Abstract
Accumulating evidence supports a therapeutic role of purinergic signaling in cardiac diseases. Previously, efficacy of systemically infused MRS2339, a charged methanocarba derivative of 2-Cl-adenosine monophosphate, was demonstrated in animal models of heart failure. We now test the hypothesis that an uncharged adenine nucleoside phosphonate, suitable as an oral agent with a hydrolysis-resistant phospho moiety, can prevent the development of cardiac dysfunction in a post-infarction ischemic or pressure overload-induced heart failure model in mice. The diester-masked uncharged phosphonate MRS2978 was efficacious in preventing cardiac dysfunction with improved left ventricular (LV) fractional shortening when administered orally at the onset of ischemic or pressure overload-induced heart failure. MRS2925, the charged, unmasked MRS2978 analog, prevented heart dysfunction when infused subcutaneously but not by oral gavage. When administered orally or systemically, MRS2978 but not MRS2925 could also rescue established cardiac dysfunction in both ischemic and pressure overload heart failure models. The diester-masked phosphate MRS4074 was highly efficacious at preventing the development of dysfunction as well as in rescuing pressure overload-induced and ischemic heart failure. MRS2978 was orally bioavailable (57–75%) giving rise to MRS2925 as a minor metabolite in vivo, tested in rats. The data are consistent with a novel therapeutic role of adenine nucleoside phosphonates in systolic heart failure.
Electronic supplementary material
The online version of this article (10.1007/s11302-020-09688-0) contains supplementary material, which is available to authorized users.
Keywords: Purinergic receptors, Adenine nucleoside phosphonate, Cardiac function, Heart failures
Introduction
Purinergic receptors mediate a number of potent and important biological functions and have attracted interest as potential targets for developing novel therapeutic drugs [1, 2]. In studies aimed at developing a new heart failure therapy, we previously showed that certain adenine nucleotides, 2-methylthio-ATP and MRS2339, can activate a cardiac myocyte P2X4 receptor (P2X4R), an ATP-gated ion channel. When MRS2339 was infused chronically in murine and canine models of heart failure, it improved cardiac function as compared with animals receiving vehicle [3–6]. MRS2339 is a ribose-modified nucleotide, i.e. it contains a bicyclo[3.1.0]hexane group (i.e. a “North (N)-methanocarba” modification) in place of the five-membered ring of native ribose. This modification tends to increase affinity at various purine receptors, including representative adenosine, P2Y and P2X receptors, compared with native ribose, by pre-establishing a receptor-preferred conformation of this ring [7–9]. However, MRS2339 is a nucleoside 5′-phosphate that is subject to hydrolysis in vivo leading to the corresponding nucleoside MRS1873 (inactive at the cardiac P2X4R), although its rate of hydrolysis by CD73 is predicted to occur at a slower rate than for the corresponding ribose derivative, based on a study of the C2-unsubstituted analogue [10]. Furthermore, MRS2339 also lacks oral bioavailability and therefore has a limitation in its potential use as an oral drug in patients with stable heart failure. Alternatively, phosphonate derivatives are not subject to hydrolysis of the phospho moiety in vivo because of the stability of the C-P bond compared with a C-O-P bond in phosphates. Nevertheless, a charged phosphonate moiety would prevent it from being orally bioavailable. By fully masking the phosphonate group as a diester, the charge is effectively removed, potentially making the molecule suitable as an oral drug. In the present study, we examined several masked, di-isopropyl ester forms of a nucleoside 5′-phosphonate and 5′-phosphate, in addition to the corresponding charged, free acids. Thus, we explored nucleoside phosph(on)ate derivatives in the (N)-methanocarba series that might have an activity similar to MRS2339 in heart failure models but would be predicted to be more stable in vivo.
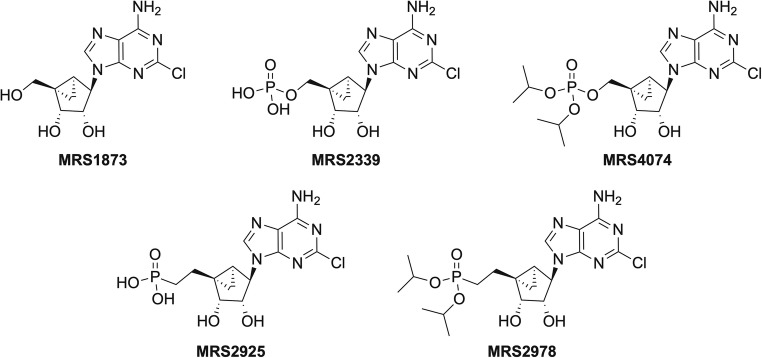
Structures of (N)-methanocarba purine derivatives are shown in Fig. 1 (upper row: nucleoside and phosphate; lower row: phosphonate derivatives). The phosphonates were examined in models of cardiac dysfunction. The possibility of formation of MRS2925 from MRS2978 by successive hydrolysis steps in vivo was examined here, although, generally, phosphonate diesters are stable and require more complex prodrug strategies [11].
Fig. 1.
Structures of (N)-methanocarba purine derivatives (upper row: nucleoside and phosphate; lower row: phosphonate derivatives) that were examined in models of cardiac dysfunction
We previously reported the beneficial effect of the unmasked free, charged phosphonate MRS2925 (compound 9 in [5]), corresponding to the masked uncharged diisopropyl ester MRS2978 (phosphonate derivative 16 in [12]), when infused subcutaneously in the CSQ (calsequestrin) model of systolic heart failure [5]. Previous studies including ours have shown that CSQ-overexpressing mice (TG) start to develop cardiac hypertrophy at a young age progressing to dilated cardiomyopathy and, eventually, premature death [13–15]. In this model, the cardiac function was already depressed when MRS2925 was applied, resulting in improved cardiac function. While the CSQ-overexpressing model was a useful model of systolic heart failure, human systolic heart failure does not have a similar etiology. The relevance of the CSQ model in mice to systolic heart failure in general is not clear. It would be therapeutically novel if masked uncharged ester phosphonates could be developed as oral agents for heart failure using the clinically more relevant models of post-infarction ischemic or aorta banding-induced pressure overload heart failure. Further, as oral agents, we hypothesize that such phosphonates may be salutary in preventing the development of cardiac dysfunction when given at the inception of ischemic or pressure overload heart failure. We reason that prevention of heart failure might be as important as rescue of established heart failure. In the present study, we used the mouse LAD ligation model of ischemic heart failure and the aorta banding pressure overload model to test this hypothesis. MRS2925, the unmasked charged free phosphonate of MRS2978, although efficacious in the CSQ model, has limited potential as an oral agent. Thus, MRS2978, which contains a masked diester form of a nucleoside phosphonate, was selected to test by oral administration for its therapeutic efficacy in preventing left ventricular (LV) dysfunction in both heart failure models. It is intriguing that MRS2925 could be formed in vivo by the successive hydrolysis of MRS2978, and it is tempting to postulate that the in vivo effects of MRS2978, possibly acting as a prodrug, may come from its ability to form MRS2925 by its diester hydrolysis. Finally, neither MRS2978 nor MRS2925 was ever tested in these two clinically relevant models of systolic heart failure.
MRS4074 (compound 8 in [12]), a phosphotriester that is a masked analogue of MRS2339, has been reported efficacious in the LAD heart failure model when it was infused systemically via a subcutaneous (SC) route [12]. Here, we further tested whether MRS4074 can also prevent the development of heart dysfunction when given orally at the inception of the same LAD ligation ischemic heart failure or at the onset of pressure overload heart failure.
Methods
LAD ligation, transverse aorta constriction and echocardiography
Permanent LAD ligation in mice was performed as previously described [16]. Cardiac function was studied using echocardiography at the indicated time after ligation. In one group, ligated mice received either vehicle (saline plus the diluted DMSO at < 0.1%) or the drug via subcutaneous infusion by an Alzet mini-pump or via oral gavage within 14.7 ± 1.2 h (total n = 74 mice) or 15.4 ± 1.2 h (n = 55) after ligation, respectively. In another group, mice received the agent at 4 weeks after ligation at which time, LV dysfunction has already developed. Administration of the agents at 4 weeks after ligation was designed to test whether any agent was capable of rescuing the heart from established LV dysfunction. For pressure overload heart failure, aorta banding or transverse aorta constriction (TAC) in mice was performed as previously described [6]. In summary, constriction was created by placing a 27 gauge needle in the transverse aorta between innominate and left carotid arteries, followed by a ligature of 7–0 Ethilon nylon suture around the needle and the aorta, with removal of the needle afterward. At 3 weeks post-banding, there was moderate LV hypertrophy with a reduced cardiac fractional shortening (FS) but no overt HF or arrhythmias. All groups of mice had similar pressure gradients across the aortic banding. Mice received the agent at 3 weeks after banding in an experiment that was designed to test whether any agent was capable of rescuing the heart from preexisting LV dysfunction induced by pressure overload.
Cardiac function was determined by echocardiography (Vevo 2100 High Resolution Imaging System from VisualSonics, Toronto, Canada) in vivo using a linear 30-MHz transducer according to the manufacturer’s instructions [6, 16]. Two-dimensional-targeted M-mode echocardiographic measurements were carried out at mid-papillary muscle level. LV end-diastolic (LVEDD) and end-systolic (LVESD) diameters were obtained to determine the fractional shortening (FS) (defined as LVEDD − LVESD/LVEDD). For TAC mice, the peak trans-TAC pressure gradient was determined by measuring the velocity across the constriction site by Doppler. The peak pressure TAC gradient was calculated using the modified Bernoulli equation (pressure gradient = 4 × velocity2). The end systolic and diastolic volumes were calculated based on LV as an ellipsoid shape using Teichholz’s formula, permitting determination of left ventricular ejection fraction (LVEF). This EF calculation becomes inaccurate when there is ventricular asynergy, which occurs in a post-infarction heart. Nevertheless, the EF data were shown with this caveat. In aorta banded animals, there was no such limitation on the utility of Teichholz formula. Thus, in mice with TAC, LVEF, LVEDD and stroke volume (SV) values were calculated and presented. All echocardiogram parameters were averaged from more than three cardiac cycles, and the echo data analyses and calculations were performed offline using the Vevo 2100 software. After echocardiography, mice were euthanized, and, when relevant, the infarct size was determined as previously described [16].
Preparation and infusion of nucleotides in vivo
MRS2978 (11 in Fig. 2, MW 473.8), the diisopropyl ester of (1′S,2′R,3′S,4′R,5′S)-4′-(6-amino-2-chloropurin-9-yl)-2′,3′-(dihydroxy)-1′-(phosphonoethylene)-bicyclo[3.1.0]hexane, was dissolved in DMSO as a stock solution at 25–50 mM. Preparing the solution for mini-osmotic pump use, a MRS2978 stock solution (25–50 mM) was diluted to the indicated concentrations of 10 μM or 1.5 mM, respectively, in 0.2 ml of normal saline, filtered, infused via subcutaneous administration at 6 μl/day for 4 weeks in LAD-ligated (10 μM) or 3 weeks in aorta-banded (1.5 mM) mice. The gavage solution was prepared by diluting MRS2978 stock solution by 33.6 to 50-fold with aqueous 0.5% methylcellulose solution of which 0.1 ml was administered, corresponding to dosing of the agent at 1, 3 or 9 mg/kg of body weight. The oral gavage was carried out for 4 weeks in LAD-ligated and for 3 weeks in aorta-banded mice.
Fig. 2.
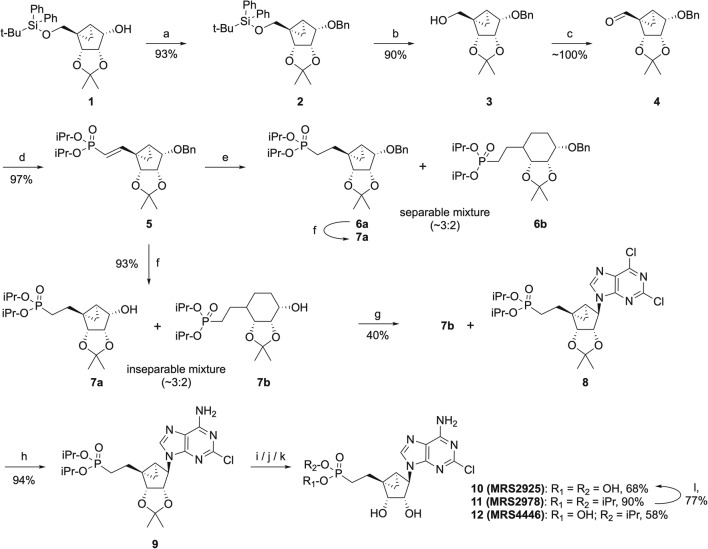
Synthesis of MRS2925 and MRS2978. Reagents and conditions: (a) DMF, NaH, BnBr; 0 °C → rt., 2 h; (b) THF, TBAF, rt., 3 h; (c) CH2Cl2, Dess-Martin reagent, rt., 3 h; (d) THF, tetra-isopropyl methylene diphosphonate, BuLi, − 40 °C → − 20 °C → rt., 1.5 h; (e) methanol, TEA, H2-Pd/C, rt., 18 h; (f) methanol, H2-Pd/C, rt., 18 h; (g) THF, 2,6-dichloropurine, PPh3, DIAD, rt., 48 h; (h) i-PrOH-NH3, 70 °C, 18 h; (i) CH2Cl2, TMSI, rt., 18 h; (j) MeOH-H2O (1:1), Dowex-50 H+-Form, 55 °C, 3 h; (k) CH2Cl2, TMSI, rt., 45 min; (l) 3 mM aq. HCl, μwave, 130 °C, 60 min
Statistics
Data obtained from each experiment were represented as mean ± SEM. Echocardiography parameters were obtained consistently at the end of the experiments before euthanasia, that is, 4 weeks after LAD ligation in the early drug administration groups, 8 weeks after ligation in the late administration groups or 6 weeks after TAC. For infusion or gavage begun early after LAD ligation, drugs or vehicle were administered less than 24 h after ligation. Because echocardiographic parameters were obtained only at one time point (at end of the experiments), we used one-way analysis of variance (ANOVA) with Newman-Keuls post-hoc test comparing differences between two individual groups. Sham operated mice served as a control group for drug vs. vehicle effects. The probability value of P < 0.05 was considered statistically significant.
Results
Chemical synthesis of nucleoside phosphonates
MRS4074 was synthesized as reported [12]. The synthetic route leading to MRS2978 and MRS2925 was modified from the reported procedure [5, 12Supporting Information]. A strategically protected (N)-methanocarba starting material 1 [17] (Fig. 2) was benzylated to protect the secondary hydroxyl, and the silyl group was removed from the primary hydroxyl at the 5 position to give 3. The primary alcohol in compound 3 was oxidized to an aldehyde using Dess-Martin periodinane (DMPI) in anhydrous methylene chloride. The phosphonate group was installed by reacting 4 with tetra(isopropyl)methylene(bis)phosphonate under Horner-Wadsworth-Emmons (HWE) olefination conditions to afford trans-alkene-bearing intermediate 5 in an excellent isolated overall yield (81%, four steps).
Initially, a palladium-catalyzed hydrogenation of compound 5 was envisaged both to reduce the double-bond and remove the benzyl protecting group in one step, to arrive at 7a, but the reaction resulted in an inseparable mixture of products. The HRMS analysis showed a mass corresponding to the desired product 7a and an unanticipated mass of 7a + 2 (identified as 7b), while the proton NMR data revealed the presence of these products in a 3:2 ratio. The addition of triethylamine to the reduction reaction of 5 attenuated the catalytic strength of the palladium, which resulted in separable products, i.e. characterized as compounds 6a,b. Compound 6a was debenzylated and confirmed as the major isomer 7a. Fortunately, subjecting the mixture of 7a,b to Mitsunobu conditions with 2,6-dichloropurine gave only the desired product 8 [12], which on heating with ammonia in a sealed tube gave adenine derivative 9. The 2′,3′-O-isopropylidene moiety in 9 was removed using acidic-DOWEX resin at slightly elevated temperature to afford MRS2978 (11) in 90% yield. However, TMSI, a strong Lewis acid and a nucleophile, removed both isopropyl and isopropylidene groups. A short reaction time (45 min) gave MRS4446 (12), while a longer time at room temperature (18 h) provided MRS2925 (10). Alternatively, compound 10 was obtained by microwave irradiation of MRS2978 in dilute aqueous hydrochloric acid at high temperature [18]. It is to be noted that MRS2925 could also be formed chemically by acid hydrolysis of MRS2978.
Prevention of LV dysfunction development after infarction
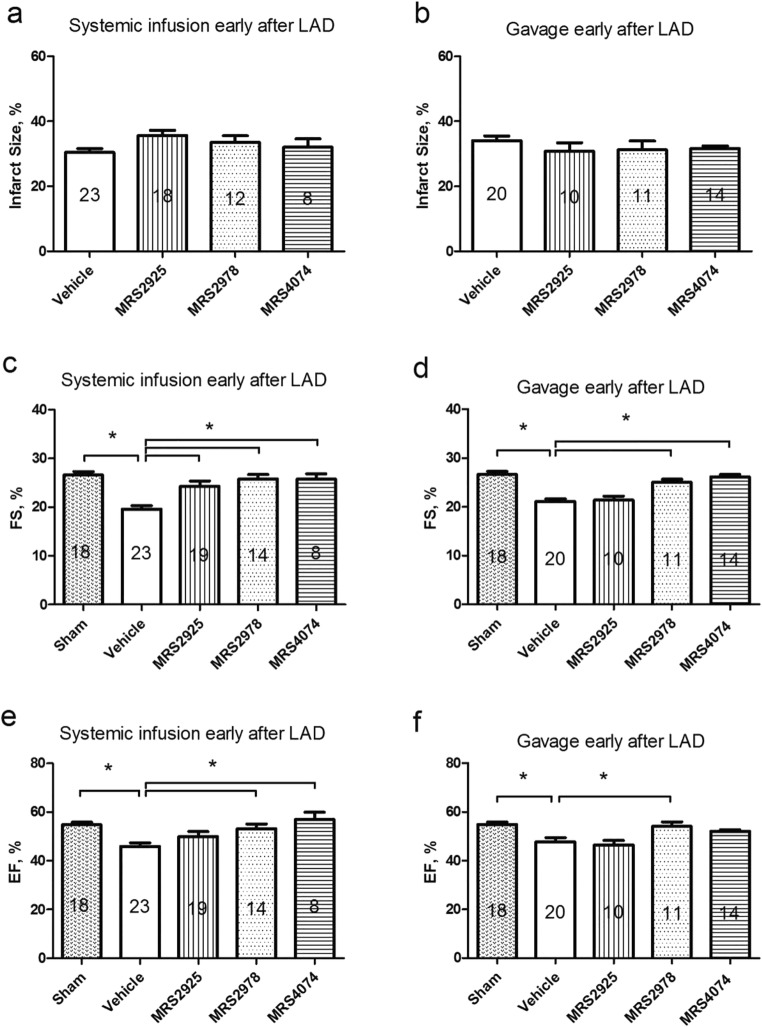
Previous studies showed that nucleoside phosphate analog MRS2339 was able to achieve a salutary effect on several animal models of systolic heart failure. Several nucleoside phosphonates (Fig. 1) were synthesized to determine whether they can prevent the development of LV dysfunction; we administered them early at the inception of heart failure within 24 h after LAD ligation by SC infusion or by oral gavage. There was no difference in the infarct size in any of the four treatment groups (Fig. 3a and b). LAD ligation resulted in significantly depressed LV function as measured by FS in ligated vs. sham mice (Fig. 3c). The nucleoside phosphonates (MRS2925, MRS2978 and MRS4074), when administered by systemic subcutaneous infusion, prevented the decrease in echocardiography-derived FS caused by cardiac infarction (Fig. 3c). However, when given by oral gavage, only MRS2978 and MRS4074 were able to prevent the decrease in FS, while MRS2925 had no effect (Fig. 3d). With the caveat that the echocardiographic EF calculation is potentially inaccurate in postinfarct hearts, EF was improved after infusion of MRS2978 or MRS4074 early after LAD ligation (Fig. 3e). Mice gavaged with MRS2978 early after ligation also showed an increased EF (Fig. 3f). It is of interest that MRS2925 was able to prevent the LV dysfunction when it was infused systemically but not when it was given orally in the same model of post-infarction heart failure. These data are consistent with the concept that MRS2925, as an unmasked and charged phosphonate derivative, may not be well-absorbed orally.
Fig. 3.
Preventive effects of systemic or orally administered nucleoside phosphonates or vehicle on development of LV dysfunction after infarction in mice. a and b Infarct sizes were determined for groups receiving infusions or oral gavages. c and e Cardiac infarction resulted in decreased FS and EF, respectively, compared with the sham group. Systemic subcutaneous infusion of MRS2925, MRS2978 or MRS4074 (10 μM in the mini-pump) caused a higher FS and EF than vehicle treatment. The data on MRS4074 were similar to those previously published [12]. d and f. When MRS2978 (1 mg/kg) and MRS4074 (3 mg/kg) and MRS2925 (3 mg/kg) were given by oral gavage, MRS2978 and MRS4074 but not MRS2925 significantly improved LVFS and LVEF, respectively. *P < 0.05
Rescue of preexisting LV dysfunction caused by LAD ligation
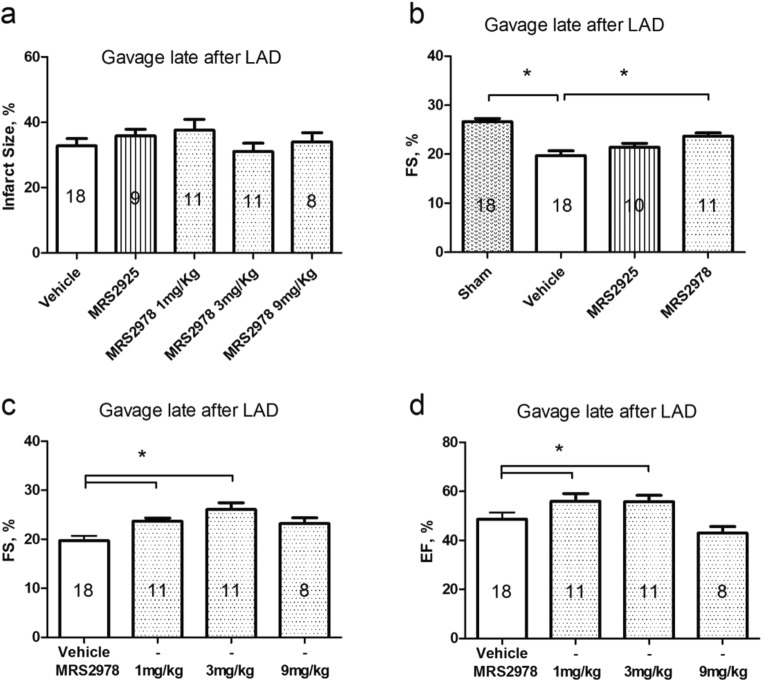
In patients with heart failure, LV dysfunction already exists. Thus, we tested whether nucleoside phosphonates were able to restore function in mice with preexisting LV dysfunction. Mice that developed LV dysfunction after 4 weeks of LAD ligation were given, beginning at 4 weeks postinfarction, either vehicle or MRS2925 or MRS2978 for 4 weeks via oral gavage. Following the end of gavage, Echo data was obtained and then infarct sizes determined. There were no differences in infarct sizes among the various treatment groups (Fig. 4a). MRS2978 was able to restore cardiac FS. However, MRS2925 was without beneficial effect despite the fact that MRS2925 was given at 3 mg/kg vs. MRS2978 dosage of 1 mg/kg (Fig. 4b). It is of interest that FS in MRS2978-gavaged mice was almost restored to a similar level as that in the sham mice. The data support the concept that MRS2925 may not be well absorbed orally to bring about an efficacy in rescuing ischemic heart failure. There appears to be a dose-dependent increase in the rescuing efficacy of MRS2978 as shown by a better cardiac FS in the 3 mg/kg treated animals (Fig. 4c). Both the 1 mg/kg and the 3 mg/kg doses showed improved EF as compared with vehicle-treated animals (Fig. 4d). At the highest dose 9 mg/kg tested, the efficacy became less, suggesting that the 3 mg/kg was an optimal dosage in rescuing the established heart failure in this ischemic model.
Fig. 4.
Effects of oral administration of nucleoside phosph(on)ates on preexisting LV dysfunction induced by 4 weeks of LAD ligation in mice. After 4 weeks of infarction, vehicle, MRS2978 or MRS2925 was given by oral gavage for 4 weeks and echocardiography-derived FS determined at the end of the gavage (end of 8th week)-groups were labeled late after LAD. a Infarct sizes were shown for the groups. b FS was depressed in vehicle-treated animals while MRS2978-treated (1 mg/kg) mice displayed restored cardaic FS. MRS2925 (3 mg/kg) was without effect. c and d The ability of MRS2978 at 1, 3 and 9 mg/kg to restore LV, FS and EF, respectively, was shown. *P < 0.05
Rescue of Preexisting LV dysfunction caused by TAC
To provide additional evidence for a salutary role of nucleoside phosphonates in subjects with preexisting LV dysfunction, mice that developed LV dysfunction after 3 weeks of TAC received vehicle, MRS4074, MRS2925 or MRS2978 for 3 weeks via either SC infusion or oral gavage. All mice subjected to TAC had similar peak pressure gradients across the constricted sites (Fig. 5a and b). After 3 weeks of drug administration, that is, after 6 weeks of banding, echocardiography-derived FS was obtained. Cardiac FS was significantly depressed in vehicle-treated TAC mice when compared with sham group. MRS2978-treated mice via the systemic infusion showed significantly better LV function in banded mice than the vehicle-treated banded mice, while MRS2925 was not efficacious in restoring heart function in these mice (Fig. 5c). On the other hand, when given orally, both MRS2978 and MRS4074 were able to rescue the depressed FS, while MRS2925 was again not able to increase FS when compared with vehicle-treated mice (Fig. 5d). Thus, in another model of established LV dysfunction, that of pressure overload heart failure, MRS2978 and MRS4074 were capable of rescuing the heart from existing dysfunction by either SC or oral route. The data suggest that MRS2978 is a promising agent to treat subjects with established heart failure. It is of interest that MRS4074, an uncharged ester of the charged phosphate MRS2339, was also highly efficacious in rescuing the heart from existing cardiac dysfunction in the pressure overload model (Fig. 5d). As another indication of the rescuing effect of MRS2978, Fig. 5e showed that the agent, when infused late (3 weeks) after TAC when LV enlargement was already evident, was able to reduce LV end-diastolic dimension (LVEDD). Both MRS2978 and MRS4074, when gavaged late (3 weeks) after TAC when LV enlargement was already evident, were also able to reduce LVEDD (Fig. 5f). On the other hand, MRS2925 was without effect on the enlarged LV, consistent with its lack of effect on FS when the agent was administered orally after TAC. Similar to the improvement in FS, there were increases in LVEF (Fig. 5h) and SV (Fig. 5j) after gavage of MRS2978 and MRS4074. While infusion of MRS2978 caused a nonstatistical trend toward an improved EF in banded animals (Fig. 5g), it significantly increased SV compared with the vehicle-infused group (Fig. 5i).
Fig. 5.
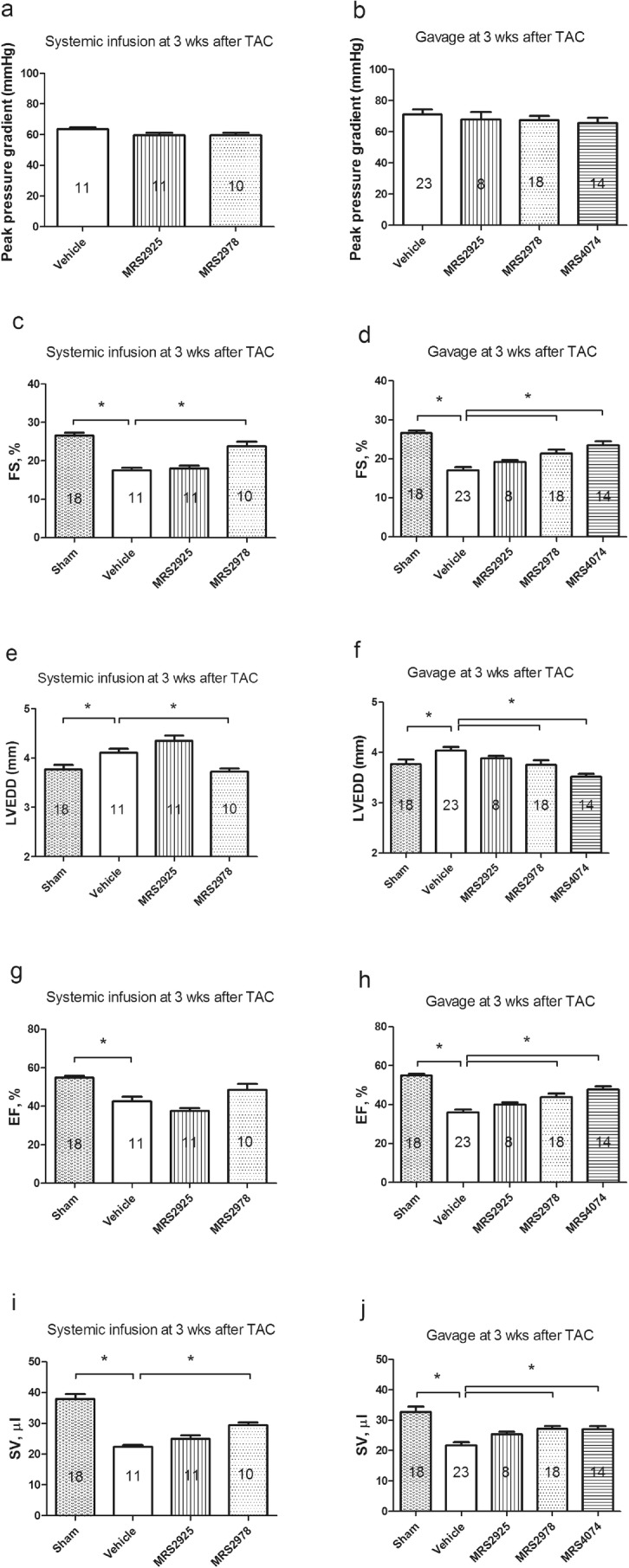
Effects of systemic or oral administration of nucleoside phosphonates on preexisting LV dysfunction induced by a total of 6 weeks of TAC in mice. a and b Peak pressure gradients across the constricted sites were similar in the various treatment groups. c MRS2978 but not MRS2925 rescued preexisting LV dysfunction when given systemically after 3 weeks of aortic banding. Both agents were present at 1.5 mM in the mini-pump. d MRS2978 and MRS4074 but not MRS2925 rescued preexisting LV dysfunction when given orally via gavage (3 mg/kg) after 3 weeks of aortic banding. e MRS2978, when its infusion was begun 3 weeks after aortic banding, was able to reduce the already enlarged LV as shown by a smaller LVEDD. f MRS2978 and MRS4074, when their oral gavage was begun 3 weeks after aortic banding, was able to reduce the already enlarged LV. g MRS2978, when infused, showed a nonstatistical trend toward an improvement in EF. h MRS2978 and MRS4074 but not MRS2925 improved EF when orally administered. i Systemic infusion of MRS2978 increased SV. j Gavage of MRS2978 and MRS4074 also increased SV compared with the vehicle group. *P < 0.05
Absorption, metabolism and off-target activities of MRS2978
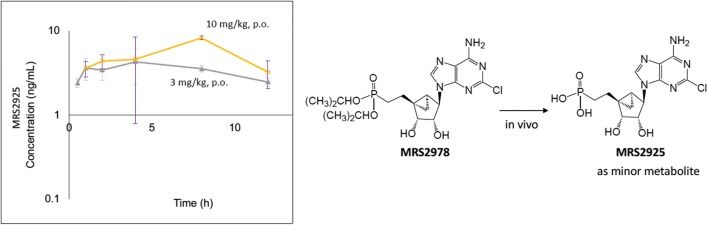
To ascertain the plasma exposure of MRS2978, the kinetics of changes in the plasma level of MRS2978 was measured at pre-determined intervals in rat after oral or intravenous administration (Fig. 6). When administered intravenously, the plasma level of MRS2978 instantly increased and then quickly degraded after about 4 h. However, when MRS2978 was orally administered, its plasma level peaked within a half-hour and then declined at a much slower rate. MRS2978 administered i.v. (0.5 mg/kg, i.v.) displayed low clearance (16.2 ± 4.8 ml/min/kg) with a high volume of distribution (12.5 l/kg) and a long half-life (11 h). The oral absorption was linear at 1 and 3 mg/kg doses. However, the oral absorption was found to be higher with a greater than proportional nonlinear increase at 10 mg/kg. The absolute oral bioavailability for MRS2978 at 1, 3 and 10 mg/kg P.O. was 58.6, 56.7 and 75.2 %F, respectively (Fig. 6). All the animals were found to be healthy throughout the study duration (24 h).
Fig. 6.
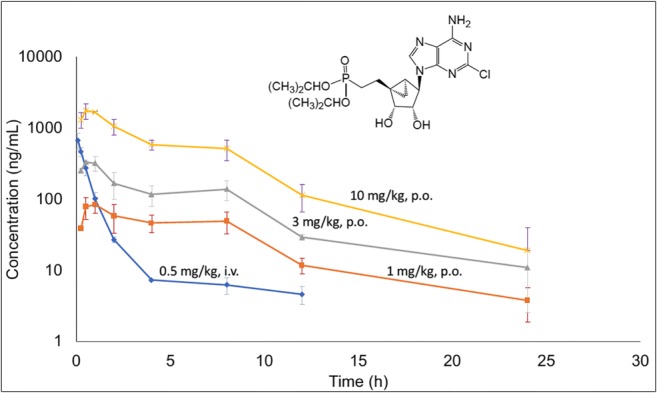
Pharmacokinetics of MRS2978 in the male Sprague-Dawley rat. MRS2978 could be classified as a low clearance compound. MRS2978 was administered in a vehicle of 10% DMSO: 90% aqueous hydroxypropyl-β-cyclodextrin (20%). The AUC (0-inf, ng.h/ml) for MRS2978 was 550 (0.5 mg/kg, i.v.), 644 (1 mg/kg, p.o.), 1870 (3 mg/kg, p.o.) and 8270 (10 mg/kg, p.o.)
In vitro characterization of MRS2978, using previously described methods [19], demonstrated it to be drug-like in its stability and off-target interactions. Using simulated gastric (SGF) and intestinal (SIF) fluid, MRS2978 was stable (100% remaining after 2 h) in both SIF and SGF. Its solubility was determined using the Pion kinetic method to be 147 ± 6 μg/mL, i.e. of suitable aqueous solubility. In a determination of its microsomal metabolic stability, MRS2978 displayed moderate clearance in human (50% remaining after 1 h, t1/2 62 min) and mouse (11% remaining after 1 h, t1/2 19 min) liver microsomes and low clearance in human hepatocytes (t1/2 6.8 h) and rat microsomes (82% remaining after 1 h, t1/2 2.4 h). MRS2978 was highly stable in human, mouse and rat plasma, with 96–100% remaining after 2 h. MRS2978 was not toxic to HEPG2 hepatocytes at 30 μM. MRS2978 was found to be moderately permeable in CACO-2 (colorectal adenocarcinoma) cells with an efflux ratio of 9.6. MRS2978 and MRS2925 did not inhibit (IC50 > 30 μM) any of five tested CYP450 enzymes (1A2, 2C9, 2C19, 2D6 and 3A4).
Minor metabolite of MRS2978
Curiously, MRS2978, a diester of a nucleoside phosphonate, was partially converted to MRS2925, which was only a minor metabolite (Fig. 7). The administered dose of MRS2978 was shown to be > 98% pure by HPLC and did not contain any measurable quantities of MRS2925. Therefore, the MRS2925 observed in the rat plasma was formed from hydrolysis of MRS2978. However, the amount of metabolite (MRS2925) formed was found to be very low (< 10% of MRS2978) and variable. A separate experiment probing the metabolites of MRS2978 in the presence of human liver microsomes showed the presence of a metabolite at mass 490 (Supporting Information), corresponding to hydroxylation of one of the isopropyl groups. Thus, the major metabolic pathway of MRS2978 in microsomes was found to be Phase I oxidation. No glucuronide metabolites were seen, which was evidenced by low human microsomal clearance (t1/2 of 2.3 h) in the presence of NADPH and UDP-glucuronic acid. However, the route of production of MRS2925 from MRS2978, in vivo or in liver, was not determined.
Fig. 7.
Appearance of unmasked MRS2925 as a metabolite of MRS2978 in the male Sprague-Dawley rat. MRS2978 was administered as in Fig. 6. The AUC (0-last, ng.h/ml) for MRS2925 (dose of MRS2978) was 27 (0.5 mg/kg, i.v.), below the level of quantification (1 mg/kg, p.o.), 37 (3 mg/kg, p.o.) and 54 (10 mg/kg, p.o.)
MRS2978 lacked affinity in radioligand binding assays [10] at three subtypes of human adenosine receptors (A1, A2A and A3), with ≤ 10% inhibition at 10 μM. Similarly, MRS4074 was tested in A1 receptor binding and found to be inactive. MRS2978 and MRS2925 were evaluated by the Psychoactive Drug Screening Program for off-target activities at forty-five diverse receptors, channels and transporters [20]. MRS2978 bound to the rat sigma2 receptor with a Ki value of 3.13 μM, and MRS2925 bound to the human dopamine transporter (DAT) with a Ki value of 7.75 μM. Thus, the few off-target activities observed for these two compounds were of low potency.
Discussion
Purine nucleosides and nucleotides have emerged as potentially novel therapeutic agents in treating a number of diseases. We previously demonstrated that a methanocarba-modified ribose nucleotide MRS2339 is efficacious in a several animal models of heart failure. MRS2339 was administered by systemic infusion and not as an oral agent given its negative charge. In search of an oral agent that can be developed to prevent heart failure, we synthesized a number of nucleoside phosph(on)ate alkyl esters and showed efficacy of MRS2978 and MRS4074 as oral agents in preventing the development of left ventricular dysfunction when it was ingested at the onset of ischemic or pressure overload-induced heart failure.
Both MRS2978 and MRS4074 emerged as an efficacious oral agent in preventing the development of LV dysfunction whether the cause was post-infarction ischemic or pressure overload heart failure. MRS2925, while capable of preventing LV dysfunction in ischemic heart failure as a systemic infusion agent, was not efficacious when it was ingested orally. This was not surprising given its multiple negative charges that would impede intestinal absorption. MRS4074, an uncharged diisopropyl ester of the phosphate MRS2339, was efficacious via systemic infusion [12] and was also able to prevent the development of LV dysfunction when given orally in the same ischemic heart failure model.
In further testing the oral efficacy of MRS2978 vs. MRS2925, we determined the effects on restoring or rescuing preexisting LV dysfunction by both agents. In the ischemic model, 4 weeks after permanent LAD ligation, mice developed depressed LV function with decreased fractional shortening (FS). MRS2978 but not MRS2925 when administered orally at 4 weeks post-LAD ligation, was able to restore FS. The most efficacious dose of MRS2978 was 3 mg/kg. While both 1 mg/kg and 3 mg/kg doses were able to significantly improve LV FS and EF, 9 mg/kg dosing did not improve FS or EF. The less therapeutic efficacy in the 9 mg/kg group was not due to lack of exposure to the drug because plasma level of MRS2978 was in fact higher in the 9 mg/kg than in the 3 mg/kg or the 1 mg/kg groups. The significance of bell-shaped MRS2978 dose-response curve is unknown. Although this biphasic response does not generalize to most other purinergic drugs, an A3 adenosine receptor agonists are reported to display a bell-shaped dose response curve. Specifically, A3 agonists in the clinic for inflammatory diseases show a similar dose-response relationship [21]. We speculate that the lower effect at high dose of MRS2978 might be from an off-target activity or from a need to only activate the receptor at low level.
In further comparing the effects of MR2978 vs. MRS2925 in rescuing preexisting LV dysfunction, we tested their effects on LV function after 3 weeks of aortic banding. After 6 weeks of banding in vehicle-treated mice, FS, EF and SV were significantly depressed. Systemic infusion of MRS2978 but not that of MRS2925 at 3 weeks post-aorta banding was able to restore these LV functional parameters. Because systemic infusion of MRS2925 was without efficacy, not unexpectedly, oral ingestion of MRS2925 was also without efficacy. Similar to its improvement effect when given by infusion, MRS2978 was efficacious as an oral agent in rescuing cardiac dysfunction in the banding model. In the same pressure overload model, when MRS4074 was given orally after LV dysfunction was already present, the agent was also able to restore cardiac function, consistent with a rescuing function. MRS4074, having the same 2-Cl substitution on the adenine ring that appears to be important based on previous study [12], was therapeutically efficacious in exerting a rescuing function. It is interesting that both uncharged esters (MRS2978 and MRS4074) are orally efficacious. In support of pharmacologic efficacy, both charged parent compounds MRS2339 and MRS2925 were active when infused systemically.
The hydrolysis of a phosphonate ester in vivo was unexpected, in light of the experience with other phosphonate antiviral agents in which simple alkyl diesters were not cleaved in vivo [22, 23]. We previously tried to identify conditions that would be predictive of in vivo hydrolysis of several phosphonate esters, including MRS2978 [12]. However, at pH of 1 or 11 and in rat plasma or upon exposure to human liver microsomes, no hydrolysis was detected. Thus, we concluded that there was no evidence that MRS2978 could be used as a prodrug of MRS2925. Given the in vivo data that MRS2925 appears to be a minor metabolite, it is unlikely that MRS2978 is a prodrug for the corresponding unmasked phosphonate MRS2925. The in vivo mechanism of action of these new nucleotide analogs is not known. It would also be interesting to understand the receptor subtype that mediates their effects. These are important questions that deserve further investigations.
Conclusions
Newly developed MRS2978, a diester of a nucleoside phosphonate, is very stable and effective when taken orally. We now have data showing the ability of oral therapy with MRS2978 to prevent the development of cardiac dysfunction when it was ingested at the inception in both ischemic and pressure overload heart failure. In addition, MRS2978 could also restore cardiac function when it was given after LV dysfunction already existed. The salutary effect of MRS2978 is not from its minor metabolite (< 10%), MRS2925, although the latter showed a preventive effect on cardiac dysfunction development when it was given via SC infusion. MRS2978 itself is a potential drug to treat HF, and it has the advantage to be taken orally on a chronic basis at the inception of heart failure. Purinergic receptors may be the targets of these nucleotides, although further study is needed.
Electronic supplementary material
(DOCX 3892 kb)
Abbreviations
- CSQ
Calsequestrin
- EF
Ejection fraction
- FS
Fractional shortening
- SV
Stroke volume
- HRMS
High resolution mass spectrometry
- LAD
Left anterior descending artery
- LVPW
Left ventricular posterior wall
- LVEDD
Left ventricular end-diastolic diameter
- LVESD
Left ventricular end-systolic diameter
- MRS2339
(1′S,2′R,3′S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-1-[phosphoryloxymethyl] bicyclo[3.1.0]hexane-2,3-diol)
- MRS2978
diisopropyl (2-((1′S,2′R,3′S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-2,3-dihydroxybicyclo[3.1.0]hexan-1-yl)ethyl)phosphonate
- MRS4074
((1′R,2′R,3′S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-2,3-dihydroxybicyclo[3.1.0]hexan-1-yl)methyl diisopropyl phosphate
- MRS2925
(2-((1′S,2′R,3′S,4′R,5′S)-4-(6-amino-2-chloro-9H-purin-9-yl)-2,3-dihydroxybicyclo[3.1.0]hexan-1-yl)ethyl)phosphonic acid
- P2XR
P2X receptor
- TAC
Transverse aorta constriction
Funding information
KAJ received support from the NIDDK Intramural Research Program (ZIADK31127). This work was supported by the Calhoun Cardiology Research Endowment and HL48225 to BTL.
Compliance with ethical standards
Conflicts of interest
Jian-Bing Shen declares that he has no conflict of interest.
Kiran S. Toti declares that he has no conflict of interest.
Saibal Chakraborty declares that he has no conflict of interest.
T. Santhosh Kumar declares that he has no conflict of interest.
Chunxia Cronin declares that she has no conflict of interest.
Bruce T. Liang declares that he has no conflict of interest.
Kenneth A. Jacobson declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. The animal protocols were reviewed and approved by the Institutional Animal Ethics Committees at each institution where animal experiments were performed.
Footnotes
Jian-Bing Shen and Kiran S. Toti are co-equal authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jian-Bing Shen, Email: jshen@uchc.edu.
Kiran S. Toti, Email: kiran.toti@nih.gov
Saibal Chakraborty, Email: sabby.doc@gmail.com.
T. Santhosh Kumar, Email: santro1112@gmail.com.
Chunxia Cronin, Email: ccronin@uchc.edu.
Bruce T. Liang, Email: bliang@uchc.edu
Kenneth A. Jacobson, Email: kennethj@niddk.nih.gov
References
- 1.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijacka W, Moraes DJA, Ratcliffe LEK, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JFR. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med. 2016;22:1151–1159. doi: 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen JB, Pappano AJ, Liang BT. Extracellular ATP-stimulated current inwild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. FASEB J. 2006;20:277–284. doi: 10.1096/fj.05-4749com. [DOI] [PubMed] [Google Scholar]
- 4.Zhou SY, Mamdani M, Qanud K, Shen JB, Pappano A, Kumar TS, Jacobson KA, Hintze T, Recchia FA, Liang BT. Treatment of heart failure by a methanocarba derivative of adenosine monophosphate. Implication for a role of cardiac P2X purinergic receptors. J Pharm Exp Therap. 2010;333:920–928. doi: 10.1124/jpet.109.164376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar TS, Zhou SY, Joshi BV, Balasubramanian R, Yang T, Liang BT, Jacobson KA. Structure activity relationship of (N)-methanocarba phosphonate analogues of 5′-AMP as cardioprotective agents acting through a cardiac P2X receptor. J Med Chem. 2010;53:2562–2576. doi: 10.1021/jm9018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T, Shen JB, Yang R, Redden J, Dodge-Kafka K, Grady J, Jacobson KA, Liang BT. Novel cardioprotective role of endogenous cardiac myocyte P2X4 receptors in heart failure. Circ Heart Fail. 2014;7:510–518. doi: 10.1161/CIRCHEARTFAILURE.113.001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA, Ji X-d, Li AH, Melman N, Siddiqui MA, Shin KJ, Marquez VE, Ravi RG. Methanocarba analogues of purine nucleosides as potent and selective adenosine receptor agonists. J Med Chem. 2000;43:2196–2203. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg A-K, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: high potency of northern ring conformation at P2Y1, P2Y2, P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn PM, Kim HS, Jacobson KA, Burnstock G. Northern ring conformation of methanocarba-adenosine 5′-triphosphate required for activation of P2X receptors. Drug Dev Res. 2004;61:227–232. doi: 10.1002/ddr.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotides analogues locked in a northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pertusati F, Serpi M, McGuigan C. Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antivir Chem Chemother. 2012;22:181–203. doi: 10.3851/IMP2012. [DOI] [PubMed] [Google Scholar]
- 12.Kumar TS, Yang T, Mishra S, Cronin C, Chakraborty S, Shen JB, Liang BT, Jacobson KA. 5′-phosphate and 5′-phosphonate ester derivatives of (N)-methanocarba adenosine with in vivo cardioprotective activity. J Med Chem. 2013;56:902–914. doi: 10.1021/jm301372c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LR, Suzuki YJ, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knollmann BC, Knollmann-Ritschel BEC, Weissmann NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525:483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y, Ferguson DG, Sako H, Dorn GW, II, Kadambi VJ, Yatani A, Hoit BD, Walsh RA, Kranias EG. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem. 1998;273:28470–28477. doi: 10.1074/jbc.273.43.28470. [DOI] [PubMed] [Google Scholar]
- 16.Sonin D, Zhou SY, Cronin C, Sonina T, Wu J, Jacobson KA, Pappano A, Liang BT. Role of P2X purinergic receptors in the rescue of ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1191–H1197. doi: 10.1152/ajpheart.00577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tosh DK, Padia J, Salvemini D, Jacobson KA. Efficient, large-scale synthesis and preclinical studies of MRS5698, a highly selective A3 adenosine receptor agonist that protects against chronic neuropathic pain. Purinergic Signal. 2015;11:371–387. doi: 10.1007/s11302-015-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansa P, Baszczyňski O, Procházková E, Dračínskýa M, Janeba Z. Microwave-assisted hydrolysis of phosphonate diesters: an efficient protocol for the preparation of phosphonic acids. Green Chem. 2012;14:2282–2288. doi: 10.1039/c2gc35547g. [DOI] [Google Scholar]
- 19.Tosh DK, Ciancetta A, Mannes P, Warnick E, Janowsky A, Eshleman AJ, Gizewski E, Brust TF, Bohn LM, Auchampach JA, Gao ZG, Jacobson KA. Repurposing of a nucleoside scaffold from adenosine receptor agonists to opioid receptor antagonists. ACS Omega. 2018;3:12658–12678. doi: 10.1021/acsomega.8b01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor (A3AR) agonists. Drug Disc Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecker SJ, Erion MD. Prodrugs of phosphates and phosphonates. J Med Chem. 2008;51:2328–2345. doi: 10.1021/jm701260b. [DOI] [PubMed] [Google Scholar]
- 23.Toti KS, Derudas M, Pertusati F, Sinnaeve D, Van den Broeck F, Margamuljana L, Martins JC, Herdewijn P, Balzarini J, McGuigan C, Van Calenbergh S. Synthesis of an apionucleoside family and discovery of a prodrug with anti-HIV activity. J Org Chem. 2014;79:5097–5112. doi: 10.1021/jo500659e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3892 kb)