Abstract
In infants, the main cause of blindness is retinopathy of prematurity that stems in a hypoxic-ischemic condition. Caffeine is a psychoactive compound that at low to moderate concentrations, selectively inhibits adenosine A1 and A2A receptors. Caffeine exerts beneficial effects in central nervous system of adult animal models and humans, whereas it seems to have malefic effect on the developing tissue. We observed that 48-h exposure (during synaptogenesis) to a moderate dose of caffeine (30 mg/kg of egg) activated pro-survival signaling pathways, including ERK, CREB, and Akt phosphorylation, alongside BDNF production, and reduced retinal cell death promoted by oxygen glucose deprivation in the chick retina. Blockade of TrkB receptors and inhibition of CREB prevented caffeine protection effect. Similar signaling pathways were described in previously reported data concerning chemical preconditioning mechanism triggered by NMDA receptors activation, with low concentrations of agonist. In agreement to these data, caffeine increased NMDA receptor activity. Caffeine decreased the levels of the chloride co-transporter KCC2 and delayed the developmental shift on GABAA receptor response from depolarizing to hyperpolarizing. These results suggest that the caffeine-induced delaying in depolarizing effect of GABA could be facilitating NMDA receptor activity. DPCPX, an A1 adenosine receptor antagonist, but not A2A receptor inhibitor, mimicked the effect of caffeine, suggesting that the effect of caffeine occurs through A1 receptor blockade. In summary, an in vivo caffeine exposure could increase the resistance of the retina to ischemia-induced cell death, by triggering survival pathways involving CREB phosphorylation and BDNF production/TrkB activation.
Keywords: Retina, Caffeine, Ischemia, NMDA, BDNF, KCC2
Introduction
The retina is a specialized tissue composed of well-established neural cell types [1]. It is part of the central nervous system (CNS), and it has most of the neurotransmitters and neuromodulators found in other CNS areas [2]. One of these neuromodulators is adenosine, a nucleoside vital for the metabolism and survival of the cell [3]. Adenosine acts through four different metabotropic receptors, named A1 and A3, which are coupled to Gi/Go protein, and A2A and A2B, coupled to Gs/Golf protein. Classically, A1 and A3 inhibit adenylyl cyclase and decrease cAMP levels, while A2A and A2B exert the opposite effect [4, 5]. In the CNS, adenosine plays a role as a neuromodulator and homeostatic regulator, protecting the tissue in harmful situations and modulating transmitter release as well as synaptic activity and plasticity [6–11]. One of the most known, and common, exogenous modulators of adenosine receptors is caffeine.
Caffeine is the most consumed psychostimulant around the world. It is found in different sources such as teas, sodas, some medications, chocolates, and coffee [12]. Under low or moderate concentrations, this methylxanthine exerts its stimulant effects by inhibiting adenosine A1 and A2A receptors. Other effects, such as the inhibition of phosphodiesterases, modulation of GABAA receptor, and calcium (Ca2+) release from internal stores, are achieved by higher/toxic doses [12, 13].
There are several studies reporting different effects of caffeine consumption in the CNS. Chronic consumption of low caffeine doses in adult animals or humans has been correlated to reduced risk for the development of neurodegenerative disease, relieving memory loss and cognitive deficit [13–19]. On the other hand, caffeine seems to affect differently the developing tissue. Series of studies using different caffeine doses in animal models reported deleterious neuronal effects when caffeine is given to pregnant female rodents or developing animals [20–25].
Ischemia is a debilitating condition in which the tissue is injured by the consequences of reduced blood supply [26]. In the retina, such event is part of several eye pathologies such as glaucoma, diabetic retinopathy, and central retinal artery occlusion (CRAO) [27–29]. A major player in ischemic damage in neurons is the neurotransmitter glutamate [30]. At high concentrations, this neurotransmitter will excessively activate its receptors at postsynaptic membrane, leading to neuronal death through a mechanism dependent on calcium entry through the N-methyl-D-aspartate receptor (NMDA), in a phenomenon called excitotoxicity [31].
During ischemia, another released molecule is adenosine [32–34]. In the nervous system, adenosine A1 receptor is known to control synaptic transmission, diminishing the release of transmitters like glutamate, a key component of excitotoxicity [33]. Accordingly, modulation of the receptor can change the tissue’s vulnerability to ischemia in adults and neonates [35–38]. Moreover, data concerning the effect of hypoxia/ischemia in A1 receptor knockout animals still need further research as conflicting data were reported [39, 40]. Interestingly, chronic treatment with adenosine A1 antagonists including caffeine can also be protective [38–40].
Data concerning the adenosine A2A receptor shows that its blockade in adult animal models [35, 41–44] and neonates [45, 46] can preserve neurons exposed to ischemic conditions, including in the retina [47]. However, it was reported beneficial effects of chronic treatment with A2A agonist [48, 49]. A2A knockouts seem to have better outcome after ischemia when neural damage was evaluated in both adults and neonates [50–52], even though in a model of chronic hypoperfusion, A2A−/− mice seem to be more vulnerable [53].
Alongside these works, there are studies that demonstrated the beneficial effects of caffeine treatment on neuronal cells when submitted to hypoxia and ischemia. Caffeine seems to ameliorate neuronal and neurological outcomes both in neonates [54–56] or in adult animals [38, 57]. Some studies showed protective effects of caffeine in the retina of adult animals submitted to ischemia as well [58].
Chemical preconditioning is a process by which repetitive nonlethal stimuli leads the tissue to a “resistant state” to a later lethal insult. This condition can be achieved by the activation of different signaling mechanisms [59, 60], including stimulation of synaptic NMDA receptors, recruiting signaling proteins such as protein kinase B (Akt), extracellular-signal responsive kinase (ERK), cyclic-AMP response element binding protein (CREB) alongside neuronal excitability, and brain-derived neurotrophic factor (BDNF) production [61, 62]. In the retina, it has been shown that cell death caused by excitotoxic concentrations of glutamate could be mimicked by NMDA application, and this toxic effect could be prevented by pre-treatment with low doses of these same compounds [63]. Moreover, this excitotoxic effect could be blocked by pre-administration of BDNF.
Thus, there are data pointing to caffeine as a neuroprotective to CNS, including retina, of adult animal models in pathological conditions. However, the data concerning the effects of caffeine in CNS during early developmental stages suggest it is malefic to the tissue. Therefore, the aim of this work was to evaluate the effects of in vivo pre-exposure to nontoxic doses of caffeine on chick embryo retinas submitted to an ex vivo ischemic insult during developmental stages, and which mechanisms could be involved.
Material and methods
Caffeine, bovine serum albumin (BSA), DL-dithiothreitol (DTT), Triton X-100, nicotinamide-adenine-dinucleotide (NAD), adenosine diphosphate (ADP), glutamate dehydrogenase (GDH), ZM241385, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), Naphthol AS-E phosphate (KG-501), anti-α-tubulin, and anti-GABA antibodies were obtained from Sigma (St Louis, MO, USA). Anti-BDNF was obtained from Preprotech (Rocky Hill, NJ, USA). HRP-anti-rabbit secondary antibody and, ECL kit were purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). Biotinylated-anti-rabbit secondary antibody and Vectastain ABC kit were obtained from Vector laboratories (Burlingame, CA, USA), HRP-anti-sheep secondary antibody was purchased from Invitrogen (Flynn Road, CA, USA). Bicinchoninic acid kit, calcium probe Fluo 3-AM and anti-GAPDH antibody were obtained from ThermoScientific (Rockford, IL, USA). [3H]-MK801 (26,4 Ci/mmol), [3H]-GABA (35 Ci/mmol) and 2,3-[3H]-D-aspartate (11.3 Ci/mmol) were obtained from PerkinElmer (Waltham, MA, USA). Antibodies against phospho-Akt (ser473), phospho-ERK and phospho-CREB were obtained from Cell Signaling technology (Beverly, MA, USA), anti-mouse secondary antibody was obtained from GE Healthcare (Little Chalfont, Buckinghamshire, UK), anti-GAT-3, anti-GluN2B, and anti-GluN2A antibodies were obtained from Abcam (Cambridge, UK), anti-VGAT was obtained from Phosphosolutions (Colorado, US), anti-GAD was obtained from NIH (Maryland, US), anti-NKCC1 antibody was obtained from Hybridoma Bank, anti-KCC2, anti-GAT-1, and anti-GluN1 antibodies were obtained from Millipore (Massachusetts, USA). Gas mixtures (95% O2/5% CO2 and 95% N2/5%CO2) (White Martins, Praxair Inc., RJ, Brazil) were used. All other reagents were of analytical grade or better.
In ovo treatment
Injection of caffeine was performed as previously described [64]. Briefly, embryonated White Leghorn eggs were obtained from a local hatchery and incubated at 38 °C and relative humidity of 80–90%. At fourteen embryonic days (E14), random eggs were assigned to receive caffeine (30 mg/kg egg) or vehicle (sterile water - VEH) injected in the eggs in sterile conditions. Other drugs injected at the same embryonic developmental age were: K252a (estimated final concentration 100 nM), an inhibitor of BDNF receptor, TrkB, KG-501 (10 μM, estimated final concentration), a CREB inhibitor and ZM241385 or DPCPX (100 nM, 1 μM or 10 μM, estimated final concentration) antagonists of A2A and A1 adenosine receptors respectively. This specific stage was chosen based on previous studies from our group showing that adenosinergic system (caffeine target) is present and functional at this age [64–67]. The staging of the embryos was systematically determined based on previous work [68]. The main developmental phenomenon of the retina occurring during the period used in the present study, from E14 to E16, is synaptogenesis in the Inner Plexiform Layer (IPL) [2, for review]. The retinal cell proliferation is finished, the natural cell death process in the GCL is already over and virtually completed in the INL [2, for review]. In addition, rod and cone outer segments appearance began at E15, and the expression of opsins and rhodopsin initiated during this period (E14–E16) [2, for review]. We also evaluated the effect of caffeine in two other periods of development, performing the injection at E12 or at E16. From E12 to E14, the retina is similar to E14-E16, natural cell death is still going on in the inner nuclear layer (INL). Finally, most of the outer retina maturation occurs from E16 to E19, during the other period studied (E16-E18). For all these experiments, the exposure to caffeine was restricted to 48 h.
Two days after caffeine injection, the retinas were dissected from the pigmented epithelium and the sclera in cold calcium magnesium free solution (CMF) (131 mM NaCl, 40.9 mM KCl, 9.2 mM Na2HPO4.7H2O, 4.5 mM KH2PO4, 12.2 mM glucose and 9.4 mM NaHCO3). The dissection always followed the order of injection which is the order in every graph from left to right. The dissected tissue was processed by different methods.
Determination of caffeine in the retina
Caffeine was determined in the retina studied by high performance liquid chromatography (HPLC). Retinas were dissected from the pigmented epithelium and the sclera in CMF. They were lysed using 1 mL of a sodium hydroxide solution (0.1 M) and then neutralized with hydrochloric acid (analytical grade, VETEC, Brazil) up to a pH = 7.0. The resulting suspension was extracted with 4 portions of 5 mL of methylene chloride (HPLC grade, Tedia, Brazil). The extracts were combined and concentrated by rotary evaporation at 55 °C, with solvent exchange to water. The resulting solution was transferred to a 2 mL volumetric flask and completed to this volume using water. The aqueous solution was filtered through disposable syringe filters (PTFE, 0.45 μm, Agilent), transferred to 2 mL vials and analyzed as described below.
The chromatographic system consisted of a vacuum degasser, a binary pump, an autosampler, a column oven and an UV-DAD detector (all Agilent 1200 Series, Santa Clara, CA, USA) and controlled by an Agilent ChemStation. A Zorbax Eclipse XDB-C18 column (150 × 4.6 mm × 5 μm, Agilent, USA) was used. The complete separation of caffeine (retention time = 7.78 min), and matrix interferents was achieved under previously optimized isocratic conditions using a mobile phase that contained 25% (v/v) of methanol (HPLC grade, J.T. Baker, PA, USA) and ultrapure water, which was ultrasonically degassed (15 min) and filtered using a PFTE membrane prior to use. The separation occurred at 35 °C at constant flow rate (0.7 mL min−1). The chromatographic run time was 13 min to allow system reconditioning, and the injection volume of sample extracts and standard solutions was 20 μL. The quantification of the analyte was carried out at 273 nm, and the external calibration method was employed, with a calibration curve ranging from 0.05 to 0.50 mg L−1, which showed determination coefficients (R2) > 0.999. The determination of caffeine in retina was free of interferences as demonstrated by the analysis of blank and caffeine spiked retinas. The concentrations of caffeine in retina samples were calculated as their ratios to the protein content, determined by the Lowry method [69], and expressed as ng caffeine per mg of protein. The analytical methodology validation followed the criteria of the Brazilian National Institute of Metrology, Quality and Technology Guide that are harmonized with international regulations.
The data obtained after transformation of caffeine concentrations (C) in natural logarithms (Ln C) was plotted against time (h) and fitted using a first-order decay model. The plot of Ln C versus time allowed estimating the parameters of the curve Ln C = −kt + ln Co and the coefficient of determination (R2) of the equation obtained. Two models were obtained, considering or not the first point (6 h) of the curve. When the first point was considered, the value of R2 was 0.977 and the decay equation was Ln C = − 0.0192t − 8.855, and when this point was excluded from the calculations, the equation was Ln C = − 0.0195t − 8.845, with R2 = 0.967.
Oxygen glucose deprivation
Retinal oxygen and glucose deprivation (OGD) for 50 min was used as a model of ischemia. E16 retinas were dissect and immersed in 1 mL of ringer solution (120 mM NaCl; 3 mM KCl; 30 mM NaHCO3; 1 mM NaH2PO4; 1 mM CaCl2; 1 mM MgCl2.6H2O; 10 mM glucose) perfused for the entire experiment with a gas mixture containing 95% O2/5% CO2 in the control (nonischemic) condition, or 95% N2/5%CO2 in the ischemic condition (OGD). The ischemic medium (OGD) had the same composition of control medium except for glucose. The pH was previously adjusted to 7.2–7.4.
When indicated, this procedure was performed in the presence of MK-801 (10 μM), a noncompetitive antagonist of NMDA receptors. Indicated groups received a 10 min preincubation with the drug and remained in its presence in proper medium for the rest of the experiment.
In OGD experiments, the normoxic conditions in the absence of caffeine were named control (CTR).
Western blot
After dissection in CMF solution, E16 retinas were processed by two different methods: first, to analyze phosphorylated proteins, the tissue was homogenized in sample buffer (10% glycerol, 5% β-mercaptoethanol, 20% sodium dodecyl sulfate, 0.5 M Tris-HCl pH 6.8) and boiled for 5 min. Protein concentration was established by Bradford reagent, using bovine serum albumin (BSA) as standard. Second, dissected retinas were homogenized in RIPA buffer (150 mM NaCl; 50 mM Tris; 5 mM EGTA, 1% triton X-100; 0.5% sodium deoxycholate (DOC); 0.1% SDS) containing 1 mM DL-dithiothreitol (DTT), centrifugated at 15000 rpm (20.627g) and 4 °C for 10 min. Supernatants were collected and kept at − 20 °C. Protein concentration was established by bicinchoninic acid protein kit assay. Before electrophoresis, denaturizing buffer (0.5 M Tris-HCL; 0.4% SDS; 30% glycerol; 10% SDS; 0.6 M DTT; 0.06 M bromophenol blue, pH = 6.8) corresponding to 1/5 of total sample volume was added to the sample. Samples containing 30 μg or 60 μg (GluN2B, VGAT, and GAT-1) of protein were submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins transferred to polyvinylidene fluoride membranes, which were blocked with 5% nonfat dry milk or 3% BSA and incubated overnight with primary antibodies at 4 °C. Then, the membranes were washed, incubated with peroxidase-conjugated secondary antibody for 1 or 2 h and developed using enhanced chemiluminescence provided by the ECL kit. The images were obtained using ChemiDoc (Bio-Rad Laboratories, Hercules, CA, USA), and optical density was analyzed with ImageJ software (version 1.38, NIH, USA).
Cell viability assay
Cell viability assay was performed as previously described [70, 71]. At the end of the 50 min OGD, retinal pieces (corresponding to the intracellular fraction) were incubated in lysis solution (0.9% Triton X-100) for 30 min at room temperature, and subsequently frozen at − 20 °C. The respective extracellular fractions were also collected and frozen. Before submitting such fractions to analysis, samples were defrosted, homogenized, and centrifuged at 2000 rpm (367g) at 25 °C for 4 min. To evaluate cell viability, a colorimetric commercial kit for identification and quantification of LDH was used (CytoTox 96® Non-Radioactive Cytotoxicity Assay, Promega). Measurements were performed in triplicates. Quantification was performed using a microplate reader (iMark, BioRad, Philadelphia, USA) at 490 nm. The percentage of LDH released was calculated as the fraction found in the extracellular medium normalized by total LDH (intracellular plus extracellular).
Immunohistochemistry
After caffeine or vehicle treatment, retinas were fixed in 4% paraformaldehyde for 1 h, and then washed in 0.16 M phosphate buffer (pH = 7.2). Next, the retinas were cryoprotected with a sucrose gradient, frozen, and sectioned in a cryostat (12 μm). Control and caffeine-treated retinal sections were collected on the same slide, to be simultaneously processed under equal conditions. Sections were incubated with 5% bovine serum albumin (BSA) for 1 h, and then with 1:7000 anti-GABA polyclonal antibody overnight. Subsequently, retinal sections were incubated with 1:200 goat-x-rabbit IgG antibody for 2 h, and then incubated with 1:50 avidin-biotin complex for 90 min, followed by an incubation with 0.05% 3.3-diaminobenzidine (Sigma-Aldrich, St. Louis, MO, USA) and 0.01% hydrogen peroxide for 10 min. After this treatment, sections were washed and mounted with buffered glycerol solution.
Glutamate measurement
This protocol performed as described previously [72]. After the 50-min OGD, the extracellular medium was collected and kept at − 20 °C until measurement. The quantification of glutamate was performed by fluorimetry using a GloMax® 20/20 Single Tube Luminometer Reader (Promega, Madison, USA) equipped with an ultraviolet excitation filter (365 nm) and a 410–450-nm emission filter. With this purpose, 1 mL of a solution containing 75 mM of Tris-acetate (pH = 8.4), 40 mM NAD and 15 mM ADP was added to 400 μL of the previously collected sample and its fluorescence was measured (i). Then, 48 units of the enzyme glutamate dehydrogenase (GDH) were added to the solution and its fluorescence was measured again 30 min later (f). The glutamate concentration was calculated by the difference between the readings (f–i) and corrected by protein concentration through Lowry method.
[3H]-MK801 binding
Each retina was dissected and divided into 2 pieces, resulting in 4 near identical pieces for each embryo. Each half would represent an experimental condition, that could be either basal (total “t” or unspecific “u”) or stimulated (total or unspecific) condition of respective control or caffeine treated animal. After dissection, each retinal half rested for 10 min in 500 μL Hank’s solution in the absence of magnesium (138 mM NaCl; 5.33 mM KCl; 5.6 mM glucose; 1.26 mM CaCl2; 20 mM HEPES; pH = 7.4) at 37 °C. Then, the tissue was washed twice and incubated in Hank’s solution containing: 2 mM glycine (basal “t” condition), 2 mM glycine and 50 μM MK801 (basal “u” condition), 2 mM glycine, and 2 mM glutamate (stimulated “t” condition) or 2 mM glycine, 2 mM glutamate, and 50 μM MK801 (stimulated “u” condition) for 30 min. Next, 5 nM of [3H]-MK801 was added to each well for 10 min. After 10 min, the tissue was washed twice and lysed with water, submitted to 3 cycles of freeze/thaw before the addition of 10 M NaOH (final concentration 0.1 M). Basal and stimulated condition final values of each group were acquired by subtracting the total values from the unspecific values. Radioactivity was measured by scintillation spectroscopy. The values obtained were corrected by protein concentration through Lowry method.
[3H]-D-aspartate uptake
Retinas were dissected in cold CMF solution, divided into 4 pieces and placed in a 24 well plate containing 500 μL of cold 4 mM glucose Hanks’ 4 solution (138 mM NaCl; 5.33 mM KCl; 4.0 mM glucose; 4 mM CaCl2; 0.5 mM MgCl2; 20 mM HEPES; pH = 7.4). The retinal pieces were acclimatized for 15 min at 37 °C. Next, the tissue was incubated with 0.05 μCi of [3H]-D-aspartate plus 20 μM of D-aspartate for different time periods (10, 20, 40, and 60 min). After the uptake time, retinas were washed 5 times with cold Hank’s 12 solution (138 mM NaCl; 5.33 KCl; 12 mM glucose; 1.26 mM CaCl2; 0.5 mM MgCl2; 20 mM HEPES; pH = 7.4) and lysed with 5% trichloroacetic acid (TCA). Then, the tissue was submitted to three freeze and thaw cycles followed by a 10 min centrifugation at 15.000 rpm (20.627g) at 4 °C. The supernatant radioactivity was measured through scintillation spectroscopy and the pellet was used for protein measurement by the Lowry method after re-suspension in 800 μL of NaOH (1 M).
[3H]-GABA uptake
Retinas were dissected in cold CMF solution and placed in a 24 well plate containing 500 μL of cold Hanks’ solution. Retinas were acclimatized for 15 min at 37 °C and incubated with 0.1 μCi of [3H]-GABA for 5 min. After incubation time, the retinas were washed 3 times with cold Hanks’ solution and lysed with TCA (5%). After lysis, tissue was submitted to three freeze thaw cycles followed by centrifugation at 15.000 rpm for 10 min at 4 °C. Supernatants were used to measure radioactivity through scintillation spectroscopy and the pellet was used for protein quantification by Lowry method after re-suspension in 800 μL of NaOH (1 M).
[3H]-GABA release
After dissection, retinas were placed in a 24-well plate containing 500 μL of CMF. After the dissection of the last retina, CMF from the wells were substituted by minimum essential medium (MEM) (Life Technologies) containing 20 mM HEPES, 1 μM GABA, and 0.1 μCi of [3H]-GABA and incubated for 40 min at 37 °C. After that, retinas were washed 3 times with Hanks’ 4 solution for 3 min each, followed by a 10 min wash. Next, the tissue was incubated in Hanks’ 4 for 10 min to allow release of [3H]-GABA. This solution was collected and named basal release 1 (B1). This procedure was repeated once to collect basal release 2 (B2). Next, tissue was exposed to 2 mM glutamate plus 2 mM D-serine for 10 min and the medium was collected and named stimulated release (S). Next, the tissue was incubated with Hanks’ 4 for 10 min twice to collect basal release 3 (B3) and 4 (B4). Finally, tissue was lysed with 5% TCA and the solution was collected to represent the total uptake. Radioactivity was measured by scintillation spectroscopy.
Mixed chick retinal cell cultures
Animals at embryonic day 10 (E10) were killed by decapitation, the eyes were removed and immediately transferred to Ca2+ and Mg2+ free balanced salt solution (CMF) where the retinas were dissected from other structures. Trypsin, at a final concentration of 0.1%, was then added to the tissues and the suspension incubated at 37 °C for 20–25 min. Trypsin solution was removed and the retinas suspended in MEM containing 5% fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Tissues were mechanically dissociated by successive aspirations of the medium. For calcium imaging experiments, cells were seeded on culture dishes (internal diameter = 22 mm), the culture medium was exchanged 1 day later and caffeine (100 uM) treatment began at 4 days in vitro (C4) until C6 (48 h). Cells were incubated at 37 °C for the indicated periods of time, in humidified atmosphere of 95% air/5% CO2.
Calcium imaging
The Fluo-3 AM indicator was used following previous published protocols. Briefly cultures were incubated for 1 h in complete MEM medium containing 5 μM Fluo-3 AM, 0.2% (v/v) Pluronic F-127, 0.5% (v/v) DMSO, and 2.5 mM probenecid. Cells were washed with Hanks’ solution with probenecid and incubated for an additional 15 min to allow complete deesterification of the AM ester. Live calcium imaging was carried out on a Leica TCS SP5 II confocal microscope, using a 488 nm laser line for excitation, images sizes of 512 × 512 pixels, and acquisition rates of 1 frame/s. Emitted fluorescence was recorded at wavelengths between 530 and 565 nm. Cells were stimulated with NMDA (100 μM) in Hanks’ without MgCl2 with glycine (2 mM) or Muscimol alone (10 μM) that were administered after baseline stabilization. (F0) represents the mean fluorescence observed at the 1 min period before stimulation of the cells and (F) represent intracellular Ca2+ fluorescence intensity after drug administration. Data were expressed as the ratio F/F0 or as the ratio Fmax/F0 with Fmax representing the maximal intensity of Fluo-3AM fluorescence emission during stimulation. At least 26 cells for each experimental condition were analyzed.
Statistical analyses
Data presented here were analyzed by GraphPad Prism 8. One-sample t test was used in the case of comparison between two groups, One-way ANOVA for comparison between more than two groups and two-way ANOVA using Sidak’s multiple comparison test for experiments comparing groups submitted to OGD procedure and other drug treatments. [3H]-GABA release was analyzed point by point using Mann-Whitney test. Data is shown as mean ± standard error of the mean (SEM) related to multiple animals in the same group. Significant differences were considered when p < 0.05. Data outside the 95% confidence interval were excluded from the analysis.
Results
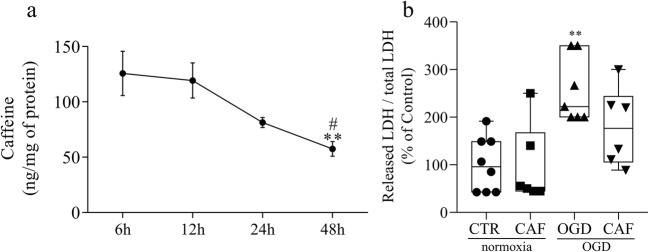
The first goal of the present study was to verify whether caffeine could reach the retina after the injection in ovo. Retinas were evaluated at 6, 12, 24, and 48 h after exposition to caffeine in six replicates for each time. By using HPLC approach, we found caffeine in retinal samples as early as 6 h (125.7 ± 19.99 ng of caffeine/mg of protein) after in ovo injection (Fig. 1a). The results obtained after the shortest exposure times (6 and 12 h) showed comparable ranges and dispersions and were shown to be equivalent by ANOVA (p > 0.812). Caffeine could still be found in the tissue 48 h after injection, whereas in a significant lower amount (57.5 ± 6.68 ng of caffeine/mg of protein, p < 0.05) compared to the other time points. Estimates of t1/2 of caffeine in this biological system indicate a value of approximately 36 h.
Fig. 1.
Caffeine reaches the retina and chronic treatment reduces LDH released from retinal cells exposed to OGD. a Time curve showing the presence of caffeine in the retina after injection in ovo measured by HPLC. At 6 h caffeine can already be detected at the higher levels (125.7 ± 19.99 ng of caffeine per mg of protein). Similar levels are found 12 h after injection (119.3 ± 15.93) before it starts to decrease at 24 h (81.38 ± 4.47) and 48 h (57.50 ± 6.68). **p < 0.01 compared to 6 h; #p < 0.05 compared to 12 h. b LDH assay of E16 retinas previously treated with caffeine (30 mg/kg) (CAF) or vehicle for 48 h, and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 22.62%, n = 7; CAF = 97.39 ± 34.01%, n = 6; OGD = 255.55 ± 25.97%, n = 7; CAF + OGD = 172.72 ± 33.29%, n = 6). **p < 0.01 compared to CTR. In all figures CTR will represent the condition in which the retina was treated with vehicle for 48 h and subsequently submitted to 50 min in normoxic conditions. Absence (−) of caffeine or OGD represents, respectively, vehicle-treated and normoxic groups. This nomenclature will be used throughout the work whenever the experimental conditions are the same. All data in this work represents the mean ± SEM of at least 3 independent experiments
Once we confirmed that caffeine reaches the retina, added to the previous observation that in ovo injection of caffeine leads to an increase in A1 receptor as well as a decrease in A2A receptor retinal content [64], we decided to study the retinal susceptibility to acute cell death induced by an ischemic event. Retinal susceptibility to cell death induced by ischemia (50 min OGD), in embryos exposed or not to caffeine for 48 h, was evaluated by LDH activity assay. OGD significantly increased retinal LDH release, showing that this ischemic event promoted cell death (Fig. 1b). Caffeine treatment did not change LDH release in normoxic condition, but it did reduce in 30% the LDH release induced by ischemia (Fig. 1b), indicating an increase in retinal cells resistance to acute cell death induced by OGD.
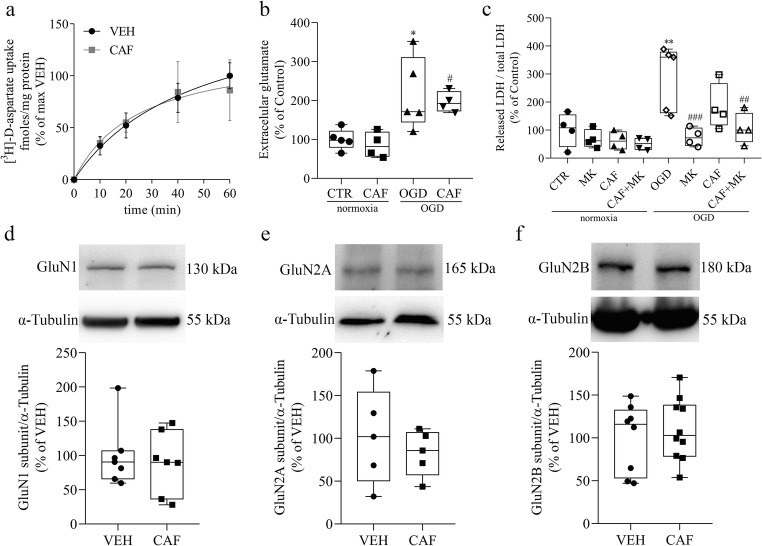
Since glutamate is a major player in the cell death induced by ischemia, it is a key target to evaluate the mechanism involved in the retinal protection promoted by caffeine. Thus, we further investigate the effect of caffeine in different components of retinal glutamatergic system. At first, we evaluated glutamate clearance capacity to investigate if caffeine could be protecting the neurons by decreasing glutamate availability. We observed that the [3H]-D-aspartate uptake over time did not change in retinas exposed to caffeine for 48 h (Fig. 2a). Extracellular glutamate content was also unaltered comparing control and caffeine-treated groups in normoxic condition, as well as after OGD, further indicating that glutamate clearance was not crucial (Fig. 2b). However, we confirmed that excitotoxicity is a major player promoting retinal cell death by ischemia since the noncompetitive antagonist of NMDA receptor (10 μM MK-801) completely blocked OGD-induced LDH release in both control and caffeine-treated groups (Fig. 2c).
Fig. 2.
Caffeine does not alter glutamate system under normal or ischemic conditions. a Time curve of [3H]-D-aspartate uptake by retinas previously treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h. Retinas were given 0.05 μCi of [3H]-D-aspartate for different time periods. VEH is the abbreviation used for groups treated with vehicle, but not submitted to OGD procedure, neither normoxia nor ischemia. This nomenclature will be used throughout the work whenever the experimental conditions are the same. b Dosage of extracellular glutamate content, released from retinas of E16 animals previously treated with vehicle (CTR) or caffeine (30 mg/kg) (CAF) for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 11.69% n = 5; CAF = 85.9 ± 16.42% n = 4; OGD = 216.21 ± 41.64% n = 5; CAF + OGD = 196.2 ± 13.27% n = 4). c LDH assay of E16 retinas previously treated with vehicle (CTR), or caffeine (30 mg/kg) (CAF) for 48 h. Before submitting of the tissue to normoxia or OGD, indicated groups received a pretreatment with 10 μM of MK-801 for 10 min in normoxia medium. Then, retinas were submitted to normoxia or OGD in the presence or not of MK-801 for the usual 50 min (CTR = 100 ± 30% n = 4; MK-801 = 68.59 ± 16.38% n = 4; CAF = 62.35 ± 16.28% n = 4; CAF + MK-801 = 51.22 ± 10.90% n = 4; OGD = 287.96 ± 52% n = 5; OGD + MK-801 = 74.80 ± 16.45% n = 4; CAF + OGD = 182.74 ± 40.85% n = 4; CAF + OGD + MK801 = 105 ± 97 + 27.99% n = 4). d–f Representative immunoblots of E16 retinas of animals treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h and analyzed for: GluN1 (VEH = 100 ± 18% n = 7; CAF = 89.35 ± 17.16% n = 7), GluN2A (VEH = 100 ± 22% n = 6; CAF = 115 ± 34% n = 6) and GluN2B (VEH = 100 ± 14.23% n = 8; CAF = 125.6 ± 18.93 n = 11) using α-Tubulin as loading control. *p < 0.05; **p < 0.01 compared to CTR; #p < 0.05 compared to CAF/normoxia; ##p < 0.01, ###p < 0.001 compared to OGD
Still regarding the glutamatergic system, we also evaluated the expression of NMDA receptor subunits, since this receptor is essential to trigger excitotoxicity. First, we measured the content of the constitutive NMDA receptor subunit, GluN1, and we found no difference in the level of this protein comparing vehicle and caffeine-treated embryos (Fig. 2d). Since this subunit is essential for the receptor function, this data indicates that the number of receptors that can potentially bind to glutamate and trigger a response is similar in vehicle and caffeine-treated groups. However, there is the possibility that not all of these receptors are located at the membrane domain. We also observed that total GluN2A and GluN2B subunits content were not altered (Fig. 2 e and f).
As described previously, low nontoxic doses of glutamate or NMDA are able to induce a protective state in a tissue by activating NMDA receptors, phenomenon known as chemical preconditioning. Based on this idea, the possibility that caffeine could act like a chemical preconditioner through NMDA receptor activation was raised. To test this idea, we performed the [3H]-MK-801 binding, which is considered a functional binding assay since the radioactive drug will bind only when the receptor is activated, and the channel pore is open, exposing the MK-801 binding site. We observed that caffeine treatment increased [3H]-MK-801 binding in 270% under basal conditions, indicating an increase in the receptor activity, whereas under exogenous stimulated conditions the binding was similar in caffeine or vehicle-treated retinas (Fig. 3d).
Fig. 3.
Caffeine increase activity of NMDA receptors. a Confocal micrographs of mixed retinal cultures treated or not with caffeine for 48 h before and after NMDA stimulation. b Fluorescence intensity (Fmax/F0) before (VEH = 1.09 ± 0.01; CAF = 1.07 ± 0.009) and after 100 μM NMDA stimulation (NMDA = 1.66 ± 0.12, CAF + NMDA = 2.25 ± 0.21) of the cultures previously treated with caffeine (CAF) or vehicle (VEH). c Representative traces (F/F0) of [Ca2+] transients, measured as peak heights, after NMDA stimulation (arrows). d Binding assay to active NMDA receptors at basal and stimulated (2 mM of glutamate) conditions of E16 retinas of animals previously treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h (VEH basal = 4.90 ± 1.84, n = 3; CAF basal = 18.11 ± 1.58, n = 3; VEH stimulated = 15.17 ± 1.10, n = 3; CAF stimulated = 19.74 ± 4.72, n = 3). Calcium data represents fluorescence intensity of 26 to 30 neuron cells recorded in seven separate experiments. Color bar from black (0) to red (255) represents intracellular calcium increase. Bar = 30 μm. *p < 0.05 related to VEH, ****p < 0.0001 related to CAF, #p < 0.05 related to NMDA
To confirm this result, we used another functional approach by using calcium imaging. Retinal cell cultures, obtained from E10 embryos and cultivated for 6 days, stimulated with 100 μM NMDA at E10C6 showed a significant increase in intracellular calcium, independent of caffeine exposure (Fig. 3a–c). However, the treatment with caffeine for 48 h induced a significantly higher calcium increase, upon stimulation with 100 μM NMDA, compared to its control, than not-treated cells (Fig. 3a–c).
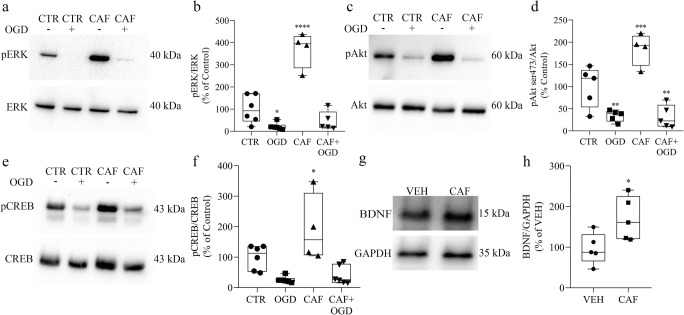
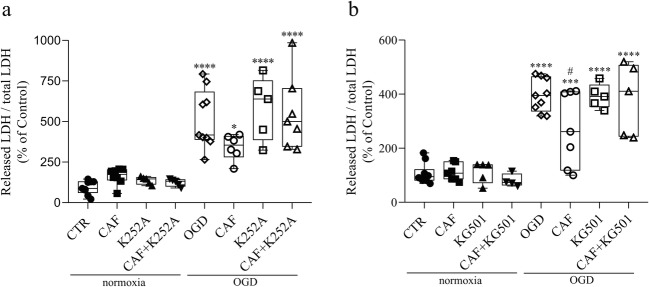
The increase of NMDA receptor activity could lead to the stimulation of survival signaling pathways, making cells exposed to caffeine more resistant to OGD insult. To address this issue, we verified the phosphorylation state of ERK, CREB and Akt, along with the production of BDNF. Remarkably, all of them were increased after 48 h of caffeine exposure (Fig. 4a–h). To evaluate the role of BDNF and CREB in the protective effect of caffeine, we co-treated the embryos with caffeine and BDNF receptor blocker (K252a) or CREB inhibitor (KG-501) (Fig. 5). We observed that, in normoxic conditions, embryos treated with K252a or KG-501 alone, or combined with caffeine, for 48 h, show no increase in cell death (Fig. 5). As observed previously, exposure to caffeine alone reduced cell death when retinas were submitted to OGD. Interestingly, this effect was completely prevented by either BDNF receptor blocker (Fig. 5a) or CREB inhibitor (Fig. 5b), suggesting that these molecules are key factors in caffeine protective outcome.
Fig. 4.
Caffeine triggers survival signaling in the retina after 48 h. a, c, e, g Representative immunoblots of E16 retinas from animals treated with vehicle (CTR) or caffeine (30 mg/kg) (CAF) for 48 h and submitted to normoxia or OGD procedure for 50 min, analyzed for a, b pERK (CTR = 100 ± 25.5%, n = 6; OGD = 21.83 ± 6.51%, n = 6; CAF = 369.6 ± 40.5%, n = 4; CAF + OGD = 46.38 ± 19.31%, n = 6); c, d pAkt (CTR = 100 ± 20.5% n = 5; OGD = 34.17 ± 5.59%, n = 5; CAF = 184.6 ± 18.2%, n = 4; CAF + OGD = 32.05 ± 11.51%, n = 5); and e-f) pCREB (CTR = 100 ± 16.3%, n = 6; OGD = 25.89 ± 4.35%, n = 6; CAF = 191.5 ± 55.97%, n = 4; CAF + OGD = 40.81 ± 12.57%, n = 6) using respective non-phosphorylated proteins as loading control. g, h Representative immunoblot of BDNF and GAPDH (loading control), using E16 retinas from animals treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h (VEH = 100 ± 17.72% n = 5; CAF = 170.4 ± 23.9% n = 5). *p < 0.05 related to CTR or VEH, **p < 0.01; ***p < 0.001; ****p < 0.0001 compared to CTR
Fig. 5.
BDNF and CREB are crucial for caffeine effect on cell survival. a LDH assay of E16 retinas previously treated with vehicle (CTR), caffeine (30 mg/kg) (CAF), K252a or CAF+K252a, for 48 h, and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 14.51%, n = 9; CAF = 163 ± 17.97%, n = 8; K252a = 136.73 ± 10.42%, n = 5; CAF + K252a = 123.49 ± 10.24%, n = 5; OGD = 514.17 ± 60.80%, n = 9; CAF + OGD = 341.57 ± 32.11%, n = 6; K252a + OGD = 583.19.3 ± 87.50%, n = 5; CAF + K252a + OGD = 552.62 ± 86.72%, n = 7). b LDH assay of E16 retinas previously treated with vehicle (CTR), caffeine (30 mg/kg) (CAF), KG-501 or CAF + KG-501, for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 10.95%, n = 10; CAF = 110.73 ± 31.39%, n = 7; KG-501 = 111.92 ± 39.33%, n = 5; CAF + KG-501 = 80.41 ± 23.97%, n = 4; OGD = 396.05 ± 61.05%, n = 9; OGD + CAF = 272.41 ± 137.44%, n = 7; OGD + KG-501 = 393.08 ± 44.96%, n = 5; OGD + CAF + KG-501 = 382.70 ± 132.97%, n = 5). *p < 0.05, ***p < 0.001, ****p < 0.0001 compared to CTR; #p < 0.05 compared to OGD
In the beginning of the central nervous system development, GABA acts as a depolarizing signal through GABAA receptor, and it is responsible for the membrane depolarization that relieves NMDA receptor inhibition by magnesium [73, 74]. As developmental progress, the GABAA receptor shift to hyperpolarizing response. In chick embryo retina, GABA shift occurs around E13 to E16 [75, 76]. Since caffeine exposure induced a higher NMDA receptor response in a period that GABA was not supposed to induce depolarization anymore, we investigate the effect of caffeine in GABAergic system. First, we found that a 48-h caffeine treatment increased GABA cell content in ganglion, amacrine, and horizontal cell bodies, as well as in the inner plexiform layer (Fig. 6a–e). To investigate the reason of the increase in GABA cell content, we evaluated GABA transporters and its synthetic enzyme. We found that GABA transporters from plasma membrane were inversely modulated by caffeine treatment, with a reduction in GABA transporter 1 (GAT-1), and an increase in GAT-3 (Fig. 7a, b). Although we found that caffeine exposure changes both GABA transporters content, we could not observe differences in [3H]-GABA release or uptake assays (Fig. 7c, d). Vesicular GABA transporter (VGAT) was also unaffected by caffeine exposure (Fig. 7e). The GABA-producing enzyme level, GAD (glutamic acid decarboxylase), was not altered after 48 h of caffeine exposure, although it showed a non-significant but 41.2% increase 24 h after caffeine injection, which could contribute to the increase of GABA cell content (Fig. 7f, g).
Fig. 6.
Caffeine modifies GABA content within the retinal tissue. a Photomicrographs of radial sections of E16 retinas treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) and immunolabeled for GABA. b–e Quantification were performed by optic densitometry in the (b) sublayers of the inner plexiform layer (IPL) (sublayer 1: VEH = 100 ± 3.96%, n = 5; CAF = 113 ± 2.55%, n = 5. Sublayer 2: VEH = 100 ± 3.28%, n = 5; CAF = 111.8 ± 2.04%, n = 5. Sublayer 3: VEH = 100 ± 4.28%, n = 5; CAF = 113.2 ± 2.82%, n = 5. Sublayer 4: VEH = 100 ± 3.75%, n = 5; CAF = 112.6 ± 3.8%, n = 4. Sublayer 5: VEH = 100 ± 3.7%, n = 5; CAF = 114.3 ± 3.31%, n = 5), in the (c) ganglion cell layer (GCL) (VEH = 100 ± 8%, n = 5; CAF = 142.4 ± 15.62%, n = 5) in the (d) inner portion of the inner nuclear layer (INL), represented by amacrine cells (VEH = 100 ± 7.03%, n = 5; CAF = 124.2 ± 7.24%, n = 5), and in the (e) outer portion of the inner nuclear layer, where horizontal cells reside (VEH = 100 ± 6.09%, n = 4; CAF = 123 ± 5.76%, n = 4). *p < 0.05, **p < 0.01 compared to VEH
Fig. 7.
Caffeine alters GABAergic system during retinal development. a, b, e, f, g Representative immunoblot of E16 retinas of animals treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h or 24 h and analyzed to a GAT-1 (VEH = 100 ± 22.14%, n = 6; CAF = 61.77 ± 12.55%, n = 6), b GAT-3 (VEH = 100 ± 33.88%, n = 4; CAF = 140.3 ± 7.35%, n = 4), e VGAT (VEH = 100 ± 16.27%, n = 6; CAF = 105 ± 17.34%, n = 6), f GAD 48 h (VEH = 100 ± 11.89%, n = 10; CAF = 101.3 ± 7.28%, n = 9) and g GAD 24 h (VEH = 100 ± 13.63%, n = 6; CAF = 141.2 ± 17.24%, n = 4) using α-tubulin as loading control. c Fractional [3H]-GABA release of E16 retinas treated with vehicle or caffeine (30 mg/kg) for 48 h. Cells were exposed to [3H]-GABA (0.1 μCi) for 40 min, and the basal (B) or stimulated (S = 2 mM of glutamate) release were measured (VEH: B1 = 2.77 ± 0.48 n = 5; B2 = 2.54 ± 0.43 n = 5; S = 7.38 ± 1.30 n = 4; B3 = 3.51 ± 0.85 n = 5; B4 = 1.96 ± 0.44 n = 5; CAF: B1 = 2.25 ± 0.41 n = 6; B2 = 1.75 ± 0.24 n = 4; E = 6.84 ± 1.11 n = 6; B3 = 3.82 ± 1.01 n = 6; B4 = 2.61 ± 0.88 n = 6). d [3H]-GABA uptake by E16 retinas treated with vehicle or caffeine (30 mg/kg) for 48 h. Cells were incubated with 0.1 μCi of [3H]-GABA for 5 min, washed, and lysed to access intracellular radioactivity (VEH = 100 ± 3%, n = 4; CAF = 110 ± 14%, n = 4). *p < 0.05 compared to VEH
As previously mentioned, it is known that in immature neurons GABA, acting through GABAA receptor, has depolarizing effect [73, 74], due to a higher expression of the chloride transporter NKCC1 than KCC2. Therefore, one possible mechanism that could lead to an increase in NMDA receptor activation, after caffeine exposure, could involve an augment in retinal excitability due to changes in chloride transporters and a delay in the shift of GABAA receptor responses. Therefore, we decided to investigate the chloride transporters and GABAA receptor responses. Caffeine exposure significantly reduced KCC2 protein levels (Fig. 8a) whereas no change in the NKCC1 transporter content was detected (Fig. 8b). Therefore, these findings raise the possibility that caffeine affects the chloride gradient delaying the shift of GABA response from depolarizing to hyperpolarizing, which could be related to increase in retinal excitability and higher NMDA receptor activation.
Fig. 8.
Caffeine alters chloride transporter pattern. Representative immunoblot of E16 retinas from animals treated with vehicle (VEH) or caffeine (30 mg/kg) (CAF) for 48 h and analyzed to NKCC1 (VEH = 100 ± 11.82%, n = 4; CAF = 106.6 ± 10.46%, n = 4) and KCC2 (VEH = 100 ± 19.16%, n = 6; CAF = 54.41 ± 11.03%, n = 6) using α-tubulin as loading control. **p < 0.01 compared to VEH
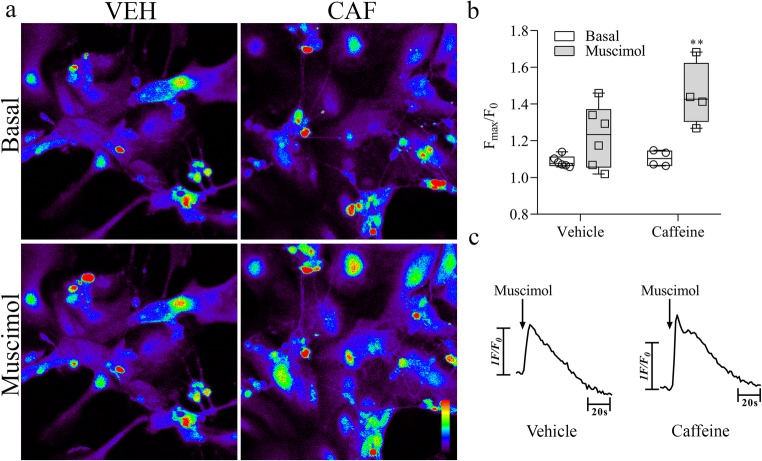
In order to test this hypothesis, we used the same approach of calcium imaging to access GABAA receptor responses by stimulation with its agonist muscimol since it is known that depolarization response of GABA induces calcium influx through voltage sensitive calcium channels [77, 78]. We observed that, in the nontreated cultures, 10 μM muscimol did not significantly affected intracellular calcium. However, caffeine pre-treated cells responded to muscimol with a significant increase in intracellular calcium, indicating a depolarizing activity of GABAA receptor (Fig. 9). Altogether, these results indicate that caffeine delayed the increase in retinal KCC2 levels keeping a residual depolarizing response of GABAA receptors in neurons, which in turn could be facilitating NMDA receptor activity.
Fig. 9.
a Representative confocal photomicrographs of vehicle (VEH) or caffeine (CAF) treated mixed retinal cell cultures before (Basal) and after Muscimol stimulation. b Intensity of fluorescence (Fmax/F0) before (Basal: VEH = 1.08 ± 0.01, CAF = 1.10 ± 0.02) and peak intensity of fluorescence after stimulation (Muscimol) of the cultures with 10 μM of Muscimol exposed or not to caffeine (Muscimol = 1.22 ± 0.06, CAF + Muscimol = 1.45 ± 0.08). c Representative traces (F/F0) of [Ca2+] transients, measured as peak heights, after 10 μM Muscimol stimulation (arrow). Data represent fluorescence intensity of 26 and 30 neuron cells recorded in at least four separate experiments. Color bar from black (0) to red (255) represents intracellular calcium increase. Bar = 30 μm. **p < 0.01 related to CAF
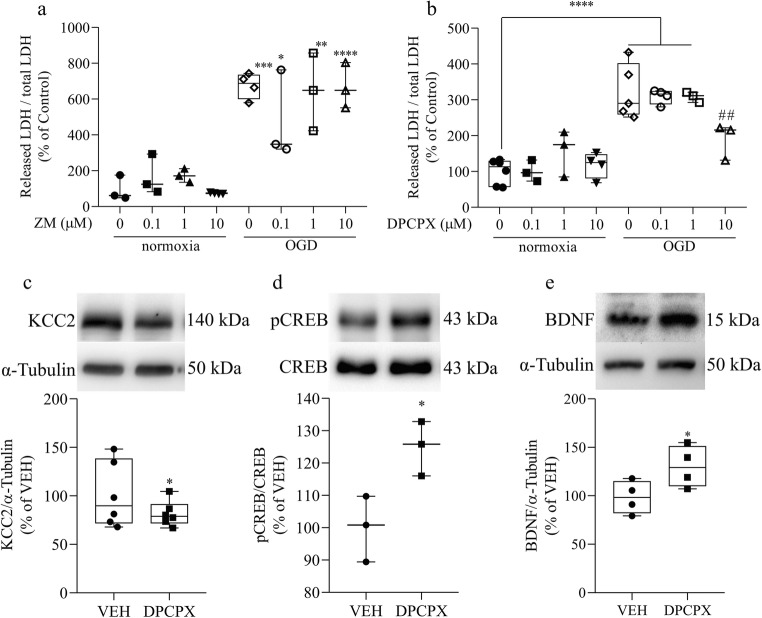
As mentioned in the introduction, the concentration of caffeine used in the present study selectively inhibits adenosine receptors. Therefore, in order to better understand the effect of caffeine, we performed experiments with adenosine A2A (ZM241385) and A1 (DPCPX) receptor antagonists to verify which receptor could be triggering the protective effect. We observed that treatment with ZM241385 for 48 h had no effect upon ischemia-induced cell death, while DPCPX, in the same conditions, reduced cell death induced by OGD, resembling caffeine effect (Fig. 10a, b). Then, we tested if DPCPX could stimulate a similar signaling response as caffeine. We observed that this compound could also reduce KCC2 content and increase both CREB phosphorylation and BDNF (Fig. 10c–e). These results suggest that caffeine would exert its protective effect by inhibiting A1 adenosine receptors.
Fig. 10.
DPCPX mimics the effect of caffeine. a LDH assay of E16 retinas previously treated with vehicle (CTR) or ZM241385 for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 43.85%, n = 3; ZM (100 nM) = 166.66 ± 64.16%, n = 3; ZM (1 μM) = 172.87 ± 21.85%, n = 3; ZM (10 μM) = 73.92 ± 1.68%, n = 4; OGD = 674.52 ± 35.46%, n = 4; OGD + ZM (100 nM) = 477.06 ± 143.34%, n = 3; OGD + ZM (1 μM) = 642.88 ± 125.37%, n = 3; OGD + ZM (10 μM) = 819.76 ± 103.83%, n = 3). b LDH assay of E16 retinas previously treated with vehicle (CTR) or DPCPX for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 14.44%, n = 6; DPCPX (100 nM) = 100.40 ± 17.05%, n = 3; DPCPX (1 μM) = 156.37 ± 37.25%, n = 3; DPCPX (10 μM) = 117.78 ± 17.83%, n = 4; OGD = 322.55 ± 34.20%, n = 5; OGD + DPCPX (100 nM) = 309.36 ± 10.16%, n = 4; OGD + DPCPX (1 μM) = 308.50 ± 8.30%, n = 3; OGD + DPCPX (10 μM) = 189.57 ± 29.14%, n = 3). c, d, e Representative immunoblot of E16 retinas from animals treated with vehicle (VEH) or DPCPX (10 μM) and analyzed to c KCC2 (VEH = 100 ± 12.09%, n = 6; DPCPX = 81.60 ± 5.32%, n = 6), d pCREB (VEH = 100 ± 5.82%, n = 3; DPCPX = 124.9 ± 4.87%, n = 3), and e BDNF (VEH = 100 ± 6.62%, n = 5; DPCPX = 127.1 ± 8.43%, n = 4) using α-tubulin or respective non phosphorylated protein as loading control. *p < 0.05, related to CTR or VEH conditions, **p < 0.01, ***p < 0.001, ****p < 0.0001 related to CTR, ##p < 0.01 related to OGD group
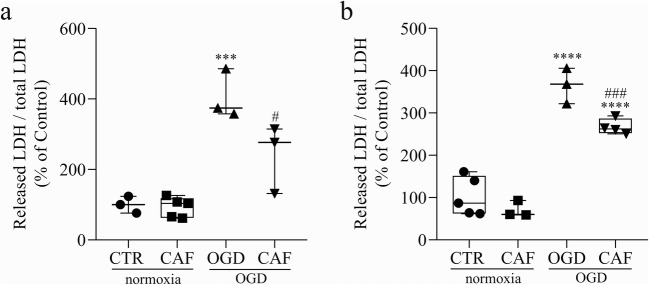
Finally, we investigate whether this protective effect of caffeine was restricted to the stage studied (E14-E16). So, we evaluated two others developmental windows, treating embryos at E12 or at E16 with caffeine and analyzing 48 h later. These periods were chosen based on the presence and functionality of adenosine receptors during retinal development. Before E12, the coupling of A2A receptors to adenylyl cyclase is virtually absent [65] and the binding to [3H]-CHA, used to detect A1 receptors, is very low [66]. Since we were using low to moderate doses of caffeine, which targets specifically the adenosine receptors, we decided to test developmental windows after E12. Caffeine treatment was able to reduce OGD-induced cell death in both additional periods evaluated (E12–E14 and E16–E18) (Fig. 11), showing that caffeine effect is not restricted to a narrow developmental window of the embryonic chick retina.
Fig. 11.
Caffeine reduces OGD induced cell death in other developmental stages. a LDH assay of E14 retinas previously treated with vehicle (CTR) or caffeine for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 13.67%, n = 3; CAF = 93.08 ± 12.56%, n = 5; OGD = 406 ± 40.13%, n = 3; CAF + OGD = 241 ± 55.83%, n = 3). b LDH assay of E18 retinas previously treated with vehicle (CTR) or caffeine for 48 h and submitted to normoxia or OGD procedure for 50 min (CTR = 100 ± 19.54%, n = 5; CAF = 70.79 ± 11.23%, n = 3; OGD = 365.3 ± 24.21%, n = 3; CAF + OGD = 267.2 ± 9.16%, n = 4). ***p < 0.001 and ****p < 0.0001 compared to CTR; #p < 0.05, ###p < 0.001 compared to OGD
Discussion
In this work, we demonstrated that exposure to caffeine for 48 h in vivo induced alterations in several neurochemical parameters leading to an increased resistance of retinal cells to an acute ischemic insult. Caffeine has been implicated in a wide range of effects for animal models and humans. This diversity correlates with variables like the consumer age, exposure time, and dose.
As mentioned before, there is growing evidence in animal models showing that caffeine can be harmful to tissues exposed during developmental stages. It was shown, for example, that chronic caffeine given to pregnant female rats during entire gestational period can stimulate enhanced activity of brain areas such as hippocampus [24] and visual cortex [79], leading to increased susceptibility to seizures in young and adult rats, as well as cognitive deficits and neuronal loss in adults. However, evidence suggests that altered susceptibility to seizures due to caffeine exposure depends on the developmental stage, dose used, and period of test [80]. Early exposure to caffeine (E1.5 or E8) in chicken embryos affects neural tube closure and reduces brain weight as analyzed at later stages [21, 22]. Similarly, caffeine given at rat gestational day 8 and 9 delayed neural tube closure and altered embryo normal development when analyzed at gestational day 10 [20]. In the developing retina, it was demonstrated that early exposure to caffeine can cause microphthalmia, reduced retinal thickness, and lens size [23]. Accordingly, in humans, caffeine intake has been associated with increased risk for low birth weight, miscarriage and stillbirth or fetal death [81–88]. However, for preterm infants, caffeine is used in the clinics as an efficient compound to ameliorate the consequences of apnea of prematurity, such as cerebral palsy and bronchopulmonary dysplasia [89, 90].
In the present study, the effect of caffeine was studied in a late period of retinal development. The main developmental phenomena occurring are differentiation and synaptogenesis. Previous study of the lab [64] has already shown that even though some neurochemical subpopulations were affected, caffeine exposure, during this period, did not induce alteration in the retinal structure, suggesting the absence of retinal damage. Here, we showed that caffeine exposure during synaptogenesis activates neurochemical changes that lead the retina to a more resistant state against an acute ischemic insult. These data suggest that the harmful effects of caffeine during development could depend on the maturation of the tissue.
Premature birth and low birth weight are the most significant risk factors for retinopathy of prematurity (ROP), one of the major causes of infant blindness [91]. Interestingly, it seems that caffeine can also help preventing this pathology progression [91, 92]. Caffeine and the A2A adenosine receptor have been investigated in the context of ROP. It has been shown that A2A receptor knockout mice exhibit a reduction in oxygen-induced damage (OID), a model for ROP [93]. In agreement, it has also been shown that caffeine can reduce the severity of ROP in mice pups treated from P7 to P12 through nursing mothers exposed to 1.0 g/L of caffeine in drinking water [94]. Here, we observe that previous exposure to caffeine, during synaptogenesis period, can consistently promote protection of retinal cells from acute ischemia induced cell death.
Some studies have previously shown a protective effect of caffeine in situations of hypoxia and ischemia. Data obtained using immature tissue demonstrated that caffeine could reduce apoptotic rates in the hippocampus and parietal cortex of 7-day-old neonatal rats, acutely exposed to caffeine before and after ischemia [55], or chronically exposed neonates treated through their dams from P0-P7 before subsequent ischemia [54]. Single dose of caffeine injected in P7 rats after ischemia also reduced behavior deficits observed later in life (P90–95) [95]. Recently, it was shown that single low dose caffeine treatment immediately after ischemia seemed to reduce infarct size and atrophy in the brain of P10 mice when analyzed 2 weeks later [56]. Moreover, using a model of chronic hypoxia to cause periventricular white matter injury, known to be related to cerebral palsy in preterm infants [96], it was observed that caffeine, given through the dam, could prevent ventriculomegaly, and reestablish myelination patterns, two factors altered as a consequence of hypoxia.
Previous data shows that adult retinal tissue can be protected from ischemic insult by chronic treatment with caffeine or the A2A receptor antagonist KW6002, and also by acute treatment with SCH 58261, another A2A receptor antagonist [47, 58]. These works demonstrated reduced apoptotic rates in rat retinas exposed to transient ischemia induced by raising intraocular pressure, alongside reduced inflammatory response. Interestingly, here we showed that the protective effect of caffeine against an acute ischemic insult (OGD), during retinal synaptogenesis window, was mimicked by A1 receptor antagonist, but not by the inhibition of A2A receptor. Thus, the mechanism of protection could differ depending on tissue developmental stage or insult type, such as ischemia/reperfusion.
The cell death induced by the ischemic insult studied here is mostly not apoptotic, and it must occur through mechanisms already described in the literature when an ischemic event takes place. The lack of oxygen and glucose leads the tissue to a depolarized state due to energy failure and the compromised capacity to regulate the ionic balance. In this context, neurotransmitters are released due to intracellular calcium increase and failure of energy dependent re-uptake [26, 97, 98]. Rapid cell death dependent on NMDA or ROS was shown to take place following these events [66, 99–103].
Data from our lab have shown that the same caffeine treatment, used in the present study, was able to increase adenosine A1 receptor expression [64]. Among other effects, this receptor can modulate neuronal excitability by decreasing calcium entry and neurotransmitter release from presynaptic terminals, attenuating excitotoxicity caused by robust glutamate signaling through NMDA receptors in ischemia [33]. Therefore, in the present study we were interested in observing possible changes in glutamate availability. We observed an increase of extracellular glutamate when retinas were exposed to the 50 min OGD. However, at least with the approach we used here, caffeine treatment did not change this response, as glutamate availability was still high after ischemia of caffeine-treated retina (Fig. 2b). These data raise the possibility that the protective mechanism induced by caffeine does not involve reduction of glutamate release.
The excitotoxicity promoted by NMDA receptor overactivation has an important role in ischemia-induced cell death [30, 63]. Compelling evidence suggest that activation of extrasynaptic NMDA receptors containing GluN2B subunits can contribute to damage the cells in ischemic or excitotoxic situations [104, 105] but synaptic NMDA receptors containing GluN2A subunits are more prone to save cells. We observed no change in the total protein content of these subunits after caffeine exposure (Fig. 2 e and f), suggesting that modulation of NMDA composition could not be crucial for the protective effect of caffeine, although, the western blot technique only detects total amount of the subunit, and we cannot exclude variations at the cell membrane level or specifically at synapses.
Despite being crucial for cellular death in ischemia, NMDA receptors are also involved in a phenomenon called preconditioning. In this case, brief periods of sub-toxic doses of glutamate or NMDA can trigger signaling events able to protect the tissue to subsequent excitotoxic/ischemic damage [106–108]. In the present study, we were interested in observing whether caffeine had any modulatory property on NMDA receptor activity. Indeed, we showed that caffeine increased NMDA receptor basal activity, through functional binding experiments, and NMDA-activated intracellular calcium increasing in neurons (Fig. 3). These results suggest that caffeine treatment modulates NMDA receptor activity. This, in turn, could be accomplished by an increase of extracellular glutamate that could be related to modulation of the transport capacity, although these effects were not detected in our present study, maybe because of the sensitivity of the techniques used. Methods that are more sensitive would need to be tested [109]. Moreover, the phosphorylation of the receptor subunits could also influence its activity as demonstrated before [110, 111].
The protective mechanism of preconditioning induced by NMDA can be achieved by activation of intracellular cascades that include phosphorylation of ERK, Akt, CREB, and BDNF production [61]. When we analyzed these parameters in the conditions used, all of them were upregulated after 48 h of exposure to caffeine (Fig. 4), and 50 min in normoxia. However, 50 min in OGD condition depleted the phosphorylation of these proteins. This observation suggests that if the activation of these proteins is the key mechanism by which caffeine is exerting cellular protection, they would not need to be activated during ischemia, but primarily before the insult. To verify whether CREB and BDNF were crucial in the signaling protective cascade, embryos were exposed to caffeine together with KG-501 or K252a, an inhibitor of CREB or a blocker of the BDNF receptor, TrkB, respectively. Indeed, we observed that caffeine protection depends on the activation of CREB and BDNF pathways previously to the ischemic insult (Fig. 5).
Next, we also evaluated the participation of the GABAergic system. Since the tissue seemed to be in a depolarized state, as indicated by the increased basal NMDA receptor activity, the main inhibitory neurotransmitter could play a role in this scenario, since it controls tissue excitability. Our data shows that GABA cell content and in the plexiform layers of the retina increases with caffeine treatment (Fig. 6). Higher intracellular GABA levels correlates well with the previous increase of glutamic acid decarboxylase (GAD) expression, the GABA synthetic enzyme (Fig. 7g).
As mentioned before, GABA causes excitatory responses through GABAA receptor in immature tissue due to a higher concentration of intracellular chloride. The modulation of this gradient is tightly controlled by chloride transporters NKCC1 and KCC2. The former predominates at the beginning of development, allowing chloride to accumulate inside the cells, making GABA excitatory. With the development progression, KCC2 levels exceed NKCC1, shifting GABA response as it becomes a hyperpolarizing neurotransmitter [76, 77, 112]. It was shown that, in the chick retina, this shift occurs from E12 until E20 [73, 74]. Thus, a strong candidate for the regulation of this supposedly retinal excitability alteration was the chloride transporters. Remarkably, we found that KCC2, the transporter that underlies the hyperpolarizing effect of GABA upon ionotropic receptor binding, was significantly downregulated after caffeine (Fig. 8a). This result suggests that the inhibitory effect of GABA could be compromised, leading the tissue to a more depolarized state, and possibly contributing to NMDA receptor increased activity. Indeed, muscimol, an agonist of GABAA receptors, was able to increase calcium entry in neurons from cultures exposed to caffeine, suggesting depolarizing activity followed by opening of voltage-gated calcium channels (Fig. 9).
Conclusion
In conclusion, the present study raises the possibility that caffeine exposure during intermediate developmental stages leads retinal cells to a more resistant state against ischemia by evoking several neurochemical changes. The precise mechanism through which caffeine protects the retina still needs further studies, but the presented data indicate that tissue excitability influenced by GABAergic and glutamatergic systems modulation seem to play an important role. This mechanism seems to be triggered by adenosine A1 receptors, CREB phosphorylation, and BDNF production (Fig. 12).
Fig. 12.
Scheme of a possible mechanism triggered by caffeine to promote retinal survival in ischemic condition. Caffeine, acting at A1 adenosine receptors, decreases KCC2 chloride co-transporters levels, delaying the shift from depolarizing to hyperpolarizing response of GABAA receptors. Caffeine also increases NMDA receptor activity, which could be consequence of an augment in retinal excitability. Intracellular calcium increased (through NMDA receptors and/or GABAA receptors) or A1 receptor blockade could be recruiting intracellular signaling pathways involving phosphorylation of ERK, Akt, and CREB. These proteins could be coupled to BDNF production and release, and further activation of TrkB receptors by BDNF would contribute to the resistance and survival of the cells. It is important to note that this is cartoon is just a strategy to show in an illustrative and simplistic manner the obtained results, and it did not exclude other possibilities
Acknowledgments
Araújo DSM, Nascimento AA and Pereira-Figueiredo, D thank CAPES/Brazil for doctoral fellowships. Calaza KC, Pereira Netto AD and Paes-de-Carvalho R thank CNPq and FAPERJ for the individual research fellowships. The anti-GAD antibody developed by Oertel et al., 1981 was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National institute of child health and human development (NICHD) and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Funding information
This work was supported by grants from National Council of Scientific and technological Development (CNPq), Coordination of Superior Level Staff Improvement (CAPES), Foundation for Research Support of the State of Rio de Janeiro (FAPERJ), The program of Nucleus of Excellence/Ministry of science and technology (PRONEX/MCT).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. Pereira-Figueiredo, Email: dannielfigueiredo@id.uff.br
R. Brito, Email: rafael.silva@icbs.ufal.br
D. S. M. Araújo, Email: daniel_araujo@id.uff.br
A. A. Nascimento, Email: alves_amandaa@hotmail.com
E. S. B. Lyra, Email: elyra@id.uff.br
A. M. S. S. Cheibub, Email: anamariacheibub@gmail.com
A. D. Pereira Netto, Email: annibal@vm.uff.br
A. L. M. Ventura, Email: almvuff@gmail.com
R. Paes-de-Carvalho, Email: robpaesuff@gmail.com
K. C. Calaza, Email: kcalaza@id.uff.br
References
- 1.Masland RH. The Neuronal Organization of the Retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calaza KC, Gardino PF. Neurochemical phenotype and birthdating of specific cell populations in the chick retina. An Acad Bras Cienc. 2010;82:595–608. doi: 10.1590/S0001-37652010000300007. [DOI] [PubMed] [Google Scholar]
- 3.Camici M, Garcia-Gil M, Tozzi M. The Inside Story of Adenosine. Int J Mol Sci. 2018;19:784. doi: 10.3390/ijms19030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredholm BB, IJzerman AP, Jacobson KA, et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. doi: 10.1124/pr.110.003285.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheth S, Brito R, Mukherjea D, et al. Adenosine receptors: Expression, function and regulation. Int J Mol Sci. 2014;15:2024–2052. doi: 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro JA. Purinergic Inhibition of Neurotransmitter Release in the Central Nervous System. Pharmacol Toxicol. 1995;77:299–305. doi: 10.1111/j.1600-0773.1995.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 7.Sebastião AM, Ribeiro JA. Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci. 2000;21:341–346. doi: 10.1016/S0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- 8.Cunha R, Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: di€erent roles, di€erent sources and di€erent receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 9.Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira JM, Paes-de-Carvalho R. Long-term activation of adenosine A2a receptors blocks glutamate excitotoxicity in cultures of avian retinal neurons. Brain Res. 2001;900:169–176. doi: 10.1016/S0006-8993(01)02279-X. [DOI] [PubMed] [Google Scholar]
- 11.De Mendonça A, Ribeiro JA. Adenosine and synaptic plasticity. Drug Dev Res. 2001;52:283–290. doi: 10.1002/ddr.1125. [DOI] [Google Scholar]
- 12.Fredholm BB, Bättig K, Holmén J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 13.Ribeiro JA, Sebastio AM (2010) Caffeine and adenosine. J Alzheimers Dis 20. 10.3233/JAD-2010-1379 [DOI] [PubMed]
- 14.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 15.Popoli P, Blum D, Martire A, et al. Functions, dysfunctions and possible therapeutic relevance of adenosine A2Areceptors in Huntington’s disease. Prog Neurobiol. 2007;81:331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Flaten V, Laurent C, Coelho JE et al (2015) What about caffeine in Alzheimer’s disease? 42:587–592. 10.1042/BST20130229 [DOI] [PMC free article] [PubMed]
- 17.Carman AJ, Dacks PA, Lane RF, et al. Current evidence for the use of coffee and caffeine to prevent age-related cognitive decline and Alzheimer’s disease. J Nutr Health Aging. 2014;18:383–392. doi: 10.1007/s12603-014-0021-7. [DOI] [PubMed] [Google Scholar]
- 18.Costenla AR, Cunha RA, De Mendonça A (2010) Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers Dis 20. 10.3233/JAD-2010-091384 [DOI] [PubMed]
- 19.Kolahdouzan M, Hamadeh MJ. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther. 2017;23:272–290. doi: 10.1111/cns.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson JM, Pollard I. In utero exposure to caffeine causes delayed neural tube closure in rat embryos. Teratog Carcinog Mutagen. 1994;14:205–211. doi: 10.1002/tcm.1770140502. [DOI] [PubMed] [Google Scholar]
- 21.Li XD, He RR, Qin Y, et al. Caffeine interferes embryonic development through over-stimulating serotonergic system in chicken embryo. Food Chem Toxicol. 2012;50:1848–1853. doi: 10.1016/j.fct.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Ma ZL, Qin Y, Wang G, et al. Exploring the caffeine-induced teratogenicity on neurodevelopment using early chick embryo. PLoS One. 2012;7:1–8. doi: 10.1371/journal.pone.0034278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma ZL, Wang G, Cheng X, et al. Excess caffeine exposure impairs eye development during chick embryogenesis. J Cell Mol Med. 2014;18:1134–1143. doi: 10.1111/jcmm.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva CG, Metin C, Fazeli W, et al. Adenosine Receptor Antagonists Including Caffeine Alter Fetal Brain Development in Mice. Sci Transl Med. 2013;5:197ra104–197ra104. doi: 10.1126/scitranslmed.3006258. [DOI] [PubMed] [Google Scholar]
- 25.Souza AC, Souza A, Medeiros LF, et al. Maternal caffeine exposure alters neuromotor development and hippocampus acetylcholinesterase activity in rat offspring. Brain Res. 2015;1595:10–18. doi: 10.1016/j.brainres.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/Reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bresnick GH, De Venecia G, Myers FL, et al. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol (Chicago, Ill 1960) 1975;93:1300–1310. doi: 10.1001/archopht.1975.01010020934002. [DOI] [PubMed] [Google Scholar]
- 28.Osborne NN, Ugarte M, Chao M, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–S128. doi: 10.1016/S0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 29.Osborne NN, Casson RJ, Wood JPM, et al. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Kostandy BB. The role of glutamate in neuronal ischemic injury: The role of spark in fire. Neurol Sci. 2012;33:223–237. doi: 10.1007/s10072-011-0828-5. [DOI] [PubMed] [Google Scholar]
- 31.Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med (Berl) 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- 32.Melani A, Corti F, Stephan H, et al. Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol. 2012;233:193–204. doi: 10.1016/j.expneurol.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 33.Pedata F, Dettori I, Coppi E, et al. Purinergic signalling in brain ischemia. Neuropharmacology. 2016;104:105–130. doi: 10.1016/j.neuropharm.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Héron A, Lekieffre D, Le Peillet E, et al. Effects of an A1 adenosine receptor agonist on the neurochemical, behavioral and histological consequences of ischemia. Brain Res. 1994;641:217–224. doi: 10.1016/0006-8993(94)90149-X. [DOI] [PubMed] [Google Scholar]
- 35.Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 36.Von Lubitz DK, Beenhakker M, Lin RC, et al. Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur J Pharmacol. 1996;302:43–48. doi: 10.3171/2009.11.JNS081052.Molecular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsson T, Cronberg T, Rytter A, et al. Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci. 2004;20:1197–1204. doi: 10.1111/j.1460-9568.2004.03564.x. [DOI] [PubMed] [Google Scholar]
- 38.Rudolphi KA, Keil M, Fastbom J, Fredholm BB. Ischaemic damage in gerbil hippocampus is reduced following upregulation of adenosine (A1) receptors by caffeine treatment. Neurosci Lett. 1989;103:275–280. doi: 10.1016/0304-3940(89)90112-2. [DOI] [PubMed] [Google Scholar]
- 39.Von Lubitz DK, Lin RC, Melman N, et al. Chronic administration of selective adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur J Pharmacol. 1994;256:161–167. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monopoli A, Lozza G, Forlani A, et al. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- 42.Melani A, Pantoni L, Bordoni F, et al. The selective A2Areceptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–250. doi: 10.1016/S0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- 43.Pugliese AM, Traini C, Cipriani S, et al. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br J Pharmacol. 2009;157:818–830. doi: 10.1111/j.1476-5381.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamed RA, Agha AM, Abdel-Rahman AA, Nassar NN. Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: Signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2) Neuroscience. 2016;314:145–159. doi: 10.1016/j.neuroscience.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 45.Bona E, Aden U, Gilland E, et al. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–1338. doi: 10.1016/S0028-3908(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang ZJ, Wang B, Kwansa H, et al. Adenosine A2A receptor contributes to ischemic brain damage in newborn piglet. J Cereb Blood Flow Metab. 2013;33:1612–1620. doi: 10.1038/jcbfm.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madeira MH, Boia R, Elvas F, et al. Selective A2Areceptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Transl Res. 2016;169:112–128. doi: 10.1016/j.trsl.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Von Lubitz DK, Lin RC, Jacobson KA. Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melani A, Corti F, Cellai L, et al. Low doses of the selective adenosine A2Areceptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res. 2014;1551:59–72. doi: 10.1016/j.brainres.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Chen JF, Huang Z, Ma J, et al. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/jneurosci.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ådén U, Halldner L, Lagercrantz H, et al. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–744. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- 52.Gui L, Duan W, Tian H, et al. Adenosine A2Areceptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res. 2009;1297:185–193. doi: 10.1016/j.brainres.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 53.Duan W, Gui L, Zhou Z, et al. Adenosine A2Areceptor deficiency exacerbates white matter lesions and cognitive deficits induced by chronic cerebral hypoperfusion in mice. J Neurol Sci. 2009;285:39–45. doi: 10.1016/j.jns.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Bona E, Adén U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38:312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Kilicdag H, Daglioglu YK, Erdogan S, Zorludemir S. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med. 2014;7058:1–6. doi: 10.3109/14767058.2013.878694. [DOI] [PubMed] [Google Scholar]
- 56.Winerdal M, Urmaliya V, Winerdal ME, et al. Single Dose Caffeine Protects the Neonatal Mouse Brain against Hypoxia Ischemia. PLoS One. 2017;12:1–7. doi: 10.1371/journal.pone.0170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland GR, Peeling J, Lesiuk HJ, et al. The effects of caffeine on ischemic neuronal injury as determined by magnetic resonance imaging and histopathology. Neuroscience. 1991;42:171–182. doi: 10.1016/0306-4522(91)90157-J. [DOI] [PubMed] [Google Scholar]
- 58.Boia R, Elvas F, Madeira MH, et al. Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell Death Dis. 2017;8:e3065. doi: 10.1038/cddis.2017.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, Roth S. Retinal ischemic preconditioning in the rat: Requirement for adenosine and repetitive induction. Investig Ophthalmol Vis Sci. 1999;40:1200–1216. doi: 10.1016/j.exer.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Vijayakumar NT, Sangwan A, Sharma B, et al. Cerebral Ischemic Preconditioning: the Road So Far??? Mol Neurobiol. 2016;53:2579–2593. doi: 10.1007/s12035-015-9278-z. [DOI] [PubMed] [Google Scholar]
- 61.Soriano FX. Preconditioning Doses of NMDA Promote Neuroprotection by Enhancing Neuronal Excitability. J Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dreixler JC, Hemmert JW, Shenoy SK, et al. The role of Akt/protein kinase B subtypes in retinal ischemic preconditioning. Exp Eye Res. 2009;88:512–521. doi: 10.1016/j.exer.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocha M, Martins RAP, Linden R. Activation of NMDA receptors protects against glutamate neurotoxicity in the retina: Evidence for the involvement of neurotrophins. Brain Res. 1999;827:79–92. doi: 10.1016/S0006-8993(99)01307-4. [DOI] [PubMed] [Google Scholar]
- 64.Brito R, Pereira-Figueiredo D, Socodato R et al (2016) Caffeine exposure alters adenosine system and neurochemical markers during retinal development. J Neurochem:557–570. 10.1111/jnc.13683 [DOI] [PubMed]
- 65.Paes de Carvalho R, de Mello FG. Adenosine-elicited accumulation of adenosine 3’, 5’-cyclic monophosphate in the chick embryo retina. J Neurochem. 1982;38:493–500. doi: 10.1111/j.1471-4159.1982.tb08655.x. [DOI] [PubMed] [Google Scholar]
- 66.Paes de Carvalho R. Development of A1 adenosine receptors in the chick embryo retina. J Neurosci Res. 1990;25:236–242. doi: 10.1002/jnr.490250212. [DOI] [PubMed] [Google Scholar]
- 67.de Carvalho RP, Braas KM, Adler R, Snyder SH. Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res. 1992;70:87–95. doi: 10.1016/0165-3806(92)90106-7. [DOI] [PubMed] [Google Scholar]
- 68.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 69.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 70.Calaza KC, De Mello FG, Gardino PF. GABA release induced by aspartatemediated activation of NMDA receptors is modulated by dopamine in a selective subpopulation of amacrine cells. J Neurocytol. 2001;30:181–193. doi: 10.1023/A:1012764422711. [DOI] [PubMed] [Google Scholar]
- 71.Miya-Coreixas VS, Maggesissi Santos R, Carpi Santos R, et al. Regulation of GABA content by glucose in the chick retina. Exp Eye Res. 2013;115:206–215. doi: 10.1016/j.exer.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Guimarães-Souza EM, Gardino PF, De Mello FG, Calaza KC. A calciumdependent glutamate release induced by metabotropic glutamate receptors I/II promotes GABA efflux from amacrine cells via a transporter-mediated process. Neuroscience. 2011;179:23–31. doi: 10.1016/j.neuroscience.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: A personal journey. Neuroscience. 2014;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Catsicas M, Mobbs P. GABAb receptors regulate chick retinal calcium waves. J Neurosci. 2001;21:897–910. doi: 10.1523/JNEUROSCI.21-03-00897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ring H, Boije H, Daniel C, et al. Increased A-to-I RNA editing of the transcript for GABA A receptor subunit a3 during chick retinal development. Vis Neurosci. 2010;27:149–157. doi: 10.1017/S0952523810000180. [DOI] [PubMed] [Google Scholar]
- 77.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-S. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 79.Fazeli W, Zappettini S, Marguet SL, et al (2017) Early-life exposure to caffeine affects the construction and activity of cortical networks in mice. Elsevier Inc [DOI] [PubMed]
- 80.Tchekalarova JD, Kubová H, Mareš P. Early caffeine exposure: Transient and long-term consequences on brain excitability. Brain Res Bull. 2014;104:27–35. doi: 10.1016/j.brainresbull.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Chen L-W, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy and risk of pregnancy loss: a categorical and dose–response metaanalysis of prospective studies. Public Health Nutr. 2016;19:1233–1244. doi: 10.1017/S1368980015002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenwood DC, Alwan N, Boylan S, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol. 2010;25:275–280. doi: 10.1007/s10654-010-9443-7. [DOI] [PubMed] [Google Scholar]
- 83.Greenwood DC, Thatcher NJ, Ye J, et al. Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose–response meta-analysis. Eur J Epidemiol. 2014;29:725–734. doi: 10.1007/s10654-014-9944-x. [DOI] [PubMed] [Google Scholar]
- 84.Matijasevich A, Barros FC, Santos IS, Yemini A. Maternal caffeine consumption and fetal death: A case-control study in Uruguay. Paediatr Perinat Epidemiol. 2006;20:100–109. doi: 10.1111/j.1365-3016.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 85.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:1–8. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 86.Hahn KA, Wise LA, Rothman KJ, et al. Caffeine and caffeinated beverage consumption and risk of spontaneous abortion. Hum Reprod. 2015;30:1246–1255. doi: 10.1093/humrep/dev063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rhee J, Kim R, Kim Y, et al. Maternal caffeine consumption during pregnancy and risk of low birth weight: A dose-response meta-analysis of observational studies. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0132334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakker R, Steegers EAP, Obradov A, et al. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study 1 – 3. Am J Clin Nutr. 2010;91:1691–1698. doi: 10.3945/ajcn.2009.28792.INTRODUCTION. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 91.Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84:83–88. doi: 10.1016/j.earlhumdev.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Chen J-F, Zhang S, Zhou R, et al. Adenosine receptors and caffeine in retinopathy of prematurity. Mol Asp Med. 2017;55:118–125. doi: 10.1016/j.mam.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Xiao-Ling XL, Zhou R, Pan QQ, et al. Genetic inactivation of the adenosine A2Areceptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Investig Ophthalmol Vis Sci. 2010;51:6625–6632. doi: 10.1167/iovs.09-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Zhou R, Li B, et al. Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J. 2017;31:3334–3348. doi: 10.1096/fj.201601285R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alexander M, Smith AL, Rosenkrantz TS, Holly R. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013;3:177–190. doi: 10.3390/brainsci3010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Back SA, Craig A, Luo NL, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 97.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1016/j.shpsa.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Wassink G, Gunn ER, Drury PP, et al. The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci. 2014;8:1–11. doi: 10.3389/fnins.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimohama S, Tamura Y, Akaike A, et al. Brain-derived neurotrophic factor pretreatment exerts a partially protective effect against glutamate-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett. 1993;164:55–58. doi: 10.1016/0304-3940(93)90856-G. [DOI] [PubMed] [Google Scholar]
- 100.Kashii S, Takahashi M, Mandai M, et al. Protective action of dopamine against glutamate neurotoxicity in the retina. Investig Ophthalmol Vis Sci. 1994;35:685–695. [PubMed] [Google Scholar]
- 101.Carloni S, Carnevali A, Cimino M, Balduini W. Extended role of necrotic cell death after hypoxia-ischemia-induced neurodegeneration in the neonatal rat. Neurobiol Dis. 2007;27:354–361. doi: 10.1016/j.nbd.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 102.Tan S, Zhou F, Nielsen VG, et al. Increased injury following intermittent fetal hypoxia-reoxygenation is associated with increased free radical production in fetal rabbit brain. J Neuropathol Exp Neurol. 1999;58:972–981. doi: 10.1097/00005072-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 103.David P, Lusky M, Teichberg VI. Involvement of excitatory neurotransmitters in the damage produced in chick embryo retinas by anoxia and extracellular high potassium. Exp Eye Res. 1988;46:657–662. doi: 10.1016/S0014-4835(88)80054-X. [DOI] [PubMed] [Google Scholar]
- 104.Brassai A, Suvanjeiev RG, Bán EG, Lakatos M. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res Bull. 2015;112:1–6. doi: 10.1016/j.brainresbull.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Chen M, Lu T-J, Chen X-J, et al. Differential Roles of NMDA Receptor Subtypes in Ischemic Neuronal Cell Death and Ischemic Tolerance. Stroke. 2008;39:3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- 106.Chuang DM, Gao XM, Paul SM. N-methyl-D-aspartate exposure blocks glutamate toxicity in cultured cerebellar granule cells. Mol Pharmacol. 1992;42:210–216. [PubMed] [Google Scholar]
- 107.Bond A, Lodge D, Hicks CA, et al. NMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischaemic tolerance in the gerbil hippocampus. Eur J Pharmacol. 1999;380:91–99. doi: 10.1016/S0014-2999(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 108.Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Socodato R, Portugal CC, Canedo T, et al. C-Src deactivation by the polyphenol 3-O-caffeoylquinic acid abrogates reactive oxygen species-mediated glutamate release from microglia and neuronal excitotoxicity. Free Radic Biol Med. 2015;79:45–55. doi: 10.1016/j.freeradbiomed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 110.Socodato R, Santiago FN, Portugal CC, et al. Dopamine promotes NMDA receptor hypofunction in the retina through D1 receptor-mediated Csk activation, Src inhibition and decrease of GluN2B phosphorylation. Sci Rep. 2017;7:1–14. doi: 10.1038/srep40912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferreira DDP, Stutz B, de Mello FG, et al. Caffeine potentiates the release of GABA mediated by NMDA receptor activation: Involvement of A1 adenosine receptors. Neuroscience. 2014;281:208–215. doi: 10.1016/j.neuroscience.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 112.Rivera C, Voipio J, Payne JA, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]