Abstract
In this study, the distribution patterns of P2X1 to P2X7 receptors in the anterior pituitary cells of rat were studied with single-, double-, and triple-labeling immunofluorescence, combined method of immunofluorescence and in situ hybridization, and Western blot. The results showed that the expression level of the P2X4 receptor protein was highest, followed by P2X5, P2X3, P2X2, P2X6, and P2X7 receptor proteins, but no P2X1 receptor protein was detected. Strong P2X4 receptor-immunoreactivity was detected in almost all the anterior pituitary cells. Different combinations of P2X receptors were detected in each individual cell type of the rat anterior pituitary. Gonadotrophs express P2X4, P2X5, and P2X6 receptors. Corticotrophs express P2X3 and P2X4 receptors. Folliculo-stellate cells express P2X2 and P2X4 receptors, and somatotrophs, lactotrophs, and thyrotrophs express only P2X4 receptors. The macrophages with Iba-1-ir expressed P2X7 receptors. The possible functions of these P2X receptors in each individual cell type of the rat anterior pituitary are discussed.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09685-y) contains supplementary material, which is available to authorized users.
Keywords: P2X receptors, Pituitary, Immunofluorescence, In situ hybridization, Rat
Introduction
The pituitary gland is often referred to as the “master” endocrine organ of the body. It is located at the base of the brain. The gland consists of two major parts: anterior pituitary (adenohypophysis) and posterior pituitary (neurohypophysis). The main cellular components of the anterior pituitary are lactotrophs, somatotrophs, corticotrophs, gonadotrophs, and thyrotrophs. These cells release prolactin (PRL), growth hormone (GH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH)/luteinizing hormone (LH), and thyroid-stimulating hormone (TSH), respectively. These hormones have very important functions in lactation in females; muscle and bone growth; maturation of sexual organs; and hormone release of adrenal cortex and growth of the thyroid gland and release of its hormone [2, 4, 5, 8].
There is increasing evidence to show that extracellular purines and pyrimidines via P2X and P2Y receptors, as well as adenosine via P1 receptors, have important physiological functions in the regulation of anterior pituitary cell secretions. Using cell calcium measurement techniques, preliminary characteristics of purine receptors in adenohypophysis cells were determined and all the secretory cells have functional purine receptors [3, 6, 9, 12, 14].
To our knowledge, there is no detailed morphological data of P2X receptor subunit expression on anterior pituitary cells. In this study, localization of expression of the seven P2X receptor subunits on the rat anterior pituitary cells was systematically studied using immunofluorescence, in situ hybridization, and Western blot techniques. The results show that the expression levels of the P2X4 receptor were highest, followed by P2X5, P2X3, P2X2, P2X6, and P2X7 receptors. Strong P2X4 receptor-immunoreactivity (-ir) was detected in almost all the anterior pituitary cells. P2X5 and P2X6 receptor-ir were detected in LH and TSH cells; P2X3 receptor-ir was detected exclusively in the ACTH cells; P2X2 receptor-ir was detected in the majority of folliculo-stellate cells.
Materials and methods
Animals and tissue preparation
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Second Military Medical University. Five Sprague Dawley rats were used. Animals were anesthetized by intraperitoneal injection with pentobarbital sodium and perfused through the aorta with a 0.9% NaCl solution and 4% paraformaldehyde in 0.05 mol/L phosphate buffer, pH 7.4 (PBS). The pituitaries were dissected out and re-fixed in 4% paraformaldehyde in PBS overnight, then transferred to 25% sucrose in PBS and kept in this solution until they sank to the bottom. Thereafter, the segment blocks were rapidly frozen and crosscut sections (20 μm in thickness) were cut with a Leica cryostat and floated in PBS.
Immunohistochemistry
Table 1 shows the primary antibodies used in this study.
Table 1.
Primary antibodies used in this study
| Primary antibodies | Host | company | Catalog no. | Dilution | ||
|---|---|---|---|---|---|---|
| IF | TSA | WB | ||||
| P2X1 | Rabbit | Alomone | APR-001 | 1:200 | 1:4000 | 1:1000 |
| P2X2 | Rabbit | Alomone | APR-003 | 1:200 | 1:4000 | 1:1000 |
| P2X3 | Rabbit | Roche | 1:400 | 1:8000 | 1:1000 | |
| P2X4 | Rabbit | Santa Cruz | ARP-002 | 1:400 | 1:8000 | 1:1000 |
| P2X5 | Rabbit | Boster | PB0339 | 1:500 | 1:8000 | 1:1000 |
| P2X6 | Rabbit | Alomone | APR-013 | 1:200 | 1:4000 | 1:1000 |
| P2X7 | Rabbit | Alomone | APR-004 | 1:400 | 1:8000 | 1:1000 |
| ACTH | Rabbit | Sigma | A1927 | 1:600 | 1:10000 | |
| TSH | Rabbit | Chemicon | AB976 | 1:400 | 1:10000 | |
| S-100 | Mouse | Abcam | ab4066 | 1:200 | ||
| Iba-1 | Rabbit | Wako | 17919741 | 1:1000 | ||
| Digoxigenin | Mouse | Jackson | 200–002-156 | 1:200 | ||
| GAPDH | Mouse | Beyotime | AG019 | 1:1000 | ||
As most of the primary antibodies are from the same host (rabbit), the double- or triple-labeling immunofluorescence protocol was used and modified based on previous reports [11, 15]. The protocol was as follows: endogenous peroxidase was blocked by 1% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% normal horse serum (NHS), 0.2% Triton X-100 in PBS for 30 min, followed by incubation with the P2X antibodies in antiserum solution overnight at 4 °C. Subsequently, the sections were incubated with biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) at a dilution of 1:500 in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1000 in PBS for 30 min at room temperature. P2X receptor-ir was visualized by the TSA (Tyramide Signal Amplification) fluorescein system (NEL701, NEN, USA). After visualization, the sections were incubated with the second primary antibodies, and S100 and Iba-1 in antiserum solution overnight at 4 °C. Subsequently, the sections were incubated with Cy3-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories) diluted 1:400 in antiserum solution for 1 h at room temperature. All the incubations and reactions were separated by 3 × 10 min washes in PBS.
As the specific antibodies for growth hormone (GH) and prolactin (PRL) could not be obtained for this study, sense– and antisense digoxigenin–labeled oligonucleotide probes were synthesized (Sagon, Shanghai, China). The sense and antisense nucleotide sequences are detailed in Table 2. In situ hybridization was carried out using the following protocol. The sections were heated in 0.01 mol/l citrate buffer pH 6.8 in a 92 °C water bath for 15 min then allowed to cool to room temperature in the same buffer. The sections were rinsed in PBS for 3 min 2 ×, then incubated in 0.25% acetic anhydride with 0.1 M triethanolamine (pH 8.0) for 10 min at room temperature, followed by washing in 2 × saline sodium citrate (SSC) for 10 min. Digoxigenin-labeled oligonucleotides (0.5 μg/ml) of either antisense or sense probe was added to the hybridization solution containing 50% formamide, 10% dextrans-sulphate, 0.3 M NaCl, 1 × Denhardt’s solution, 0.05 M Tris-HC (pH 8.0), 1 mM EDTA, and 250 μg/ml Herring sperm DNA (Sigma). Hybridization was carried out for 12–16 h at 45 °C in a hybridization oven. The sections were washed in 4 × SSC for 20 min, in 2 × SSC for 20 min, in 1 × SSC for 20 min, and in 0.1 × SSC for 20 min at 37 °C. The following method was used to detect hybridization signals and P2X receptors. Endogenous peroxidase was blocked by 3% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% NHS, 0.2% Triton X-100 in PBS for 30 min, followed by incubation with two primary antibodies (mouse anti-digoxigenin IgG and one of the P2X receptor antibodies) diluted in antibody dilution solution overnight at 4 °C. Subsequently, the sections were incubated with two secondary antibodies of biotinylated donkey anti-rabbit IgG for P2X and Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) for digoxigenin in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1000 in PBS for 30 min at room temperature. P2X receptor subunit-ir was visualized by the TSA Fluorecein system. All the incubations and reactions were separated by 10 min × 3 washes in PBS. Some sections were counter-stained with 5 μg/ml Hoechst 33342.
Table 2.
GH and PRL oligonucleotide probe sequences
| Gene | Probe | Sequences 5′-3′ | GenBank accession |
|---|---|---|---|
| GH | antisense 1 | Dig-GGGCATGGCAGGGAAAGCACCAGCCTCTTGAGGCCACAGCAGGCAGAGC | U62779.1 |
| GH | antisense2 | Dig-GCTGGGCCTCCTCCTTGCCGGTGGGGGCTGGGATGGTCTCTGAGAAGCAG | U62779.1 |
| GH | sense 1 | Dig-GCTCTGCCTGCTGTGGCCTCAAGAGGCTGGTGCTTTCCCTGCCATGCCC | U62779.1 |
| GH | sense2 | Dig-CTGCTTCTCAGAGACCATCCCAGCCCCCACCGGCAAGGAGGAGGCCCAGC | U62779.1 |
| PRL | antisense 1 | Dig-CCGGGCTGACACTTGGCTGTTCATGGTGATGACCACTGGGACCACACTTC | NM_012629.1 |
| PRL | antisense2 | Dig-CCGGGAGAGGTGTCTGGCAGTCGCCACCAGAACAGACTGGCAGGGTCTGC | NM_012629.1 |
| PRL | sense 1 | Dig-GAAGTGTGGTCCCAGTGGTCATCACCATGAACAGCCAAGTGTCAGCCCGG | NM_012629.1 |
| PRL | sense2 | Dig-GCAGACCCTGCCAGTCTGTTCTGGTGGCGACTGCCAGACACCTCTCCCGG | NM_012629.1 |
Triple-labeling immunofluorescence was carried out to detect P2X5 and P2X6 and LH or P2X5, P2X6, and TSH in the same section at the same time. Firstly, the TSA fluorescein system was used to detect P2X6 receptors. The protocol was as follows: endogenous peroxidase was blocked by 3% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% NHS, 0.2% Triton X-100 in PBS for 30 min, followed by incubation with the P2X6 receptor antibody in antibody dilution solution overnight at 4 °C. Subsequently, the sections were incubated with biotinylated donkey anti-rabbit IgG in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1000 in PBS for 30 min at room temperature. P2X6 receptor-ir was visualized by the TSA Fluorecein system. All the incubations and reactions were separated by 10 min × 3 washes in PBS. After P2X6 receptor visualization, the sections were incubated with 3H2O2 in PBS for 30 min at room temperature to inactivate the activity of the extravidin peroxidase used above, incubated with 1 μm streptoavidin in PBS for 20 min, and with 1 μm biotin for another 20 min to extinguish endogenous biotin. All the incubations were separated by 5 min × 3 washes in PBS. Then the TSA-Cy3 (Tyramdie Signal Amplification-cyanine 3) system was used to detect P2X5 receptors, the protocol of which was the same as above. Finally, AMCA-conjugated Donkey anti-rabbit IgG was used to detect LH or TSH.
Western blot
Western blot analysis was carried out to further study the expression of P2X receptors in the rat anterior pituitary. The three rats were deeply anesthetized by sodium pentobarbital (60 mg/kg) and killed by decapitation. The anterior pituitaries were rapidly removed, washed with ice-cold PBS and lysed with 20 mM Tris-HCl buffer, pH 8.0, containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (Sigma). Protein concentration was determined by the BCA protein assay method using bovine serum albumin as a standard (BCA protein assay kit from beyotime). Protein samples (50 μg) were loaded per lane, separated by SDS-PAGE (10% polyacrylamide gels), and then were electrotransferred onto nitrocellulose membranes. The membranes were blocked with 10% nonfat dry milk in Tris-buffered saline for 1 h and incubated overnight at 4 °C with P2X antibodies in 2% BSA in PBS. The membranes were then incubated with alkaline phosphatase–conjugated goat anti-rabbit IgG (Sigma) diluted 1:5000 in 2% BSA in PBS for 1 h at room temperature. The color development was performed with 400 μg/ml nitro-blue tetrazolium and 200 μg/ml 5-bromo-4-chloro-3-indolyl phosphate in TSM (0.1 mol/l Tris–HCl2 buffer, pH 9.5, 0.1 mol/l NaCl, and 0.05 mol/l MgCl2) in the dark. Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories). Western blot analysis was repeated three times with different rat pituitary extracts.
Control experiments were carried out as follow: at first, the P2X antibodies were diluted in antibody solution as Table 1, then their own peptides were added separately (5 μg/ml), and incubated at 4 °C overnight. Afterwards, control experiments for immunofluorescence and Western blot were carried out by pre-absorbing the antibodies with the individual peptides. A further negative control was carried out by omitting the primary antibodies. No staining was observed in the control preparations.
Quantitative analysis
Quantitative analysis for the P2X receptors immunostaining in the anterior pituitary was performed as follows: five random fields (each area was 0.36 mm2) for one section were chosen and the number of positive cells was counted and expressed as the positive cell numbers/mm2. Five fields for each of the five sections from each of the five rats were used. The mean number of positive cells/mm2 from each rat was calculated and expressed as mean ± standard error of the mean (n = number of rat).
Photomicroscopy
Images were taken with a Nikon digital camera DXM1200 (Nikon, Japan) attached to a Nikon Eclipse E600 microscope (Nikon). Images were imported into a graphics package (Adobe Photoshop).
Results
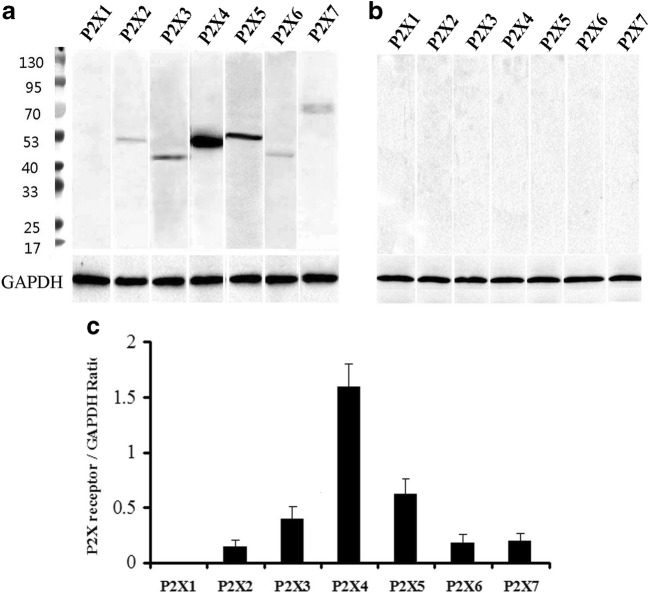
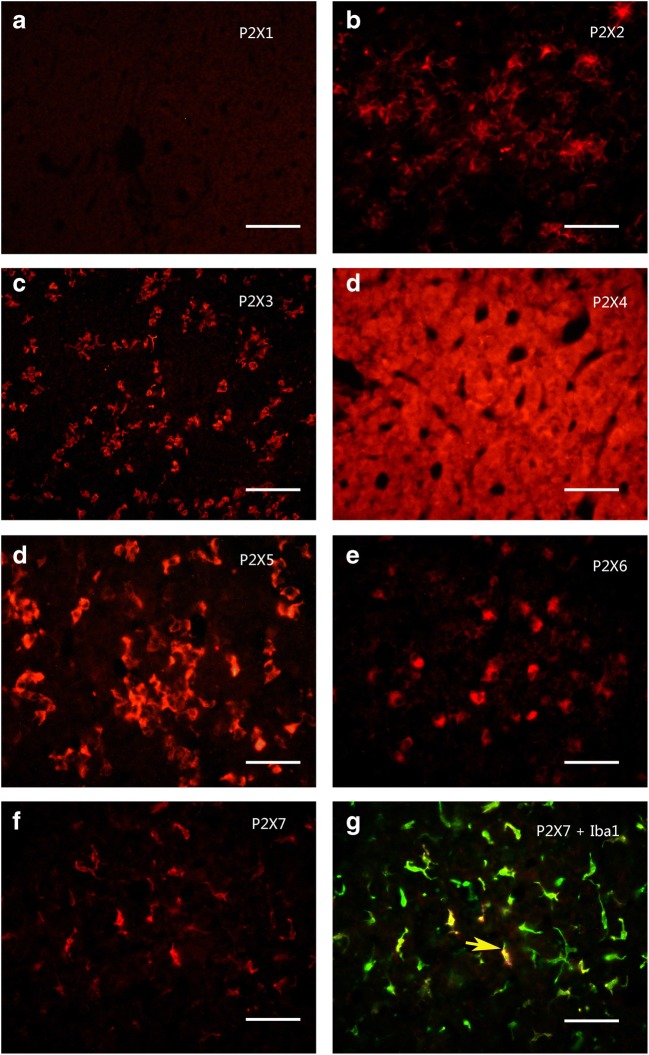
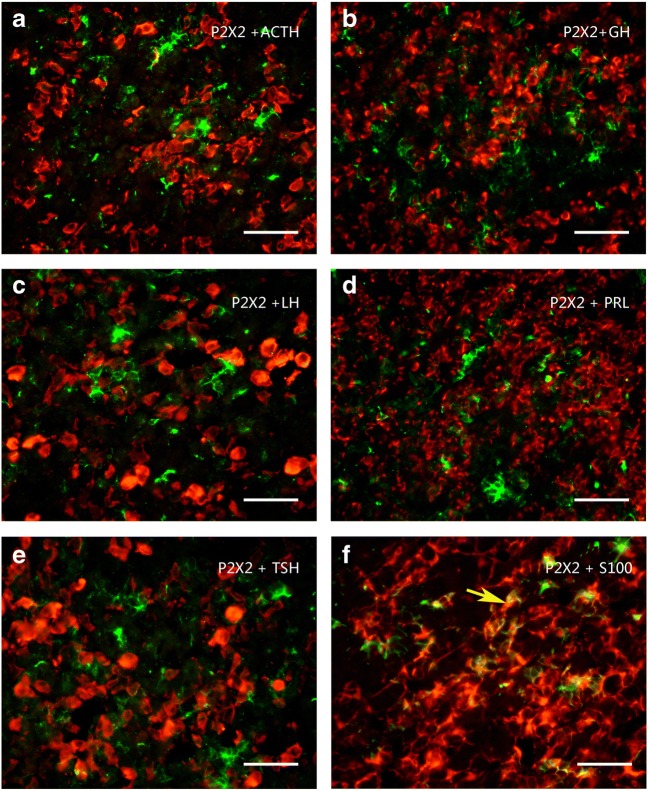
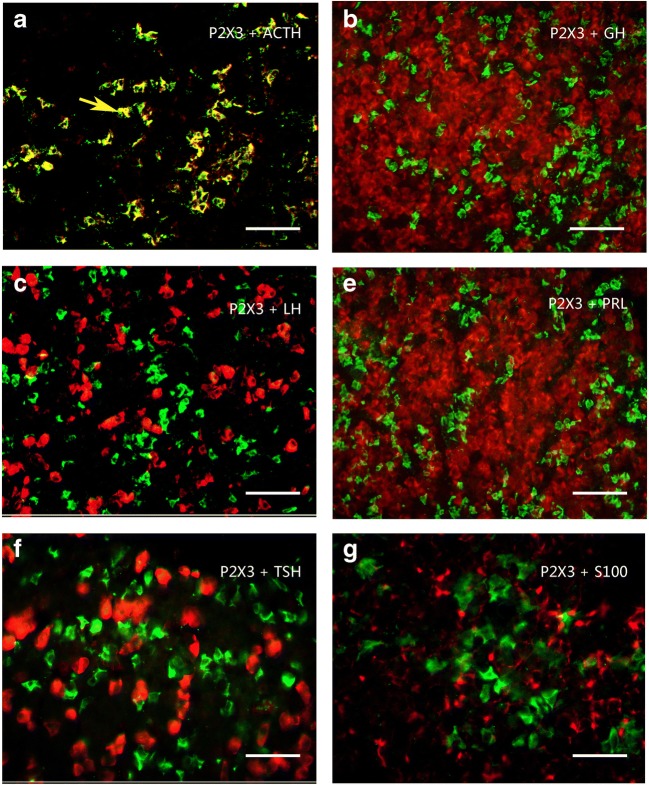
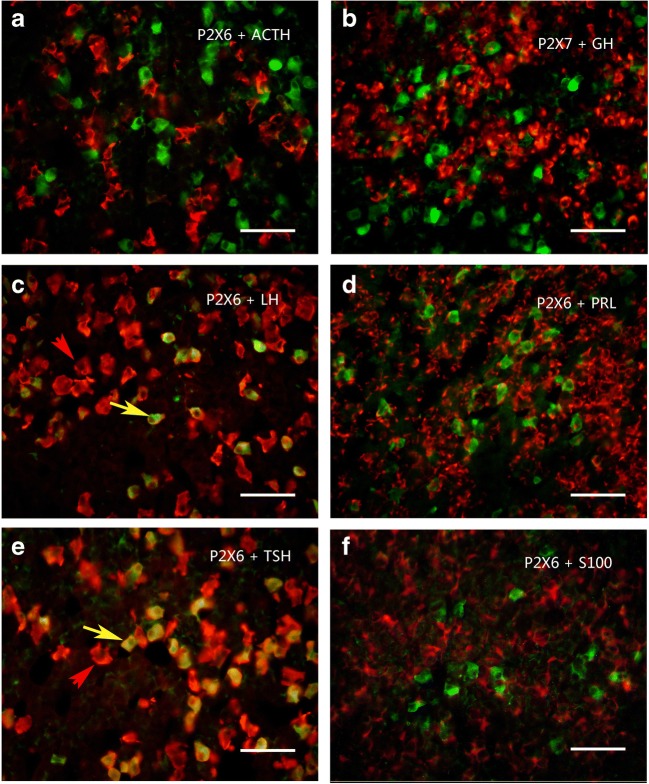
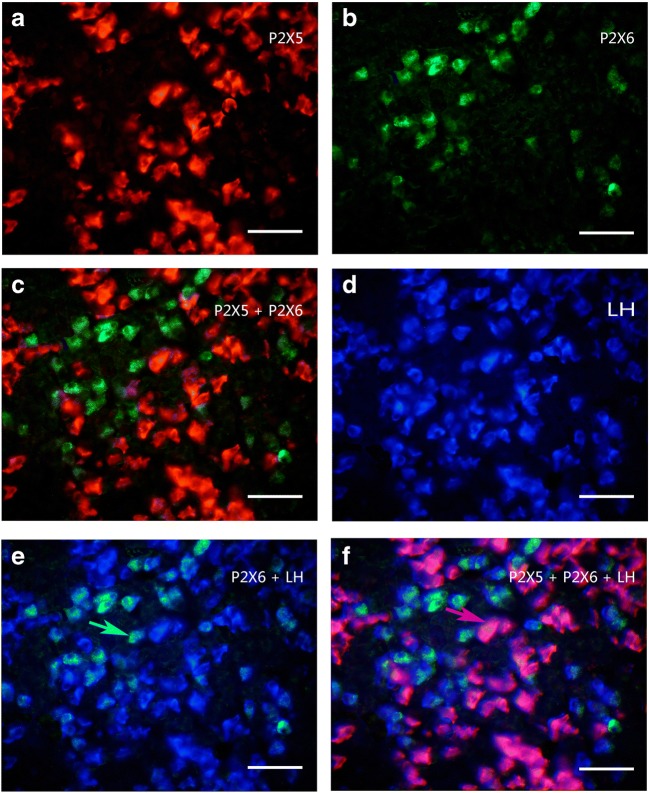
Using Western blot techniques, the protein bands for P2X2–P2X7 receptors, but not for P2X1, were detected in the rat anterior pituitary extracts. Among these seven receptors, P2X4 receptor protein had the highest expression level, the band being very strong, with a molecular weight of about 50 kd. The expression level of P2X5 receptor protein was less than the P2X4 receptor protein, the molecular weight of the band was demonstrated to be about 52 kd. The expression level of P2X3 receptor protein was less than the P2X5 receptor protein, the molecular weight was found to be about 44 kd. P2X2, P2X6, and P2X7 receptor protein expression levels were similar and lower. The molecular weights of the bands for P2X2, P2X6, and P2X7 receptor antibodies were about 50 kd, 43 kd, and 70 kd, respectively (Fig. 1a). The bands for the P2X receptor antibodies disappeared after the antibodies have been pre-absorpted by their respective peptides (Fig. 1b), which indicates that these antibodies specifically recognize the corresponding protein bands. Figure 1c shows the band density ratio of P2X receptors over GAPDH. No P2X1 receptor-ir cells were detected in the rat anterior pituitary (Fig. 2a). P2X2-ir receptor cells were generally ovoid in shape with weak to moderate immunostaining, some of P2X2 receptor-ir cells were angular in shape with processes. Double-labeling immunofluorescence showed that the majority of P2X2-ir cells were also immunoreactive for S100-ir cells, but none of them were immunoreactive for ACTH, LH, or TSH in the anterior pituitary of the rat (Fig. 3). Growth hormone (GH) and prolactin (PRL) mRNA hybridization signals were also not detected in the P2X2 receptor-ir cells in the anterior pituitary (Fig. 3). P2X3 receptor-ir cells were distributed widely in the rat anterior pituitary (Fig. 2c). Double-labeling immunofluorescence showed that all the P2X3 receptor-ir cells were also immunoreactive for ACTH, but none of them were immunoreactive for LH, TSH, and S100. GH and PRL mRNA hybridization signals were also not detected in the P2X3 receptor-ir cells in the anterior pituitary (Fig. 4). P2X4-ir was detected in almost all the cells in the anterior pituitary. The boundary between P2X4-ir cells was unclear, although some oval-shaped positive cells could be distinguished (Fig. 2d). Double-labeling immunofluorescence showed that almost all of the P2X4 receptor-ir cells were found to be immunoreactive for ACTH, LH, and TSH, and also to contain GH and PRL mRNA hybridization signals (Fig. 5). The majority of the S100-ir cells were also labeled with P2X4-ir (Fig. 5f). Moderate to strong immunostaining of P2X5 receptor cells were found widely in the rat anterior pituitary (Fig. 2e). All of these P2X5 receptor-ir cells were immunoreactive for LH or TSH. The majority of the LH or TSH-ir cells were immunoreactive for P2X5 (Fig. 6). No ACTH-ir and S100-ir cells, and GH and PRL mRNA hybridization signals cells were found to be immunoreactive for P2X5 (Fig. 6). Weak to moderate immunostaining of P2X6 receptor-ir cells were detected and scattered in the rat anterior pituitary. The number of P2X6 receptor-ir cells was relatively low (Fig. 2f). All of the P2X6 receptor-ir cells were immunoreative for LH or TSH, but only a minority of the LH or TSH-ir cells were immunoreactive for P2X6 (Fig. 7). No ACTH-ir and S100-ir cells, and GH and PRL mRNA hybridization signals cells were found to be immunoreactive for P2X6 (Fig. 7). As P2X5 and P2X6 receptors were found to coexist with LH or TSH, it implies that P2X5 and P2X6 receptors, and FSH and TSH may coexist in the same endocrine cells. As such, triple-labeling immunostaining experiments were carried out to clarify this issue. The results showed that none of the P2X5 receptor-ir cells were also labeled by the P2X6 receptor antibody and vice versa, although both P2X5 and P2X6-ir cells were labeled by LH or TSH antibodies (Figs. 8 and 9). Figures 8f and 9f show that all the LH or TSH-ir cells were labeled by P2X5 and P2X6 antibodies. Quantitative data of the coexistence between P2X5 and P2X6 receptor-ir cells, and LH-ir and TSH-ir cells are summarized in Table 3. Moderate immunostaining of P2X7 receptors in cells with 2 to 3 long processes were detected widely in the anterior pituitary, all of them were labeled by Iba-1, but not by ACTH, LH, TSH, S100, or GH, and PRL mRNA hybridization signals (Fig. 2g, h). Localization of P2X1 to P2X7 receptor-ir in ACTH-ir LH-ir, TSH-ir, S100-ir, Iba-1-ir cells, and GH and PRL mRNA hybridization signals in cells are summarized in Table 4.
Fig. 1.
Western blot analysis of P2X receptor expression in the anterior pituitary. a Lane P2X1 to P2X7 are the results detected by P2X1 to P2X7 antibodies, respectively, lane M is the molecular weight marker, note that a strong immunostained band was detected by the P2X4 antibody, followed by the P2X5, P2X3, P2X2, P2X7, and P2X6 receptor antibodies, no band was detected by P2X1 receptor antibody. b Lane P2X1 to P2X7 are the results of the antibody pre-absorption control experiments for P2X1 to P2X7, note that no bands were detected. c The band density ratio of each P2X receptor over GAPDH
Fig. 2.
Expression of P2X1 to P2X7 receptor-ir (red) in the rat anterior pituitary. a to f P2X1 to P2X7 receptor immunoreactive cells respectively. Note that strong immunostaining was detected using the P2X4 receptor antibody, followed by P2X5, P2X3, P2X6, P2X2, and P2X7 receptor antibodies. No obvious immunostaining was detected by the P2X1 antibody. g A merged image from f and an image of Iba-1-ir cells (green) (a macrophage/microglia marker) in the same field of f. Note that all the P2X7 receptor reactive cells were labeled by Iba-1-ir. All scale bars = 120 μm
Fig. 3.
Colocalization of P2X2 receptor-ir (green) with ACTH-ir, LH-ir, TSH-ir (red), and GH and PRL mRNA hybridization signals (red) in rat anterior pituitary. Note that majority of P2X2 receptor-ir cells were labeled by S100-ir f, no P2X2-ir cells were labeled by ACTH-ir, LH-ir, TSH-ir, and GH and PRL mRNA hybridization signals. An arrow indicates a cell double labeled (yellow) with P2X2-ir and S100-ir. All scale bars = 120 μm
Fig. 4.
Colocalization of P2X3 receptor-ir (green) with ACTH-ir, LH-ir, TSH-ir (red), and GH and PRL mRNA hybridization signals (red) in rat anterior pituitary. Note that all the P2X3 receptor-ir cells were labeled by ACTH-ir. No P2X3 receptor-ir cells were detected that were also LH-ir, TSH-ir, or S100-ir, and GH and PRL mRNA hybridization signals. An arrow indicates a cell double labeled (yellow) with P2X3-ir and ACTH in a. All scale bars = 120 μm
Fig. 5.
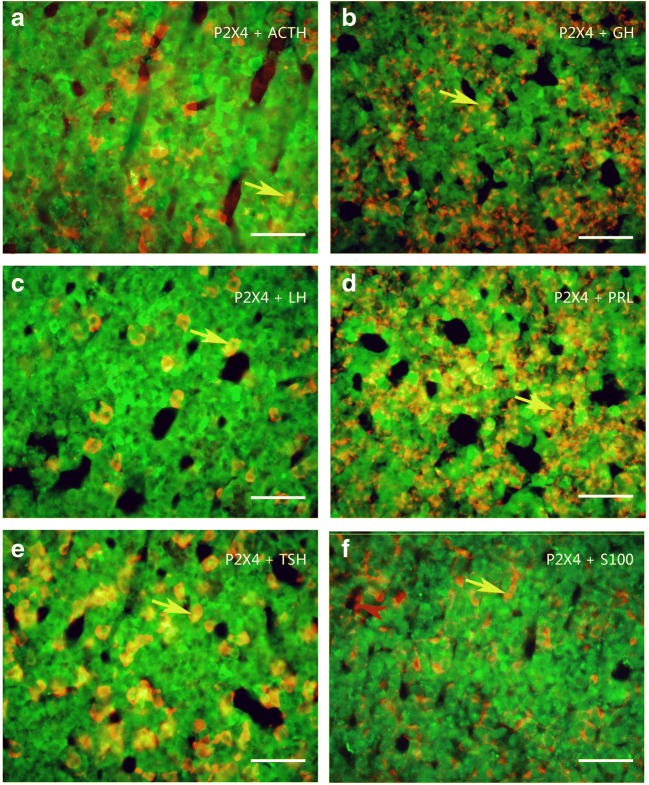
Colocalization of P2X4 receptor-ir (green) with ACTH-ir, LH-ir, TSH-ir (red), and GH and PRL mRNA hybridization signals (red) in rat anterior pituitary. Note that the majority of P2X4 receptor-ir cells were labeled by ACTH-ir, LH-ir, TSH-ir, S100-ir, and GH and PRL mRNA hybridization signals, only a few S100-ir cells were not labeled by P2X4-ir, indicated by an arrowhead in f. An arrow indicates a cell double labeled with P2X4-ir and ACTH, GH mRNA hybridization signals, LH-ir, PRL mRNA hybridization signals, and TSH-ir in a to e, respectively. All scale bars = 120 μm
Fig. 6.
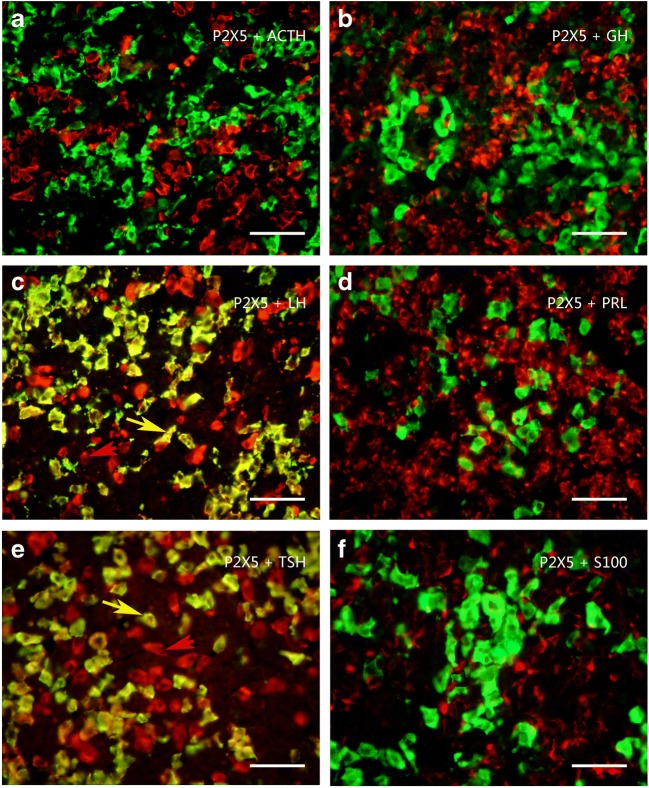
Colocalization of P2X5 receptor-ir (green) with ACTH-ir, LH-ir, TSH-ir (red), and GH and PRL mRNA hybridization signals (red) in rat anterior pituitary. Note that all P2X5 receptor-ir cells were labeled by LH-ir or TSH-ir, and the majority of LH-ir or TSH-ir cells were also labeled by P2X5-ir. No P2X5 receptor-ir cells were detected with ACT-ir, S100-ir, and GH and PRL mRNA hybridization signals. An arrow indicates a cell double labeled with P2X5-ir and LH-ir or TSH-ir in c and e, respectively. All scale bars = 120 μm
Fig. 7.
Colocalization of P2X6 receptor-ir (green) with ACTH-ir, LH-ir, TSH-ir (red), and GH and PRL mRNA hybridization signals (red) in rat anterior pituitary. Note that all P2X6 receptor-ir cells were labeled by LH-ir or TSH-ir, but only a minority of LH-ir or TSH-ir cells were also labeled by P2X6-ir. No P2X6 receptor-ir cells were detected with ACT-ir, S100-ir, and GH and PRL mRNA hybridization signals. An arrow indicates a cell double labeled with P2X6-ir and LH-ir or TSH-ir in c and e, respectively. All scale bars = 120 μm
Fig. 8.
Colocalization of P2X5 receptor-ir (a, red), P2X6 receptor-ir (b, green) and LH-ir (d, blue) in rat anterior pituitary. c The merged image from a and b, note that no P2X5 receptor-ir cells were labeled by P2X6-ir. e The merged image from b and d, note that all the P2X6 receptor-ir cells were labeled with LH-ir, although only a minority of LH-ir cells were labeled with P2X6 receptor-ir. f The merged image from a, b, and d, note that all LH-ir cells were labeled with P2X5 and P2X6 receptor-ir. No LH-ir cells were single labeled. An arrow indicates a cell double labeled with P2X6-ir and LH-ir or P2X5 receptor-ir and LH-ir in e and f, respectively. All scale bars = 120 μm
Fig. 9.
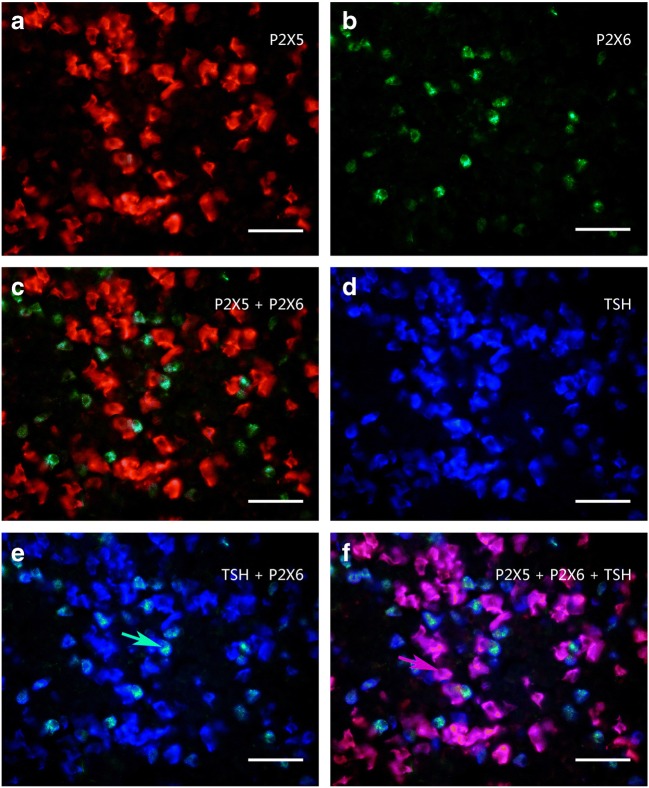
Colocalization of P2X5 receptor-ir (a, red), P2X6 receptor-ir (b, green), and TSH-ir (d, blue) in rat anterior pituitary. c The merged image from a and b, note that no P2X5 receptor-ir cells were labeled by P2X6-ir. e The merged image from b and d, note that all the P2X6 receptor-ir cells were labeled with TSH-ir, although only a minority of TSH-ir cells were labeled with P2X6 receptor-ir. f The merged image from a, b, and d, note that all TSH-ir cells were labeled with P2X5 and P2X6 receptor-ir. No TSH-ir cells were single labeled. An arrow indicates a cell double labeled with P2X6-ir and TSH-ir or P2X5 receptor-ir and TSH-ir in e and f, respectively. All scale bars = 120 μm
Table 3.
Quantitative analysis of the coexistence between P2X5 and P2X6 receptor-ir cells, and LH-ir and TSH-ir cells (the positive cell number/0.36 mm2)
| Total no | P2X5 receptor-ir | P2X6 receptor-ir | |||
|---|---|---|---|---|---|
| + | – | + | – | ||
| LH+ | 55 ± 6 | 46 ± 8 | 8 ± 3 | 8 ± 3 | 46 ± 8 |
| TSH | 60 ± 9 | 50 ± 9 | 10 ± 4 | 10 ± 4 | 50 ± 9 |
Table 4.
Coexistence between P2X receptors and pituitary hormones or S100
| P2X | ACTH | LH | GH | PRL | TSH | S100 |
|---|---|---|---|---|---|---|
| P2X1 | – | – | – | – | – | – |
| P2X2 | – | – | – | – | – | + |
| P2X3 | + | – | – | – | – | – |
| P2X4 | + | + | + | + | + | + |
| P2X5 | – | + | – | – | + | – |
| P2X6 | – | + | – | – | + | – |
| P2X7 | – | – | – | – | – | – |
+ coexistence; − no coexistence
Control experiments were also carried out with P2X1 to P2X7 antibodies pr-eabsorbed with P2X1 to P2X7 receptor peptides. No staining was observed in the preparations incubated with antiserum solutions pre-absorbed with the P2X1 to P2X7 peptides (supplementary Fig. 1). No hybridization signals to GH and PRL mRNA were detected in the sections incubated with sense GH and PRL oligonucleotide probes (supplementary Fig. 2).
Discussion
In this study, the expression of all seven P2X receptor subunits and their colocalization with typical pituitary hormone and stem cell markers were studied systematically in the rat anterior pituitary with single, double, and triple immunofluorescence, a combined method of in situ hybridization and immunofluorescence, and Western blot. To our knowledge, this is the first report of expression of all seven P2X receptor subunits in each cell type of the pituitary with immunofluorescence in vivo.
The present study showed that the P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 receptor subunits were detected in the anterior pituitary. Corticotroph, gonadotroph, somatotroph, lactotroph, thyrotroph, and folliculo-stellate cells (stem cell) in the anterior pituitary expressed at least one of the P2X receptor subunits. The results presented in this study were validated by rigorous control experiments.
Few publications about the expression or function of P2X receptors in corticotrophs of the anterior pituitary could be found. Previous data showed that ATP and its derivatives induce POMC gene expression in a mouse pituitary corticotroph cell line and mRNAs for P2X1, P2X3, P2X4, P2X6, P2X7, P2Y1, P2Y2, P2Y4, A1, and A2A receptors were identified by RT-PCR in this cell line [18]. In the present study, expression of P2X3 and P2X4 receptors was identified in corticotroph cells of the rat anterior pituitary, which further supported that ATP via P2X3 or P2X4 receptors promotes the expression of the POMC gene.
No data of the expression and function of P2X receptors in somatotrophs have been reported. In the present study, P2X4 receptors were detected in GH cells, which implies that P2X4 receptors may have a role, although further experiments will need to be carried out to confirm this role.
Lactotrophs and GH3 cells were shown to express transcripts for P2X3, P2X4, and P2X7 receptors [6]. A heteropolymeric P2X2 receptor has been claimed to mediate hormone release from lactotrophs [10]. In the present study, only P2X4 receptor-ir was detected in lactotrophs, whereas P2X2, P2X3, and P2X7 receptor-ir were detected in folliculo-stellate cells, corticotrophs, and macrophages respectively. The prolactin secretory studies clearly indicate that P2X4Rs are present in lactotrophs and activation of these receptors potentiates basal prolactin release [17], the presence of which are further confirmed by the present study.
Zemkova et al. [17] reported that extracellular ATP induced an inward depolarizing current in the majority of thyrotropin-releasing hormone-responsive pituitary cells, which resembled the current profile generated by recombinant P2X4 receptors with single-cell patch-clamp recordings. And they also reported mRNA transcripts for the P2X4 receptor subunit are the most abundant in rat anterior pituitary tissue using quantitative RT-PCR, although further studies are needed to clarify whether thyrotrophs also express P2X4 receptors [17]. In the present study, the highest expression level of P2X receptor protein was for P2X4 receptors, which was expressed in all the cells examined, including thyrotrophs. So P2X4 receptors may have a functional role in thyrotrophs.
The folliculo-stellate cells are glia-like cells in the anterior pituitary, which express nervous tissue–specific S100 protein [7]. The physiological functions of the folliculo-stellate cells are heterogeneous. They are involved in regulating the activity of the pituitary endocrine cells, act as immune cells such as macrophages and dendritic cells, and represent an adult stem cell population of the pituitary [1]. Previous data showed that both ATP and UTP increased the intracellular Ca2+ concentration of primary cultured folliculo-stellate cells of the pituitary in a concentration-dependent manner in a range between 0.1 and 10 μM. The response was completely suppressed by thapsigargin, an inhibitor of endoplasmic reticulum Ca2+-ATPase, and was significantly suppressed by U-73122, an inhibitor of PLC. These results indicate that ATP increases the intracellular Ca2+ concentration of folliculo-stellate cells by activating PLC via P2Y2 receptors [13], previous morphological data supported this results [16]. In present study, P2X2 receptor-ir was detected in the folliculo-stellate cells with S100-ir in the rat anterior pituitary. This result implies that ATP, released by autocrine or paracrine mechanisms, acting via P2X2 receptors, is also involved in modulating the activity of folliculo-stellate cells.
In conclusion, the present study showed that P2X receptors are widely expressed in the anterior pituitary. All cell types, corticotrophs, gonadotrophs, somatotrophs, lactotrophs, thyrotrophs, and folliculo-stellate cells, express at least one P2X receptor subunit, which implies that ATP could regulate the functions of these cells; but so far, functional P2X2 and P2X4 receptor channels have been documented only in gonadotrophs and lactotrophs, respectively. Further functional work should be focused on the identification of other receptor subtypes expressed in other secretory cell types, as well as in non-secretory folliculostrellate cells.
Electronic supplementary material
control experiment of immunofluorescence. A to G show the results in the sections incubated with the P2X1 to P2X7 antibodies pre-absorbed with their relative peptides. Note that no obvious immunofluorescence signals were detected in the sections. All scale bars = 120 μm. (JPG 899 kb)
control experiment of in situ hybridization. A and B show the results of the sections incubated with GH and PRL sense oligonucleotide probes. Note that no obvious hybridization signals were detected. All scale bars = 120 μm. (JPG 689 kb)
Funding information
This work was financially supported by the National Natural Science Foundation of P. R. China (81471260 to Z Xiang).
Compliance with ethical standards
Conflicts of interest
Wenqi Zhao declares that she has no conflict of interest.
Yu Zhang declares that she has no conflict of interest.
Ruihua Ji declares that she has no conflict of interest.
Gillian E. Knight declares that she has no conflict of interest.
Geoffrey Burnstock declares that he has no conflict of interest.
Hongbin Yuan declares that he has no conflict of interest.
Zhenghua Xiang declares that he has no conflict of interest.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Second Military Medical University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenqi Zhao, Yu Zhang and Ruihua Ji contributed equally to this work.
Contributor Information
Hongbin Yuan, Email: jfjczyy@aliyun.com.
Zhenghua Xiang, Email: xiang-zhenghua@smmu.edu.cn.
References
- 1.Allaerts W, Vankelecom H. History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol. 2005;153:1–12. doi: 10.1530/eje.1.01949. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signalling in endocrine organs. Purinergic Signal. 2014;10:189–231. doi: 10.1007/s11302-013-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca(2+)](i) changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–1852. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florio T. Adult pituitary stem cells: from pituitary plasticity to adenoma development. Neuroendocrinology. 2011;94:265–277. doi: 10.1159/000330857. [DOI] [PubMed] [Google Scholar]
- 5.Herlant M. The cells of the adenohypophysis and their functional significance. Int Rev Cytol. 1964;17:299–382. doi: 10.1016/S0074-7696(08)60409-X. [DOI] [PubMed] [Google Scholar]
- 6.Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology. 2000;141:4091–4099. doi: 10.1210/endo.141.11.7737. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima T, Yamaguchi H, Takahashi K. S100 protein in folliculostellate cells of the rat pituitary anterior lobe. Brain Res. 1980;191:523–531. doi: 10.1016/0006-8993(80)91300-1. [DOI] [PubMed] [Google Scholar]
- 8.Nakane PK. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem. 1970;18:9–20. doi: 10.1177/18.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Nunez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Phys. 1997;272:E1117–E1123. doi: 10.1152/ajpendo.1997.272.6.E1117. [DOI] [PubMed] [Google Scholar]
- 10.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/S1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- 11.Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, Klein G. Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Methods. 1998;73:89–97. doi: 10.1016/S0166-0934(98)00048-2. [DOI] [PubMed] [Google Scholar]
- 12.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama M, Nakajima Y, Sakuma Y, Kato M. Purinergic regulation of intracellular Ca2+ concentration of rat pituitary folliculo-stellate cells in primary culture. J Neuroendocrinol. 2001;13:378–385. doi: 10.1046/j.1365-2826.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Phys. 1997;273:C1963–C1971. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Z, Burnstock G. Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol. 2005;124:379–390. doi: 10.1007/s00418-005-0043-7. [DOI] [PubMed] [Google Scholar]
- 16.Yu Q, Guo W, Song X, Liu X, Xiang Z, He C, Burnstock G. Expression of P2Y receptors in the rat anterior pituitary. Purinergic Signal. 2011;7:207–219. doi: 10.1007/s11302-011-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–E651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao LF, Iwasaki Y, Oki Y, Tsugita M, Taguchi T, Nishiyama M, Takao T, Kambayashi M, Hashimoto K. Purinergic receptor ligands stimulate pro-opiomelanocortin gene expression in AtT-20 pituitary corticotroph cells. J Neuroendocrinol. 2006;18:273–278. doi: 10.1111/j.1365-2826.2006.01416.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
control experiment of immunofluorescence. A to G show the results in the sections incubated with the P2X1 to P2X7 antibodies pre-absorbed with their relative peptides. Note that no obvious immunofluorescence signals were detected in the sections. All scale bars = 120 μm. (JPG 899 kb)
control experiment of in situ hybridization. A and B show the results of the sections incubated with GH and PRL sense oligonucleotide probes. Note that no obvious hybridization signals were detected. All scale bars = 120 μm. (JPG 689 kb)