately result in the opening of non-selective and irreversible macropores in the plasma membrane. The cell membranes then become permeable to molecules with a molecular mass of up to 900 Da (Fig. 1) [77]. The development of these macropores in the membranes of cervical cells, due to the interaction with extracellular ATP for a long time, is associated with the progression of cervical cell apoptosis [78].
Fig. 1.
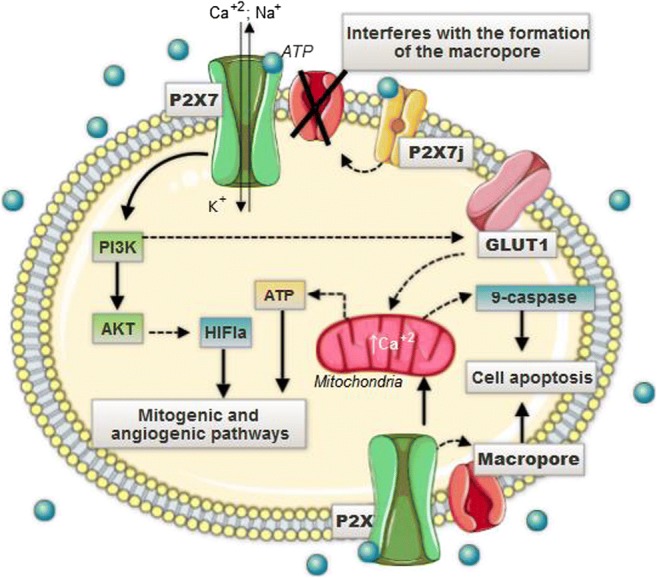
The high concentration of extracellular ATP, characteristic of the microenvironment of CeCa, activates the P2X7 receptor, which triggers the increase of Ca2+ inflow. Nonetheless, situations in which the extracellular ATP interacts with the P2X7 for long periods cause the appearance of non-selective and irreversible pores that allow up to 900-Da molecules to pass through. The opening of such pores would collaborate for intracellular ionic deregulation and, consequently, for the apoptotic process. The influx of Ca2+ in these conditions would induce the activation of the mitochondrial caspases pathway, whose function is to conduct the apoptotic process, and the GLUT1 upregulation, related to the increase in oxidative phosphorylation and the consequent production of EROS. The result of these factors is, therefore, the increased ATP production, decreased pH, and, finally, cell apoptosis tumoral cervicals through immunosuppression generated by extracellular ATP and its activation with P2RX7. Cancerous cells of the uterine cervix, on the other hand, express a P2X7j variant that affects the formation of non-selective pores, decreasing cell permeability and cellular death, which facilitates the widespread proliferation of cells
P2X7 when activated promotes the disruption of the inner potential mitochondrial transmembrane due to hyper-oxidation and increased intracellular Ca+2. As a result, mitochondrial pores appear. Apoptosis in human cervical epithelial cells and CeCa cells, for that matter, involves calcium-induced mitochondrial dysfunction [27]. The increase in the concentration of Ca+2 mitochondrial, via the P2X7R, leads to a higher intracellular ATP generation and metabolites for the synthesis of cellular structures—through the increase of the oxidative phosphorylation (Fig. 1) [79].
P2X7R, when activated, triggers the phosphatidylinositol-3-OH kinase (PI3K)/protein kinase B (Akt) pathway, which leads to inadequate oxygen supply to tumor cells based on HIF1α signaling. HIFα is responsible for promoting mitogenic and angiogenic pathways [7]. Such events favor the biochemical composition of the TME and the progression of cancerous cells [80]. Concurrently, the activation of P2X7R boosts the expression of the glucose transporter 1 (GLUT1)—overexpressed in CeCa [81]—takes possibly via the PI3K-AKT pathway (Fig. 1). Overexpression of GLUT1 provides either ATP or metabolic intermediates for the synthesis of cellular structural constituents. In addition, it leads to a decrease in pH and consequent immunosuppression of the TME of CeCa [7]. The P2X7R, given its importance, for that matter, is a pharmaceutical target for the prevention and treatment of epithelial neoplasia due to its ability to induce apoptosis [82].
The P2X7j variant in cervical cancer cells
P2X7R, as discussed, is capable of inducing programmed cell death both in normal cervical cells and in tumor cells of the cervix; however, such effects are more prevalent in non-tumor cells, when compared to cancer cells, indicating that tumor cells of the cervix contain intrinsic defense mechanisms against apoptotic pathways mediated by P2X7 activation. One of these tumor protection mechanisms may be associated with a variant of the P2X7 receptor, which is called P2X7j [71, 83]. The P2X7j receptor is a protein of 258 amino acids that is characterized by not presenting the intracellular C-terminal part and not presenting the second transmembrane domain, as well as the distal third of the extracellular loop—which are present in the structure of the P2X7 receptor [71, 84]. This variant P2X7j, when interacting with P2X7, not only affects the formation of pores and disturbs the permeability of cell membranes, but also interferes with the induction of cell death that is mediated by P2X7 [27]. The expression of P2X7j in tumor cells of the cervix seems to favor the uncontrolled growth of abnormal cells in the female genital tract since it prevents programmed cell death (Fig. 1) [71].
The effect of 17β-estradiol on apoptosis induced by P2X7
Studies conducted by Wang et al. [27] and by Gorodeski [85] demonstrated that estrogen acts as an anti-apoptotic factor in cancer cells of the uterine cervix. This mechanism occurs by blocking the influx of Ca+2 after the activation of P2X7R. As a result, it was observed that in the healthy cells of the human cervical epithelium (hECEs) lineage and cell lines infected by HPV carcinogenic types, such as CaSki, the action of estrogen blocked the activation of P2X7R.
Moreover, the anti-apoptotic effect of estrogen was not observed in SiHa and HeLa cell lines. For this reason, the author suggests that these cells may have evolved mechanisms of resistance to the described anti-apoptotic action [27, 85]. However, these results imply that the level of estrogen, the age of the woman and menopause, do not influence the expression of P2X7R in healthy tissues and tumors—since these effects would be associated with the modulation of events distant from the site of expression of these receptors [72]. Therefore, there is a need for additional research that relates estrogen to the apoptotic mechanism of P2X7, in order to clarify the possible mechanisms that exist in the action of this essential female hormone with CeCa.
P2Y receptors and the tumor microenvironment of cervical cancer
Extracellular ATP can also activate some purinergic P2Y receptors (P2YR). Bukhari et al. conducted a study in cancer cell lines of the uterine cervix (CXT cell), where it was noted that ATP, through interaction with specific P2YR, was able to permeabilize the plasma membrane of CXT cell to cytotoxic polyatomic cationic substances—such as Hoechst 33258 (Pibenzimol). The initial hypothesis of the study was that Hoechst 33258 would enter the cells through P2X7R—which are permeable to larger cationic substances. However, it was evidenced that, in CeCa cells, cationic cytotoxins are not transferred to the intracellular medium by this process [43]. That is because, as previously exposed, CeCa cells present a P2X7j variant that interacts with the P2X7R and disturbs the permeability of the cell membrane [77, 86]. Nevertheless, these substances can still penetrate the plasma membrane of CeCa cells through P2YR activated by extracellular ATP [86].
P2YR, coupled to protein G, can trigger the phospholipase C (PLC) pathway, that is responsible for forming inositol 1,4,5-triphosphate (InsP3) [49], a substance associated with the formation of diacylglycerol (DAG)—an endogenous activator of protein C kinases (PKCs) [87, 88]. The formation of InsP3, through the activation of the PLC, occurs from the binding of extracellular nucleotides with P2YR which, subsequently, increases the concentration of cytoplasmic calcium of adjacent and non-adjacent cells to those initially stimulated—this increase in intracellular Ca+2 takes place at several intensities, according to which extracellular nucleotide sensitized the P2YR [89, 90].
Okuda et al. [89] conducted a study which showed that HeLa cells —he first line of laboratory-cultured cells that were originated from an adenocarcinoma of the cervix [91]—presents oscillations in the concentration of intracellular Ca+2 ions when submitted to specific concentrations of ATP, UTP, UDP, and ADP. These variations in concentration were expressed with different intensities in comparison to the type of nucleotide and dosage used. The study also demonstrated that the oscillation pattern has shifted over time. After a long period of perfusion, the sustained phase of calcium influx was suppressed. A reason for the variation in oscillations may be due to the desensitization of purinergic receptors. Another possibility could be the intracellular pathway desensitization, given that the binding of nucleotides to P2YR activates a G protein, which activates the PLC and IP3 pathways, which are responsible for the liberation of Ca+2 [89].
The ATP and UTP nucleotides, in a study conducted by Muscella et al. [92], showed similar potential in the promotion of intracellular calcium through activation of P2Y2R. Moreover, ATP and UTP, in another research conducted by Muscella et al. [87], have also shown an inhibitory effect on the activity of Na+/K+ATPase via activation of PKC-α, PKC-β, and ε. The enzyme Na+/K+ATPase is involved mainly in the regulation of the electrochemical gradient through the plasma membrane; however, recent studies have also associated it as an essential factor of initiation, growth, and development of cancerous cells through numerous regulatory pathways of cell maintenance and death [93]. The inhibition of Na+/K+ ATPase activity by ATP and UTP, via P2Y2, consequently, corroborates the tumor progression [94]. Moreover, when activated, the P2Y2R takes part in the process of cellular proliferation through pathways that involve mitogen-activated protein kinases (MAPKs) (Fig. 2) [92].
Fig. 2.
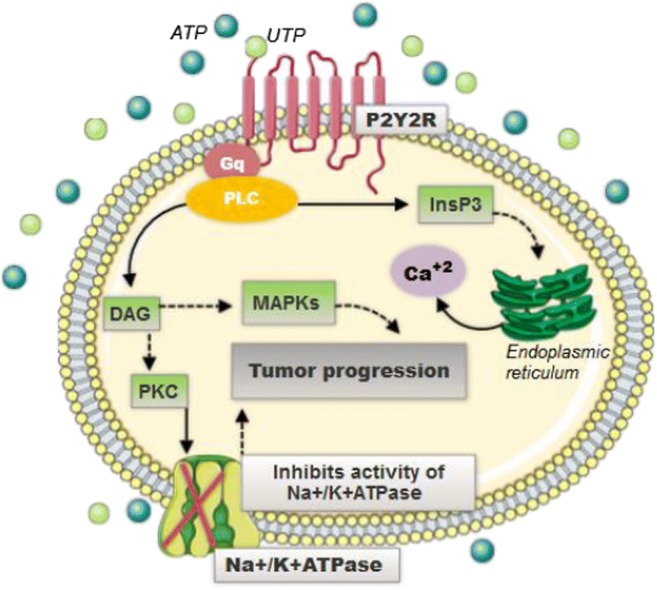
P2Y2 receptors are coupled to G protein and activate the PLC pathway. The PLC leads to the formation of InsP3, which in turn leads to the release of intracellular Ca+2. When the PLC is activated, it also causes the generation of DAG, which in turn is a PKC activator that promotes the inhibition of the Na+/K+ATPase activity. Besides, the P2Y2 receptor also activates the MAPK pathway. All these intracellular effects caused by the activation of P2Y2 are promoters of tumor progression
Buvinic et al. [95] highlighted in his research that P2Y1R can mediate mitogenic signals of endogenously released nucleotides in HeLa cells, through transactivating the epidermal growth factor receptor (EGFR) pathway. The activation of the EGFR pathway mediated by the interaction of agonists with P2Y1R supports the understanding that extracellular nucleotides in the TME constitute powerful mitogenic signals. EGFR consists of a complex network of protein interactions that works as a control system for cell proliferation and differentiation. Mutations in the EGF complex occur in association with carcinogenic development [96]. Thus, P2Y1R, when activated, promotes the establishment of a state mediated by highly proliferative EGFR, together with an increase in the expression of EGFR (Fig. 3) [95].
Fig. 3.
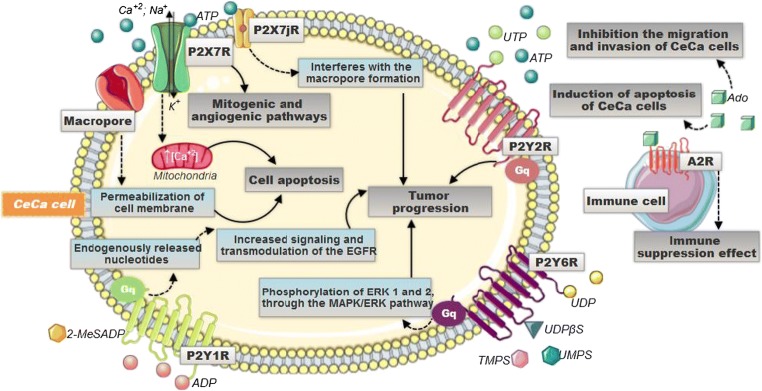
Different mechanisms are triggered when stimulated by the different nucleotides in the CeCa TME. P2X7R, when activated, promotes an increase in intracellular ATP production and, via PI3K-AKT, through the signaling dependent on HIF1α, triggers mitogenic and angiogenic pathways, stimulating tumor progression. P2Y-type receptors are coupled to protein G and activate the PLC pathway. When the P2Y2R is activated, it leads to an increase in cell proliferation, while the P2Y6R, through the MAPK/EK1,2 pathway, also participates in processes that promote cellular proliferation. On the other hand, P2Y1R mediates the mitogenic signals of endogenously released nucleotides through transactivating the epidermal growth factor receptor (EGFR) pathway—which is related to cell differentiation and proliferation and, ultimately, also promotes tumor progression. Adenosine, present in high amounts in the TME, leads to the activation of A2a receptor in T lymphocyte cells, which promotes the decrease of proliferation, activation, and effector function of these cells
Throughout the proliferation process, there is an increase in the expression of P2Y4 and P2Y6 receptors during the proliferation of CeCa cells [44]. These receptors are activated respectively by UTP and UDP [49]. P2Y6 receptors present in HeLa cells are activated by UDP or by molecules analogous to thymine 5′-O-monophosphorothioate, as demonstrated by Gendaszewska-Darmach and Szustak [97]. They also are related to the stimulation of cellular proliferation involving phosphorylation pathways of kinases regulated by extracellular signals (ERKs) 1 and 2, through the MAPK/ERK pathway and the expression of the c-Fos protein (Fig. 3) [98]. In some circumstances, these properties can be very advantageous, especially for the induction of long-term effects of cellular proliferation and migration mechanisms that occur in TME [97].
Hydrolysis of ATP and platelet aggregation
In a clinical study carried out on platelets of CeCa patients, Maldonado et al. [28] demonstrated that the balance of the components of purinergic signaling suffers changes in different degrees of CINs and in cervical carcinoma stage. It was noted that ADP and AMP are more sensitive to the stage of cancer when compared to ATP since the hydrolysis rates of these two nucleotides suffered more significant changes between the groups observed. For ATP, results showed that there was a gradual decrease in the hydrolysis activity of this nucleotide between the groups of CINs until the cancer stage.
A decrease in the rate of ATP hydrolysis was observed in patients with CIN I, CIN II, and CIN III, and in patients allocated to the cancer group compared to the results obtained with individuals in the control group. This ATP hydrolysis decrease occurs due to lower enzymatic activity of CD39. According to the authors, this is a form of defense strategy against uncontrolled platelet aggregation, since ADP—which is the immediate product of ATP—acts as a facilitator of platelet aggregation [28]. However, despite the authors’ conclusions that ATP would function as an inhibitor of ADP interaction with receptors present in platelets, several studies have demonstrated that ATP also plays a role as a platelet aggregating agent [99–102]. Hence, further studies are needed to clarify if the decline in ATP hydrolysis among CINs groups to the cancer stage relates or not to a cellular defense mechanism in the CeCa.
Purinergic system and the immune system in the tumor microenvironment of cervical cancer
The development of CeCa follows several mechanisms of immune response suppression and evasion [103]. Purinergic signaling is also able to adjust and modulate the function of immune cells through cell-cell interactions, cytokine, and chymosin secretion, the release of cell surface antigens, removal of intracellular pathogens, and generation of reactive oxygen species [58]. Such effects occur through the interaction of purinergic mediators—such as ATP and Ado—with specific receptors that lead to the transmission of efferent signals that affect some immune responses [104].
Ectonucleotidases control and modulate ATP and Ado levels in TME [48, 105]. Among them, CD39 and CD73 act on the regulation of the duration, magnitude, and composition of the purinergic pool that involves the immune cells and, therefore, have their expressions and enzymatic activity altered according to the pathophysiological context in which they are inserted [58].
This balance between extracellular ATP and Ado is an indispensable factor in immunologic homeostasis due to the role played by these two molecules while interacting with purinergic receptors expressed on the surface of immunologic cells. As previously mentioned, ATP acts as a danger signal when released by injured cells or under stress, through the interaction with P2 type purinergic receptors [106, 107]—behaving as a potent DAMP and trigger inflammatory responses [108, 109]. At the same time, extracellular ATP can also act as a source of the Ado immunosuppressant via CD39 and CD73 ectonucleotidases [10, 106].
ATP has a variety of pro-inflammatory effects in high concentrations in TME due to the activation of P2 receptors. However, the triggering of P2 receptors—as a result of the high levels of extracellular ATP released by abnormal cells present in TME of CeCa—may also initiate adaptive immune responses in other sites [55, 106, 110]. Therefore, extracellular ATP, via activation of P2X7R in immune cells, promotes the release of inflammatory cytokines such as IL-1β and IL-18—which sensitizes immune cells to increase immune responses [106, 111]. [106, 111]. IL-1β promotes the maturation of macrophages and stimulates the release of inflammatory cytokines by activated macrophages. Similarly, natural killer (NK) cells are activated by the IL-18, which results in an enhancement of the interferon γ (IFN-γ) secretion and the cytotoxic state [106]. High levels of ATP extracellular can also, by binding to purinergic receptors, amplify T Cell receptor (TCR) signaling in lymphocytes and promote inflammasome activation in macrophages and dendritic cells [103].
The enzymatic activity of CD39 and CD73 are then able to modulate the duration and magnitude of purinergic signaling through the ATP-CD39-CD73-Ado cascade since CD39 hydrolyzes extracellular ATP in AMP and CD73 dephosphorylates AMP in Ado—a critical regulator in both innate immune response mechanisms and adaptive immune response [7, 58, 59]—where CD39 acts as a limiting enzyme for this cascade [37]. Thus, the high expression of these ectonucleotidases involved in ATP catabolism is considered a sign of a poor clinical outcome [53].
Finally, Ado formed by the hydrolysis of extracellular ATP—abundant in the TME [7]—attaches to A2AR, the primary receptor of Ado [38]. These nucleosides caused, through the A2AR, the accumulation of cytosolic cAMP, which not only inhibits the activation of T lymphocytes but also reduces the secretion of inflammatory cytokines [59].
In this sense, Bahreyni et al. [29] describe that the activation of adenosinergic molecules, including the enzymes CD39, CD73, and the A2a and A2b receptors, presented a reductive action in the immune responses throughout the development of CeCa (Fig. 3). The impairment of the immune response occurs mainly due to the overexpression of IL-10 and the downregulation of HLAI. Besides, the interaction between Ado and these receptors induces the production of the TGF-β [39], which also suppresses antitumor immune responses [112]. TGF-β levels are high in patients with CeCa not only due to the action of Ado but also because of the influence of persistent HPV infection. Hence, TGF-β behaves as an essential defense mechanism in healthy cervix cells that aim to decrease and limit the progression of HPV-infected cells [112].
A study conducted by Mora-García et al. [34] showed that HPV-infected CeCa cell lines (CaSki, SiHa HeLa cells) could contribute significantly to the formation of Ado in CeCa’s TME, as the hypoxia characteristic of TME induces expression of CD73 ectonucleotidase. CD73 overexpression favors rapid hydrolysis of AMP and consequently produces large amounts of Ado—responsible for inhibition of the effector function of cytotoxic T lymphocytes. Aside from the suppressive effect on T cells, high concentrations of Ado in TME could suppress the immune response by other pathways, which include the block of the NK cell lytic activity and deregulation of mononuclear phagocyte cell differentiation and maturation [113]. As a consequence of this adrenergic pathway, an immunosuppressive microenvironment favorable to tumor growth is formed [39].
The combined activity of CD39 and CD73 can inhibit the action of pro-inflammatory immune cells—enabled by extracellular ATP—as it directs the TME to an anti-inflammatory state mediated by the formation of extracellular Ado [58]. Hence, targeting ATP or Ado extracellular signaling could be a new strategy to increase antitumor immunity in TME, and many researchers have suggested blocking this type of signaling for future immunotherapy treatments against CeCa [55, 105].
High concentrations of adenosine in the tumor microenvironment of cervical cancer
Recent reports indicate that the adenosinergic pathway has an essential effect on the pathogenesis of gynecological cancer [29]. Hypoxia caused by tumor growth in the cervix, as a consequence of inadequate vascularization, promotes the degradation of adenine nucleotides, resulting in the release of extracellular Ado excess [114]. Also, the concentration of extracellular Ado becomes much higher when associated with the hydrolysis of extracellular ATP due to the high rates of CD39 and CD73 enzymes in the TME [29].
Beyond the immune suppression effect, extracellular Ado also stimulates tumor angiogenesis through increased blood flow through vasodilation. Moreover, Ado-rich TME promotes endothelial progenitor recruitment of endothelial progenitor cells in response to wound healing, and it stimulates endothelial cell tube formation and promotes the release of pro-angiogenic factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) [115].
In contrast to the immunosuppressive effect of Ado on immune cells, where Ado has substantial immunosuppressive activities concerning the mechanisms present in the tumor cells, Ado can either stimulate or inhibit tumor growth, depending on the cell type and receptor expressed on tumor surface [46, 47]. Gao et al. [113] showed that extracellular Ado could inhibit the migration and invasion of CeCa cells by repressing the epithelial to mesenchymal transition progress.
Extracellular Ado in high concentrations is also associated with the apoptosis induction of apoptosis in cells from a variety of cancerous cells [29]. Mello et al. [12] conducted a study on SiHa cell cultures, a cell line of human cervical carcinoma infected by HPV-16, and found that such cells, when exposed to significant amounts of extracellular AMP—which is not a ligand of any purinergic P2 receptor—enter into the apoptotic process. The cytotoxic effect occurs because AMP, after its release into the extracellular environment, is properly degraded to Ado due to the action of CD73.
Gao et al. [113] also found that extracellular Ado may lead to disturbed balance between pro- and anti-apoptotic factors. This may cause the release of a cascade of a pro-apoptotic signaling molecule (caspase-3 being the most relevant), which played the role—via their proteolytic activities—of inducing multiple cellular changes, and finally leading to programmed cell death. The activation of pro-apoptotic cascades shows that Ado induces the death of CeCa cells by activating the mitochondrial apoptosis pathway. This fact demonstrates the vital role played by Ado in the induction of cellular death of cancerous cells of the uterine cervix.
Prognostic impact of purinergic regulators in cervical cancer
Most new anti-cancer drugs interfere with the cellular components involved in cell survival and the apoptosis process. As outlined in this review, extracellular nucleotides and other components of the purinergic system can regulate proliferation, differentiation, and apoptosis of CeCa cells. The role played by purinergic signaling components in TME makes them important therapeutic targets [10, 44, 48].
HeLa cells from human cervical cancer are widely used to study purinergic involvement during tumor progression [44]. P2Y1R agonists such as ADP and 2-MeSADP were able to stimulate cell proliferation in cancerous HeLa cells [95] as well as the activation of P2Y2R with UTP and ATP promoted proliferation of those cell types [92]. Extracellular ATP can also activate other P2YR, such as P2Y4R and P2Y6R [44]. P2Y6R is implicated in the stimulation of CeCa cells migration (Fig. 3) [97]. However, there are no studies that explain the functions performed by the activation of P2Y4R in cervical tumor cells, although Okuda et al. [89] have shown that HeLa cells express P2Y4R mRNA—which makes further studies necessary. That being said, such effects occasioned by the purinergic receptors expressed in CeCa cells suggest that specific antagonists of P2Y1R, P2Y2R, and P2Y6R could be beneficial as promising future therapies.
The P2Y6R is highly selective for 5′-diphosphate derivatives; therefore, Uridine 5′-O-(2-thiodiphosphate) (UDPβS) is a potent agonist of this receptor. Although they are weaker agonists compared to UDPβS, thymidine 5′-Omonophosphorothiolate (TMPS) and uridine 5′-O-monophosphorothiolate (UMPS) also stimulate P2Y6R-mediated cell migration from HeLa cells. Moreover, it was observed that UMPS is a more powerful agonist—when compared to TMPS—however, it is more easily degraded by CD73 ectonucleotidases. It is also understood that improving the stability of TMPS and UMPS, as well as their affinity with P2Y6R, could result in specific long-term effects mediated by this receptor. Despite the understanding that P2Y6R expressed in CeCa cells facilitates the migration of cervical tumor cells [97], the potential role of UDP and P2Y6R in the regulation of tumor formation in the CeCa has not been deeply investigated. Nevertheless, the knowledge of the properties of P2Y6 agonists offers excellent potential in the development of alternative therapies, especially when inducing long-term effects provided by the activation of P2Y6R.
CeCa cells also express P2X7R, which interacts with ATP and are responsible for the control and induction of the programmed cell death. The expression of P2X7R in CeCa cells is considerably lower in cancer and pre-cancerous epithelial cells when compared to healthy cells [70–72], so using the expression of P2X7R as a biomarker for CeCa could be an alternative way to determine the prognosis of the disease [75, 82, 85].
P2X7R plays an essential role as a pro-apoptotic modulator in human cervical epithelial cells (Fig. 3) [82, 85]. Therefore, the P2X7R could be an appealing therapeutic target for chemoprevention and in CeCa treatment [116]. Adinolfi et al. [79] stimulated P2X7R in HeLa cells with benzoyl ATP (BzATP)—a potent P2X7R agonist—and observed the loss of mitochondrial membrane potential with subsequent fragmentation of mitochondria and cell death. Fu et al. [117], in another study, used BzATP to upregulate P2X7-mediated apoptosis and modulate the growth of epidermal neoplasia. These data suggest that the activation of P2X7R-dependent apoptosis with BzATP could be used as a chemotherapeutic treatment to avoid cell growth of pre-cancerous lesions and early cervical epithelial lesions [116].
Besides the functions mediated by the activation or inactivation of purinergic receptors in CeCa cells, the control of ectonucleotidases activity has also emerged as an important therapeutic target during cancer growth and metastasis. Among the enzymatic cascade, CD39 and CD73 are overexpressed in TME at CeCa and in early intraepithelial lesions [8, 35, 118, 119]. Mora-García et al. [34] demonstrated that high-risk HPV infection might benefit the CD73 constitutive expression in CeCa’s TME in order to suppress the immune response through the generation of large amounts of Ado. Moreover, the role of CD73 in TME extends far beyond its enzymatic activity of nucleosidase—when it catalyzes AMP hydrolysis in Ado since CD73 is also implicated as a critical regulatory molecule involved in cancer growth and metastasis—performing both enzymatic and non-enzymatic functions in TME [115, 120]. Gao et al. [120] demonstrated that this promotive effect of cancer growth and metastasis is mediated through the non-enzymatic functions of CD73 overexpression on CeCa cells proliferation and migration is independent of its enzyme activity. Therefore, CD73 overexpression promotes Hela and SiHa cells proliferation by, to some extent, boosting the EGFR/Akt and VEGF/Akt signaling pathway. Thus, as previously mentioned in this review, ATP conversion into Ado in CeCa TME by CD73 and CD39 ultimately promotes an ideal condition for the spread of cancer cells with no robust antitumor response [48, 56, 119] implying that the CD73 blockage could be a promising new therapeutic strategy [121].
Hence, despite the increasing advances in the pursuit of new anti-cancer treatments, there are still few studies that seek to evaluate the real therapeutic potential of molecules (agonists or receptor antagonists; enzyme inhibitors or activators) that target the components of the purinergic system involved in the progression of CeCa specifically. Therefore, further studies are needed on this subject.
Conclusion
In this review, we have highlighted the interaction between CeCa and the purinergic system. Cervical tumor cells are characterized by an abnormal purinergic network, as they express a higher activity of ectonucleotidases and the overexpression or not of specific receptors. The oncogenic processes leading to accumulation of ATP and Ado of the CeCa TME are still not fully understood; however, the infection of high-risk HPV-type invasion in epithelial cells of the cervix results in disruption in normal cell-cycle control and the promotion of uncontrolled cell division leading to the accumulation of genetic damage and an inflammatory condition—which ultimately leads to cellular stress in this site. Cells under stress often end up releasing large amounts of ATP into the extracellular environment. Extracellular ATP then functions as a DAMP while it interacts with the P2 receptors and the ectonucleotidases that degrade it to Ado. Such facts end up contributing to the high concentrations of extracellular ATP and Ado in the CeCa TME.
These molecules affect tumor growth, immune cell functions, and tumor-host interaction in different ways. Extracellular ATP can activate, in CeCa cells, some purinergic P2YR, such as P2Y1, P2Y2, and P2Y6, which contribute to tumor progression and are a promising target for future therapies. The P2X7R, when activated promotes programmed cell death. However, CeCa cells are characterized by their low expression of that receptor when compared to normal cervical cells at the same time that exhibit a variant P2X7j that interacts with P2X7 and thereby disrupts the apoptotic process.
Ado also plays an essential role in CeCa’s TME. This nucleotide acts in distinct ways at specific sites. The high concentrations of Ado in TME affect the host immune system, which leads to suppression of the immune response—aiding tumor progression. However, Ado itself also acts locally in TME by stimulating angiogenesis tumors through increased blood flow through vasodilation. In addition to these propitious effects on tumor progression, extracellular Ado is able to inhibit the migration and invasion of CeCa cells through repressing the epithelial to mesenchymal transition progress. It can also induce pro-apoptotic mechanisms. Finally, it is essential to emphasize that the knowledge of purinergic signaling in tumor cells of CeCa and the understanding of cervical cell biology and tumorigenesis may contribute to future treatments and studies that help in a better understanding of the advancement and proliferation of the disease.
Compliance with ethical standards
Conflicts of interest
MS Pfaffenzeller declares that she has no conflict of interest. MLM Franciosi declares that she has no conflict of interest. AM Cardoso declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marta Schmidt Pfaffenzeller, Email: mspfaffenzeller@gmail.com, Email: marta.schmidt@estudante.uffs.edu.br.
Maria Luiza Mukai Franciosi, Email: maria.franciosi@estudante.uffs.edu.br.
Andréia Machado Cardoso, Email: andreia.cardoso@uffs.edu.br.
References
- 1.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 2.Campion M, Canfell K. Gynecologic oncology. 6. Filadélfia: Wolters Kluwer; 2015. Cervical Cancer screening and Preinvasive disease; pp. 242–325. [Google Scholar]
- 3.Chen L, Luan S, Xia B, Liu Y, Gao Y, Yu H, Mu Q, Zhang P, Zhang W, Zhang S, Wei G, Yang M, Li K. Integrated analysis of HPV-mediated immune alterations in cervical cancer. Gynecol Oncol. 2018;149:248–255. doi: 10.1016/j.ygyno.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 6.Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination—review of current perspectives. J Oncol. 2019;2019:3257939. doi: 10.1155/2019/3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Virgilio F, Sarti AC, Falzoni S, et al. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 8.Gao Z, Dong K, Zhang H. The roles of CD73 in Cancer. Biomed Res Int. 2014;2014:460654. doi: 10.1155/2014/460654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari D, Malavasi F, Antonioli L. A purinergic trail for metastases. Trends Pharmacol Sci. 2017;38:277–290. doi: 10.1016/j.tips.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72:5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Gong Z, Zhang L, Zhao C, Zhao X, Gu X, Chen H. Autophagy knocked down by high-risk HPV infection and uterine cervical carcinogenesis. Int J Clin Exp Med. 2015;8:10304–10314. [PMC free article] [PubMed] [Google Scholar]
- 12.de Andrade Mello P, Filippi-Chiela EC, Nascimento J, et al. Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell. 2014;25:2905–2918. doi: 10.1091/mbc.e14-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SG, Choi J-K, Choi BH, et al. The effect of adenosine 5′-triphosphate on calcium mobilization and cell proliferation in cervical cancer cells. Eur J Obstet Gynecol Reprod Biol. 2006;127:110–114. doi: 10.1016/j.ejogrb.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36:293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14:1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Jiang Y, Lu X, Zhao H, Chen C, Wang Y, Hu W, Zhu Y, Yan H, Yan F. Genomic characterization of cervical cancer based on human papillomavirus status. Gynecol Oncol. 2018;152:629–637. doi: 10.1016/j.ygyno.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes JV, de Medeiros Fernandes TAA, de Azevedo JCV, et al. Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncol Lett. 2015;9:1015–1026. doi: 10.3892/ol.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alizon S, Murall CL, Bravo IG. Why human papillomavirus acute infections matter. Viruses. 2017;9:293. doi: 10.3390/v9100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boda D, Docea A, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, Mamoulakis C, Tzanakakis G, Spandidos DA, Drakoulis N, Tsatsakis AM. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (review) Int J Oncol. 2018;52:637–655. doi: 10.3892/ijo.2018.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okunade KS (2019) Human papillomavirus and cervical cancer. J Obstet Gynaecol:1–7. 10.1080/01443615.2019.1634030 [DOI] [PubMed]
- 22.Georgescu SR, Mitran CI, Mitran MI, et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. J Immunol Res. 2018;2018:1–10. doi: 10.1155/2018/5315816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krump NA, You J. Molecular mechanisms of viral oncogenesis in humans. Nat Rev Microbiol. 2018;16:684–698. doi: 10.1038/s41579-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee NS, Wang H-K, Broker TR, Chow LT. Human papillomavirus (HPV) E7 induces prolonged G2 following S phase reentry in differentiated human keratinocytes. J Biol Chem. 2011;286:15473–15482. doi: 10.1074/jbc.M110.197574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Futur Virol. 2011;6:45–57. doi: 10.2217/fvl.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbster S, Paladino A, Freitas S, Boccardo E (2018) Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics 73:–e551s. 10.6061/clinics/2018/e551s [DOI] [PMC free article] [PubMed]
- 27.Wang Q, Wang L, Feng Y-H, et al. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol-Cell Physiol. 2004;287:1349–1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado PA, Pimentel VC, Negrini LA, Morsch VM, Schetinger MR. Role of the purinergic system in patients with cervical intraepithelial neoplasia and uterine cancer. Biomed Pharmacother. 2012;66:6–11. doi: 10.1016/j.biopha.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Bahreyni A, Samani SS, Ghorbani E, Rahmani F, Khayami R, Toroghian Y, Behnam-Rassouli R, Khazaei M, Ryzhikov M, Parizadeh MR, Hasanzadeh M, Avan A, Hassanian SM. Adenosine: an endogenous mediator in the pathogenesis of gynecological cancer. J Cell Physiol. 2018;233:2715–2722. doi: 10.1002/jcp.26056. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G. Purinergic signalling in the reproductive system in health and disease. Purinergic Signal. 2014;10:157–187. doi: 10.1007/s11302-013-9399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcocer-González JM, Berumen J, Taméz-Guerra R, Bermúdez-Morales V, Peralta-Zaragoza O, Hernández-Pando R, Moreno J, Gariglio P, Madrid-Marina V. In vivo expression of immunosuppressive cytokines in human papillomavirus-transformed cervical cancer cells. Viral Immunol. 2006;19:481–491. doi: 10.1089/vim.2006.19.481. [DOI] [PubMed] [Google Scholar]
- 32.Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, Madrid-Marina V. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol. 2014;5:753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora-García ML, Ávila-Ibarra LR, García-Rocha R, Weiss-Steider B, Hernández-Montes J, Don-López CA, Gutiérrez-Serrano V, Titla-Vilchis IJ, Fuentes-Castañeda MC, Monroy-Mora A, Jave-Suárez LF, Chacón-Salinas R, Vallejo-Castillo L, Pérez-Tapia SM, Monroy-García A. Cervical cancer cells suppress effector functions of cytotoxic T cells through the adenosinergic pathway. Cell Immunol. 2017;320:46–55. doi: 10.1016/j.cellimm.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, Soto-Cruz I. Cervical Cancer cells express markers associated with immunosurveillance. J Immunol Res. 2019;2019:1242979. doi: 10.1155/2019/1242979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009;21:54–59. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Matosevic S. Adenosinergic signaling as a target for natural killer cell immunotherapy. J Mol Med (Berl) 2018;96:903–913. doi: 10.1007/s00109-018-1679-9. [DOI] [PubMed] [Google Scholar]
- 38.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Rocha R, Monroy-García A, Hernández-Montes J, Weiss-Steider B, Gutiérrez-Serrano V, del Carmen Fuentes-Castañeda M, Ávila-Ibarra LR, Don-López CA, Torres-Pineda DB, de Lourdes Mora-García M. Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine. 2019;118:71–79. doi: 10.1016/j.cyto.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 41.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Bagatini MD, dos Santos AA, Cardoso AM, et al. The impact of purinergic system enzymes on noncommunicable, neurological, and degenerative diseases. J Immunol Res. 2018;2018:4892473. doi: 10.1155/2018/4892473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 44.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graner MW. Extracellular vesicles in cancer immune responses: roles of purinergic receptors. Semin Immunopathol. 2018;40:465–475. doi: 10.1007/s00281-018-0706-9. [DOI] [PubMed] [Google Scholar]
- 46.Gessi S, Merighi S, Sacchetto V, et al. Adenosine receptors and cancer. Biochim Biophys Acta Biomembr. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 48.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson KA, Paoletta S, Katritch V, Wu B, Gao ZG, Zhao Q, Stevens RC, Kiselev E. Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol. 2015;88:220–230. doi: 10.1124/mol.114.095711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Vigano S, Alatzoglou D, Irving M, Ménétrier-Caux C, Caux C, Romero P, Coukos G. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol. 2019;10:925. doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 55.de Andrade MP, Coutinho-Silva R, Savio LEB. Multifaceted effects of extracellular adenosine triphosphate and adenosine in the tumor–host interaction and therapeutic perspectives. Front Immunol. 2017;8:1526. doi: 10.3389/fimmu.2017.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6:57. doi: 10.1186/s40425-018-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Gu Z, Fan Y, Zhai G, Zhao X, Sun Q, Shi Y, Lin G. Inhibition of the adenosinergic pathway: the indispensable part of oncological therapy in the future. Purinergic Signal. 2019;15:53–67. doi: 10.1007/s11302-018-9641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorodeski GI, Burfeind P, Gan SU, et al. Regulation by retinoids of P2Y2 nucleotide receptor mRNA in human uterine cervical cells. Am J Physiol. 1998;275:C758–C765. doi: 10.1152/ajpcell.1998.275.3.C758. [DOI] [PubMed] [Google Scholar]
- 62.Welter-Stahl L, da Silva CM, Schachter J, et al. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNγ in human epithelial cells. Biochim Biophys Acta Biomembr. 2009;1788:1176–1187. doi: 10.1016/j.bbamem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Gorodeski GI. Expression, regulation, and function of P2X4 purinergic receptor in human cervical epithelial cells. Am J Physiol-Cell Physiol. 2002;282:C84–C93. doi: 10.1152/ajpcell.2002.282.1.C84. [DOI] [PubMed] [Google Scholar]
- 64.Gorodeski GI, Hopfer U, De Santis BJ, et al. Biphasic regulation of paracellular permeability in human cervical cells by two distinct nucleotide receptors. Am J Physiol-Cell Physiol. 1995;268:C1215–C1226. doi: 10.1152/ajpcell.1995.268.5.C1215. [DOI] [PubMed] [Google Scholar]
- 65.Gorodeski GI, Goldfarb J. Extracellular ATP regulates transcervical permeability by modulating two distinct paracellular pathways. Am J Physiol-Cell Physiol. 1997;272:C1602–C1610. doi: 10.1152/ajpcell.1997.272.5.C1602. [DOI] [PubMed] [Google Scholar]
- 66.Gorodeski GI (2008) Regulation of paracellular permeability in low-resistance human vaginal-cervical epithelia. In: Ehrhardt C, Kim K-J (eds) Drug Absorption Studies: In Situ, In Vitro and In Silico Models, 1st edn. Springer Science & Business Media, pp 339–367
- 67.Darville T, Welter-Stahl L, Cruz C, et al. Effect of the purinergic receptor P2X7 on chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 68.Roger S, Jelassi B, Couillin I, et al. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta Biomembr. 2015;1848:2584–2602. doi: 10.1016/j.bbamem.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Ding J, Wang L. The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci. 2019;64:388–394. doi: 10.1016/j.advms.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Li X, Qi X, Zhou L, Catera D, Rote NS, Potashkin J, Abdul-Karim FW, Gorodeski GI. Decreased expression of P2X7 in endometrial epithelial pre-cancerous and cancer cells. Gynecol Oncol. 2007;106:233–243. doi: 10.1016/j.ygyno.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng Y-H, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Zhou L, Feng Y-H, Abdul-Karim FW. The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomark Prev. 2006;15:1906–19013. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackenzie AB, Young MT, Adinolfi E, Surprenant A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem. 2005;280:33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- 74.Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R. The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol. 2018;9:52. doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y-C, Chang T-Y, Chen T-C, Lin WS, Chang SC, Lee YJ. Functional variant of the P2X7 receptor gene is associated with human papillomavirus-16 positive cervical squamous cell carcinoma. Oncotarget. 2016;7:82798–82803. doi: 10.18632/oncotarget.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sluyter R. The P2X7 receptor. Adv Exp Med Biol. 2017;1051:17–53. doi: 10.1007/5584_2017_59. [DOI] [PubMed] [Google Scholar]
- 77.Di Virgilio F, Giuliani AL, Vultaggio-Poma V, et al. Non-nucleotide agonists triggering P2X7 receptor activation and pore formation. Front Pharmacol. 2018;9:39. doi: 10.3389/fphar.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pevarello P, Bovolenta S, Tarroni P, Za L, Severi E, Torino D, Vitalone R. P2X7 antagonists for CNS indications: recent patent disclosures. Pharm Pat Anal. 2017;6:61–76. doi: 10.4155/ppa-2016-0044. [DOI] [PubMed] [Google Scholar]
- 79.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.e04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Marchi E, Orioli E, Dal Ben D, Adinolfi E. Advances in protein chemistry and structural biology. 1. Epub: Elsevier; 2016. P2X7 receptor as a therapeutic target; pp. 39–79. [DOI] [PubMed] [Google Scholar]
- 81.Mendez LE, Manci N, Cantuaria G, Gomez-Marin O, Penalver M, Braunschweiger P, Nadji M. Expression of glucose transporter-1 in cervical cancer and its precursors. Gynecol Oncol. 2002;86:138–143. doi: 10.1006/gyno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 82.Gorodeski GI. P2X7 receptors and epithelial cancers. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:349–371. doi: 10.1002/wmts.33. [DOI] [Google Scholar]
- 83.Feng YH, Li X, Zeng R, Gorodeski GI. Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids. 2006;25:1271–1276. doi: 10.1080/15257770600890921. [DOI] [PubMed] [Google Scholar]
- 84.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 85.Gorodeski GI. Estrogen attenuates P2X7-R-mediated apoptosis of uterine cervical cells by blocking calcium influx. Nucleosides Nucleotides Nucleic Acids. 2004;23:1287–1293. doi: 10.1081/NCN-200027549. [DOI] [PubMed] [Google Scholar]
- 86.Bukhari M, Deng H, Jones N, Towne Z, Woodworth CD, Samways DS. Selective permeabilization of cervical cancer cells to an ionic DNA-binding cytotoxin by activation of P2Y receptors. FEBS Lett. 2015;589:1498–1504. doi: 10.1016/j.febslet.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 purinoceptor inhibits the activity of the Na+/K+-ATPase in HeLa cells. Cell Signal. 2003;15:115–121. doi: 10.1016/S0898-6568(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 88.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:789–803. doi: 10.1002/wmts.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okuda A, Furuya K, Kiyohara T. ATP-induced calcium oscillations and change of P2Y subtypes with culture conditions in HeLa cells. Cell Biochem Funct. 2003;21:61–68. doi: 10.1002/cbf.992. [DOI] [PubMed] [Google Scholar]
- 90.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Landecker H. Biotechnology and culture: bodies, anxieties, ethics. 1. Indiana: Indiana University Press; 2000. Immortality, in vitro: a history of the HeLa cell line creator; pp. 53–72. [Google Scholar]
- 92.Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J Cell Physiol. 2003;195:234–240. doi: 10.1002/jcp.10242. [DOI] [PubMed] [Google Scholar]
- 93.Durlacher CT, Chow K, Chen X-W, He ZX, Zhang X, Yang T, Zhou SF. Targeting Na+/K+-ATPase in cancer treatment. Clin Exp Pharmacol Physiol. 2015;42:427–443. doi: 10.1111/1440-1681.12385. [DOI] [PubMed] [Google Scholar]
- 94.Blok LJ, Chang GTG, Steenbeek-Slotboom M, van Weerden W, Swarts HG, de Pont JJ, van Steenbrugge G, Brinkmann AO. Regulation of expression of Na+,K+-ATPase in androgen-dependent and androgen-independent prostate cancer. Br J Cancer. 1999;81:28–36. doi: 10.1038/sj.bjc.6690647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buvinic S, Bravo-Zehnder M, Boyer JL, Huidobro-Toro JP, González A. Nucleotide P2Y1 receptor regulates EGF receptor mitogenic signaling and expression in epithelial cells. J Cell Sci. 2007;120:4289–4301. doi: 10.1242/jcs.03490. [DOI] [PubMed] [Google Scholar]
- 96.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gendaszewska-Darmach E, Szustak M. Thymidine 5’-O-monophosphorothioate induces HeLa cell migration by activation of the P2Y6 receptor. Purinergic Signal. 2016;12:199–209. doi: 10.1007/s11302-015-9492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S. Differential signalling of purinoceptors in HeLa cells through the extracellular signal-regulated kinase and protein kinase C pathways. J Cell Physiol. 2004;200:428–439. doi: 10.1002/jcp.20033. [DOI] [PubMed] [Google Scholar]
- 99.Nurden AT. Does ATP act through P2X 1 receptors to regulate platelet activation and thrombus formation?: Does ATP act through P2X1receptors? J Thromb Haemost. 2007;5:907–909. doi: 10.1111/j.1538-7836.2007.02456.x. [DOI] [PubMed] [Google Scholar]
- 100.Koupenova M, Ravid K. Biology of platelet purinergic receptors and implications for platelet heterogeneity. Front Pharmacol. 2018;9:37. doi: 10.3389/fphar.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macfarlane DE, Mills DC. The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood. 1975;46:309–320. doi: 10.1182/blood.V46.3.309.bloodjournal463309. [DOI] [PubMed] [Google Scholar]
- 102.Packham MA, Mustard JF. Platelet aggregation and adenosine diphosphate/adenosine triphosphate receptors: a historical perspective. Semin Thromb Hemost. 2005;31:129–138. doi: 10.1055/s-2005-869518. [DOI] [PubMed] [Google Scholar]
- 103.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 104.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao H, Bo C, Kang Y, Li H. What else can CD39 tell us? Front Immunol. 2017;8:727. doi: 10.3389/fimmu.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marchi ED, Orioli E, Pegoraro A, et al. The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene. 2019;38:3636–3650. doi: 10.1038/s41388-019-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38:1017–1028. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 110.Di Virgilio F, Dal Ben D, Sarti AC, et al. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 111.Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. The P2X7 receptor-interleukin-1 liaison. Front Pharmacol. 2017;8:123. doi: 10.3389/fphar.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karunagaran D, Jinesh G. TGF-β, Smads and cervical cancer. In: Jakowlew SB, editor. Cancer drug discovery and development: growth factor-β in Cancer therapy. Totowa, NJ: Humana Press; 2008. pp. 33–49. [Google Scholar]
- 113.Gao ZW, Wang HP, Dong K, Lin F, Wang X, Zhang HZ. Adenosine inhibits migration, invasion and induces apoptosis of human cervical cancer cells. Neoplasma. 2016;63:201–207. doi: 10.4149/204_150723N407. [DOI] [PubMed] [Google Scholar]
- 114.Merighi S, Mirandola P, Varani K, et al. A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Ther. 2003;100:31–48. doi: 10.1016/S0163-7258(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 115.Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134:1466–1473. doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- 116.Gorodeski GI. P2X7-mediated chemoprevention of epithelial cancers. Expert Opin Ther Targets. 2009;13:1313–1332. doi: 10.1517/14728220903277249. [DOI] [PubMed] [Google Scholar]
- 117.Fu W, McCormick T, Qi X, Luo L, Zhou L, Li X, Wang BC, Gibbons HE, Abdul-Karim FW, Gorodeski GI. Activation of P2X7-mediated apoptosis inhibits DMBA/TPA-induced formation of skin papillomas and cancer in mice. BMC Cancer. 2009;9:114. doi: 10.1186/1471-2407-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Lourdes M-GM, García-Rocha R, Morales-Ramírez O, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14:302. doi: 10.1186/s12967-016-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Lourdes M-GM, López-Cisneros S, Gutiérrez-Serrano V, et al. HPV-16 infection is associated with a high content of CD39 and CD73 ectonucleotidases in cervical samples from patients with CIN-1. Mediat Inflamm. 2019;2019:4651627. doi: 10.1155/2019/4651627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao Z, Wang H, Lin F, Wang X, Long M, Zhang HZ, Dong K. CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer. 2017;17:1–8. doi: 10.1186/s12885-017-3128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghalamfarsa G, Kazemi MH, Raoofi Mohseni S, Masjedi A, Hojjat-Farsangi M, Azizi G, Yousefi M, Jadidi-Niaragh F. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Targets. 2019;23:127–142. doi: 10.1080/14728222.2019.1559829. [DOI] [PubMed] [Google Scholar]