Abstract
Background
Studies suggest that intranasal vaccination can stimulate nonspecific immunity against agents not contained within the vaccine, but this effect is not reported for cats.
Hypothesis
A modified live feline herpesvirus‐1 (FHV‐1) and feline calicivirus (FCV) intranasal vaccine will reduce clinical signs of disease caused by experimental infection with Bordetella bronchiseptica.
Animals
Twenty specific pathogen‐free 12‐week‐old kittens.
Methods
Experimental study. Cats were randomized into 2 groups of 10 cats each. The vaccinated group was administered a single intranasal dose of a commercially available vaccine containing modified live strains of FHV‐1 and FCV, and the control group remained unvaccinated. All 20 cats were administered B. bronchiseptica by nasal inoculation 7 days later and were observed daily for clinical signs of illness for 20 days.
Results
In the first 10 days after B. bronchiseptica challenge, vaccinated cats were less likely to be clinically ill than control cats with a median clinical score of 0/180 (range 0–5) versus 2/180 (range 0–8) (P = .01). Nine of 10 control cats and 2 of 10 vaccinated cats were recorded as sneezing during days 1–10 after challenge (P = .006).
Conclusions and Clinical Importance
Intranasal vaccination against FHV‐1 and FCV decreased signs of illness due to an infectious agent not contained in the vaccine. This nonspecific immunity could be beneficial for protection against organisms for which vaccines are not available and as protection before development of vaccine‐induced humoral immunity.
Keywords: Cat, Innate, Immunity, Respiratory, Virus
Abbreviations
- AcNPV
Autographa californica nuclear polyhedrosis baculovirus
- FCV
feline calicivirus
- FHV‐1
feline herpesvirus
- FVRC
FHV‐1 and FCV
- FVRCP
FHV‐1, FCV, and panleukopenia
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- PCR
polymerase chain reaction
- SPF
specific pathogen free
Upper respiratory infections are one of the most common syndromes affecting cats in shelters, boarding facilities, and multiple cat households. The infectious agents implicated as primary causes of rhinitis in cats include feline herpesvirus 1 (FHV‐1), feline calicivirus (FCV), Bordetella bronchiseptica, Chlamydophila felis, Mycoplasma spp., and some strains of Pasteurella spp. Vaccines are available only for FHV‐1, FCV, B. bronchiseptica, and C. felis, and no single product is available that induces protection against all 4 agents. In addition, as with FCV, different strains of each agent can respond differently to vaccination, and it is unlikely that any product confers complete protection.1, 2, 3, 4
There are different formulations of the respiratory agents contained in commercially available vaccines in the United States. The majority of available vaccines are formulated for parenteral administration; however, there are 2 vaccines containing attenuated FHV‐1 and FCV that are formulated for intranasal administration.1 , 2 , 5 There are potential differences between immunologic responses induced by intranasal inoculation and parenteral inoculation. For example, in one study, intranasal administration of a FHV‐1, FCV, and panleukopenia (FVRCP) vaccine induced more rapid serologic responses to FHV‐1 and FCV than a modified live FVRCP for parenteral administration.6 In other studies, intranasal administration of a FVRCP vaccine induced protection against FHV‐1 challenge as early as 4 days after a single vaccination,7 and administration of an intranasal B. bronchiseptica vaccine induced protection against B. bronchiseptica as early as 3 days after a single vaccination,8 suggesting protection mediated at least in part by nonspecific immune stimulation. In addition, intranasal administration of a FVRCP vaccine induced greater lymphoblast responses to concanavalin A (a nonspecific mitogen) and to FHV‐1 antigens than a modified live FVRCP for parenteral administration.3 It therefore seems possible that nonspecific immune stimulation induced by intranasal vaccination might lessen clinical signs of disease when the vaccinate is exposed to infectious agents not contained within the vaccine. This is the case in murine studies in which intranasal administration of B. pertussis or Autographa californica nuclear polyhedrosis baculovirus (AcNPV) protected against challenge with influenza virus.9, 10
The purpose of the present study was to investigate whether intranasal administration of a commercially available vaccine containing FHV‐1 and FCVa confers protection against intranasal challenge with an infectious agent (B. bronchiseptica) not contained in the vaccine.
Materials and Methods
Study Design and Animals
Young (12 week old), mixed sex kittens (n = 20) from a barrier facility known to be negative for FHV‐1 and FCV were purchased. On arrival at the research facility, samples were collected by gently rubbing a sterile cotton swab against the oropharynx at the level of the molar teeth, placed in transport media,4 and cultured for aerobic bacteria, including B. bronchiseptica, and Mycoplasma spp.5 Total DNA and RNA were extracted from a second pharyngeal swab and evaluated for DNA of FHV‐1 and DNA of GAPDH (control DNA) by polymerase chain reaction (PCR) as well as RNA of FCV by reverse transcriptase PCR assay as previously described.4 Before vaccination, serum was collected and assayed for FHV‐1 and FCV antibodies by serum neutralization.6 The kittens were not tested for Chlamydophila felis.
The kittens were randomized utilizing a random number generator into 2 groups of 10, group‐housed in different areas of the facility, and acclimatized for 14 days. After the kittens were shown to be negative for antibodies against FHV‐1 and FCV, negative for FHV‐1 and FCV nucleic acids from the pharyngeal swabs, and negative for B. bronchiseptica by culture, one group was administered a single dose of the intranasal FVRC vaccine according to the manufacturer's guidelines1 on Day 0, and the other group was not vaccinated. After vaccination, facility staff members used barrier precautions to avoid cross‐infection of the kittens with the modified live vaccine strains of FHV‐1 and FCV. The protocols were approved by the Institutional Animal Care and Use Committee at the research facility.
Challenge Inoculation
The D‐2 strain of B. bronchiseptica was grown from freezing solution at Iowa State University.8 Once successfully cultured, the isolate was shipped overnight to Colorado State University in broth and subcultured to provide adequate challenge inoculum. The B. bronchiseptica isolate was subcultured onto a TSA (tryptic soy agar) with 5% sheep's blood agar plate to ensure the organism was in pure culture. Once a pure culture was confirmed, the isolate was put into 5 mL of TSB (tryptic soy broth) and allowed to grow for 24 hours at 37°C in ambient air. On day 7, an investigator (ML) administered 0.5 mL of the inoculum into each naris of all kittens. Quantitative culture of the isolate performed the morning of the challenge inoculation showed that approximately 1012 CFU of B. bronchiseptica were administered to each kitten.
Clinical Monitoring
Clinical scoring was performed daily for 20 days after inoculation with B. bronchiseptica. A previously designed upper respiratory disease scoring system7 was adapted and applied to each cat daily by trained individuals blinded to the treatment groups (Table 1). Using this system, a total of 18 points were possible per day; accordingly, a total of 360 points were possible for the 20‐day study period. The clinical observers spent 30 minutes in each cat room at the same time each day applying the clinical score parameters to each kitten, including whether or not sneezing was observed for each kitten during the observation period. Aural temperatures were collected to estimate changes in body temperature to reduce the stress on the kittens induced by repeated rectal temperature measurement.8 An aural body temperature of >95°F accompanied by lethargy or inappetance was considered evidence of fever. On Day 7 after B. bronchiseptica challenge, a pharyngeal swab was collected from each kitten for aerobic bacterial culture and Mycoplasma culture to determine whether the kittens had been colonized by the B. bronchiseptica inoculum.4 , 5
Table 1.
Clinical scoring system used to monitor for clinical evidence of upper respiratory disease
| Clinical Sign | |
|---|---|
| Conjunctivitis | 0 = None |
| 1 = Mild conjunctival hyperemia | |
| 2 = Moderate to severe conjunctival hyperemia | |
| 3 = Moderate to severe conjunctival hyperemia and chemosis | |
| Blepharospasm | 0 = None |
| 1 = Eye <25% closed | |
| 2 = Eye 25–50% closed | |
| 3 = Eye 50–75% closed | |
| 4 = Eye completely closed | |
| Ocular discharge | 0 = None |
| 1 = Minor serous discharge | |
| 2 = Moderate mucoid discharge | |
| 3 = Marked mucopurulent discharge | |
| Sneezing | 0 = None |
| 1 = Observed | |
| Nasal discharge | 0 = None |
| 1 = Minor serous discharge | |
| 2 = Moderate mucoid discharge | |
| 3 = Marked mucopurulent discharge | |
| Nasal congestion | 0 = None |
| 1 = Minor congestion (barely audible) | |
| 2 = Moderate congestion (easily audible) | |
| 3 = Marked congestion with open mouth breathing | |
| Body temperature (aural) | 0 = ≤95°F |
| 1 = >95°F | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Statistical Analysis
The observation periods were divided into Days 1–10 post inoculation and Days 11–20 post inoculation. The Days 1–20 cumulative results also were compared between groups. The total clinical score for the vaccinated cats was compared to that of the control cats within each observation period using the Mann‐Whitney U‐test.9 The proportion of individual cats that were observed to be sneezing within the observation periods was compared between groups by Fisher's exact test. Lastly, the percentage of total observation points with sneezing recorded was calculated for both groups within each observation period (10 cats per group; 10 observation points per 10 day period) and compared by Fisher's exact test.9 Significance was defined as P < .05 for all analyses.
Results
Before vaccination and B. bronchiseptica inoculation, all of the kittens were negative for antibodies against FHV‐1 and FCV, negative for nucleic acids of FHV‐1 and FCV on pharyngeal swabs, and negative for B. bronchiseptica and Mycoplasma spp. on pharyngeal swabs by culture. On Day 7 after B. bronchiseptica inoculation, 19 of 20 cats were positive for B. bronchiseptica, and all 20 cats were negative for Mycoplasma spp. by culture. The one B. bronchiseptica negative kitten was in the vaccinated cat group.
Sneezing was not recorded by the observers or the facility staff members during the equilibration period or the first 7 days after vaccination. After inoculation with B. bronchiseptica, clinical signs of disease were generally mild in both groups of cats. Sneezing was the predominant clinical sign of disease in both groups of cats. Aural temperatures of >95°F were rarely detected, and none of the cats were ever considered inappetant or lethargic, which suggests that fever did not occur. In addition, ocular manifestations of disease were uncommon, and so total ocular scores between groups were not compared statistically. None of the cats developed clinical signs severe enough to require supportive care or antibiotic therapy.
In the control cats, the Day 1–20 median cumulative clinical score for each cat after B. bronchiseptica challenge was 4.5 (out of 360 possible points; range 1–24). In the vaccinated cats, the Day 1–20 median cumulative clinical score for each cat was 2.5 (range 0–9). When Day 1–10 results after challenge were analyzed, the median cumulative clinical score for each control cat was 2 (out of 180 possible points; range 0–8). In the vaccinated cats, the Day 1–10 median cumulative clinical score for each cat was 0 (range 0–5). The control cats had significantly higher cumulative clinical scores per cat than the vaccinated cats during the Day 1–10 observation period (P = .01, Fig 1).
Figure 1.
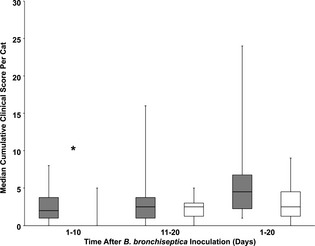
Box and whisker plots of cumulative clinical scores for individual cats after challenge with B. bronchiseptica for 10 control cats (gray boxes) and 10 cats vaccinated once with FELOMUNE CVR (white boxes). Central lines represent the median, boxes represent 25th and 75th percentiles, and whiskers represent minimum and maximum. Mann‐Whitney U‐test, *P < .05.
Overall, 9 of 10 control cats and 2 of 10 vaccinated cats sneezed at least once during observation periods in Days 1–10 after inoculation with B. bronchiseptica (P = .006). This difference between groups was no longer apparent during Days 11–20 when 9 of 10 control cats and 8 of 10 vaccinated cats sneezed at least once. The percentage of observation points with sneezing recorded (Fig 2) was significantly greater in control cats than in vaccinated cats over Days 1–10 (P < .0001) and Days 1–20 (P = .02).
Figure 2.
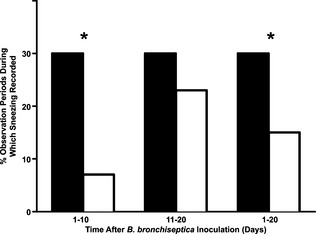
Percentage of observation periods during which sneezing was observed after challenge with B. bronchiseptica for 10 control cats (black columns) and 10 cats vaccinated once with FELOMUNE CVR (white columns). Fisher's exact test, *P < .05.
Discussion
In this study, cats administered a single dose of a modified life FHV‐1 and FCV vaccine intranasally had lower total clinical scores than control cats over the first 10 days after challenge with a mildly pathogenic strain of B. bronchiseptica. Results were unlikely affected by prior immunity or infection, as SPF kittens were used, and infection with B. bronchiseptica, Mycoplasma spp, FHV‐1 and FCV was ruled out with negative tests before inoculation. These findings support the hypothesis that intranasal vaccination against FHV‐1 and FCV confers cross‐protection against challenge with an infectious agent (B. bronchiseptica) not contained in the vaccine. However, the measured effects were lost during the second 10 days of observation after challenge, suggesting that the protection was short‐lived. In the aforementioned study of the efficacy of an intranasal B. bronchiseptica vaccine, there was both rapid protection against B. bronchiseptica challenge at 72 hours after vaccination and a 12‐month duration of immunity against B. bronchiseptica.8 It seems plausible, then, that the rapid onset of protection observed was because of nonspecific immunity induced by the process of intranasal vaccination or an unintentional component of the vaccine, whereas the longer term protection was because of a specific acquired immune response against B. bronchiseptica. Based on the current study, it does not appear that vaccination against FHV‐1 and FCV will confer a long‐term duration of immunity against organisms not contained within the vaccine.
One of the vaccinated cats was culture‐negative for B. bronchiseptica after inoculation. It is plausible that this cat was able to clear the infection, possibly with the assistance of nonspecific immunity conferred by the intranasal FHV‐1 and FCV vaccine. However, it is also possible that the culture was falsely negative because of imperfect sampling or culture technique. Regardless, as these were purpose‐bred SPF cats of the same age that were housed and handled identically and administered a standardized dose of inoculum, it is likely that the cat was exposed to but not colonized with B. bronchiseptica. Accordingly, the data from this cat were included in the analysis.
The mechanisms of nonspecific immunity induced by intranasal vaccination are not fully understood. It is unknown whether it is because of the infectious agents within the vaccine or some other component, such as cell culture proteins. One theory is that prior vaccination might dampen cytokine‐mediated inflammation caused by respiratory infection, lessening clinical signs of disease. However, in a study demonstrating the cross‐protective effect of B. pertussis vaccination against influenza in mice, this effect lagged at least 3 weeks behind vaccination, and so this mechanism seems an unlikely explanation for the findings described here.9 Stimulation of the innate immune system by intranasal vaccination seems a more likely explanation. Rapid boosting of innate immunity occurs after administration of AcNPV10 and chitin microparticles in rodent models of influenza immunity.11 These effects are attributed to activation of natural killer cells, as well as regulation of inflammatory cytokines. It seems plausible, then, that if one component of the innate immune system (natural killer cells) can be recruited by an unrelated intranasal treatment, so could other components of the innate immune system that would protect against the effects of bacterial infection. Immune responses of the kittens were not measured in the present study, and further research is required to determine the mediation of this nonspecific immunity.
Regardless of the mechanism, stimulation of nonspecific immunity via intranasal vaccination would have several benefits. First, it might provide protection against organisms for which vaccinations are not available or routinely administered, such as Mycoplasma spp. in cats and dogs and canine herpesvirus, canine respiratory coronavirus, or canine influenza virus in dogs. Second, nonspecific immunity likely becomes active while specific immunity is still developing, thereby conferring protection more quickly after vaccination. While the B. bronchiseptica challenge in this study was on Day 7 after intranasal vaccination with FHV‐1 and FCV, and timing of onset of the nonspecific immune response was not investigated, previous work demonstrated a partial immune response as early as 2 days after intranasal vaccination of cats against FHV‐1.7 This type of early protection would be of particular value in the event of unforeseen exposure to potential infectious organisms (for example, emergency boarding) and in shelter environments, where the typical “stray hold” period is 5 days, and there might be financial limitations on the number of vaccines that can be administered.
The present study was limited by the relatively mild clinical signs observed in both the vaccinated and control cats. While the cats in the study described here were administered a larger dose of the same strain of B. bronchiseptica than administered in a previous study, the cats in this study had much milder clinical signs of disease.8 This strain of B. bronchiseptica has been studied for approximately 27 years, and it is possible that long‐term storage, multiple passages, or freezing and thawing of the agent have rendered it less pathogenic.8 In addition, in the study described here, B. bronchiseptica was purposely administered to the cats via direct inoculation rather than aerosolization to avoid the need for sedation and to attempt to minimize the potential for severe systemic illness. It is likely that this limited the inoculum to the upper respiratory tissues, which might have lessened systemic clinical signs of disease like fever and cough.8
Finally, this study studied the cross‐protection effects induced by one vaccine administered intranasally. Whether similar effects would be recognized after administration of other intranasal vaccines or by parenteral vaccines is unknown, and further research in these areas is warranted.
Acknowledgments
The authors thank Dr Valeria Scorza, Dr Sukallaya Assarasakorn, Amber Caress, Kris Obssuth, and Jennifer Hawley for technical assistance.
Conflict of Interest Declaration: This project was financially supported by an unrestricted donation from Pfizer Animal Health, New York, NY and the Center for Companion Animal Studies at Colorado State University.
Footnotes
FELOMUNE CVR, Pfizer Animal Health, New York, NY
Feline UltraNasal FVRC and FVRCP Vaccines, Heska Corporation, Loveland, CO
Lappin MR, Veir J, Sebring R, Radecki SV. Feline lymphocyte blastogenesis in response to feline herpesvirus 1 antigens and concanavalin A after vaccination with five FVRCP vaccines. J Vet Intern Med 2005;19:467 (abstract)
BBL CultureSwab Plus Amies Gel, BD Diagnostics, Sparks, MD
Veterinary Diagnostic Laboratory, Colorado State University, Fort Collins, CO
New York State Veterinary Diagnostic Laboratory, Ithaca, NY
Center for Veterinary Biologics and National Veterinary Services Laboratories Testing Protocol: Supplemental Assay Method for Scoring Feline Rhinotracheitis Virus in Cats Following Challenge and Supplemental Assay Method for Scoring Feline Calicivirus in Cats Following Challenge
Pet infrared ear thermometer, B‐care Technology Corp, Taiwan
GraphPad Prism, GraphPad Software, La Jolla, CA
References
- 1. Johnson LR, Foley JE, De Cock HE, et al. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc 2005;227:579–585. [DOI] [PubMed] [Google Scholar]
- 2. Pesavento PA, Chang KO, Parker JSL. Molecular virology of feline calicivirus. Vet Clin Small Anim 2008;38:775–786. [DOI] [PubMed] [Google Scholar]
- 3. Maggs DJ, Clarke HE. Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. Am J Vet Res 2005;66:1550–1555. [DOI] [PubMed] [Google Scholar]
- 4. Veir JK, Ruch‐Gallie R, Spindel ME, Lappin MR. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J Feline Med Surg 2008;10:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richards JR, Elston TH, Ford RB, et al. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report. J Am Vet Med Assoc 2006;229:1405–1441. [DOI] [PubMed] [Google Scholar]
- 6. Lappin MR, Veir J, Hawley J. Feline panleukopenia virus, feline herpesvirus‐1, and feline calicivirus antibody responses in seronegative specific pathogen‐free cats after a single administration of two different modified live FVRCP vaccines. J Feline Med Surg 2009;11:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lappin MR, Sebring RW, Porter M, et al. Effects of a single dose of an intranasal feline herpesvirus 1, calicivirus, and panleukopenia vaccine on clinical signs and virus shedding after challenge with virulent feline herpesvirus 1. J Feline Med Surg 2006;8:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams J, Laris R, Gray AW, Jacobs AAC. Studies of the efficacy of a novel intranasal vaccine against feline bordetellosis. Vet Rec 2002;150:439–442. [DOI] [PubMed] [Google Scholar]
- 9. Li R, Lim A, Phoon MC, et al. Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm. J Virol 2010;84:7105–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abe T, Takahashi H, Hamazaki H, et al. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol 2003;171:1133–1139. [DOI] [PubMed] [Google Scholar]
- 11. Ichinohe T, Nagata N, Strong P, et al. Prophylactic effects of chitin microparticles on highly pathogenic H5N1 influenza virus. J Med Virol 2007;79:811–819. [DOI] [PubMed] [Google Scholar]


