Abstract
In this study, we aimed to assess whether women are able to withstand more tau before exhibiting verbal memory impairment. Using data from 121 amyloid-β-positive Alzheimer’s Disease Neuroimaging Initiative participants, we fit a linear model with Rey Auditory Verbal Learning Test score as the response variable and tau-PET standard uptake value ratio as the predictor and took the residuals as an estimate of verbal memory reserve for each subject. Women demonstrated higher reserve (i.e. residuals), whether the Learning (t = 2.78, P = 0.006) or Delay (t = 2.14, P = 0.03) score from the Rey Auditory Verbal Learning Test was used as a measure of verbal memory ability. To validate these findings, we examined 662 National Alzheimer’s Coordinating Center participants with a C2/C3 score (Consortium to Establish a Registry for Alzheimer’s Disease) at autopsy. We stratified our National Alzheimer’s Coordinating Center sample into Braak 1/2, Braak 3/4 and Braak 5/6 subgroups. Within each subgroup, we compared Logical Memory scores between men and women. Men had worse verbal memory scores within the Braak 1/2 (Logical Memory Immediate: β = −5.960 ± 1.517, P < 0.001, Logical Memory Delay: β = −5.703 ± 1.677, P = 0.002) and Braak 3/4 (Logical Memory Immediate: β = −2.900 ± 0.938, P = 0.002, Logical Memory Delay: β = −2.672 ± 0.955, P = 0.006) subgroups. There were no sex differences in Logical Memory performance within the Braak 5/6 subgroup (Logical Memory Immediate: β = −0.314 ± 0.328, P = 0.34, Logical Memory Delay: β = −0.195 ± 0.287, P = 0.50). Taken together, our results point to a sex-related verbal memory reserve.
Keywords: tau, verbal memory, sex differences, Alzheimer’s disease
In this study, we demonstrated in two independent cohorts that, for a given level of tau pathology, women perform better than men on verbal memory tests. Our findings suggest that women exhibit cognitive reserve, such that they are able to tolerate more tau before showing verbal memory impairment.
Graphical Abstract
Graphical Abstract.
Introduction
Cognitive reserve describes the phenomenon where individuals vary in cognitive performance despite harbouring similar amounts of Alzheimer’s disease pathology (Stern, 2002). Cognitive reserve has been attributed to factors such as education (Stern et al., 1992), overall intellectual ability (Alexander et al., 1997), diet (Scarmeas et al., 2006) and social network size (Bennett et al., 2006).
Sex may also play a role in reserve, with women demonstrating higher reserve in verbal memory (Beinhoff et al., 2008, Chapman et al., 2011). This is supported by a pair of recent imaging studies, which reported that women, while expressing similar levels of neurodegeneration (Sundermann et al., 2016a, b), outperform men in verbal memory. Further evidence comes from an investigation demonstrating that sex can moderate the relationship between amyloid-β (Aβ) and verbal memory performance (Caldwell et al., 2017).
Recent studies have revealed sex differences in tau pathology. Post-mortem data indicate that women have more tau at autopsy (Liesinger et al., 2018; Oveisgharan et al., 2018). Ante-mortem examination of brain tau is now available through positron emission tomography (PET) (Marquié et al., 2015). A recent tau-PET study reported that, among cognitively normal individuals with elevated Aβ, women harboured more tau (Buckley et al., 2019). A potential corollary to these findings is that women can withstand more tau before exhibiting verbal memory impairment. In other words, women may exhibit more reserve, but this hypothesis has not been explored in vivo.
A useful approach for estimating cognitive reserve is the residual framework (Reed et al., 2010; Zahodne et al., 2013; Hohman et al., 2016; van Loenhoud et al., 2017). Under this framework, a model is fitted to the data, where cognitive performance is the response variable and Alzheimer's disease pathology is the predictor. This model provides a predicted level of cognition for a given level of pathology. Those that display higher than predicted cognitive performance (i.e. positive residual) can be characterized as having high cognitive reserve and vice versa.
In this study, we applied this residual approach to PET and verbal memory data from Alzheimer’s Disease Neuroimaging initiative (ADNI) to estimate reserve. We then assessed sex differences in reserve, hypothesizing that women would demonstrate higher reserve than men. We further aimed to characterize how women’s verbal memory advantage varies by disease stage. For validation, we examined autopsy and verbal memory data subjects from the National Alzheimer’s Coordinating Center (NACC).
Methods and materials
Study 1: ADNI tau-PET analysis
ADNI sample
We included ADNI participants who underwent Aβ-PET, flortaucipir (FTP)-PET and magnetic resonance imaging, completed the ADNI neuropsychological battery and had APOE genotyping. Recruitment details for ADNI are detailed elsewhere (Aisen et al., 2010; Weiner et al., 2017). We restricted our sample to Aβ-positive subjects (based on previously derived thresholds; Landau et al., 2012, 2013) to focus on the Alzheimer's disease spectrum.
ADNI neuroimaging processing
For each participant, we downloaded the first available FTP-PET in its most preprocessed form (Joshi et al., 2009) and the magnetic resonance imaging acquired temporally closest to this FTP-PET. Magnetic resonance imaging was processed with FreeSurfer (Dale et al., 1999, Fischl et al., 1999). FTP volumes were first co-registered to each subject’s magnetic resonance imaging. Then, standard uptake value ratio volumes were generated by normalizing to average FTP signal in the cerebellar grey. Regional tau values were derived from mean standard uptake value ratio within each Desikan-Killiany region (Desikan et al., 2006). Tau load was defined as the average regional tau from entorhinal, parahippocampal, fusiform, inferior temporal and middle temporal cortex (Jack et al., 2017).
Aβ pathology was assessed using summary cortical standard uptake value ratio (whole cerebellum reference) data generated by the Jagust Lab (Landau et al., 2012, 2013).
ADNI memory measures
To assess verbal memory, we used Rey Auditory Verbal Learning Test (RAVLT) scores acquired closest in time to the FTP-PET (time between FTP-PET and RAVLT date: mean: 0.639 years, SD: 0.783). We used the sum of words across the first five trials (RAVLT Learning) and the number of words recalled after a 30-minute delay (RAVLT Delay).
Statistical analysis
Subject characteristics
We used Welch t-tests to assess sex differences in age, education and summary Aβ and χ2 tests to examine sex differences in ε4 status.
Reserve analyses
We took a residual approach to estimate reserve. First, we fit a linear regression model with RAVLT score as the response variable and age, education, ε4 status and tau load as predictors. This model provides an individual’s predicted RAVLT score for a certain level of tau load. Since ‘reserve’ is defined as having better or worse cognition than is predicted by pathology, we took each individual’s residual in the model as an estimation of their reserve. Welch t-tests were then performed to test for a difference in residuals (i.e. reserve) between women and men. This procedure was done for two separate models, using either RAVLT Learning or RAVLT Delay as the response variable.
Subgroup stratified analysis
To further explore these sex differences in tau and verbal memory, we stratified our sample into two groups: cognitively normal participants [preclinical Alzheimer’s disease (preAD)] and mild cognitive impairment/Alzheimer's disease participants [prodromal/probable Alzheimer's disease (proAD)]. Within each subgroup, we performed the following linear model analyses. First, we assessed sex differences in tau load, RAVLT Learning and RAVLT Delay after correcting for age, education and ε4 status. Then, we tested for sex differences in RAVLT Learning and RAVLT Delay, while controlling for age, education, ε4 status and tau load.
Study 2: NACC post-mortem analysis
NACC sample
For NACC analyses, we utilized data from the December 2018 freeze. We included participants with a clinical diagnosis of normal cognition, amnestic mild cognitive impairment or dementia (with Alzheimer's disease as presumptive etiology) at last clinical visit and autopsy data within 5 years of that visit. Our sample was restricted to individuals 60 years or older at baseline and had at least two visits prior to autopsy. We selected only participants with a Consortium to Establish a Registry for Alzheimer’s Disease neocortical neuritic plaque rating of C2 or C3, indicating moderate to frequent plaques (Mirra et al., 1991), to focus on participants on the Alzheimer's disease spectrum and to parallel our ADNI analyses, which included only Aβ-positive individuals.
NACC neuropsychology measures
The NACC neuropsychological battery does not include the RAVLT or similar list-learning task, so we instead used scores from the Logical Memory (LM) test, which assesses immediate (LM Immediate) and 20-minute delayed recall of a brief story (LM Delay). The memory scores from the last test administration prior to death were used.
Statistical analysis
Subject characteristics
To assess sex differences in age, education and time between last clinical visit and death, we used Welch two-sample t-tests. To compare carriage of the ε4 allele between men and women, we used χ2 tests.
Pathology analyses
We first stratified our NACC cohort into three subgroups: Braak 1/2, Braak 3/4 and Braak 5/6 subgroups. We then used linear models to examine sex differences in LM Immediate and LM Delay scores within each subgroup. In these models, we corrected for time between last clinical visit and death, age at clinical visit and ε4 status.
Data availability
The ADNI demographic, genetic, neuroimaging and neuropsychology data that were used in our analyses are available for eligible users for access and download at the ADNI data repository (adni.loni.usc.edu). The NACC demographic, genetic, neuropathology and neuropsychology data that were used can be accessed freely by eligible researchers through the NACC website (alz.washington.edu).
Results
Study 1: ADNI tau-PET analysis
Subject characteristics
A total of 121 ADNI participants met criteria for our study. Summary statistics are displayed in Table 1. Across the sample, women were younger [t(119) = −2.37, P = 0.02] and had fewer years of education [t(119) = −3.40, P < 0.001]. No sex difference in ε4 status [χ2 (1) = 0.0476; P = 0.83] was observed. In our preAD group (23 men and 26 women), the women were not different with respect to age [t(44) = −1.50, P = 0.14], education [t(47) = −1.35, P = 0.18] or ε4 status [χ2(1) = 0.0137; P = 0.91]. In the proAD group (40 men and 32 women), the men were marginally older than women [t(65) = −1.77, P = 0.08] and had higher education than proAD women [t(73) = 3.21, P = 0.002] but were not different with respect to ε4 status [χ2 (1) < 0.001; P > 0.99]. We observed no sex differences in summary Aβ standard uptake value ratio across the whole group [t(115) = 0.946, P = 0.35], within the preAD [t(46) = 1.298, P = 0.20] or within proAD [t(65) = 0.376, P = 0.71].
Table 1.
Demographic characteristics and memory tests scores of participants included in ADNI tau-PET analyses
| Variable | Women | Men |
|---|---|---|
| Number (% of ADNI sample) | 58 (47.9) | 63 (52.1) |
| Age (years)* | 76.7 (6.80) | 79.7 (6.98) |
| Education (years)* | 15.4 (2.41) | 16.9 (2.46) |
| Number of (%) APOE ε4 carriers | 32 (55) | 36 (57) |
| Race (% white) | 94.8 | 96.8 |
| Number of preAD | 26 | 23 |
| Number of proAD (MCI/Alzheimer's disease) | 32 (17/15) | 40 (27/13) |
| RAVLT Learning | 38.1 (14.1) | 34.2 (12.0) |
| RAVLT Delay | 5.14 (5.01) | 3.97 (4.63) |
Cells are formatted as mean (SD) unless otherwise noted.
MCI = mild cognitive impairment.
Significant difference (P < 0.05) between women and men across the entire sample.
Reserve analysis
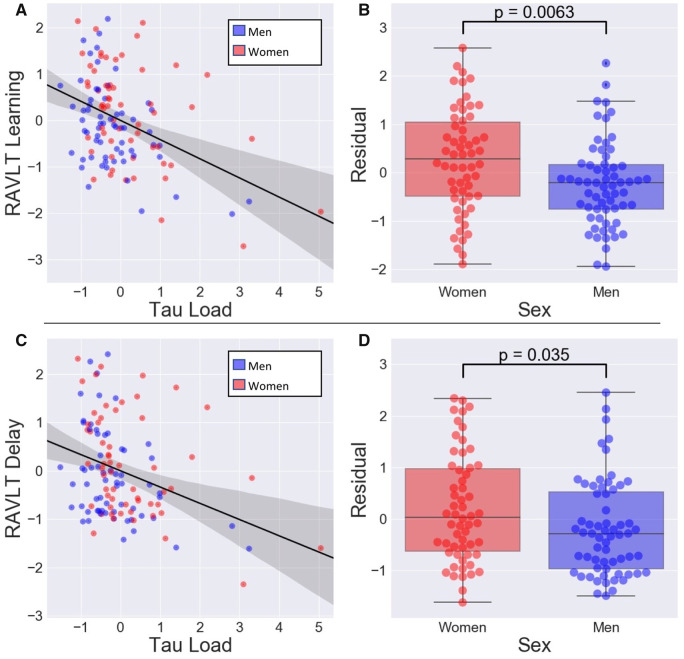
We first fit a linear regression model with RAVLT Learning as the response variable and with age, education, ε4 status and tau load as predictors. In this model (R-squared of model: 0.258), age (β = −0.591, SE = 0.156, P < 0.001), ε4 status (β = −5.03, SE = 2.19, P = 0.02) and tau load (β = −21.6, SE = 3.86, P < 0.001) were independently associated with RAVLT Learning. Education was not significantly associated with RAVLT Learning (β = 0.681, SE = 0.414, P = 0.10). Analysing the residuals with Welch’s t-tests revealed that women had significantly higher residuals (i.e. more reserve) than men in the RAVLT Learning [t(111) = 2.78, P = 0.006] (Fig. 1B).
Figure 1.
Women demonstrate higher reserve to tau than men. Scatter plots (A) between RAVLT Learning and tau load or (C) between RAVLT Delay and tau load. RAVLT Learning, RAVLT Delay and tau load were regressed onto age, years of education and ε4 status before plotting. Here, tau load is the average of regional SUVRs from a set of Alzheimer's disease-vulnerable regions in temporal cortex. The boxplots with swarm plot overlays are residuals from a linear model predicting (B) RAVLT Learning or (D) RAVLT Delay from tau load, age, years of education and ε4 status. Women have significantly higher residuals than men. SUVRs = Standard uptake value ratios.
When this analysis was repeated with RAVLT Delay as the response variable, rather than RAVLT Learning, similar results were observed (R-squared of model: 0.262). Tau load (β = −5.57, SE = 1.449, P < 0.001), age (β = −0.256, SE = 0.0574, P < 0.001), ε4 status (β = −2.41, SE = 0.804, P = 0.003) and education (β = 0.338, SE = 0.152, P = 0.03) were related to RAVLT Delay. Furthermore, analysis of the residuals demonstrated that women also had higher reserve in this model [t(114) = 2.14, P = 0.04] (Fig. 1D).
The significant age difference between men and women in our ADNI sample may have potentially confounded the results of our reserve analysis. Thus, we re-performed this analysis using a subset of our ADNI participants (N = 106; 53 women, 53 men) that were matched for age across sexes. In these age-matched analyses, we found similar results.
Subgroup stratified analysis
After correcting for age, education and ε4 status, men in the preAD group performed worse on RAVLT Learning (β = −6.75, SE = 3.16, P = 0.04) than women, but comparably on RAVLT Delay (β = −1.74, SE = 1.41, P = 0.23). In addition, preAD men had less tau load than women (β = −0.0921, SE = 0.0362, P = 0.01), after accounting for age, education and ε4 status. Lastly, after correcting for age, education, ε4 status and tau load, men performed marginally worse on RAVLT Learning (β = −6.54, SE = 3.42, P = 0.06) but comparably on RAVLT Delay (β = −1.76, SE = 1.53, P = 0.26).
Within the proAD group, women and men did not perform differently on RAVLT Learning (β = −0.861, SE = 2.64, P = 0.75) or RAVLT Delay (β = 0.281, SE = 0.833, P = 0.74) after controlling for age, education and ε4 status. However, proAD men had lower tau (β = −0.191, SE = 0.0819, P = 0.02) than women. In models controlling for age, education, ε4 status and tau load, we found no sex differences in RAVLT Learning (β = −1.96, SE = 2.46, P = 0.43) or RAVLT Delay performance (β = −0.436, SE = 0.811, P = 0.59).
Study 2: NACC post-mortem analysis
Subject characteristics
There were 662 subjects in the NACC database who met criteria for our study and had complete data. The summary statistics are presented in Table 2. There were no sex differences in any demographic variables within the Braak 1/2 group or within the Braak 3/4 group. In the Braak 5/6 subgroup, there were differences in age [t(419) = 3.447, P < 0.001] and education [t(446) = −6.570, P < 0.001], with women being older and men having more educational attainment.
Table 2.
Demographic characteristics and memory tests scores of participants included in NACC post-mortem analyses
| Braak 1/2, N = 46 |
Braak 3/4, N = 153 |
Braak 5/6, N = 463 |
||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Number (% of Braak subgroup) | 24 (52) | 22 (48) | 67 (44) | 86 (56) | 198 (43) | 265 (57) |
| Age (years)c | 84.9 (7.6) | 84.4 (7.9) | 85.9 (7.9) | 83.7 (7.5) | 82.9 (8.3)* | 80.2 (8.1) |
| Education (years)c | 15.0 (2.2) | 15.1 (3.7) | 15.1 (2.6) | 15.5 (3.3) | 14.2 (2.7) | 16.0 (3.0)* |
| Number of (%) APOE ε4 carriers | 6 (25) | 6 (27) | 32 (48) | 41 (48) | 114 (58) | 165 (62) |
| Race (% white) | 100 | 95.5 | 98.5 | 93.0 | 89.9 | 95.5 |
| Time between last clinical visit and death (years) | 1.0 (0.8) | 0.9 (0.8) | 1.0 (0.9) | 1.3 (0.9) | 1.9 (1.4) | 1.8 (1.3) |
| LM Immediatea,b | 13.8 (5.3)* | 8.0 (5.7) | 8.7 (6.0)* | 6.0 (5.7) | 2.5 (3.7) | 2.2 (3.0) |
| LM Delaya,b | 12.5 (6.0)* | 7.1 (6.3) | 7.4 (6.2)* | 4.9 (5.8) | 1.3 (3.3) | 1.2 (2.6) |
Cells are formatted as mean (SD) unless otherwise noted.
Significant sex difference (P < 0.05) in the Braak 1/2 subgroup.
Significant sex difference (P < 0.05) in the Braak 3/4 subgroup.
Significant sex difference (P < 0.05) in the Braak 5/6 subgroup.
*Asterisk indicates higher value for women than men in that Braak category.
Pathology analysis
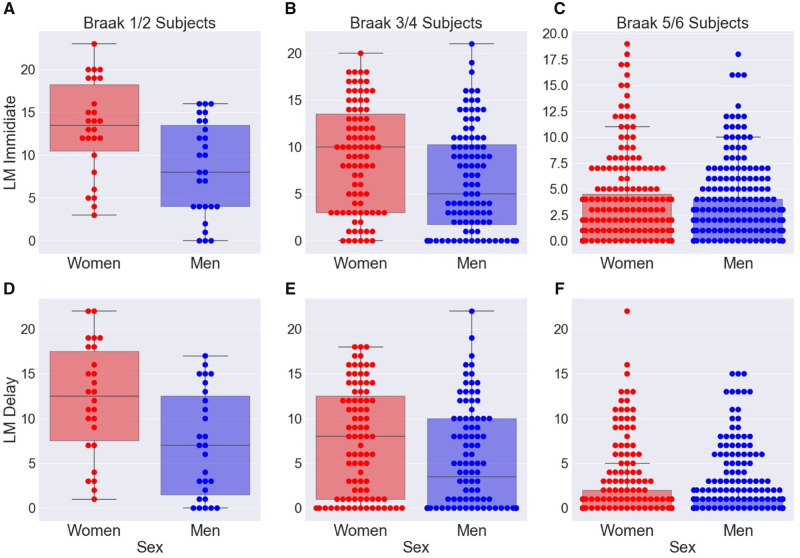
In the Braak 1/2 group, men had lower scores on both the LM Immediate (β = −5.960, SE = 1.517, P < 0.001) and LM Delay (β = −5.703, SE = 1.677, P = 0.001) after controlling for age at clinical visit, time between last clinical visit and death date, education and ε4 status. In a similar model within the Braak 3/4 group, we observed similar results. Men had lower scores on both LM Immediate (β = −2.900, SE = 0.938, P = 0.002) and LM Delay (β = −2.672, SE = 0.955, P = 0.006) (Fig. 2B and D). In contrast, there were no sex differences in LM Immediate (β = −0.314, SE = 0.328, P = 0.34) or LM Delay (β = −0.195, SE = 0.287, P = 0.50) performance within the severe Alzheimer's disease group.
Figure 2.
Among participants with similar levels of Alzheimer's disease-related pathology, women perform better on verbal memory tests. On the y-axis are raw scores for (A–C) LM Immediate and (D–F) LM Delay. Within the Braak 1/2 group and within the Braak 3/4 group, women had significantly higher scores on both LM Immediate and LM Delay. We observed no significant differences in LM score in the Braak 5/6 group.
Discussion
We examined the relationship between sex, tau and verbal memory in two different cohorts. Using ADNI data, we applied a residual approach to estimate verbal memory reserve to tau pathology for each subject. We found that women demonstrate higher verbal memory reserve. These findings were validated using data from the NACC, where we found that, among individuals within Braak 1/2 or Braak 3/4, women had superior verbal memory. Taken together, our findings point to a sex-related verbal memory reserve in the face of tau pathology.
The residual framework has been used extensively to estimate reserve in the presence of brain changes associated with Alzheimer's disease, such as neurodegeneration and Aβ (Hohman et al., 2016). We are aware of no prior tau imaging studies that have explored sex-related reserve. However, a series of recent studies suggested that for similar levels of neurodegeneration, women performed better on the RAVLT (Sundermann et al., 2016a, b). Furthermore, another study found that the relationship between Aβ and RAVLT performance can be moderated by sex (Caldwell et al., 2017). Our findings, in combination with these studies, indicate that women can sustain more Alzheimer's disease-related brain insult before showing impaired RAVLT performance.
Apart from these imaging investigations, our results are compatible with clinical and neuropsychological studies. The verbal memory advantage for cognitively normal women over men that we observed is consistent with prior clinical investigations (Beinhoff et al., 2008, Chapman et al., 2011). Furthermore, these studies, like ours, showed that the advantage disappears with the progression of disease into dementia. Taken together, these observations are congruent with the following interpretation of how Alzheimer's disease may progress in men and women. Women start with a premorbid (i.e. prior to the onset of Alzheimer's disease pathology) advantage in verbal memory abilities. During the early phases of tau accumulation, memory abilities begin to decline in both men and women, but the premorbid advantage for women persists during this early phase, such that women still perform superiorly in verbal memory for a given level of tau (consistent with the apparent reserve that we found in our study). Then, after a critical point in the Alzheimer's disease course, women begin to show a faster decline in memory abilities and ultimately ‘catch up’ to the memory impairment of men (in line with our lack of verbal memory sex differences in the later AD stages). The notion that women begin to decline more rapidly is supported by in vivo studies showing that women progress faster from mild cognitive impairment to Alzheimer's disease and exhibit greater rates of Alzheimer's disease-related cognitive decline (Lin et al., 2015, Koran et al., 2017). Even further evidence comes from a post-mortem study indicating that women are more likely than men to express Alzheimer's disease pathology as dementia (Barnes et al., 2005). Lastly, it was recently reported that women are more susceptible to tau-related hypometabolism (Ramanan et al., 2019), proposing a potential underlying mechanism for this rapid decline seen in women. Despite this burden of evidence, however, our finding of a lack in verbal memory sex differences among the more progressed stages of Alzheimer's disease can alternatively be attributed to a floor effect in the verbal memory scores rather than a rapid decline in women.
Our results from the NACC post-mortem analyses bolster our conclusions from the ADNI tau-PET analyses. First, the finding that, within Braak 1/2 and Braak 3/4 subgroups, women performed better on verbal memory is consistent with our interpretation of a sex-related reserve that we derived from ADNI results. Furthermore, for our NACC analyses, we used scores from a different memory test. The harmony in results across ADNI and NACC analyses indicates that the sex-related reserve is not specific to RAVLT or LM but reserve in verbal memory abilities in general.
The sex-related verbal memory reserve would have several implications for clinical research. Much of our understanding about the evolution of Alzheimer's disease is garnered from large observational cohorts, such as ADNI and NACC. These cohorts often rely heavily on assessing memory with verbal tests. Our findings contribute to the mounting evidence that it is critical to take into account sex differences when considering cut points for verbal memory tests (Sundermann et al., 2019). They also endorse the use of additional non-verbal memory tests in cohort studies of aging to better characterize the memory changes associated with Alzheimer's disease.
Although the residual approach has been shown to be a suitable proxy for reserve by many groups, it clearly does not account for all variability in cognition. For example, men might have worse cognition than predicted by tau because they have more co-morbidities, working in concordance with tau, to impair cognition. Incorporating in vivo markers for pathologies that commonly co-occur with Alzheimer's disease would be helpful to further characterize sex differences in the ability to tolerate tau.
Though this study is unable to fully explain the underpinnings of reserve, it demonstrates that sex plays a role in conferring apparent cognitive reserve in the face of tau. As such, we feel these results and others call for the end of treating sex as a variable of no interest and, instead, suggest thoughtful consideration into the role of sex in the expression of Alzheimer's disease.
Funding
Research support was provided by the Shiley-Marcos Alzheimer’s Disease Research Center (P30 AG062429). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering and generous contributions from the following: AbbVie; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai, Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer, Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory of NeuroImaging at the University of Southern California. The NACC database is funded by NIA/NIH (Grant U01 AG016976). NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD) and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Competing interests
J.B.B. has served on advisory boards for Elan, Bristol-Myers Squibb, Avanir, Novartis, Genentech and Eli Lilly and holds stock options in CorTechs Labs, Inc., and Human Longevity, Inc. The terms of these arrangements have been reviewed and approved by UCSD in accordance with its conflict of interest policies.
Supplementary Material
Glossary
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- FTP
flortaucipir
- LM
Logical Memory
- NACC
National Alzheimer’s Coordinating Center
- PET
positron emission tomography
- preAD
preclinical AD
- proAD
prodromal/probable AD
- RAVLT
Rey Auditory Verbal Learning Test
Appendix I
ADNI Investigators: Michael Weiner, MD (UC San Francisco, Principal Investigator, Executive Committee); Paul Aisen, MD (UC San Diego, ADCS PI and Director of Coordinating Center Clinical Core, Executive Committee, Clinical Core Leaders); Ronald Petersen, MD, PhD (Mayo Clinic, Rochester, Executive Committee, Clinical Core Leader); Clifford R. Jack, Jr., MD (Mayo Clinic, Rochester, Executive Committee, MRI Core Leader); William Jagust, MD (UC Berkeley, Executive Committee; PET Core Leader); John Q. Trojanowki, MD, PhD (U Pennsylvania, Executive Committee, Biomarkers Core Leader); Arthur W. Toga, PhD (USC, Executive Committee, Informatics Core Leader); Laurel Beckett, PhD (UC Davis, Executive Committee, Biostatistics Core Leader); Robert C. Green, MD, MPH (Brigham and Women’s Hospital, Harvard Medical School, Executive Committee and Chair of Data and Publication Committee); Andrew J. Saykin, PsyD (Indiana University, Executive Committee, Genetics Core Leader); John Morris, MD (Washington University St. Louis, Executive Committee, Neuropathology Core Leader); Leslie M. Shaw (University of Pennsylvania, Executive Committee, Biomarkers Core Leader); Enchi Liu, PhD (Janssen Alzheimer Immunotherapy, ADNI 2 Private Partner Scientific Board Chair); Tom Montine, MD, PhD (University of Washington); Ronald G. Thomas, PhD (UC San Diego); Michael Donohue, PhD (UC San Diego); Sarah Walter, MSc (UC San Diego); Devon Gessert (UC San Diego); Tamie Sather, MS (UC San Diego,); Gus Jiminez, MBS (UC San Diego); Danielle Harvey, PhD (UC Davis;); Michael Donohue, PhD (UC San Diego); Matthew Bernstein, PhD (Mayo Clinic, Rochester); Nick Fox, MD (University of London); Paul Thompson, PhD (USC School of Medicine); Norbert Schuff, PhD (UCSF MRI); Charles DeCArli, MD (UC Davis); Bret Borowski, RT (Mayo Clinic); Jeff Gunter, PhD (Mayo Clinic); Matt Senjem, MS (Mayo Clinic); Prashanthi Vemuri, PhD (Mayo Clinic); David Jones, MD (Mayo Clinic); Kejal Kantarci (Mayo Clinic); Chad Ward (Mayo Clinic); Robert A. Koeppe, PhD (University of Michigan, PET Core Leader); Norm Foster, MD (University of Utah); Eric M. Reiman, MD (Banner Alzheimer’s Institute); Kewei Chen, PhD (Banner Alzheimer’s Institute); Chet Mathis, MD (University of Pittsburgh); Susan Landau, PhD (UC Berkeley); Nigel J. Cairns, PhD, MRCPath (Washington University St. Louis); Erin Householder (Washington University St. Louis); Lisa Taylor Reinwald, BA, HTL (Washington University St. Louis); Virginia Lee, PhD, MBA (UPenn School of Medicine); Magdalena Korecka, PhD (UPenn School of Medicine); Michal Figurski, PhD (UPenn School of Medicine); Karen Crawford (USC); Scott Neu, PhD (USC); Tatiana M. Foroud, PhD (Indiana University); Steven Potkin, MD UC (UC Irvine); Li Shen, PhD (Indiana University); Faber Kelley, MS, CCRC (Indiana University); Sungeun Kim, PhD (Indiana University); Kwangsik Nho, PhD (Indiana University); Zaven Kachaturian, PhD (Khachaturian, Radebaugh & Associates, Inc and Alzheimer’s Association’s Ronald and Nancy Reagan’s Research Institute); Richard Frank, MD, PhD (General Electric); Peter J. Snyder, PhD (Brown University); Susan Molchan, PhD (National Institute on Aging/National Institutes of Health); Jeffrey Kaye, MD (Oregon Health and Science University); Joseph Quinn, MD (Oregon Health and Science University); Betty Lind, BS (Oregon Health and Science University); Raina Carter, BA (Oregon Health and Science University); Sara Dolen, BS (Oregon Health and Science University); Lon S. Schneider, MD (University of Southern Califor nia); Sonia Pawluczyk, MD (University of Southern California); Mauricio Beccera, BS (University of Southern California); Liberty Teodoro, RN (University of Southern California); Bryan M. Spann, DO, PhD (University of Southern California); James Brewer, MD, PhD (University of California San Diego); Helen Vanderswag, RN (University of California San Diego); Adam Fleisher, MD (University of California San Diego); Judith L. Heidebrink, MD, MS (University of Michigan); Joanne L. Lord, LPN, BA, CCRC (University of Michigan); Ronald Petersen, MD, PhD (Mayo Clinic, Rochester); Sara S. Mason, RN (Mayo Clinic, Rochester); Colleen S. Albers, RN (Mayo Clinic, Rochester); David Knopman, MD (Mayo Clinic, Rochester); Kris Johnson, RN (Mayo Clinic, Rochester); Rachelle S. Doody, MD, PhD (Baylor College of Medicine); Javier Villanueva Meyer, MD (Baylor College of Medicine); Munir Chowdhury, MBBS, MS (Baylor College of Medicine); Susan Rountree, MD (Baylor College of Medicine); Mimi Dang, MD (Baylor College of Medicine); Yaakov Stern, PhD (Columbia University Medical Center); Lawrence S. Honig, MD, PhD (Columbia University Medical Center); Karen L. Bell, MD (Columbia University Medical Center); Beau Ances, MD (Washington University, St. Louis); John C. Morris, MD (Washington University, St. Louis); Maria Carroll, RN, MSN (Washington University, St. Louis); Sue Leon, RN, MSN (Washington University, St. Louis); Erin Householder, MS, CCRP (Washington University, St. Louis); Mark A. Mintun, MD (Washington University, St. Louis); Stacy Schneider, APRN, BC, GNP (Washington University, St. Louis); Angela Oliver, RN, BSN, MSG; Daniel Marson, JD, PhD (University of Alabama Birmingham); Randall Griffith, PhD, ABPP (University of Alabama Birmingham); David Clark, MD (University of Alabama Birmingham); David Geldmacher, MD (University of Alabama Birmingham); John Brockington, MD (University of Alabama Birmingham); Erik Roberson, MD (University of Alabama Birmingham); Hillel Grossman, MD (Mount Sinai School of Medicine); Effie Mitsis, PhD (Mount Sinai School of Medicine); Leyla deToledo-Morrell, PhD (Rush University Medical Center); Raj C. Shah, MD (Rush University Medical Center); Ranjan Duara, MD (Wien Center); Daniel Varon, MD (Wien Center); Maria T. Greig, HP (Wien Center); Peggy Roberts, CNA (Wien Center); Marilyn Albert, PhD (Johns Hopkins University); Chiadi Onyike, MD (Johns Hopkins University); Daniel D’Agostino II, BS (Johns Hopkins University); Stephanie Kielb, BS (Johns Hopkins University); James E. Galvin, MD, MPH (New York University); Dana M. Pogorelec (New York University); Brittany Cerbone (New York University); Christina A. Michel (New York University); Henry Rusinek, PhD (New York University); Mony J. de Leon, EdD (New York University); Lidia Glodzik, MD, PhD (New York University); Susan De Santi, PhD (New York University); P. Murali Doraiswamy, MD (Duke University Medical Center); Jeffrey R. Petrella, MD (Duke University Medical Center); Terence Z. Wong, MD (Duke University Medical Center); Steven E. Arnold, MD (University of Pennsylvania); Jason H. Karlawish, MD (University of Pennsylvania); David Wolk, MD (University of Pennsylvania); Charles D. Smith, MD (University of Kentucky); Greg Jicha, MD (University of Kentucky); Peter Hardy, PhD (University of Kentucky); Partha Sinha, PhD (University of Kentucky); Elizabeth Oates, MD (University of Kentucky); Gary Conrad, MD (University of Kentucky); Oscar L. Lopez, MD (University of Pittsburgh); MaryAnn Oakley, MA (University of Pittsburgh); Donna M. Simpson, CRNP, MPH (University of Pittsburgh); Anton P. Porsteinsson, MD (University of Rochester Medical Center); Bonnie S. Goldstein, MS, NP (University of Rochester Medical Center); Kim Martin, RN (University of Rochester Medical Center); Kelly M. Makino, BS (University of Rochester Medical Center); M. Saleem Ismail, MD (University of Rochester Medical Center); Connie Brand, RN (University of Rochester Medical Center); Ruth A. Mulnard, DNSc, RN, FAAN (University of California, Irvine); Gaby Thai, MD (University of California, Irvine); Catherine Mc Adams Ortiz, MSN, RN, A/GNP (University of California, Irvine); Kyle Womack, MD (University of Texas Southwestern Medical School); Dana Mathews, MD, PhD (University of Texas Southwestern Medical School); Mary Quiceno, MD (University of Texas Southwestern Medical School); Ramon Diaz Arrastia, MD, PhD (University of Texas Southwestern Medical School); Richard King, MD (University of Texas Southwestern Medical School); Myron Weiner, MD (University of Texas Southwestern Medical School); Kristen Martin Cook, MA (University of Texas Southwestern Medical School); Michael DeVous, PhD (University of Texas Southwestern Medical School); Allan I. Levey, MD, PhD (Emory University); James J. Lah, MD, PhD (Emory University); Janet S. Cellar, DNP, PMHCNS BC (Emory University); Jeffrey M. Burns, MD (University of Kansas, Medical Center); Heather S. Anderson, MD (University of Kansas, Medical Center); Russell H. Swerdlow, MD (University of Kansas, Medical Center); Liana Apostolova, MD (University of California, Los Angeles); Kathleen Tingus, PhD (University of California, Los Angeles); Ellen Woo, PhD (University of California, Los Angeles); Daniel H. S. Silverman, MD, PhD (University of California, Los Angeles); Po H. Lu, PsyD (University of California, Los Angeles); George Bartzokis, MD (University of California, Los Angeles); Neill R. Graff Radford, MBBCH, FRCP (London) (Mayo Clinic, Jackson ville); Francine Parfitt, MSH, CCRC (Mayo Clinic, Jacksonville); Tracy Kendall, BA, CCRP (Mayo Clinic, Jacksonville); Heather Johnson, MLS, CCRP (Mayo Clinic, Jacksonville); Martin R. Farlow, MD (Indiana University); Ann Marie Hake, MD (Indiana University); Brandy R. Matthews, MD (Indiana University); Scott Herring, RN, CCRC (Indiana University); Cynthia Hunt, BS, CCRP (Indiana University); Christopher H. van Dyck, MD (Yale University School of Medicine); Richard E. Carson, PhD (Yale University School of Medicine); Martha G. MacAvoy, PhD (Yale University School of Medicine); Howard Chertkow, MD (McGill Univ., Montreal Jewish General Hospital); Howard Bergman, MD (McGill Univ., Montreal Jewish General Hospital); Chris Hosein, Med (McGill Univ., Montreal Jewish General Hospital); Sandra Black, MD, FRCPC (Sunnybrook Health Sciences, Ontario); Dr. Bojana Stefanovic (Sunnybrook Health Sciences, Ontario); Curtis Caldwell, PhD (Sunnybrook Health Sciences, Ontario); Ging Yuek Robin Hsiung, MD, MHSc, FRCPC (U.B.C. Clinic for AD & Related Disorders); Howard Feldman, MD, FRCPC (U.B.C. Clinic for AD & Related Disorders); Benita Mudge, BS (U.B.C. Clinic for AD & Related Disorders); Michele Assaly, MA Past (U.B.C. Clinic for AD & Related Disorders); Andrew Kertesz, MD (Cognitive Neurology St. Joseph’s, Ontario); John Rogers, MD (Cognitive Neurology St. Joseph’s, Ontario); Dick Trost, PhD (Cognitive Neurology St. Joseph’s, Ontario); Charles Bernick, MD (Cleveland Clinic Lou Ruvo Center for Brain Health); Donna Munic, PhD (Cleveland Clinic Lou Ruvo Center for Brain Health); Diana Kerwin, MD (Northwestern University); Marek Marsel Mesulam, MD (Northwestern University); Kristine Lipowski, BA (Northwestern University); Chuang Kuo Wu, MD, PhD (Northwestern University); Nancy Johnson, PhD (Northwestern University); Carl Sadowsky, MD [Premiere Research Inst (Palm Beach Neurology)]; Walter Martinez, MD [Premiere Research Inst (Palm Beach Neurology)]; Teresa Villena, MD [Premiere Research Inst (Palm Beach Neurology)]; Raymond Scott Turner, MD, PhD (Georgetown University Medical Center); Kathleen Johnson, NP (Georgetown University Medical Center); Brigid Reynolds, NP (Georgetown University Medical Center); Reisa A. Sperling, MD (Brigham and Women’s Hospital); Keith A. Johnson, MD (Brigham and Women’s Hospital); Gad Marshall, MD (Brigham and Women’s Hospital); Meghan Frey (Brigham and Women’s Hospital); Jerome Yesavage, MD (Stanford University); Joy L. Taylor, PhD (Stanford University); Barton Lane, MD (Stanford University); Allyson Rosen, PhD (Stanford University); Jared Tinklenberg, MD (Stanford University); Marwan N. Sabbagh, MD (Banner Sun Health Research Institute); Christine M. Belden, PsyD (Banner Sun Health Research Institute); Sandra A. Jacobson, MD (Banner Sun Health Research Institute); Sherye A. Sirrel, MS (Banner Sun Health Research Institute); Neil Kowall, MD (Boston University); Ronald Killiany, PhD (Boston University); Andrew E. Budson, MD (Boston University); Alexander Norbash, MD (Boston University); Patricia Lynn Johnson, BA (Boston University); Thomas O. Obisesan, MD, MPH (Howard University); Saba Wolday, MSc (Howard University); Joanne Allard, PhD (Howard University); Alan Lerner, MD (Case Western Reserve University); Paula Ogrocki, PhD (Case Western Reserve University); Leon Hudson, MPH (Case Western Reserve University); Evan Fletcher, PhD (University of California, Davis Sacramento); Owen Carmichael, PhD (University of California, Davis Sacramento); John Olichney, MD (University of California, Davis Sacramento); Charles DeCarli, MD (University of California, Davis Sacramento); Smita Kittur, MD (Neurological Care of CNY); Michael Borrie, MB ChB (Parkwood Hospital); T. Y. Lee, PhD (Parkwood Hospital); Dr. Rob Bartha, PhD (Parkwood Hospital); Sterling Johnson, PhD (University of Wisconsin); Sanjay Asthana, MD (University of Wisconsin); Cynthia M. Carlsson, MD (University of Wisconsin); Steven G. Potkin, MD (University of California, Irvine BIC); Adrian Preda, MD (University of California, Irvine BIC); Dana Nguyen, PhD (University of California, Irvine BIC); Pierre Tariot, MD (Banner Alzheimer’s Institute); Adam Fleisher, MD (Banner Alzheimer’s Institute); Stephanie Reeder, BA (Banner Alzheimer’s Institute); Vernice Bates, MD (Dent Neurologic Institute); Horacio Capote, MD (Dent Neurologic Institute); Michelle Rainka, PharmD, CCRP (Dent Neurologic Institute); Douglas W. Scharre, MD (Ohio State University); Maria Kataki, MD, PhD (Ohio State University); Anahita Adeli, MD (Ohio State University); Earl A. Zimmerman, MD (Albany Medical College); Dzintra Celmins, MD (Albany Medical College); Alice D. Brown, FNP (Albany Medical College); Godfrey D. Pearlson, MD (Hartford Hosp, Olin Neuropsychiatry Research Center); Karen Blank, MD (Hartford Hosp, Olin Neuropsychiatry Research Center); Karen Anderson, RN (Hartford Hosp, Olin Neuropsychiatry Research Center); Robert B. Santulli, MD (Dartmouth Hitchcock Medical Center); Tamar J. Kitzmiller (Dartmouth Hitchcock Medical Center); Eben S. Schwartz, PhD (Dartmouth Hitchcock Medical Center); Kaycee M. Sink, MD, MAS (Wake Forest University Health Sciences); Jeff D. Williamson, MD, MHS (Wake Forest University Health Sciences); Pradeep Garg, PhD (Wake Forest University Health Sciences); Franklin Watkins, MD (Wake Forest University Health Sciences); Brian R. Ott, MD (Rhode Island Hospital); Henry Querfurth, MD (Rhode Island Hospital); Geoffrey Tremont, PhD (Rhode Island Hospital); Stephen Salloway, MD, MS (Butler Hospital); Paul Malloy, PhD (Butler Hospital); Stephen Correia, PhD (Butler Hospital); Howard J. Rosen, MD (UC San Francisco); Bruce L. Miller, MD (UC San Francisco); Jacobo Mintzer, MD, MBA (Medical University South Carolina); Kenneth Spicer, MD, PhD (Medical University South Carolina); David Bachman, MD (Medical University South Carolina); Elizabether Finger, MD (St. Joseph’s Health Care); Stephen Pasternak, MD (St. Joseph’s Health Care); Irina Rachinsky, MD (St. Joseph’s Health Care); John Rogers, MD (St. Joseph’s Health Care); Andrew Kertesz, MD (St. Joseph’s Health Care); Dick Drost, MD (St. Joseph’s Health Care); Nunzio Pomara, MD (Nathan Kline Institute); Raymundo Hernando, MD (Nathan Kline Institute); Antero Sarrael, MD (Nathan Kline Institute); Susan K. Schultz, MD (University of Iowa College of Medicine, Iowa City); Laura L. Boles Ponto, PhD (University of Iowa College of Medicine, Iowa City); Hyungsub Shim, MD (University of Iowa College of Medicine, Iowa City); Karen Elizabeth Smith, RN (University of Iowa College of Medicine, Iowa City); Norman Relkin, MD, PhD (Cornell University); Gloria Chaing, MD (Cornell University); Lisa Raudin, PhD (Cornell University); Amanda Smith, MD (University of South Floriday: USF Health Byrd Alzheimer’s Institute); Kristin Fargher, MD (University of South Floriday: USF Health Byrd Alzheimer’s Institute); and Balebail Ashok Raj, MD (University of South Floriday: USF Health Byrd Alzheimer’s Institute).
Contributor Information
Alzheimer’s Disease Neuroimaging Initiative:
Michael Weiner, Paul Aisen, Ronald Petersen, Clifford R Jack, William Jagust, John Q Trojanowki, Arthur W Toga, Laurel Beckett, Robert C Green, Andrew J Saykin, John Morris, Leslie M Shaw, Enchi Liu, Tom Montine, Ronald G Thomas, Michael Donohue, Sarah Walter, Devon Gessert, Tamie Sather, Gus Jiminez, Danielle Harvey, Michael Donohue, Matthew Bernstein, Nick Fox, Paul Thompson, Norbert Schuff, Charles DeCArli, Bret Borowski, Jeff Gunter, Matt Senjem, Prashanthi Vemuri, David Jones, Kejal Kantarci, Chad Ward, Robert A Koeppe, Norm Foster, Eric M Reiman, Kewei Chen, Chet Mathis, Susan Landau, Nigel J Cairns, Erin Householder, Lisa Taylor Reinwald, Virginia Lee, Magdalena Korecka, Michal Figurski, Karen Crawford, Scott Neu, Tatiana M Foroud, Steven Potkin, Li Shen, Faber Kelley, Sungeun Kim, Kwangsik Nho, Zaven Kachaturian, Richard Frank, Peter J Snyder, Susan Molchan, Jeffrey Kaye, Joseph Quinn, Betty Lind, Raina Carter, Sara Dolen, Lon S Schneider, Sonia Pawluczyk, Mauricio Beccera, Liberty Teodoro, Bryan M Spann, James Brewer, Helen Vanderswag, Adam Fleisher, Judith L Heidebrink, Joanne L Lord, Ronald Petersen, Sara S Mason, Colleen S Albers, David Knopman, Kris Johnson, Rachelle S Doody, Javier Villanueva Meyer, Munir Chowdhury, Susan Rountree, Mimi Dang, Yaakov Stern, Lawrence S Honig, Karen L Bell, Beau Ances, John C Morris, Maria Carroll, Sue Leon, Erin Householder, Mark A Mintun, Stacy Schneider, Angela Oliver, Randall Griffith, David Clark, David Geldmacher, John Brockington, Erik Roberson, Hillel Grossman, Effie Mitsis, Leyla deToledo-Morrell, Raj C Shah, Ranjan Duara, Daniel Varon, Maria T Greig, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Daniel D’Agostino II, Stephanie Kielb, James E Galvin, Dana M Pogorelec, Brittany Cerbone, Christina A Michel, Henry Rusinek, Mony J de Leon, Lidia Glodzik, Susan De Santi, P Murali Doraiswamy, Jeffrey R Petrella, Terence Z Wong, Steven E Arnold, Jason H Karlawish, David Wolk, Charles D Smith, Greg Jicha, Peter Hardy, Partha Sinha, Elizabeth Oates, Gary Conrad, Oscar L Lopez, MaryAnn Oakley, Donna M Simpson, Anton P Porsteinsson, Bonnie S Goldstein, Kim Martin, Kelly M Makino, M Saleem Ismail, Connie Brand, Ruth A Mulnard, Gaby Thai, Catherine Mc Adams Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Ramon Diaz Arrastia, Richard King, Myron Weiner, Kristen Martin Cook, Michael DeVous, Allan I Levey, James J Lah, Janet S Cellar, Jeffrey M Burns, Heather S Anderson, Russell H Swerdlow, Liana Apostolova, Kathleen Tingus, Ellen Woo, Daniel H S Silverman, Po H Lu, George Bartzokis, Neill R Graff Radford, Francine Parfitt, Tracy Kendall, Heather Johnson, Martin R Farlow, Ann Marie Hake, Brandy R Matthews, Scott Herring, Cynthia Hunt, Christopher H van Dyck, Richard E Carson, Martha G MacAvoy, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Andrew Kertesz, John Rogers, Dick Trost, Charles Bernick, Donna Munic, Diana Kerwin, Marek Marsel Mesulam, Kristine Lipowski, Chuang Kuo Wu, Nancy Johnson, Carl Sadowsky, Walter Martinez, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A Sperling, Keith A Johnson, Gad Marshall, Meghan Frey, Jerome Yesavage, Joy L Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan N Sabbagh, Christine M Belden, Sandra A Jacobson, Sherye A Sirrel, Neil Kowall, Ronald Killiany, Andrew E Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O Obisesan, Saba Wolday, Joanne Allard, Alan Lerner, Paula Ogrocki, Leon Hudson, Evan Fletcher, Owen Carmichael, John Olichney, Charles DeCarli, Smita Kittur, Michael Borrie, T Y Lee, Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M Carlsson, Steven G Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Adam Fleisher, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W Scharre, Maria Kataki, Anahita Adeli, Earl A Zimmerman, Dzintra Celmins, Alice D Brown, Godfrey D Pearlson, Karen Blank, Karen Anderson, Robert B Santulli, Tamar J Kitzmiller, Eben S Schwartz, Kaycee M Sink, Jeff D Williamson, Pradeep Garg, Franklin Watkins, Brian R Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J Rosen, Bruce L Miller, Jacobo Mintzer, Kenneth Spicer, David Bachman, Elizabether Finger, Stephen Pasternak, Irina Rachinsky, John Rogers, Andrew Kertesz, Dick Drost, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Susan K Schultz, Laura L Boles Ponto, Hyungsub Shim, Karen Elizabeth Smith, Norman Relkin, Gloria Chaing, Lisa Raudin, Amanda Smith, Kristin Fargher, and Balebail Ashok Raj
References
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, et al. Clinical core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement J Alzheimers Assoc 2010; 6: 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry 1997; 154: 165–72. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA.. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 2005; 62: 685–91. [DOI] [PubMed] [Google Scholar]
- Beinhoff U, Tumani H, Brettschneider J, Bittner D, Riepe MW.. Gender-specificities in Alzheimer’s disease and mild cognitive impairment. J Neurol 2008; 255: 117–22. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS.. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006; 5: 406–12. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 2019; 76: 542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JZK, Berg J-L, Cummings JL, Banks SJ; for the Alzheimer’s Disease Neuroimaging Initiative. Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume. Alzheimers Res Ther 2017; 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, Mapstone M, Gardner MN, Sandoval TC, McCrary JW, Guillily MD, et al. Women have farther to fall: gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc 2011; 17: 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–94. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM.. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL; for the Alzheimer's Disease Neuroimaging Initiative. Asymptomatic Alzheimer disease: defining resilience. Neurology 2016; 87: 2443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2017; 13: 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Koeppe RA, Fessler JA.. Reducing between scanner differences in multi-center PET studies. Neuroimage 2009; 46: 154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran MEI, Wagener M, Hohman TJ; for the Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav 2017; 11: 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med off Publ Soc Nucl Med 2013; 54: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol 2012; 69: 623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol 2018; 136: 873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM; Alzheimer's Disease Neuroimaging Initiative. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement Transl Res Clin Interv 2015; 1: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015; 78: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41: 479–86. [DOI] [PubMed] [Google Scholar]
- Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA.. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol 2018; 136: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Castillo AM, Knopman DS, Graff-Radford J, Lowe VJ, Petersen RC, et al. Association of apolipoprotein E ɛ4, educational level, and sex with tau deposition and tau-mediated metabolic dysfunction in older adults. JAMA Netw Open 2019; 2: e1913909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain J Neurol 2010; 133: 2196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Tang M-X, Mayeux R, Luchsinger JA.. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006; 59: 912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002; 8: 448–60. [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R.. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 1992; 32: 371–5. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Biegon A, Rubin LH, Lipton RB, Mowrey W, Landau S, et al. ; for the Alzheimer's Disease Neuroimaging Initiative. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 2016a; 86: 1368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Maki P, Biegon A, Lipton RB, Mielke MM, Machulda M, et al. ; Alzheimer's Disease Neuroimaging Initiative. Sex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology 2019; 93: e1881–e1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Maki PM, Rubin LH, Lipton RB, Landau S, Biegon A; for the Alzheimer's Disease Neuroimaging Initiative. Female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology 2016b; 87: 1916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenhoud A, Wink AM, Groot C, Verfaillie SCJ, Twisk J, Barkhof F, et al. A neuroimaging approach to capture cognitive reserve: application to Alzheimer’s disease. Hum Brain Mapp 2017; 38: 4703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. ; Alzheimer's Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement J Alzheimers Assoc 2017; 13: 561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, DeCarli C, Stern Y.. Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc 2013; 19: 854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ADNI demographic, genetic, neuroimaging and neuropsychology data that were used in our analyses are available for eligible users for access and download at the ADNI data repository (adni.loni.usc.edu). The NACC demographic, genetic, neuropathology and neuropsychology data that were used can be accessed freely by eligible researchers through the NACC website (alz.washington.edu).