Abstract
Purpose
To analyse the high-resolution computed tomography (HRCT) early imaging features and the changing trend of coronavirus disease 2019 (COVID-19) pneumonia.
Materials and Methods
Forty-six patients with COVID-19 pneumonia who had an isolated lesion on the first positive CT were enrolled in this study. The following parameters were recorded for each lesion: sites, sizes, location (peripheral or central), attenuation (ground-glass opacity or consolidation), and other abnormalities (supply pulmonary artery dilation, air bronchogram, interstitial thickening, etc.). The follow-up CT images were compared with the previous CT scans, and the development of the lesions was evaluated.
Results
The lesions tended to be peripheral and subpleural. All the lesions exhibited ground-glass opacity with or without consolidation. A higher proportion of supply pulmonary artery dilation (89.13 % [41/46]) and air bronchogram (69.57 % [32/46]) were found. Other findings included thickening of the intralobular interstitium and a halo sign of ground glass around a solid nodule. Cavitation, calcification or lymphadelopathy were not observed. The reticular patterns were noted from the 14 days after symptoms onset in 7 of 20 patients (45 %). At 22–31 days, the lesions were completely absorbed only in 2 of 7 patients (28.57 %).
Conclusion
The typical early CT features of COVID-19 pneumonia are ground-glass opacity, and located peripheral or subpleural location, and with supply pulmonary artery dilation. Reticulation was evident after the 2nd week and persisted in half of patients evaluated in 4 weeks after the onset. Long-term follow-up is required to determine whether the reticulation represents irreversible fibrosis.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; 2019-nCoV, 2019 Novel coronavirus; CT, computed tomography; HRCT, high resolution computed tomograph; GGO, ground-glass opacity; WHO, World Health Organization; RT-PCR, real-time fluorescence polymerase chain reaction
Keywords: Early diagnosis, Computed tomography, Follow-up, Pneumonia, Coronavirus
1. Introduction
At the end of December 2019, a series of viral pneumonia cases caused by an unidentified microbial agent emerged in Wuhan, Hubei, China. A novel betacoronavirus was subsequently identified as the causative pathogen, which was named severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) [[1], [2], [3]]. By March 26, 2020, there were 469938 confirmed 2019-nCoV cases globally, and an additional 81985 cases suspected in China have been reported. On March 11, 2020, World Health Organization (WHO) declared the COVID-19 as a pandemic. Symptoms resulting from COVID-19 include fever, cough, myalgia, fatigue, diarrhoea and vomiting, which are similar to those of the regular human flu [[2], [3], [4]]. Anecdotal evidence suggested that some patients were asymptomatic [3,4]. Computed tomography (CT) of the chest is one of the major imaging modalities according to World Health Organization and CDC guidelines [5]. The typical findings from chest CT images of patients are bilateral multiple lobular and subsegmental areas of consolidation and ground-glass opacity [2,[6], [7], [8]]. However, the early imaging features of COVID-19 infection are not typical. We aimed to describe the early CT characteristics of COVID-19 pneumonia based on an isolated lesion on initial CT scans. Thus far, this information has not been previously reported. In this study, we conducted a comprehensive evaluation of the isolated lesion on the first positive CT of patients with COVID-19 pneumonia. Additionally, we presented temporal lung changes in the follow-up chest CT scans.
2. Materials and methods
2.1. Patients and CT imaging
SARS-Cov-2 is the subject of a continuing global public health outbreak investigations. Therefore, patient consent for this retrospective study was considered exempted by our institutional review board. We chose the patients who had an isolated lesion on the first positive chest CT and who underwent the follow-up chest CT from 1 January 2020 to 28 February 2020 in this single center study. All patients had recent travel history to or lived in Wuhan, China (the epicenter of the COVID-19 outbreak). Certain patients had contact with other patients with a diagnosis of COVID-19 pneumonia. The patients underwent CT for fever or other symptoms including cough, myalgia, fatigue, vomiting or diarrhoea. All cases were later confirmed with a positive result to real-time fluorescence polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 nucleic acid, with throat or nasopharyngeal swab specimens. Forty-six patients were ultimately included in the study.
All patients underwent non-contrast CT scanning (GE Healthcare, Philips, or Toshiba Medical Systems) of the thorax in the supine position during end-inspiration
(80–120 kVp, automated tube current modulation, mA ranges from 60 to 300,rotate time 0.5 s, itch 0.984:1,a slice thickness of 1.25 mm.(some diffences according to the machine types).
2.2. Review of CT images
All CT images were reviewed by two radiologists. Decisions were reached by consensus. The lesions were analysed based on sites and sizes, and the lesion size was described as small (diameter, <1 cm), medium (diameter, 1 to<3 cm), or large (diameter, ≥3 cm) [9]. The CT images were also analysed for peripheral or central location, subpleural, ground-glass opacity (GGO) or consolidation, a halo sign of ground glass around a solid nodule, supply pulmonary artery dilation, air bronchogram, interstitial thickening, and other abnormalities(pleural effusion, cavitation, lymphadenopathy, etc). The major CT terminologies were described using internationally standard defined by the Fleischner Society glossary [10]. The location of the lesion was defined as peripheral if it was located in the outer one-third of the lung, and otherwise, it was defined as central. Supply pulmonary artery of the lesion area was compared supply pulmonary artery of the lesion area with other pulmonary artery at the same or similar normal segment.
2.3. Follow-up CT images
The intervals of the follow-up CT ranged from 3 days to 31 days after the onset of initial symptoms. The CT images were compared with previous CT scans and were evaluated for lesion development.
2.4. Statistical analysis
All statistical analyses were performed using SPSS 21.0. Quantitative data were presented as the mean ± standard deviation (minimum-maximum), and the counting data were presented as the percentage of the total.
3. Results
3.1. Patient characteristics
The average age of the 27 male and 19 female patients was 39.17 ± 10.03 years old (range, 23−60 years). The most common symptom was fever (40/46, 86.96 %) (Table 1 ). Other non specific symptoms included cough, myalgia, fatigue, myalgia, vomiting and diarrhoea. In all, 36 of 46 patients (78.26 %) had assigned to common type, and 10 of 46 patients (21.74 %) had progressed to severe type [10]. None of the patients had died at the time of this writing.
Table 1.
Demographics and clinical characteristics of patients with COVID-19 Pneumonia (N = 46).
| Characteristics | Number |
|---|---|
| Ages(years) | |
| Range | 23−60 |
| Mean | 39.2 |
| Standard deviation | 9.6 |
| Sex | |
| Men | 27(58.70) |
| Wemen | 19(41.30) |
| Sign and symptoms | |
| Fever | 40(86.96) |
| Cough | 10(21.74) |
| Myalgia | 5(10.86) |
| Fatigue | 6(10.04) |
| Vomiting/Diarrhea | 3(6.5) |
Note.—Number in parentheses are percentages.
3.2. Sites and sizes of Lesions on the initial positive chest CT
The first chest CT scan was performed 1–5 days (3.2 ± 1.3) after the onset of symptoms. Two cases were negative according to the first CT and were positive on the second/follow-up CT. A total of 46 lesions were analysed on the initial positive chest CT. All lobes of the lung can be involved, and the affected segments were located in the lower lobes (54.35 % [25/46]) and in the right lobe (56.52 % [26/46]) (Table 2 , Fig. 1 ). A difference was observed between the sizes of the lesions (5−60 mm). A higher proportion of medium lesions (diameter, 1 to <3 cm) was noted (60.87 % [28/46]), and five lesions had sizes less than 10 mm (10.87 %% [5/46]) (Fig. 2 , Table 3 ).
Table 2.
Number of patients with affected segments in particular lung regions.
| Location | Number |
|---|---|
| Right upper lobe | 8(17.39) |
| Right middle lobe | 4(8.69) |
| Right lower lobe | 14(30.43) |
| Left upper lobe | 9(19.57) |
| Left lower lobe | 11(23.91) |
Note.—Number in parentheses are percentages.
Fig. 1.
49-year old male presenting with fever, cough, myalgia, and fatigue. (a) At present (day 4) ground-glass opacity lesion locates peripheral and subpleural region, in the posterior basal segment of right lower lobe. Air bronchogram (a, arrow) and Intralobular interstitium is clearly visible. (b) day 9, progressive ground-glass opacity in the posterior basal segment of right lower lobe was seen. (c) day 14 and (d) day 19 the lesion was gradually absorbed.
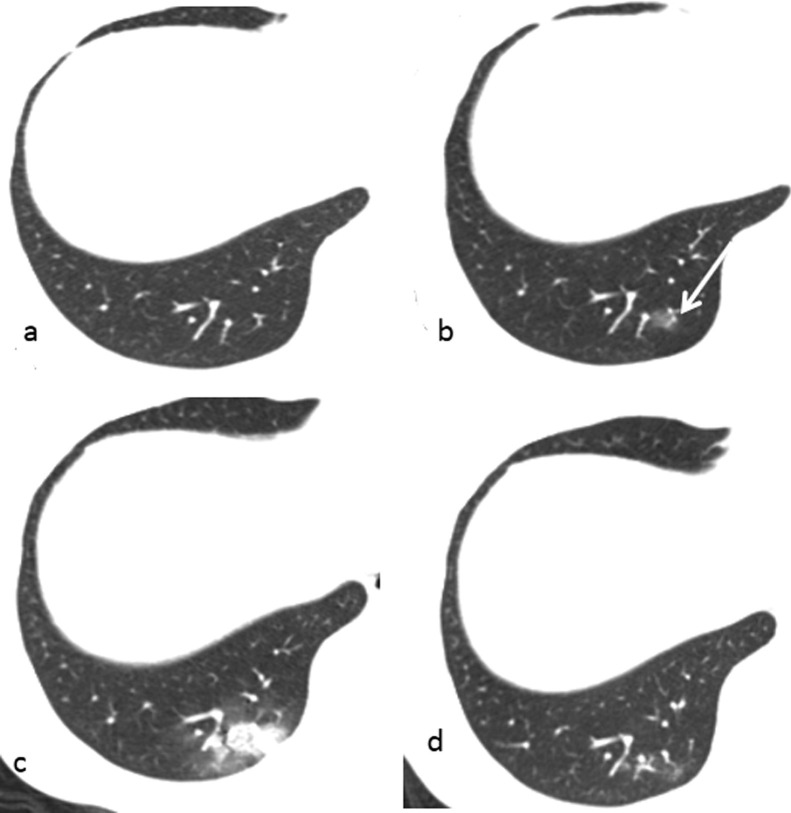
Fig. 2.
33-year old male presenting with cough. (a) shows normal lung on first CT. (b) showed a new ground-glass opacity lesion in the right lower lobe (arrow) after 2 days. (c) follow-up image obtained 6 days later showed the lesion was larger and expanding in the posterior segment of right lower lobe and a new small lesions appeared in left lower lobe. (d) follow-up image obtained 14 days later showed the lesions were gradually absorbed.
Table 3.
Number of sizes of Lesions on the first positive CT.
| Lesion Diameter | Number |
|---|---|
| <1 cm | 5(10.87) |
| 1 to <3 cm | 28(60.87) |
| ≥3 cm | 13(28.26) |
Note.—Number in parentheses are percentage.
3.3. Characteristics of Lesions on the initial positive chest CT
In terms of location within a lung segment, the lesions tended to be peripheral (65.22 % [30/46]) and subpleural (78.26 % [36/46]) on the first positive chest CT. Six lesions had interlobar pleural locations and had no peripheral distribution, and 34.78 % of isolated lesions (16/46) were located in the central region (Table 4 ).
Table 4.
Number of patients with particular characteristics on the first positive CT.
| Characteristics | Number |
|---|---|
| Location | |
| peripheral | 30(65.22) |
| subpleural | 36(78.26) |
| Opacification | |
| Pure ground-glass opacification | 18(39.13)) |
| Mixed ground-glass and consolidation | 28(60.83) |
| Pure well-defined solid nodule | 0 |
| a halo sign of ground glass around a solid nodule | 12(26.08) |
| Supply pulmonary artery dilation | 41(89.13) |
| Air bronchogram | 32(69.57) |
| Intralobular interstitium thickening | 13(28.26) |
| Interlobular septal thickening | 1(2.17) |
| Pleural effusion | 1(2.17) |
| Cavitation | 0 |
| Calcification | 0 |
| Lymphadenopathy | 0 |
Note.—Number in parentheses are percentages.
No pure solid nodule with a well-defined boundary was observed. The main chest CT findings were pure ground glass opacity (GGO) (39.13 % [18/46]) (Fig. 2b, 4a), and mixed GGO lesions with consolidations (60.87 % [28/46]) (Figs. 3,5 and 6 ). A halo sign of ground glass around a solid nodule was observed in 12 of 46 patients (26.08 %) (Fig. 6a). Supply pulmonary artery dilation was found in 41 of 46 patients (89.13 %) and air bronchogram was observed in 32 of 46 patients (69.57 %). Other findings included the thickening of intralobular interstitium (28.26 % [13/46]) and interlobular septa (2.17 % [1/46]) (Table 3). Pleural effusion was noted in only 1 of 46 patients (2.17 %). No cavitation, calcification, or lymphadenopathy was observed in this study.
Fig. 4.
32-year old male presenting with fever. (a) At present (day 1) showed a ground-glass lesion in the left upper lobe. (b) day 3, there were several mixed ground-glass opacity and consolidation lesions in the left and right lobe. (c) day 11 and (d) day17, the lesion is absorbed. Reticulation was seen on 17 days after the onset of initial symptoms.
Fig. 3.
41-year old male presenting with fever. (a) At present (day 3) A mixed ground-glass opacity and small consolidation lesion was showed, located in central region of lateral basal segment of right lower lobe. Air bronchogram, supply pulmonary artery dilation (a, arrow), and intralobular interstitium were seen. (b) day 6, image showed expanding lesion with involvement of the perihilar regions, and ground glass changed to be consolidation. (c) day 13 and (d) day 30, the lesion is absorbed. Ground-glass opacities with superimposed irregular linear opacities were seen in 30 day after the onset of initial symptoms.
Fig. 6.
30-year old male presenting with fever. (a) At present (day 1), a halo sign of ground-glass opacity around a solid nodule in the right lower lobe was seen. (b) Follow-up image obtained 2 days later showed expanding lesion. (c) and (d) Images day 9 showed diffuse lesions in most lobes(white lung).
3.4. Follow-up chest CT images
A total of 145 pulmonary CT scans were performed and each patient underwent an average of 3 ± 1 CT scans (range: 2–7). The average CTDIvols were 2.37 ± 1.11 mGy, DLPs were 93.89 ± 45.61 mGy.cm, and the effective dose after ICRP guideline was 2.01 ± 0.67 mSv for each CT scan. Longitudinal changes in specific abnormalities were documented in 20 patients with serial scans obtained in the 2nd week (8−14days), in 14 patients with serial scans obtained in the 3rd weeks (15–21 days) and in 7 patients in the 4th weeks or later (22–31days)after the onset. At 3–14 days after the onset of initial symptoms, the lesions were larger and expanding, and new lesions were observed at 6–14 days after onset of all patients, which might indicate a progressive stage. Diffuse lesions in most lobes (white lung) were observed at 9 days after onset in one patient (Fig. 6c,d). In 7–31 days after onset, the lesions were gradually absorbed and became irregularly linear and reticular structure, and were even completely absorbed in two patients (Fig. 5). However, the absorbed lesions were accompanied by new lesions at 10–18 days. After 10 day (the 2nd week), 12 (60 %) of 20 patients had irregular linear opacities with or without associated ground-glass opacity or consolidation. Mixed and predominantly reticular patterns were noted from the 14th day in 7 of 20 patients (45 %). At 22–31 days after the onset of initial symptoms, the lesions were completely absorbed in only 2 of 7 patients (28.57 %, Fig. 7 ). Of 16 in whom the isolated lesion was located the central region on the first CT, 12 cases (75 %) showed involvement of the central and perihilar regions by expanding on the follow-up chest CT (Fig. 3).
Fig. 5.
31-year old male presenting with fever, myalgia, and fatigue. (a) At present (day 2) showed a ground-glass opacity lesion in right middle lobe. (b) day 3 and (c) day 8, the lesion became larger and changed to consolidation. (d) day 26, the lesion was completely absorbed. The image was normal.
Fig. 7.
Stacked-bar graph shows distribution of different patterns of lung changes on HRCT scans at various time points from onset of symptoms. gray = reticular pattern, white = irregular linear opacities, black = ground-glass opacity or consolidation, striped = normal.
4. Discussion
Herein we reported the early CT features of patients diagnosed with COVID-19 pneumonia. We assessed the isolated lesion on the initial positive CT and had progression on the follow-up CT. We described the CT findings on the initial positive CT in details, when patients are suggested to be in an earlier stage of the disease. Additionally, we presented the temporal lung changes on the follow-up chest CT.
Lei et al. [8]. introduced the CT findings of COVID-19 pneumonia as a case report, which showed multiple ground-glass opacities in the bilateral upper lobe lungs. Our
initial experience has shown that the typical findings from chest CT images of COVID-19 pneumonia were bilateral multiple lobular consolidations and ground-glass opacity, predominantly in the lower lobes, similar to previous reports [3,7,12]. We found that the typical early pulmonary CT images of COVID-19 pneumonia were ground-glass opacity with or without consolidation, predominantly located peripheral or subpleural location with pulmonary artery dilation and air bronchogram. However, the early image performance of some cases of the COVID-19 infection is not typical. Sixteen of 46 isolated lesions (34.78 %) were located in the central region. In total, 12 of 16 cases (75 %) showed involvement of the central and perihilar regions by expanding on the follow-up chest CT. A halo sign of ground glass around a solid nodule was observed in 12 of 46 patients. Pleural effusion was uncommon. No cavitation, calcification or lymphadenopathy was found in this study. In our cases, five lesions had sizes less than 10 mm. We advised that the small lesions, and especially new lesions, that contained an area of ground-glass opacity required follow-up to eliminate the possibility of COVID-19 pneumonia in these high-risk groups. Particularly, it should be noted that two cases in this study were negative on the first CT and positive on the follow-up CT. Therefore, CT re-examination might be recommended for the high-risk population with a history of epidemic condition exposure. We hope that our study findings can help to ensure triage and early recognition of the COVID-19 pneumonia.
The dynamic changes in lesion manifestation were closely monitored by analyzing multiple follow-up CT scans. Although lesion development progressed in a time-dependent manner, we observed that at 3–14 days after disease onset, patients exhibited increased lung involvement accompanied by lesion enlargement and expansion, or new lesions were observed, indicating a potential progressive stage of the infection. This stage might be critical for treatment intervention and requires close clinical observations. During the reconstruction stage, which occurred at 7–31 days after disease onset in our studies, the lesions were absorbed and formed irregular linear opacities. In further analysis, we found that the lesions were absorbed but that patients developed new lesions at 10–18 days. Additionally, mixed and predominantly reticular patterns were noted from 14 day in 7 of 20 patients (45 %) and the lesions were completely absorbed only in 2 of 7 patients (28.57 %) within 22–31 days after the onset of initial symptoms. Long-term follow-up with CT and concomitant functional studies are required to determine the long-term pulmonary sequelae of COVID-19 pneumonia. Several studies have suggested that COVID-19 enters into host cells via cell receptor angiotensin converting enzyme II (ACE2) [[13], [14], [15]], and excessive activation of immune cells leads to the production of a large number of inflammatory cytokines, such as IL-6, causing diffuse damage to pulmonary capillary endothelial cells and alveolar epithelium. Though with meticulous treatments, it takes a time for the immune response to build and produce antibodies to suppress virus replication. A large sample study is needed to establish the evolution mechanism of CT characteristics during the disease.
This study indeed has some limitations. The limitations of this study include its retrospective nature. Secondly, the full range of COVID-19 pneumonia appearance and distribution might not have been reflected. To further elucidate the early CT imaging features and changes in the images associated with COVID-19 pneumonia, a larger sample size is needed in our next study. Besides, we evaluated the reticulation in 4 weeks after the onset. Long-term follow-up is required in future to determine whether the reticulation represents irreversible fibrosis.
In summary, the typical early CT image features of COVID-19 pneumonia were ground-glass opacity, predominantly located peripheral or subpleural location and pulmonary artery dilation. Additionally, a new small lesion that contained an area of ground-glass opacity might require follow-up CT to eliminate the possibility of COVID-19 infection in high-risk groups. Reticulation is evident after the 2nd week and persists in half of patients evaluated after 4 weeks. Long-term follow-up is required to determine whether the reticulation represents irreversible fibrosis. We hope that our study findings can facilitate early identification and management of cases of suspected COVID-19 pneumonia.
Declaration of Competing Interest
All authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Acknowledgments
We express our gratitude to Qihang Chen (Department of Radiology, Beijing Hospital) for consultation on CT images.
Contributor Information
Yueying Pan, Email: xpyy02@sina.com.
Liming Xia, Email: xialiming2017@outlook.com.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;(January (29)) doi: 10.1056/NEJMoa2001316. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201(4):P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 5.2020. Infection Prevention and Control during Health Care When Novel Coronavirus (nCoV) Infection Is Suspected. Interim Guidance. 25 January(2020). WHO/2019-nCoV/IPC/v2020.2. [Google Scholar]
- 6.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;(February (4)) doi: 10.1148/radiol.2020200241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung M., Bernheim A., Mei X.Y. CT imaging features of 2019 novel coronavirus(2019-nCoV) Radiology. 2020;(February (4)) doi: 10.1148/radiol.2020200230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;2020(January (31)) doi: 10.1148/radiol.2020200236. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonio G.E., Wong K.T., Hui D.S. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228(3):810–815. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 10.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Muller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 12.Pan Y., Guan H., Zhou S. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020;(February (13)) doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59(1–3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Hao, Kang Zijian, Gong Haiyi. The Digestive System Is a Potential Route of 2019-nCov Infection: A Bioinformatics Analysis Based on Single-cell Transcriptomes. https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1.full.pdf+html
- 15.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. Epub 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]