Abstract
Background
In this systematic review and meta-analysis, we aimed to explore the association between cardiac injury and mortality, the need for intensive care unit (ICU) care, acute respiratory distress syndrome (ARDS), and severe coronavirus disease 2019 (COVID-19) in patients with COVID-19 pneumonia.
Methods
We performed a comprehensive literature search from several databases. Definition of cardiac injury follows that of the included studies, which includes highly sensitive cardiac troponin I (hs-cTnl) >99th percentile.The primary outcome was mortality, and the secondary outcomes were ARDS, the need for ICU care, and severe COVID-19. ARDS and severe COVID-19 were defined per the World Health Organization (WHO) interim guidance of severe acute respiratory infection (SARI) of COVID-19.
Results
There were a total of 2389 patients from 13 studies. This meta-analysis showed that cardiac injury was associated with higher mortality (RR 7.95 [5.12, 12.34], p < 0.001; I2: 65%). Cardiac injury was associated with higher need for ICU care (RR 7.94 [1.51, 41.78], p = 0.01; I2: 79%), and severe COVID-19 (RR 13.81 [5.52, 34.52], p < 0.001; I2: 0%). The cardiac injury was not significant for increased risk of ARDS (RR 2.57 [0.96, 6.85], p = 0.06; I2: 84%). The level of hs-cTnI was higher in patients with primary + secondary outcome (mean difference 10.38 pg/mL [4.44, 16.32], p = 0.002; I2: 0%).
Conclusion
Cardiac injury is associated with mortality, need for ICU care, and severity of disease in patients with COVID-19.
Keywords: Cardiac injury, Coronavirus, COVID-19, Troponin, Mortality
1. Introduction
A series of pneumonia cases of unknown origin emerged in Wuhan, the Hubei province of China, in December 2019, and the clinical presentations were most similar to viral pneumonia [1]. This pneumonia is a newly recognized illness that has spread rapidly across the country and around the world. A few days after the initial outbreak, Chinese scientists managed to identify a novel coronavirus [1], which was later named severe acute respiratory syndrome—coronavirus 2 (SARS-CoV-2). This virus is classified as a ß CoV of group 2B and has at least similarity in genetic sequence to severe acute respiratory syndrome—coronavirus (SARS-CoV-1). SARS is a zoonosis caused by SARS-CoV-1, which first appeared in China in 2002 and spread to 29 countries in 2003, causing a global outbreak with 8903 cases [2].
Coronaviruses are viruses with single-stranded RNA enveloped by a fat-coated substance. This virus belongs to the Coronaviridae family and is encountered in humans and mammals. Although coronavirus infection is generally mild, the previous two betacoronavirus epidemics, namely SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), has caused a cumulative case of 10,000 patients with a 10% case fatality rate for SARS-CoV-1 and 37% for MERS-CoV [3,4].
The clinical spectrum of SARS-CoV-2 pneumonia ranges from mild to critically ill cases. The previous studies reported only the epidemiological findings, the clinical presentation, and the clinical outcomes. However, more specific information identifying critically ill patients remains unknown. Recently, cardiac injury has been reported to be associated with mortality [5]. The mortality of critically ill patients with SARS-CoV-2 pneumonia is substantial [6]. Older patients with comorbidities and adult respiratory distress syndrome (ARDS) are at increased risk of death [6]. Therefore, studying the association of acute cardiac injury with the mortality in COVID-19 is essential and justified for prevention and preparation in the hospitals facing these global pandemics.
In this systematic review and meta-analysis, we aimed to explore the association between acute cardiac injury and mortality, the need for Intensive Care Unit (ICU) care, acute respiratory distress syndrome (ARDS), and severe COVID-19 in patients with COVID-19.
2. Methods
This meta-analysis was accomplished in agreement with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [7].
2.1. Eligibility criteria
We included all research articles in adult patients diagnosed with COVID-19 with information on hs-cTnl, cardiac injury, and clinical grouping or outcome of the clinically validated definition of mortality, the need for ICU care, acute respiratory distress syndrome (ARDS), or severe COVID-19. The following types of the article were excluded: articles other than original research (e.g., case report or series, review articles, letters to editor, editorials or commentaries), duplicate publication, and non-English articles.
2.2. Search strategy and study selection
We systematically searched PubMed, SCOPUS, EuropePMC, ProQuest, and Cochrane Central Databases with the search terms “COVID-19” or “SARS-CoV-2” and “Cardiac Disease” and “Cardiovascular Disease” and “Acute Cardiac Injury” and “ARDS” and “critically ill COVID-19”; search results were limited to the year 2020. Duplicate results were removed. The remaining articles were independently screened for relevance by its abstracts with two authors. The remaining investigators read full selected articles that met the requirements and provided final suggestions. These articles were thoroughly read, and those that fulfilled our criteria were included in the study. The final inclusion of studies was merely based on the agreements of all investigators; then, any disagreement was resolved by consensus.
The full text of residual articles was assessed according to the inclusion and exclusion criteria. The search was finalized on March 29th, 2020.
2.3. Data extraction
Data extraction was performed independently by two authors, and we used standardized forms that include authors, year of the study, study design, age, gender, hs-cTnl (including its cut-off point), history of hypertension, history of coronary artery/cardiovascular diseases, cardiac injury, mortality, ARDS, need for ICU care, and severe COVID-19.
Definition of cardiac injury follows that of the included studies, which includes highly sensitive cardiac troponin I (hs-cTnl) >99th percentile, regardless of electrocardiography and echocardiography. As a result, studies that reported elevation of hs-cTnl above the 99th percentile were considered as a cardiac injury.
The primary outcome was mortality, and the secondary outcomes were ARDS, the need for ICU care, and severe COVID-19. Acute respiratory distress syndrome was defined as per the World Health Organization (WHO) interim guidance on Severe Acute Respiratory Infection (SARI) of COVID-19, including the acute onset, chest imaging, origin of pulmonary infiltrates, and oxygenation impairment [8]. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to the fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and/or multiple organ dysfunction/failure) [9].
2.4. Statistical analysis
To perform a meta-analysis, Review Manager 5.3 (Copenhagen: The Cochrane Collaboration, 2014) and Stata version 16 (StataCorp LP, Texas 77845, USA) were used. The continuous variables were reported as means with standard deviations (SDs) and were calculated using the inverse-variance method. Dichotomous variables were calculated using the Mantel-Haenszel formula. Random effects models were used regardless of heterogeneity. Mean differences (MDs) and risk ratios (RRs) were reported with 95% confidence intervals (CIs) for continuous and dichotomous variables, respectively. The P-value was two-tailed, and the statistical significance set at ≤0.05. Sensitivity analysis by leave-one-out was performed to single out heterogeneity.
Heterogeneity was assessed with the Q-statistic test and the I2 test. The I2 statistic measured the percentage of total variation across the studies due to clinical or methodological heterogeneity instead of chance. If the significant Q statistics (P < 0.05) indicated heterogeneity across the studies, a random effect model was utilized for meta-analysis. Otherwise, a fixed-effect model was utilized. Substantial heterogeneity was represented by I2 for >50% [10].
To assess the small-study effect and publication bias, we performed the regression-based Egger's test for continuous variable and Harbord's test for a binary outcome. We also performed qualitative assessment for publication bias by using inverted plot analysis, an asymmetrical shape indicates publication bias.
3. Results
3.1. Baseline characteristics and study selection
We found a total of 157 records, and 147 remained after the removal of duplicates. One hundred and twenty-one records were excluded after screening the title/abstracts. After assessing 26 full-text for eligibility, we excluded 13 full-text articles because: 1) no dichotomous outcome of interest (elevated troponin/cardiac injury) and/or hs-cTnI (n = 11), and 2) specific population (myocarditis and cardiovascular manifestation) (n = 2). We included 13 studies in qualitative synthesis and 12 in meta-analysis (Fig. 1 ). There were a total of 2389 patients from 13 studies [5,[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. The baseline characteristics of the included studies are presented in Table 1 . All of the studies were retrospective observational. Most of the included studies defined cardiac injury as hs-cTnI elevation above 99th percentile. There are studies that did not specify their definition of cardiac injury, however, these studies presumably used similar definition to the existing studies (Fig. 2 ).
Fig. 1.
PRISMA study flow diagram.
Table 1.
Characteristics of the included studies
| Authors | Study design | Samples | Cardiac injury definition | Troponin | Troponin cut-off | Male (%) | Age | HTN (%) | CAD (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen T 2020 | Observational retrospective | 274 (113/161) | hs-cTnl above 99th percentile | hs-cTnl | >15.6 pg/mL | 73 vs 55 | 68.0 (62.0–77.0) vs 51.0 (37.0–66.0) | 48 vs 24 | 16 vs 7 | Mortality |
| Li K 2020 | Observational retrospective | 32 (11/21) | Unspecified | hs-cTnl | >34.2 pg/mL | 73 vs 22 | 69 (57–78) vs 51 (33–70) | 45 vs 19 | 9 vs 0 | Mortality |
| Luo XM 2020 | Observational retrospective | 403 (100/303) | Unspecified | hs-cTnl | >40 pg/mL | 57 vs 44.9 | 71 (65–80) vs 49 (37–62) | 60 vs 17.5 | 16 vs 6.6 | Mortality |
| Shi S 2020 | Observational retrospective | 416 | hs-cTnl above 99th percentile | hs-cTnl | Unspecified | N/A | N/A | N/A | N/A | Mortality, ARDS, severe COVID-19 |
| Wu C 2020a | Observational retrospective | 188 | Unspecified | hs-cTnl | ≥6.126 pg/mL | N/A | N/A | N/A | N/A | Mortality, ICU care, ARDS, |
| Wang D 2020 | Observational retrospective | 138 (36/102) | hs-cTnl above 99th percentile | hs-cTnl | ≥26.2 pg/mL | 61.1 vs 52 | 66 (57–78) vs 51 (37–62) | 58.3 vs 21.6 | 25 vs 10.8 (CVD) | Need for ICU care |
| Zhang F 2020 | Observational retrospective | 48 (17/31) | hs-cTnl above 99th percentile (>26 pg/mL) | hs-cTnl | >26 pg/mL | 70.6 vs 67.7 | 78.65 ± 8.31 vs 66.16 ± 13.66 | 70.6 vs 64.5 | 23.5 vs 29 | Mortality |
| Zhang Guqin 2020 | Observational retrospective | 221 (55/166) | hs-cTnl above 99th percentile | hs-cTnl | >26.2 pg/mL | 63.6 vs 44 | 62.0(52.0–74.0) vs 51.0 (36.0–64.3) | 47.3 vs 16.9 | 23.6 vs 5.4 | Severe COVID-19 |
| Zhou 2020 | Observational retrospective | 191 | hs-cTnl above 99th percentile | hs-cTnl | >28 pg/mL | 70 vs 59 | 69.0 (63.0–76.0) vs 52.0 (45.0–58.0) | 48 vs 23 | 24 vs 1 | Mortality |
| Huang 2020 | Observational retrospective | 41 (13/28) | hs-cTnl above 99th percentile | hs-cTnl | >28 pg/mL | 85 vs 68 | 49.0 (41.0–61.0) vs 49.0 (41.0–57.5) | 15 vs 14 | 23 vs 11 (CVD) | ICU Care |
| Hu L 2020 | Observational retrospective | 323 (172/151) | Unspecified | hs-cTnl | >0.04 pg/mL | 52.9 vs 49.7 | 65 vs 56 | 38.3 vs 25.8 | 19.2 vs 5.3 (CVD) | Severe COVID-19 |
| Hu B 2020 | Observational retrospective | 36 (16/20) | Unspecified | hs-cTnl | N/A | 68.8 vs 65 | 66.5 (61.3–75.0) vs 56.0 (48.5–67.5) |
50 vs 40 | 43.8 vs 0 (CVD) | Mortality |
| Zhao W 2020 | Observational retrospective | 78 (20/58) | Unspecified | hs-cTnl | >50 pg/mL | 55 vs 40.4 | 69 ± 15 vs 45 ± 17 | 40 vs 14 | 30 vs 5.3 (CVD) | Severe COVID-19 |
CAD: Coronary artery disease; COVID-19: Coronavirus disease 2019; cTnI: Cardiac troponin I; CVD: Cardiovascular Disease; hs-cTnI: Highly sensitive cardiac troponin I; ICU: Intensive Care Unit; N/A: Not available.
Group was not poor outcome vs good outcome (high troponin vs low-moderate troponin; cardiac injury vs no cardiac injury).
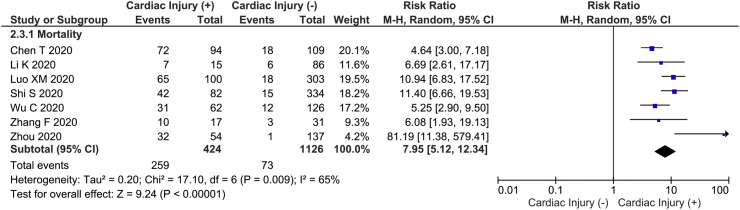
Fig. 2.
Cardiac injury and mortality. Cardiac injury was associated with increased mortality.
3.2. Cardiac injury and mortality
This meta-analysis showed that cardiac injury was associated with higher mortality (RR 7.95 [5.12, 12.34], p < 0.001; I2: 65%, p = 0.009). Sensitivity analysis showed that heterogeneity for mortality outcomes could be reduced by removal of Zhou 2014 et al. study (RR 7.22 [4.97, 10.47], p < 0.001: I2: 54%, p = 0.05).
3.3. Cardiac injury and secondary outcome
Cardiac injury was associated with a higher need for ICU care (RR 7.94 [1.51, 41.78], p = 0.01; I2: 79%, p = 0.009) and severe COVID-19 (RR 13.81 [5.52, 34.52], p < 0.001; I2: 0%, p = 0.38) (Fig. 3 ). Cardiac injury was not significant for an increased risk of ARDS (RR 2.57 [0.96, 6.85], p = 0.06; I2: 84%, p = 0.01). The removal of the Wu et al. study reduced heterogeneity for the need for ICU care (RR 16.85 [4.93, 57.62], p < 0.001; I2: 0%, p = 0.36).
Fig. 3.
Cardiac injury and secondary outcome. Cardiac injury was associated with an increased need for ICU care and severe COVID-19. The association was not significant for ARDS. ARDS: Acute Respiratory Distress Syndrome, ICU: Intensive Care Unit.
3.4. Highly sensitive cardiac troponin I and primary + secondary outcome
The level of hs-cTnI was higher in patients with primary + secondary outcome (mean difference 10.38 pg/mL [4.44, 16.32], p = 0.002; I2: 0%, p = 0.92) (Fig. 4 ).
Fig. 4.
Highly-sensitive troponin I and primary + secondary outcome. Elevated hs-cTnI was associated with poor outcome. hs-cTnI: Highly-sensitive Troponin I.
3.5. Publication bias
The funnel-plot analysis showed an asymmetrical shape for all outcomes (Fig. 5A, B, and C), indicating possible publication bias. Regression-based Harbord's test showed no indication of small-study effects for mortality (p = 0.882). Egger's test showed an indication of small-study effects for sensitive troponin I and primary + secondary outcome (p = 0.035).
Fig. 5.
Funnel-plot analysis. Funnel-plot analysis showing asymmetrical funnel plot for mortality.
4. Discussion
This meta-analysis demonstrated that acute cardiac injury, represented by elevated troponin concentration, was associated with increased mortality, the need for ICU care, and severe COVID-19. Although the association between cardiac injury and ARDS did not show statistical significance, it is essential from a clinical standpoint. These findings are beneficial and should be considered in clinical management, prevention, and preparation for patient safety issues in the hospital in the setting of the COVID-19 global pandemic. As previously reported, the mortality of critically ill patients with COVID-19 pneumonia is high. The survival period of the non-survivors is likely to be within 1 to 2 weeks after ICU admission. Elderly patients with comorbidities and ARDS are at increased risk of mortality. Consequently, the severity of COVID-19 pneumonia poses a high burden to hospital care resources, and a shortage of medical personnel [25].
Previously, a letter to the editor reporting a meta-analysis of 4 studies showed that troponin levels were associated with the severity of the disease (presented as standardized mean difference). The result from the subgroup analysis of cardiac injury and severe COVID-19 further supported this notion. To the best of the authors' knowledge, this study is the first meta-analysis that evaluates the relationship between cardiac injury and mortality in COVID-19 and presents the effect estimate in dichotomous form as RR (also applicable for the secondary outcome). The present meta-analysis showed that cardiac injury is associated with RR of 8 for mortality. This result gave clinicians more information on the impact and clinical importance of cardiac injury.
Mortality from COVID-19, as shown in the study, is likely due to cytokine storm syndrome and fulminant myocarditis. Fulminant myocarditis is primarily caused by a viral infection. It arises quickly, progresses rapidly, and results in severe heart failure and circulatory failure. The clinical presentation is hypotension and cardiogenic shock, with a mortality rate as high as 50%–70% [26,27]. In a portion of patients with COVID-19, interstitial mononuclear cells were shown to infiltrate myocardium in autopsies [28]. Case reports on COVID-19-induced myocarditis are also available [29,30]. While the exact mechanism is still unknown, myocardial damage might be due to the direct injury from the virus and exacerbated by the host's secondary immune response. Such a phenomenon has been observed in viral myocarditis of other causes and possibly also applies to COVID-19 [31,32].
Following spike protein activation by transmembrane protease serine 2 (TMPRSS2), the viral surface spike (S) protein binds to Angiotensin-converting Enzyme 2 (ACE2) [33]. The receptor-binding domain (RBD) in the SARS-CoV-2 S protein has a higher binding affinity for human ACE2 and is significantly higher compared to SARS-CoV-1 [34,35]. Although ACE2 is only slightly expressed in the cardiomyocyte, it was highly expressed in the pericytes. COVID-19 may attack pericytes, which is essential for endothelial stability, causing capillary endothelial dysfunction, which leads to microcirculatory disorders [36]. This explains why, although ACE2 is only slightly expressed in the cardiomyocytes, COVID-19 may cause cardiac injury. Patients with cardiovascular comorbidity such as heart failure are thought to be more susceptible to cardiac injury due to significantly increased ACE2 expression [36]; this is further reflected by a meta-analysis of six studies showing that patients with cardiovascular, metabolic disease were at risk for increased severity [37]. However, the rise of troponin in cardiac injury was also paralleled by the increase in inflammatory biomarkers which may indicate the role of cytokine storm in addition to direct cardiac injury [38]. Such manifestation may explain why the cardiac injury is potentially linked to ARDS, which might a be a surrogate marker for cytokine storm or vice versa [39].
4.1. The implication for clinical practice
Our meta-analysis suggests elevated troponin and cardiac injury were associated with poor outcomes. Nevertheless, troponin and cardiac injury can be a marker of poor prognosis in patients with COVID-19. We simply encourage the inclusion of troponin when constructing a prognostication model for a patient with COVID-19. During a pandemic, risk stratification in triage is necessary, and troponin can be a potential indicator of high-risk patients.
4.2. Limitation
The limitation of this study is first, the presence of publication bias; this is possibly due to the shortage of studies pertinent to the issues. Most of the articles included in the study were preprints; nevertheless, the authors have made exhaustive efforts to ensure that only sound studies were included. Most of the studies are from China; the patients might overlap across the reports. Second, the included studies were also mostly retrospective in design.
5. Conclusion
Cardiac injury is associated with mortality, need for ICU care, and severity of disease in patients with COVID-19. The high mortality in COVID-19 is very likely due to cytokine storm and fulminant myocarditis.
CRediT authorship contribution statement
Anwar Santoso:Conceptualization, Methodology, Data curation, Investigation, Writing - original draft, Writing - review & editing, Supervision.Raymond Pranata:Conceptualization, Methodology, Data curation, Formal analysis, Investigation, Writing - original draft.Arief Wibowo:Data curation, Writing - original draft.Makhyan Jibril Al-Farabi:Data curation, Writing - original draft.Ian Huang:Data curation, Investigation, Writing - original draft, Project administration.Budhi Antariksa:Investigation, Writing - review & editing.
References
- 1.World Health Organization Novel coronavirus – China. 2020. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 2.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2020. https://www.who.int/csr/sars/country/table2004_04_21/en/
- 4.World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV) 2020. https://www.who.int/emergencies/mers-cov/en/
- 5.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas-Rüddel D., Winning J., Dickmann P., et al. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist. 2020 doi: 10.1007/s00101-020-00760-3. March. [DOI] [PubMed] [Google Scholar]
- 7.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected; pp. 1–21.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 9.World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). vol. 2019. https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19).
- 10.Deek J., Higgins J., Altman D. In: Cochrane handbook for systematic reviews of interventions. Higgins J., Green S., editors. John Wiley & Sons Ltd; West Sussex, PO198SQ, England: 2008. Analysing data and undertaking meta-analysis; pp. 244–296. [Google Scholar]
- 11.Bai T., Tu S., Wei Y., et al. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. SSRN Electron J. 2020;6 doi: 10.2139/ssrn.354611. [DOI] [Google Scholar]
- 12.Zhang F., Yang D., Li J., et al. Myocardial injury is associated with in - hospital mortality of confirmed or suspected COVID - 19 in Wuhan, China : a single center retrospective cohort study. medRxiv. 2020;17 doi: 10.1101/2020.03.21.20040121. [DOI] [Google Scholar]
- 13.Zhang G., Hu C., Luo L., et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.02.20030452. 2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W., Yu S., Zha X., et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medRxiv. 2017;21(1):1–9. [Google Scholar]
- 15.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736(20):1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:m1091. doi: 10.1136/bmj.m1091. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao M., Zhang D., Wang Y., et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020 doi: 10.1101/2020.03.04.20030395. 2020.03.04.20030395. [DOI] [Google Scholar]
- 18.Hu B., Wang D. Clinical features of critically ill patients with COVID-19 infection in China. ResearchSquare. 2020:1–21. doi: 10.21203/rs.3.rs-16250/v1. [DOI] [Google Scholar]
- 19.Hu L., Ph D., Chen S., et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K., Chen D., Chen S., Feng Y., Chang C. Radiographic findings and other predictors in adults with Covid-19. medRxiv. 2020;2 doi: 10.1101/2020.03.23.20041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X., Xia H., Yang W., et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 23.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Hu X., Song J., et al. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) 2020;Vol 2019 doi: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 25.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;February doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yajima T., Knowlton K.U. Viral myocarditis from the perspective of the virus. Circulation. 2009;119(19):2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 32.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circ Res. 2016;118(3):496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0400-4. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W., Moore M.J., Vasllieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa078. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clerkin K.J., Fried J.A., Raikhelkar J., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;March doi: 10.1161/circulationaha.120.046941. CIRCULATIONAHA.120.046941. [DOI] [Google Scholar]
- 39.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/mmbr.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]