It has been more than 1 month since the first 2019‐nCoV infected person was diagnosed. So far, 42 638 people have been diagnosed as 2019‐nCoV pneumonia, which also known as Wuhan pneumonia, among them, 7333 are severe cases, 1016 patients died, and only 3996 patients have been cured in the mainland of China (Hong Kong, Macao, and Taiwan were excluded). 1 All 31 provincial‐level regions and the Xinjiang Production Corps have the confirmed cases. 1 Hubei province, where believed to be the origin of Wuhan pneumonia outbreak, suffers the most, with 31 728 confirmed cases and 974 death cases. 2 The trend of cumulative cases is soaring, including 24 other countries outside of China had confirmed cases. With a remarkable notice that the total number of cases has far exceeded the number during severe acute respiratory syndrome (SARS) period. The first case of 2019‐nCoV pneumonia was linked to wild animal trading in the Huanan seafood market in the city of Wuhan, the capital of Hubei province. 3 Zhou et al 4 found the initial source of 2019‐nCoV probably originated from the Chinese horseshoe bat since the novel coronavirus was 96% identical to a bat coronavirus at a whole genome level. This coronavirus, which has not been identified in humans previously, had been detected from patients in Wuhan 5 and it was the first case for human infection with 2019‐nCoV from wild animals. 6 Recently, Wu et al 7 estimated 75 815 individuals would be infected in Wuhan, with an uncertainty range from 37 304 to 130 330 by using the susceptible‐exposed‐infectious‐recovered model. While the variation of the virus is still under surveillance.
Coronaviruses are a huge family of viruses, of which severe acute respiratory syndrome coronavirus (SARS‐nCoV) and Middle East respiratory syndrome coronavirus (MERS‐nCoV), and so on have already known by humans. 8 The 2019‐nCoV has an incubation period of 3 to 7 days in general, with a maximum of 14 days. 9 The most common symptoms at the onset of illness are fever, cough, and myalgia or fatigue. 9 , 10 While a few patients might have stuffiness, pharyngalgia, or diarrhea. 9 By far, the main sources of infection are 2019‐nCoV infected humans, however, the asymptomatic infection might also be a source of infection. 9 These patients are not easy to be detected among the population when initially infected but might have headache, fever, dyspnea, fatigue, and so on later. 11 This type of coronavirus has been proved could spread from person to person through the respiratory tract and contact transmission. 9 , 12 Zhang et al 13 pointed out the digestive system might also be a potential route of 2019‐nCoV infection based on bioinformatics analysis. Wu et al 7 found R 0, the basic reproduction number is around 2.68 with 2.47 to 2.86 of 95% credible interval, by applied Markov Chain Monte Carlo methods, where transmission characteristics appear to be similar of SARS. 14 Mild and occult cases could be the major concerns for disease prevention. 15 , 16 The World Health Organization (WHO) recommends wearing a medical mask when leaving homes 17 and ethanol and the high temperature had been proved could prevent disease occurrence. 9 The event has been determined as a Public Health Emergency of International Concern by WHO. 18
Humans are generally susceptible to coronavirus. 9 The youngest infected patient is only 1 month after birth. 19 Death cases were mostly found in the elderly and who already have chronic conditions. 10 , 20 The confirmed case is defined as either positive of novel coronavirus nucleic acid by real‐time fluorescent reverse transcription‐polymerase chain reaction detection of respiratory or blood specimens or highly homologous to any known novel coronaviruses through virus gene sequencing of respiratory or blood specimens. Only when body temperature returns to normal for more than 3 days, respiratory symptoms significantly improved and two consecutive tests negative of the nucleic acid of respiratory pathogens, with at least 1 day on sampling interval, people could be allowed to discharge from hospital 9 (Figure 1).
Figure 1.
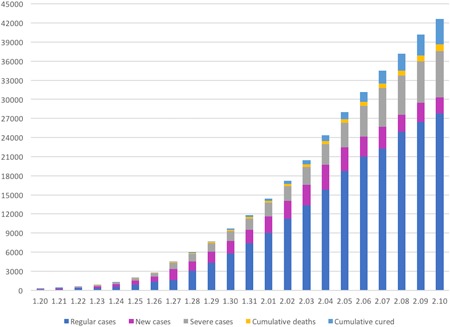
The trend of 2019‐nCoV pneumonia cases
The Chinese government has taken emergency measures to support population prevention and clinical treatment after the outbreak and made remarkable progress in responding to disease control comparing with SARS time (Figure 3). 8 , 21 The National Health Commission has set 2019‐nCoV pneumonia as a category B infectious disease with A‐class management on 20 January. 22 Provincial Bureau of health from Shanghai, Anhui, Zhejiang, and so on have organized medical teams, mainly including medical staff from the respiratory department, infections department and critical care medication, hurried to Wuhan for medical support. 23 “Huo Shen Shan” had been established for the centralized treatment of Wuhan pneumonia patients by reference to the model of Xiao Tang Shan hospital in Beijing during the SARS period. Although the cure rate has been rising up, compared with the fast‐growing cases and fatality rate from the updated collected data, it is still required to improve (Table 1). Through the efforts of scientists, Chinese Center for Disease Control and Prevention (CDC) has obtained the full‐length genome of 2019‐nCoV from clinical specimens and found the sequence of the genome has significant differences from any known coronavirus, 24 which is most related to the nucleotide sequence of bat‐SL‐CoVZC45 with 85% sequence similarity. 24 , 25 All the found genome sequence of 2019‐nCoV has been published as open data for scientific research as disease prevention and control. 25 CDC is also conducting the medication screening for Wuhan pneumonia and developing a vaccine for 2019‐nCoV. University of Queensland, GeoVax Labs Inc, and other world‐class research teams have also been joining in the research of vaccine development. 26 , 27 The Ministry of Health Disease Control Bureau also takes psycho crisis intervention and assistance for the population in need. Figure 2 showed the key nodes in the Wuhan pneumonia event, including government measures.
Figure 3.
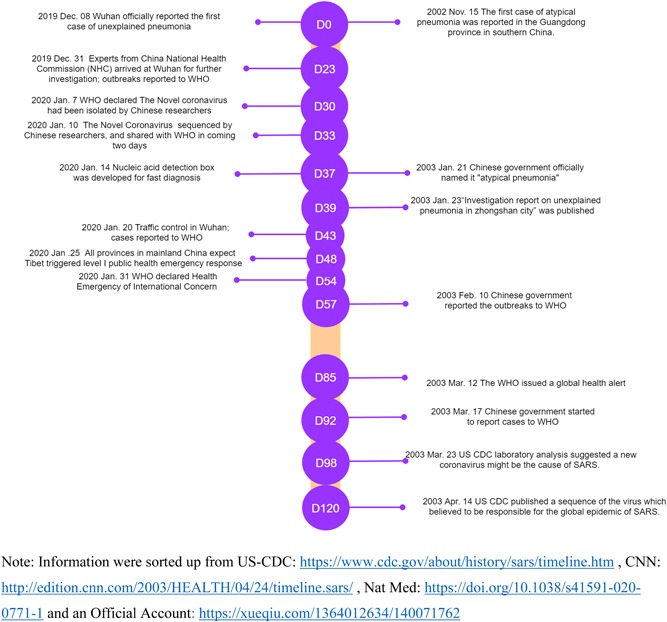
The comparison between 2019‐nCoV and severe acute respiratory syndrome event development
Table 1.
The updated cases of 2019‐nCoV pneumonia
| Date | Cumulative cases | Regular cases | New cases | Severe cases | Cumulative deaths | Cumulative cured | Fatality rate | Cure rate |
|---|---|---|---|---|---|---|---|---|
| 1.20 | 291 | 139 | 77 | 44 | 6 | 25 | 2.062% | 8.591% |
| 1.21 | 440 | 155 | 149 | 102 | 9 | 25 | 2.045% | 5.682% |
| 1.22 | 571 | 303 | 131 | 95 | 17 | 25 | 2.977% | 4.378% |
| 1.23 | 830 | 335 | 259 | 177 | 25 | 34 | 3.012% | 4.096% |
| 1.24 | 1287 | 527 | 444 | 237 | 41 | 38 | 3.186% | 2.953% |
| 1.25 | 1975 | 858 | 688 | 324 | 56 | 49 | 2.835% | 2.481% |
| 1.26 | 2744 | 1383 | 769 | 461 | 80 | 51 | 2.915% | 1.859% |
| 1.27 | 4515 | 1602 | 1771 | 976 | 106 | 60 | 2.348% | 1.329% |
| 1.28 | 5974 | 3041 | 1459 | 1239 | 132 | 103 | 2.210% | 1.724% |
| 1.29 | 7711 | 4310 | 1737 | 1370 | 170 | 124 | 2.205% | 1.608% |
| 1.30 | 9692 | 5799 | 1982 | 1527 | 213 | 171 | 2.198% | 1.764% |
| 1.31 | 11 791 | 7392 | 2102 | 1795 | 259 | 243 | 2.197% | 2.061% |
| 2.01 | 14 380 | 9048 | 2590 | 2110 | 304 | 328 | 2.114% | 2.281% |
| 2.02 | 17 205 | 11 244 | 2829 | 2296 | 361 | 475 | 2.098% | 2.761% |
| 2.03 | 20 438 | 13 358 | 3235 | 2788 | 425 | 632 | 2.079% | 3.092% |
| 2.04 | 24 324 | 15 836 | 3887 | 3219 | 490 | 892 | 2.014% | 3.667% |
| 2.05 | 28 018 | 18 749 | 3694 | 3859 | 563 | 1153 | 2.009% | 4.115% |
| 2.06 | 31 161 | 21 021 | 3143 | 4821 | 636 | 1540 | 2.041% | 4.942% |
| 2.07 | 34 546 | 22 274 | 3399 | 6101 | 722 | 2050 | 2.090% | 5.934% |
| 2.08 | 37 198 | 24 894 | 2656 | 6188 | 811 | 2649 | 2.180% | 7.121% |
| 2.09 | 40 171 | 26 436 | 3062 | 6484 | 908 | 3281 | 2.260% | 8.168% |
| 2.10 | 42 638 | 27 815 | 2478 | 7333 | 1016 | 3996 | 2.383% | 9.372% |
Note: ; cumulative cases = regular cases + new cases + severe cases + cumulative deaths + cumulative cured.
The above data only represented cases in mainland of China, Hong Kong, Taiwan, and Macau were excluded. Data were collected from National Health Commission of the People's Republic of China. Available from http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
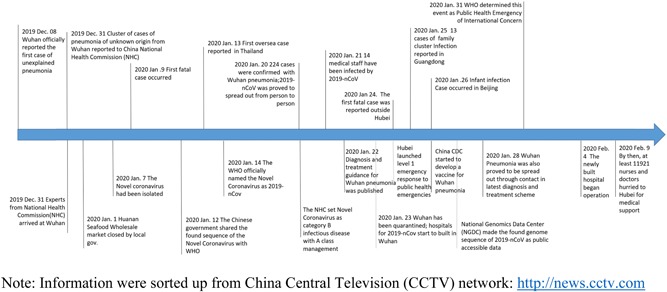
The key nodes in 2019‐nCoV pneumonia event
It has turned out to be urgent for epidemiologists, virologists, biologists, clinical doctors, and pharmaceutical researchers working together to battle against coronavirus outbreak. Human beings should engrave this zoonotic disease in mind for better dealing with “One‐Human‐Environmental‐Animal‐Health.” 28 We believe this novel coronavirus could be wiped out under the joint efforts of scientists around the world soon.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors express their deep thanks to the medical staff who are fighting in the front line. The authors appreciate their contributions very much. They also thank all the scientists and international cooperative organizations for supporting their country.
Wang G, Jin X. The progress of 2019 novel coronavirus event in China. J Med Virol. 2020;92:468–472. 10.1002/jmv.25705
Guan Wang and Xian Jin contributed equally to this study.
REFERENCES
- 1. National Health Commission of the People's Republic of China . Prevention and control of novel coronavirus infected pneumonia. http://www.nhc.gov.cn. Accessed 11 February 2020.
- 2. Health Commission of Hubei Province . Epidemic situation of 2019‐nCoV pneumonia in Hubei Province on Feb. 10th, 2020. http://wjw.hubei.gov.cn/. Accessed 11 February 2020.
- 3. Mahase E. China coronavirus: what do we know so far? BMJ. 2020;368:m308. [DOI] [PubMed] [Google Scholar]
- 4. Zhou P, Yang XL, Wang XG, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020. 10.1101/2020.01.22.914952 Available from: https://www.biorxiv.org/content/10.1101/2020.01.22.914952v2 [DOI] [Google Scholar]
- 5. Overview of 2019 novel coronavirus (2019‐nCoV). BMJ. https://bestpractice.bmj.com/topics/en-gb/3000165. Accessed 9 February 2020.
- 6. Catharine IP, Hilary DM, Anthony SF. Coronavirus infections—more than just the common cold. JAMA. 2020. 10.1001/jama.2020.0757 Available from: https://jamanetwork.com/journals/jama/fullarticle/2759815 [DOI] [PubMed] [Google Scholar]
- 7. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020. 10.1016/S0140-6736(20)30260-9 Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30260-9/fulltext [DOI] [Google Scholar]
- 8. Kickbusch I, Leung G. Response to the emerging novel coronavirus outbreak. BMJ. 2020;368:m406. [DOI] [PubMed] [Google Scholar]
- 9. National Administration of Traditional Chinese Medicine . Diagnosis and treatment of novel coronavirus infected pneumonia (The fifth edition). http://www.satcm.gov.cn. Accessed 9 February 2020.
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019‐nCov infection: a bioinformatics analysis based on single‐cell transcriptomes. bioRxiv. 2020. 10.1101/2020.01.30.927806 Available from: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1 [DOI] [Google Scholar]
- 14. Riou J, Althaus CL. Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4), 10.2807/1560-7917.ES.2020.25.4.2000058 Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.4.2000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Paper . Zhaohui Tong, the Critical Care Medicine expert: The biggest difference between SARS‐nCoV and 2019‐nCoV. https://www.thepaper.cn/newsDetail_forward_5641344. Accessed 9 February 2020.
- 16. Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020. 10.1056/NEJMp2000929 Available from: https://www.nejm.org/doi/full/10.1056/NEJMp2000929 [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Health topics: Coronavirus. https://www.who.int/health-topics/coronavirus. Accessed 9 February 2020.
- 18. World Health Organization . Newsroom, detail. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Accessed 9 February 2020.
- 19. The Paper . A neonatal has been confirmed with 2019‐nCoV pneumonia 30 hours after birth in Wuhan. https://www.thepaper.cn/newsDetail_forward_5798570. Accessed 9 February 2020.
- 20. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020. 10.1002/jmv.25689 Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nkengasong J. China's response to a novel coronavirus stands in a stark contrast to the 2002 SARS outbreak response. Nat Med. 2020. 10.1038/s41591-020-0771-1 Available from: https://www.nature.com/articles/s41591-020-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Central Government of the People's Republic of China . http://www.gov.cn. Accessed 9 February 2020.
- 23. Sina finance . The national health commission sent medical staffs to support Wuhan city. https://baijiahao.baidu.com/s?id=1656612972558028424&wfr=spider&for=pc. Accessed 9 February 2020.
- 24. National Microbiology Data Center . http://nmdc.cn/#/nCoV. Accessed 9 February 2020.
- 25. National Genomics Data Center . https://bigd.big.ac.cn/?lang=zh. Accessed 9 February 2020.
- 26. Chinese Center for Disease Control and Prevention . http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/. Accessed 9 February 2020. [DOI] [PMC free article] [PubMed]
- 27. Tencent News . Challenges are remaining with the development of novel coronavirus. At least six research teams around the world are racing against time. https://wxn.qq.com/cmsid/20200128A096A500. Accessed 9 February 2020.
- 28. Hui DS, Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic treat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]


