Abstract
The improvement of cryopreserved oocyte survival is imperative for the preservation of female fertility. In this study, we investigate whether P2Y2 receptors (P2Y2R) can be directly implicated in calcium (Ca2+) homeostasis misbalances observed during the cryopreservation process of cumulus oocyte complexes (COC). Firstly, RNA was extracted from bovine immature and mature oocytes and cumulus cells and real-time PCR performed to identify P2Y2R transcripts (experiment 1). Changes in intracellular calcium concentration [Ca2+]i of mature COC and oocytes (experiment 2) were measured upon exposure to cryoprotectants (CPA), UTP (P2Y2R stimulator, 100 μM), and/or suramin (P2Y2R inhibitor, 100 and 300 μM). The functional role of P2Y2R was investigated by analyzing the effect on oocyte viability of its modulation prior and during oocyte exposure to CPA (experiment 3). Mature COC were randomly divided into groups, and exposed to CPA and different P2Y2 modulators. Oocytes’ viability, cortical granules location, and competence for development were assessed. Results showed that P2Y2R mRNAs are expressed in both oocytes and cumulus cells. Stimulation with UTP and CPA led to [Ca2+]i increase, and this effect was totally or partially blocked by suramin (P2Y2R inhibitor). Oocyte exposure to CPA and UTP reduced embryo rates compared with control and suramin100μM (P ≤ 0.04). The observed enhanced premature zona hardening in oocytes exposed to CPA (P = 0.04) and UTP (P = 0.005) stimulus was inhibited by suramin 100 μM. In conclusion, inhibition of P2Y2R during cryoprotectant exposure reduces premature intracellular Ca2+ release and significantly improves the developmental competence of exposed bovine oocytes.
Keywords: Oocyte, Cryopreservation, P2Y2 receptors, Cryoprotectants, Calcium, Fertility
Introduction
Preservation of female fertility depends largely on the survival of cryopreserved oocytes [1, 2]. In fact, preservation of female gametes is a crucial issue determining the posterior viable reproduction in women who are at risk of losing ovarian function due to several pathologies. It is also important for the preservation of highly valuable females of any species, as well as for commercial purposes [3–5].
Artificial reproductive technologies (ART) are used to preserve the structural and functional integrity of gametes, thus attempting to maintain their developmental competence. However, there are still several limitations, in particular those related to oocyte cryopreservation [1–3]. Several approaches have been developed in the quest for the best protocol for mammal oocyte cryopreservation [6–8].
Cryoinjuries affecting the survival of oocytes after the cryopreservation process have been extensively reported [9–12]. A number of studies have investigated the important role of calcium (Ca2+) on cryopreservation and fertilization. Interestingly, damage to organelles that modulate intracellular calcium concentrations [Ca2+]i during cryopreservation, leads to reduced developmental competence [6]. Moreover, Ca2+ concentrations in the vitrification media positively correlate to oocyte spontaneous parthenogenetic activation and negatively correlate with cleavage rate after fertilization of ovine oocytes [13]. In addition, mature mouse oocytes were shown to retain their developmental competence when cryopreserved in the presence of cumulus cells and in a Ca2+-free media [14] Absence of Ca2+ in the media was also shown to improve zona pellucida (ZP) penetration by sperm cells [15]. Furthermore, some studies showed that oocytes exposed to solutions containing cryoprotectants (e.g., dimethylsulphoxide, ethylene glycol, and propylene glycol), suffer rises on the levels of [Ca2+]i [16] resulting in premature oocyte activation and subsequent ZP hardening [8, 17, 18]. Notwithstanding differences among species, related to concentrations, chemical nature, and permeability of cryoprotectants (CPA) may also be expected [5, 8, 19].
One significant modulator of [Ca2+]i is the purinergic receptor P2Y2 (P2Y2R), which was identified in the mouse cumulus cell-enclosed oocytes and might be relevant for follicular growth and oocyte maturation [20]. P2Y2Rs are G protein–coupled receptors that, when activated by extracellular nucleotides, lead to a stimulation of inositol triphosphate (IP3) receptors in the endoplasmic reticulum (ER) resulting in the release of stored Ca2+ to the intracellular space [21]. P2Y2Rs could be equally stimulated by UTP and ATP, and are inhibited by suramin. When stimulated, these receptors cause the release of Ca2+ from the ER [22, 23]. An important side effect of CPAs seems to be the increase of [Ca2+]i concentrations in the oolema. We hypothesize that P2Y2Rs could be affected by CPA exposure during the cryopreservation procedure, thereby contributing to the increase of [Ca2+]i levels and subsequent premature oocyte activation and ZP hardening [8, 18]. The potential of these receptors for cryopreservation has never been explored before. Therefore, our goal was to investigate the functional expression of P2Y2R in bovine cumulus oocyte complexes by measuring dynamic changes in [Ca2+]i upon stimulation or inhibition with pharmacological tools. Once expression was confirmed, we investigated whether P2Y2R modulation prior to cryopreservation could potentially significantly improve oocyte viability.
Materials and methods
All chemicals used were purchased from Sigma Aldrich Chemical Co. (St. Louis, USA) unless otherwise specified.
Oocyte collection and in vitro maturation
Ovaries were collected from a local slaughterhouse, and kept at 35–37 °C, in a phosphate-buffered saline (PBS) supplemented with 0.15% of bovine serum albumin (w/v, BSA) and 0.05 mg mL−1 of kanamycin. From each ovary, follicles of 2–8 mm in diameter were aspirated with a 19-gauge needle [5, 9]. The COC aspirated from the follicles with this size are coordinated at the same meiotic stage presenting also similar developmental potential. Collected oocytes with at least three layers of compact cumulus cells and an evenly granulated cytoplasm were washed and selected for maturation [24]. Maturation was accomplished for 22–24 h, in an incubator at 39 °C with humidified air and 5% CO2. The maturation medium composition was tissue culture medium 199 (TCM) with 10% of fetal bovine serum, 0.2 mM sodium pyruvate, 10 ng mL−1 of epidermal growth factor, and 10 μL mL−1 of gentamicin.
RNA extraction from bovine oocytes and cumulus cells
In order to investigate the expression of P2Y2R genes in bovine oocytes and cumulus cells, total RNA was extracted from 20 to 40 immature and mature COC. After physical separation of oocytes and the respective cumulus cells, the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems) was used to extract RNA according to the manufacturer’s instructions. This protocol included the DNase treatment to remove genomic DNA from RNA, while the RNA is bound on the Spin Cartridge. After extraction, the purified RNA was stored at − 80 °C. RNA concentration was measured, using Nanodrop 2000c (Thermo Scientific).
Reverse-transcription polymerase chain reaction
RNA was reverse transcribed using 20 ng (oocytes) or 20 ng (cumulus cells) of RNA in 20 μL of total volume, by means of Maxima 1st strand cDNA synthesis kit for RT-qPCR (Thermo Scientific), according to the manufacturer’s protocol. Each reaction mix was incubated for 10 min at 25 °C and for 15 min at 50 °C, followed by heating at 85 °C for 5 min. The cDNAs obtained were stored at − 20 °C until further investigation.
Real-time PCR
Primer design and validation
The housekeeping genes used in this study were cyclophilin B (CICLOB) and β-2-microglobulin (B2M). Primers for P2Y2R were designed using the bovine gene sequence data in GenBank (NM_001166525.1). The primer design software Primer 3 [25] was used, with amplicon sizes in the range of 80 to 160 bp. The adequacy of primer sequences was tested by using Primer Express Software (Thermo Fisher Scientific), and the best primers were aligned against a publicly available database using the Primer BLAST software of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) to rule out the possibility of any non-specific priming. Specificity of the primers was evaluated by the observation of a single peak in the melting curve. Sequences of primers for the reference genes and the target genes are shown in Table 1.
Table 1.
Characterization of the selected primers used in the real-time PCR assay
| Gene symbol | Full gene name | GenBank accession name | Primer sequence | bp | Annealing temperature (°C) |
GC content (%) |
Amplicon size (bp) |
|---|---|---|---|---|---|---|---|
| CICLOB | Cyclophilin B | NC_007308.3/281419 |
F: TCCGTCTTCTTCCTGCTGTTG R:CCAATTCGCAGGTCAAAGTAC |
21 21 |
59.9 56.0 |
52 48 |
98 |
| B2M | β-2-Microglobulin | NC_007308.3/280729 |
F: GGGCTGCTGTCGCTGTCT R: TCTTCTGGTGGGTGTCTTGAGT |
18 22 |
58.7 57.4 |
67 50 |
80 |
| P2RY2R 1 | Purinergic receptor P2Y2 | NM_001166525.1 |
F: AGCATCCTGACAGCAAGAGCA R: GCCATTGGTGGTGCCATT |
21 18 |
59.7 58.7 |
52 56 |
79 |
| P2RY2R 2 | Purinergic receptor P2Y2 | NM_001166525.1 |
F: TGAGTGAGGAACCTGAGCTGG R: GCCATTCCAGGTGTCCAGG |
21 19 |
59.0 59.9 |
57 63 |
93 |
Real-time quantitative PCR
Real-time PCR analyses were performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) in a real-time PCR cycler (Applied Biosystems Step-one Instrument). Two gigagrams of cDNA were used as template for each real-time PCR with 10 μL SYBR Green and 40 nM of specific forward and reverse primers, in a final volume of 20 μL. Each sample was analyzed in duplicate and reactions containing water instead of template were included as negative controls. The following real-time PCR protocol was applied: a denaturation step at 95 °C for 10 min, a 40-cycle stage, including denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min, and a melting curve program (60–95 °C) with continuous fluorescence measurement. The products were separated in 2% agarose gels to confirm the right size of the fragments obtained. In parallel, 5-fold serial dilutions of total cDNA from cumulus cells were run as a calibration curve, and for each sample, the amounts of target gene mRNA were determined using the threshold cycle (Ct) values, with relation to the standard curves. In addition, the level of transcripts for the chosen reference genes was measured for each sample to control for sample to sample differences in RNA concentration. Levels of target genes mRNA were then expressed as a ratio to the mRNA values obtained for the reference genes.
Evaluation of intracellular calcium levels
The measurement of [Ca2+]i in oocytes was performed in the Centre for Neuroscience and Cell Biology (Coimbra University) facilities. Briefly, mature denuded oocytes or cumulus oocytes complexes (COC) were immobilized in 8 well plates (1 μ slide 8 well Ibitreated, Ibidi, cat.No: 80826) coated with poly-L-lysine and incubated with a Ca2+-sensitive fluorescence probe (4 μM in SOF + BSA, Fluro4-AM probe, molecular probes Life Technologies F14201) for 40 min. Then, the plate was mounted on the stage of an inverted fluorescence microscope (Carl Zeiss Axio Observer Z1 equipped with an incubator and CCD camera) and subjected to different experimental conditions. All experiments were conducted at 38.8 °C and preceded by the identification of the basal [Ca2+]i during 3–6 min. Subsequently oocytes or COC in each well were subjected to different stimulus injected to the media, namely (i) a 100 μM UTP solution to stimulate P2Y2R and the release of Ca2+ from the endoplasmic reticulum [23, 26], (ii) a 100 or 300 μM suramin solution to inhibit P2Y2R [20, 27], and/or (iii) different solutions of CPA, according to the experimental design, and [Ca2+]i levels were recorded. Each individual well contained either 3–5 oocytes or COC. For time lapse analysis, microscopic optical images were acquired (× 20 magnification) with a CCD digital camera (Axiocam HRm) and a CMOS digital camera (ORCA Flash 4.0), every 10 s for up to 3 min long and every 3 s afterwards, until needed. Sequences were analyzed by means of Zen Blue software 2012 and Image J Fiji [28]. Then fluorescence intensities were calculated by normalizing the intensity after addition of CPA and/or P2Y2R modulators, against oocyte, or COC basal levels.
Oocyte cryopreservation
The vitrification method was based on the protocol used in Matos et al. [5]. Mature oocytes with at least one layer of cumulus cells (COCs) were immersed for 3 min in 100 μL droplets of holding medium (HM), consisting of TCM199 and 20% fetal calf serum (FCS). Afterwards COC were transferred to the equilibration medium, holding medium supplemented with 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethylsulfoxide (DMSO) for 5 min. Oocytes were then transferred to 100 μL droplets of vitrification solution, containing HM supplemented with 15% EG, 15% DMSO, and 0.5 M sucrose. In total, oocytes were exposed to vitrification solution for 30–45 s prior to cryopreservation. All media supplemented or not with P2Y2R modulators according to the experimental design were kept at room temperature. Then the COC were immersed during 1 min in a warming solution (0.5 M sucrose in HM) at 38.5 °C and transferred to a serial of diluted solutions (0.25, 0.1 and 0 M sucrose in HM) at a 5 min interval. The COC were then washed and maintained in HM until further transfer to fertilization medium.
Cortical granules labeling
Cumulus-oocyte complexes exposed either to CPA and P2Y2R modulators were washed several times in PBS without calcium chloride to eliminate cumulus cells and fixed with freshly prepared 4% (v/v) paraformaldehyde for 1 h at room temperature. Fixed oocytes were then washed twice in a solution of PBS-PVP (0.1 M PBS, pH 7.4, with 0.3% (w/v) PVP) and permeated with PBSS (0.1 M PBS, pH 7.4, with 0.1% saponin) for 10 min. Samples were finally incubated at room temperature in the dark with 5 μg mL−1 fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) in PBS for 30 min [18]. After staining, oocytes were washed and transferred to 2 μL Mowiol mounting media (Sigma-Aldrich) in a clean slide. Samples were kept in the dark at 4 °C until assessment within 48 h. Oocytes were then observed under a fluorescence microscope (Olympus BX40, BP 470–490 filter, UPlanFI 20×/0.50 objective lens) [8, 29]. After labeling, and according to the localization of the cortical granules, oocytes were classified into three categories as in Marques et al. [8]: (A) peripheral cortical granules location (line), where they were all visible near the oolema in the form of a continuous peripheral fluorescent ring; (B) incomplete exocytosis, where a part of the cortical granules was no longer visible because a fusion with the oolema was already in course and a discontinuous fluorescent ring was present; and (C) total exocytosis, where no fluorescence was visible.
Oocyte fertilization and embryo culture
In each session or replicate, fertilization was performed with frozen-thawed semen following Percoll gradient procedure as in Marques et al. [8]. In vitro fertilization medium consisted of modified Tyrode’s medium supplemented with 5.4 USPmL−1 of heparin, 10 mM of penicillamine, 20 mM of hypotaurine, and 0.25 mM of epinephrine. Sperm concentration was adjusted to 2 × 106 spermatozoa mL−1. Sperm and oocyte were co-incubated for 18 h and, following this co-incubation, presumptive zygotes were placed in 25 μL droplets of synthetic oviductal fluid (SOF) supplemented with BME and MEM amino acids, glutamine, glutathione, and BSA, cultured at 38.8 °C in a humidified atmosphere with 5% O2, 5% CO2, and 90% N2. After assessing cleavage at 48 h, embryo development proceeded in SOF BSA plus 10% FCS (IVF = day 0). Cleavage and day 7/8 embryo rates were calculated considering only viable oocytes (intact mature oocytes showing no signs of degeneration and with at least one layer of expanded cumulus cells). Cleavage (2–4 cell embryos) rates were calculated as a proportion of viable oocytes, while day 7/8 embryo (morula and blastocysts, being most embryos at the blastocyst stage) development rates were calculated as a proportion of cleaved embryos.
Experimental design
This study was approved by the Animal Care Committee of the National Veterinary Authority (No. 08965DGAV), following European Union guidelines (No. 86/609/EEC). Three experiments were designed:
Experiment no. 1
This experiment was designed to investigate if the purinergic receptor (P2Y2R) genes are expressed in bovine cumulus cells and oocytes in both immature and mature stages. After physical separation of oocytes and the respective cumulus cells, mRNAs were extracted from 20 to 40 immature and mature COC, and mRNA transcript levels of P2Y2Rs were evaluated by using real time-PCR (total, n = 273; 4 sessions).
Experiment no. 2
This experiment was conducted in two trials (Table 2), with 4 sessions each, in order to investigate alterations in resting [Ca2+]i of COC and denuded oocytes subjected to different stimulus [P2Y2R inhibitor (two doses of suramin 100 μM [20] and 300 μM [27]) and/or stimulator (100 μM UTP [23, 26]) and CPAs]. Therefore, in the first trial, after the incubation with Fluro4-AM probe and acquisition of the [Ca2+]i base line levels, mature oocytes (3–8 each treatment, totalizing 28), or COCs (4–9 each treatment, totalizing 35) were exposed to (1) 100 μM of UTP, (2) 100 μM of suramin, (3) 300 μM of suramin, (4) 100 μM of suramin and after 3 min to 100 μM of UTP, (5) 300 μM of suramin and after 3 min to 100 μM of UTP, and (6) CPA 7.5 EG% + 7.5% DMSO, and [Ca2+]i levels were recorded as previously stated.
Table 2.
Experimental design of experiment 2. Mature denuded oocytes or cumulus oocyte complexes (COC) were previously incubated with a Ca2+ sensitive fluorescence probe (Fluro4-AM) for 40 min. Then after the identification of the basal [Ca2+]i during 3–6 min using inverted fluorescence microscope they were subjected to different experimental conditions as described below and [Ca2+]i levels registered
| Trial | Cell type (n) | Group | Treatment |
|---|---|---|---|
| 1 | Oocytes (7), COC (4) | UTP | 100 μM of UTP |
| 1 | Oocytes (8), COC (9) | Suramin100 | 100 μM of suramin |
| 1 | Oocytes (4), COC (9) | Suramin300 | 300 μM of suramin |
| 1 | Oocytes (3), COC (4) | Suramin100 + UTP | 100 μM of suramin and after 3 min to 100 μM of UTP |
| 1 | Oocytes (3), COC (4) | Suramin300 + UTP | 300 μM of suramin and after 3 min to 100 μM of UTP |
| 1 | Oocytes (3), COC (5) | CPA | 7.5 EG% + 7.5% DMSO |
| 2 | COC (5) | CPA + CPA | CPA (7.5% EG + 7.5% de DMSO) and after 5 min another CPA (15% EG + 15% DMSO) exposure (30–45 s) |
| 2 | COC (5) | Suramin100 + CPA + CPA | 100 μM of suramin and after 3 min CPA (7.5% EG + 7.5% DMSO) and then after 5 min another CPA (15% EG + 15% DMSO) exposure (30–45 s) |
| 2 | COC (5) | Suramin300 + CPA + CPA | 100 μM of suramin and after 3 min CPA (7.5% EG + 7.5% DMSO) and then after 5 min another CPA (15% EG + 15% DMSO) exposure (30–45 s) |
The second trial was performed to test the effect of 100 μM and 300 μM of the P2Y2R inhibitor suramin prior to the protocol of cryopreservation on the [Ca2+]i of COCs. Thus, after the incubation with Fluro4-AM probe and acquisition of the [Ca2+]i baseline, mature COCs (5 each group, totalizing 15) were expose to (1) CPA (7.5% EG + 7.5% de DMSO) and after 5 min another CPA (15% EG + 15% DMSO) exposure; (2) 100 μM of suramin and after 3 min CPA (7.5% EG + 7.5% DMSO) and then after 5 min another CPA (15% EG + 15% DMSO) exposure (30–45 s); or (3) 300 μM of suramin and after 3 min CPA (7.5% EG + 7.5% DMSO) and then after 5 min another CPA (15% EG + 15% DMSO) exposure (30–45 s). Changes in [Ca2+]i were recorded.
Experiment no. 3
Experiment no. 3 was performed to investigate whether the modulation of the function of the P2Y2R upon stimulation with UTP in the presence and absence of suramin can improve oocyte developmental competence following cryopreservation procedures. Therefore, the effects of the osmotic stress and toxicity induced by the CPAs in the presence of P2Y2R modulators on oocyte developmental competence were studied (6 sessions). In this trial, all steps of the vitrification/warming process were performed, without plunging the COC in liquid nitrogen (LN2). Prior to this procedure, mature COC were randomly distributed into seven experimental groups (1) control, non-exposed COC (n = 203); (2) CPA, COC were exposed to EG- and DMSO-containing solutions and then to solutions with decreased concentrations of sucrose according to the above described protocol (n = 162); (3) suramin100, COC were exposed to 100 μM of suramin in the holding solution during 3 min, then to EG- and DMSO-containing solutions also supplemented with 100 μM of suramin and finally to solutions with decreased concentrations of sucrose (n = 151); (4) UTP, COC were exposed to CPA-containing solutions supplemented with 100 μM of UTP and then to solutions with decreased concentrations of sucrose (n = 177); (5) suramin100 + UTP, COC were exposed to 100 μM of suramin in the holding solution during 3 min, then to CPA-containing solutions supplemented with 100 μM of UTP plus 100 μM of suramin and finally to solutions with decreased concentrations of sucrose (n = 196); (6) suramin300, COC were exposed 300 μM of suramin during 3 min in the holding solution, then to CPA-containing solutions supplemented with 300 μM of suramin and finally to solutions with decreased concentrations of sucrose (n = 141); and (7) suramin300 + UTP, COC were exposed to 300 μM of suramin in the holding solution during 3 min, then to CPA-containing solutions supplemented with 100 μM of UTP plus 300 μM of suramin and finally to solutions with decreased concentrations of sucrose (n = 130). Viable oocytes were inseminated with frozen/thawed bovine semen and embryo development evaluated (cleavage and day 7/8 embryo development rates). Samples of oocytes from all groups (control, n = 27; CPA, n = 26; suramin100, n = 26; UTP, n = 28; suramin100 + UTP, n = 26; suramin300, n = 20; suramin300 + UTP, n = 21) were labeled for cortical granules location evaluation.
Statistical analysis
Data representing 6 replicates of post warming oocyte viability and of embryo production rates post exposure/removal to CPA (experiment no. 3) were analyzed with GLIMMIX procedure of Statistical Analysis Systems Institute (SAS Inst., Inc., Cary, NC, USA) using the binary distribution and the logit as link function. The generalized linear mixed model included treatment as fixed effect and replicates as random effect. In addition, the means for each treatment were calculated, and comparisons were performed using the PDIFF test. Mann–Whitney U test (NPAR1WAY procedure) was used to analyze data of cortical granules location in oocytes classified into three categories (line, incomplete, and total exocytosis). Finally, data representing four replicates of COC and denuded oocytes [Ca2+]i (experiment no. 2) and of P2Y2R mRNA transcript levels (experiment no. 1) were analyzed using the MIXED procedure of SAS with a model including treatment and time or cell type and maturation stage as fixed effects, respectively. The session was considered as a random effect. Differences were considered significant when P ≤ 0.05.
Results
Expression of P2Y2R
Expression of RNA transcripts of P2Y2R was determined in both oocytes and cumulus cells either in immature (n = 136) or mature (n = 137) stages using real-time PCR (Fig. 1). The highest abundance of P2Y2R mRNA transcript level was identified in immature cumulus cells (P < 0.0001). This abundance was higher in mature cumulus cells compared with mature oocytes (P = 0.04).
Fig. 1.
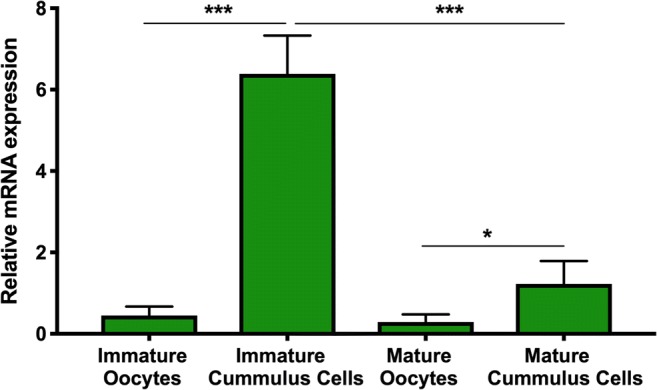
Endogenous expression of bovine P2Y2R mRNA in bovine oocytes and cumulus cells obtained by real-time PCR. Results are expressed as mean ± SD and were normalized to CICLOB and B2M reference genes. Asterisks indicate significant differences (*P < 0.05, mature cumulus cells vs. mature oocytes; ***P < 0.0001 immature cumulus cells vs. other groups)
Evaluation of intracellular calcium
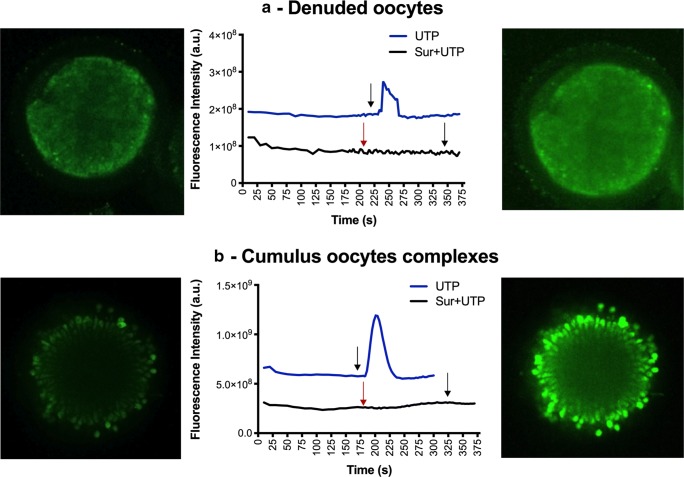
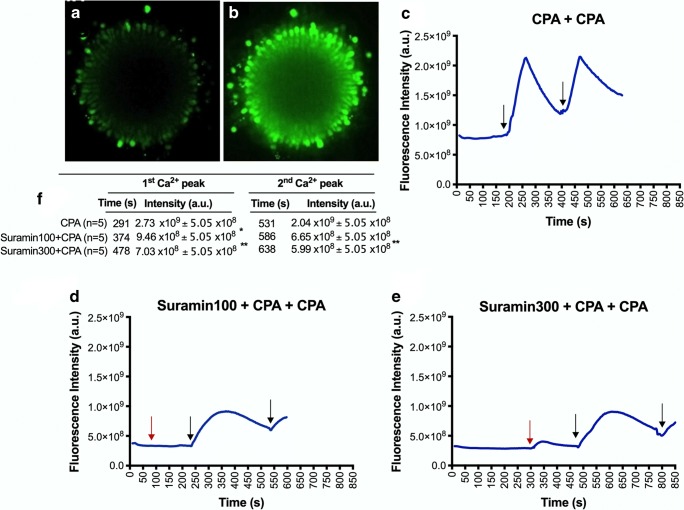
The P2Y2Rs were stimulated in both COC and denuded oocytes by UTP (Fig. 2). It was observed a larger [Ca2+]i as a response to the UTP stimulus in COC (7.87 × 108 ± 5.59 × 108 u.a.) compared to denuded oocytes (1.20 × 108 ± 6.50 × 107 u.a.). Moreover, this effect was inhibited by both doses of suramin (Fig. 2). An increase of [Ca2+]i was identified in denuded oocytes (7.33 × 108 ± 9.94 × 107 u.a.) and COC (2.32 × 109 ± 5.24 × 108 u.a.) stimulated by CPA with a higher magnitude compared with the UTP stimulus (P ≤ 0.008). In the second trial, to simulate the protocol of vitrification, COC were twice exposed to CPAs using different concentrations (7.5% and 15%) (Fig. 3). Time lapse analysis of Ca2+ responses to CPAs in COC demonstrated [Ca2+]i elevations immediately after the initial stimulus, reaching a peak at 291 s and 500 s, respectively. A full recovery to basal levels was not achieved within 5 min. In fact, after the administration of CPAs, a doubled baseline COC [Ca2+]i was registered (Fig. 3). However, when the COC were incubated with suramin prior to the addition of CPAs, [Ca2+]i changes were significantly reduced (Fig. 3, P = 0.03).
Fig. 2.
Time course of intracellular calcium elevations in response to P2Y2R stimulator (UTP) or inhibitor (suramin) in mature denuded oocytes or cumulus-oocyte complexes (COC). a Mature denuded oocytes prior to (left) and after (right) administration of UTP (100 μM) stimulus. The graphs in the center represent an example of the intensity of fluorescence measured in response to UTP alone or suramin100 plus UTP stimulus. B) Mature cumulus oocyte complexes prior to (left) and after (right) administration of UTP (100 μM) stimulus. In the center, the graph represents the time course of fluorescence intensity after stimulation with UTP alone or suramin100 plus UTP. Red and black arrows represent suramin100 and UTP stimulus, respectively. The fluorescence intensity was measured after incubation with a fluorescence probe (Fluro4-AM). Then after the identification of the basal intracellular calcium during 3–6 min using an inverted fluorescence microscope oocytes or COC were subjected to different experimental conditions and [Ca2+]i levels registered. For time lapse analysis, microscopic optical images were acquired with digital cameras. Sequences were analyzed by means of Zen Blue software 2012 and Image J Fiji. Then fluorescence intensities were calculated by normalizing the intensity after addition of P2Y2R modulators, against oocyte or COC basal levels. It was observed that the P2Y2Rs were stimulated in both COC and denuded oocytes by UTP, although this response was higher in COC compared with denuded oocytes (P < 0.05). Suramin inhibited the response induced by the UTP stimulus (a and b)
Fig. 3.
Time course of intracellular calcium concentration in response to P2Y2R inhibitor and cryoprotectants (CPA) in mature cumulus oocyte complexes (COC). a Basal levels of [Ca2+]i after COC incubation with Fluro4-AM probe; b COC response to cryoprotectant stimulus resulting in increased fluorescence intensity; c Effect of the protocol used for COC cryopreservation on [Ca2+]i (1st peak results from exposure to 7.5% ethylene glycol and 7.5% dimethylsulfoxide; 2nd peak results of 15% ethylene glycol and 15% dimethylsulfoxide exposure 5 min later); d and e Examples of the effect on [Ca2+]i of 100 μM (suramin100) or 300 μM of suramin (suramin300) exposure (3 min) prior to the protocol for COC cryopreservation, respectively. Red and black arrows represent suramin and CPA stimulus, respectively. f The table represents the intensity of fluorescence analysis, summarizing the average response obtained in different experimental conditions (4 sessions, totalizing 15 COC). Fluorescence intensities were calculated by normalizing the intensity after addition of CPA and/or P2Y2R inhibitors, against COC basal levels. A partial inhibition of the response induced by the CPA stimulus on [Ca2+]i was consistently observed when P2Y2 receptor were inhibited by suramin prior to the application of the cryopreservation protocol. Asterisks indicate significant differences (CPA + CPA vs. other groups: *P < 0.05, **P < 0.01)
Oocyte competence for fertilization and embryo production
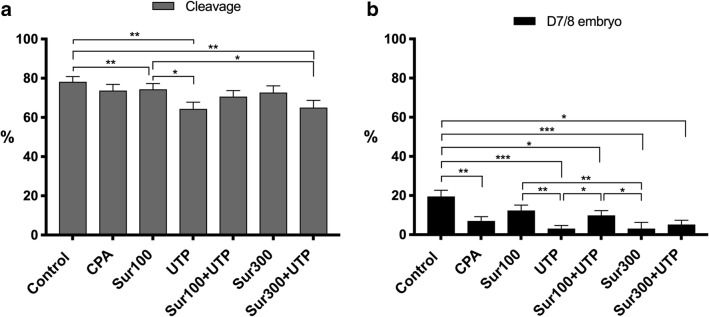
Effects of P2Y2R modulation on cortical granules location and developmental competence were investigated in mature oocytes exposed to the osmotic stress and toxicity induced by CPAs (Figs. 4 and 5). Supplementation of the vitrification media with UTP reduced the cleavage rate (64.4 ± 3.4%) compared with control (78.2 ± 2.7%, P = 0.003), CPA (73.7 ± 3.2%, P = 0.059), and suramin100 (74.4 ± 2.9%, P = 0.033). These latter groups also achieved higher cleavage rates compared with suramin300 + UTP (65.0 ± 3.7%, P ≤ 0.05) (Fig. 4a).
Fig. 4.
Effect of P2Y2R modulation (inhibitor—suramin and stimulator—UTP) on the embryo production rates (A, cleavage and B, day 7/8 embryos) of bovine oocytes exposed to the osmotic stress/toxicity of cryoprotectants (CPA). Cleavage rate (2–4 cell embryos/viable inseminated oocytes × 100); day 7/8 embryo rate (morula and blastocysts/cleaved embryos × 100); Sur100, 100 μM suramin; Sur300, 300 μM suramin; Sur100 + UTP, 100 μM suramin plus 100 μM UTP; Sur300 + UTP, 300 μM suramin plus 100 μM UTP; data (6 sessions, experiment no. 3, n = 1160) with asterisks are significantly different (4A cleavage rate *P < 0.05 Sur100 vs. UTP and Sur300, **P < 0.01 control vs. UTP and Sur300; 4B day 7/8 embryo rate: *P < 0.05 control vs. Sur100 + UTP and Sur300 + UTP, Sur100 + UTP vs. Sur300 and UTP; **P < 0.01 control vs. CPA, Sur100 vs. UTP and Sur300; ***P < 0.001 control vs. UTP and Sur300)
Fig. 5.
Effect on cortical granules location and zona pellucida hardening of bovine oocytes exposed to the osmotic stress/toxicity of cryoprotectants (CPA) and to P2Y2 modulators (inhibitor- suramin and stimulator - UTP) containing solutions (6 sessions, n = 174). Oocytes were classified into three categories and scored for zona pellucida hardening: a Line, total peripheral cortical granules location (1 point); b incomplete exocytosis, a part of the granules was no longer visible (2 points); and c Total exocytosis, no visible cortical granules (3 points). CPA, cryoprotectants; Sur100, 100 μM suramin; Sur300, 300 μM suramin; Sur100 + UTP, 100 μM suramin plus 100 μM UTP; Sur300 + UTP, 300 μM suramin plus 100 μM UTP; Asterisks above bars indicate significant differences in the score of zona pellucida hardening among treatments (*P < 0.05 control vs Sur300, Sur100 + UTP vs. CPA; **P < 0.01, control vs. UTP and CPA, Sur100 vs. UTP, Sur300 and CPA, Sur100 + UTP vs. UTP, Sur300 + UTP vs. UTP and CPA)
Oocyte exposure to CPA had a detrimental action on D7/8 embryo rates (CPA 7.0 ± 2.2% vs. control 19.5 ± 3.2%, P = 0.006). Interestingly the supplementation of CPA with suramin100 (12.4 ± 2.7%) was able to avoid this detrimental effect (Fig. 4). The stimulation of P2Y2R by UTP in the presence of CPA (3.1 ± 1.6%) reduced D7/8 embryo rates when compared with control (19.5 ± 3.2%, P = 0.001), suramin100 (12.4 ± 2.7%, P = 0.01), and suramin100 + UTP (9.9 ± 2.4%, P = 0.04). The highest dose of suramin (300 μM, 3.1 ± 3.2%)) also induced a lower blastocyst development compared with suramin100 (P = 0.01), suramin100 + UTP (P = 0.04), and control (P = 0.001). The highest D7/8 embryo rate was achieved in the control group (P ≤ 0.03), although no differences were identified when compared to the suramin100 group.
The exposure of oocytes to CPA and UTP (Fig. 5) enhanced the ZP hardening compared with suramin100 (P = 0.008 and P = 0.006, respectively), suramin100 + UTP (P = 0.02 and P < 0.0001, respectively), and control (P = 0.003 and P = 0.005, respectively). Similar results were obtained in the suramin300 + UTP group (P ≤ 0.007). No differences were obtained between suramin100 and suramin300 (P > 0.05), although the latter had an enhanced ZP hardening compared with control (P = 0.02) and suramin100 + UTP (P = 0.002).
Discussion
Our results showed for the first time the presence of P2Y2R mRNA transcripts in both bovine oocytes and cumulus cells and confirmed that these receptors might be stimulated with UTP and inhibited by suramin. Indeed, upon stimulation of P2Y2R with UTP, a release of Ca2+ was registered with higher magnitude in the COC compared with denuded oocytes. Moreover, the inhibition of P2Y2R by suramin blocked this increase of [Ca2+]i and reduced the transient increase of [Ca2+]i induced by CPA exposure. These results seem to confirm our hypothesis that during the vitrification process, CPA affect P2Y2R leading to the increase in [Ca2+]i.
Due to improved cryosurvival outcomes, vitrification is so far the best strategy for the cryopreservation of both oocytes and embryos in clinical ART [3, 6, 30, 31]. The vitrification process comprises two main steps: CPAs exposure/removal and chilling after plunging into the liquid nitrogen. In our study, we only performed the CPAs exposure/removal experiment and not the plunging, in order to avoid the overlap of the effect of liquid nitrogen over the effect of CPA exposure. Current vitrification protocols usually employ both DMSO and EG [3, 5, 6, 8, 9]. However, as previously shown in mouse [16] and confirmed herein in bovine oocytes, these CPAs cause transient increases in [Ca2+]i very similar to the initial increase caused by sperm entrance during the fertilization process. Our results confirm an association between the increase in [Ca2+]i and premature oocyte activation, as previously hypothesized by others [8, 17, 18]. Our results also suggest that exposure of COC to the vitrification protocol including increasing concentrations of DMSO and EG triggered the cortical granules exocytosis with ZP hardening, thus impairing the developmental competence of oocytes to the blastocyst stage. The reduced developmental competence of cryopreserved oocytes remains a major setback for the successful use of this technology in different species [1, 2].
Previously, by using electrophysiological and molecular biology techniques, Arellano et al. [20] studied the responses generated by purinergic stimulation of mouse COC. These authors showed that the activation of G protein–coupled receptors by extracellular nucleotides (ATP, UTP or ADP) leads to the stimulation of Ca2+ release from the endoplasmic reticulum. Also, upon ATP stimulation of the Xenopous follicular cells, phospholipase C was activated, which in turn elicited a [Ca2+]i increase in the cytoplasm through the Ca2+ release from intracellular reservoirs and the induction of Ca2+ influx [32]. In addition, Arellano et al. [20] managed to identify the expression of P2Y2R in the mouse follicular cells specifically through the detection of their transcripts in the granulosa and cumulus cells. Accordingly, this expression was also found in bovine cumulus cells in this study. Furthermore, we also identified P2Y2R transcripts in the oocyte, although in a smaller proportion compared with the cumulus cells. Interestingly, the highest level of these receptor transcripts was found in immature cumulus cells. Nonetheless, as mature oocytes are broadly accepted to be easier to cryopreserve due to a higher permeability to CPAs than immature oocytes [33, 34], our study on the modulation of P2Y2R was conducted on mature oocytes and COC. Our results show that the stimulation of both mature denuded oocytes and COC with UTP induced a prompt and transient increase in [Ca2+]i (Fig. 2). Moreover, the observed higher magnitude of the response in COC when compared with denuded oocytes is in accordance with the higher level of P2Y2R transcripts found in the cumulus cells. However, the bi-directional exchanges between the oocyte and the surrounding cumulus cells are crucial for the acquisition of oocyte developmental competence. In fact, cumulus cytoplasmic projections embedded in the zona pellucida intimately connect cumulus cells to the oocyte cytoplasm, allowing the transfer of molecules essential for oocyte metabolism and support of oocyte development [35, 36], as well as for the regulation of meiotic arrest or resumption [37]. Ca2+ is an ubiquitous intracellular messenger involved in the regulation of these processes. Therefore, the activation of P2Y2R resulting in the release of stored Ca2+ to the oocyte intracellular space [21] will certainly affect the development of the oocyte as demonstrated in the present study.
Following the administration of CPAs, simulating the protocol of vitrification, a doubled transient increase of [Ca2+]i was registered in COC (Fig. 3). In an attempt to reduce the amount of Ca2+ being released, COC were incubated with different doses of suramin (100 and 300 μM) prior to the addition of CPAs. There are several reports demonstrating that P2Y2R blockage induced by 100 to 300 μM of suramin, effectively inhibit the effect of ATP or UTP in different cells [23, 27, 38]. In addition, the data from the present study demonstrated for the first time that suramin can also effectively inhibit the [Ca2+]i surge induced by CPA stimulus. Our findings demonstrated that the COC preincubation with suramin for 3 min significantly reduced the doubled increase of [Ca2+]i induced by CPA exposure. These data strongly suggested, as hypothesized, that CPA exposure during COC vitrification stimulates P2Y2R and the premature release of intracellular Ca2+. Several authors have previously reported that the cryopreservation process causes a series of ultrastructural and functional damage compromising oocyte Ca2+ signaling and developmental competence [6, 10]. Our data is in strong agreement with those previous findings.
On the other hand, previous studies indicated that suramin toxicity could not be excluded above 100 μM, at least in bovine nasal cartilage cells [38]. This effect is confirmed in the present study as the addition of the highest dose of suramin had a deleterious action on oocyte developmental competence, although not interfering in its ability to inhibit the release of Ca2+ induced by UTP or CPA. Conversely, the lowest dose effectively blocked the activation of P2Y2R by UTP in both oocytes and COC, thus inhibiting the increase of [Ca2+]i. Suramin is more effective in blocking the release of Ca2+ induced by UTP than with CPA, (Figs. 2 and 3, respectively), since suramin was identified as an inhibitor of P2Y2R, while UTP is known to be a specific stimulator of these receptors. P2Y2 receptors are G protein–coupled receptors that, when activated, lead to the stimulation of IP3 in the endoplasmic reticulum resulting in the release of stored Ca2+ to the intracellular space [21]. But suramin100 addition reduced the doubled increase of [Ca2+]i triggered by CPA, preventing both the zona hardening and the impaired developmental competence which usually results from the exposition of COC to the protocol of cryopreservation. As suramin was only added to the oocyte cryopreservation media during 8 min and 45 s, in this particular protocol, it is not expected to have an effect on inhibiting P2Y2R release of Ca2+ during embryo development. The study of the effect of suramin added to the culture media during embryo development was never performed, but it should be included in a future work.
In conclusion, our data point out to the possibility of improving bovine oocyte developmental competence through the inhibition of P2Y2R during the exposure to cryoprotectants, in the course of the cryopreservation process. Moreover, the expression of these purinergic G protein–coupled P2Y2 receptors was identified for the first time in both oocytes and cumulus cells of bovine cumulus oocyte complexes. Also, the UTP-specific activation of P2Y2R and consequent induction of intracellular Ca2+ release was blocked by suramin. Cryoprotectants have been previously shown to be implicated in the misbalances in Ca2+ homeostasis caused during the cryopreservation procedures and leading to impaired oocyte developmental competence. In the present study, these negative effects are shown to be partially mitigated through the inhibition of P2Y2R. These findings open important avenues for the development and implementation of new and more successful cryopreservation protocols that include P2Y2R modulation. In the future, the modulation of these receptors on immature oocytes could also be investigated, since the highest level of these receptor transcripts was found in immature cumulus cells.
Abbreviations
- ART
Artificial reproductive technologies
- BSA
Bovine serum albumin
- Ca2+
Calcium
- COC
Cumulus oocyte complexes
- CPA
Cryoprotectants
- DMSO
Dimethylsulfoxide
- EG
Ethylene glycol
- ER
Endoplasmic reticulum
- FITC-PNA
Fluorescein isothiocyanate-conjugated peanut agglutinin;
- IP3
Inositol triphosphate
- [Ca2+]i
Intracellular calcium concentration
- HM
Holding medium
- NCBI
National Center for Biotechnology Information
- PBS
Phosphate-buffered saline
- P2Y2R
P2Y2 receptor
- PVP
Polyvinylpyrrolidone
- SOF
Synthetic oviductal fluid
- TCM
Tissue culture medium 199
- UTP
Uridine 5′-triphosphate
- ZP
Zona pellucida
Funding information
This work was supported by funding from Fundação para a Ciência e Tecnologia, Portugal (PTDC/CVT-REP/2863/2012 and Project UID/CVT/00276/2019) and Alentejo2020, Portugal2020 and UE2020 under project ALT20-03-0246-FEDER-000021.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prates EG, Nunes JT, Pereira RMLN. (2014). A role of lipid metabolism during cumulus-oocyte complex maturation: impact of lipid modulators to improve embryo production. Mediators of Inflammation, volume 2014, Article ID 692067, 11 pages [DOI] [PMC free article] [PubMed]
- 2.De Munck N, Vajta G. Safety and efficiency of oocyte vitrification. Cryobiology. 2017;78:119–127. doi: 10.1016/j.cryobiol.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Pereira RM, Marques CC. Animal oocyte and embryo cryopreservation. Cell Tissue Bank. 2008;9:267–277. doi: 10.1007/s10561-008-9075-2. [DOI] [PubMed] [Google Scholar]
- 4.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141:1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 5.Matos JE, Marques CC, Moura TF, Baptista MC, Horta AEM, Soveral G, Pereira RMLN. Conjugated linoleic acid improves oocyte cryosurvival through modulation of the cryoprotectants influx rate. Reprod Biol Endocrinol. 2015;13:60. doi: 10.1186/s12958-015-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26:2452–2460. doi: 10.1093/humrep/der210. [DOI] [PubMed] [Google Scholar]
- 7.Pimenta J, Pessa P, Marques CC, Azevedo R, Fonseca E, Almeida-Santos T, Pereira RMLN (2017) Medical grade honey in ovarian cortex preservation: an in vitro animal model approach for studying angiogenesis. 5th World Congress of the International Society for Fertility Preservation. Vienna, Austria, November 16-18, p.22. http://www.isfp2017.cme-congresses.com/posters
- 8.Marques CC, Santos-Silva C, Rodrigues C, Matos JE, Moura T, Baptista MC, Horta AEM, Bessa RJB, Alves SP, Soveral G, Pereira RMLN. Bovine oocyte membrane permeability and cryosurvival: effects of different cryoprotectants and calcium in the vitrification media. Cryobiology. 2018;81:4–11. doi: 10.1016/j.cryobiol.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Morato´ R, Izquierdo D, Paramio MT, Mogas T (2008) Cryotops versus open pulled straws (OPS) as carriers for the cryopreservation of bovine oocytes: effects on spindle and chromosome configuration and embryo development. Cryobiology 57:137–141 [DOI] [PubMed]
- 10.Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertility Sterility. 2009;91:1023–1034. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 11.Arav A. Cryopreservation of oocytes and embryos. Theriogenology. 2014;81:96–102. doi: 10.1016/j.theriogenology.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Romão R, Marques CC, Bettencourt E, Pereira RMLN (2016) Cryopreservation of sheep produced embryos: current and future perspectives. In: insights in animal reproduction. Editor: Rita Payan-Carreira. InTech Europe, Croatia ", pp.1-38. ISBN: 978-953-51-4629-2
- 13.Succu S, Berlinguer F, Leoni GG, Bebbere D, Satta V, Marco-Jimenez F, Pasciu V, Naitana S. Calcium concentration in vitrification medium affects the developmental competence of in vitro matured ovine oocytes. Theriogenology. 2011;75:715–721. doi: 10.1016/j.theriogenology.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Kohaya N, Fujiwara K, Ito J, Kashiwazaki N. High developmental rates of mouse oocytes cryopreserved by an optimized vitrification protocol: the effects of cryoprotectants, calcium and cumulus cells. J Reprod Dev. 2011;57:675–680. doi: 10.1262/jrd.11-066H. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara K, Sano D, Seita Y, Inomata T, Ito J, Kashiwazaki N. Ethylene glycol-supplemented calcium-free media improve zona penetration of vitrified rat oocytes by sperm cells. J Reprod Dev. 2010;56:169–175. doi: 10.1262/jrd.09-107H. [DOI] [PubMed] [Google Scholar]
- 16.Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant-induced zona pellucid hardening and increases fertilization rates in mouse oocytes. Reproduction. 2006;131:53–61. doi: 10.1530/rep.1.00878. [DOI] [PubMed] [Google Scholar]
- 17.Tian SJ, Yan CL, Yang HX, Zhou GB, Yang ZQ, Zhu SE. Vitrification solution containing DMSO and EG can induce parthenogenetic activation of in vitro matured ovine oocytes and decrease sperm penetration. Anim Reprod Sci. 2007;101:365–371. doi: 10.1016/j.anireprosci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Tsai PS, van Haeften T, Gadella BM. Preparation of the cortical reaction: maturation-dependent migration of SNARE proteins, clathrin and complexin to the porcine oocyte's surface blocks membrane traffic until fertilization. Biol Reprod. 2011;84:327–335. doi: 10.1095/biolreprod.110.085647. [DOI] [PubMed] [Google Scholar]
- 19.Larman MG, Katz-Jaffe MG, Sheehan CB, Gardner DK. 1,2-propanediol and the type of cryopreservation procedure adversely affect mouse oocyte physiology. Hum Reprod. 2007;22:250–259. doi: 10.1093/humrep/del319. [DOI] [PubMed] [Google Scholar]
- 20.Arellano RO, Martínez-Torres A, Garay E. Ionic currents activated via purinergic receptors in the cumulus cell-enclosed mouse oocyte. Biol Reprod. 2002;67:837–846. doi: 10.1095/biolreprod.102.003889. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahidullah M, Wilson WS. Mobilisation of intracellular calcium by P2Y2 receptors in cultured, non-transformed bovine ciliary epithelial cells. Curr Eye Res. 1997;16:1006–1016. doi: 10.1076/ceyr.16.10.1006.9018. [DOI] [PubMed] [Google Scholar]
- 23.Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol. 2005;564:269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira RM, Baptista MC, Vasques MI, Horta AEM, Portugal PV, Bessa RJ, Silva JC, Pereira MS, Marques CC. Cryosurvival of bovine blastocysts is enhanced by culture with trans-10, cis-12 conjugated linoleic acid. Anim Reprod Sci. 2007;98:293–301. doi: 10.1016/j.anireprosci.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 26.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda T, Kubo A, Taguchi T, Mizumura K. ATP decreases mechanical sensitivity of muscle thin-fiber afferents in rats. Neurosci Res. 2015;97:36–44. doi: 10.1016/j.neures.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Gualtieri R1, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26:2452–2460. doi: 10.1093/humrep/der210. [DOI] [PubMed] [Google Scholar]
- 29.Bello OD, Cappa AI, Paola M, Zanetti MN, Fukuda M, Fissore RA, Mayorga LS, Michaut MA. Rab3A, a possible marker of cortical granules, participates in cortical granule exocytosis in mouse eggs. Exp Cell Res. 2016;347(1):42–51. doi: 10.1016/j.yexcr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira RMLN, Marques CC, Pimenta J, Barbas JP, Batista MC, Torres AC, Lopes da Costa L (2019) Assisted reproductive technologies (ART) directed to germplasm preservation. In: A Portrait of the State-of-the-Art Research at CIISA. Editor: CIISA – FMV, Springer, (accepted)
- 32.Arellano RO, Robles-Martínez L, Serrano-Flores B, Vázquez-Cuevas F, Garay E. Agonist-activated Ca2+ influx and Ca2+ −dependent Cl- channels in Xenopus ovarian follicular cells: functional heterogeneity within the cell monolayer. J Cell Physiol. 2012;227:3457–3470. doi: 10.1002/jcp.24046. [DOI] [PubMed] [Google Scholar]
- 33.Diez C, Duque P, Gomez E, Hidalgo CO, Tamargo C, Rodriguez A, Fernández L, de la Varga S, Fernández A, Facal N, Carbajo M. Bovine oocyte vitrification before or after meiotic arrest: effects on ultrastructure and developmental ability. Theriogenology. 2005;64:317–333. doi: 10.1016/j.theriogenology.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Diez C, Munoz M, Caamano JN, Gomez E. Cryopreservation of the bovine oocyte: current status and perspectives. Reprod Domest Anim. 2012;47:76–83. doi: 10.1111/j.1439-0531.2012.02029.x. [DOI] [PubMed] [Google Scholar]
- 35.Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139:685–695. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- 36.Prates EG, Alves SP, Marques CC, Baptista MC, Horta AE, Bessa RJ, Pereira RM. Fatty acid composition of porcine cumulus oocyte complexes (COC) during maturation: effect of the lipid modulators trans-10, cis-12 conjugated linoleic acid (t10,c12 CLA) and forskolin. In Vitro Cell Dev Biol Anim. 2013;49:335–345. doi: 10.1007/s11626-013-9624-2. [DOI] [PubMed] [Google Scholar]
- 37.Park Y, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 38.Lewis CL, Frazer A, Russell RGG, Bunning RA. The effect of suramin on the resorption of bovine nasal cartilage. Inflammopharmacol. 1999;7:387. doi: 10.1007/s10787-999-0032-x. [DOI] [PubMed] [Google Scholar]