Abstract
In primary interferon‐α (IFN‐α) nonresponders with chronic hepatitis C, retreatment with IFN‐α has only limited efficacy with sustained response rates below 10%. Therefore, the aims of the present study were to compare the efficacy and safety of IFN‐α alone or in combination with amantadine sulphate in nonresponders to previous IFN‐α monotherapy. Fifty‐five IFN‐α nonresponders with chronic hepatitis C (mean age: 46.6 years) received IFN‐α 6 MIU thrice weekly for 24 weeks followed by 3 MIU thrice weekly for additional 24 weeks. Amantadine sulphate (n=26) or a matched placebo (n=29) was given orally twice daily for 48 weeks. Because of a low initial response rate at week 12 (13/55 patients) and a high breakthrough rate (8/13 patients) after IFN‐α dose reduction in week 24, a virological end‐of‐treatment response with undetectable serum HCV‐RNA (< 1000 copies/mL) was achieved in only five patients (IFN‐α/amantadine sulphate, one patient; IFN‐α/placebo, four patients). After 24 weeks follow‐up a sustained virological response was observed in only two patients receiving IFN‐α and placebo. Health‐related quality‐of‐life analysis showed a substantial improvement of the Profile of Mood States (POMS) scale concerning the subscales fatigue (P < 0.05) and vigor (P < 0.05) in patients receiving combined IFN‐α/amantadine sulphate treatment compared with those treated with IFN‐α alone. IFN‐α/amantadine sulphate combination therapy was well tolerated without any serious adverse events. In conclusion, retreatment with IFN‐α and amantadine sulphate does not increase the low sustained virological response rates of IFN‐α therapy in primary IFN‐α nonresponders with chronic hepatitis C, but may lead to a sustained improvement of health‐related quality‐of‐life.
Keywords: chronic hepatitis C, interferon nonresponder, interferon‐α/amantadine retreatment
Abbreviations
- HCV, hepatitis C virus
IFN‐α, interferon‐α
IFN‐α2a, interferon‐α2a
INTRODUCTION
Hepatitis C virus (HCV) infection frequently leads to chronic hepatitis and may progress to liver cirrhosis and possibly hepatocellular carcinoma [1, 2, 3, –4]. HCV‐related end‐stage liver disease is currently one of the leading indications for liver transplantation. Treatment with interferon‐α (IFN‐α) alone leads only to a sustained viral clearance in approximately 15–20% of patients [5,6]. The addition of ribavirin to standard interferon‐α treatment improves the sustained virological response rate to approximately 40% in previously untreated patients [7,8].
Several treatment strategies have been investigated in patients with chronic hepatitis C not responding to previous interferon‐α monotherapy. However, retreatment of previous interferon‐α nonresponders with standard regimens of interferon‐α alone (3 × 3–6 MIU/week s.c.) or in combination with ribavirin (1000–1200 mg/day orally) showed only limited therapeutic efficacy with sustained viral response rates below 10% [9,10].
Amantadine (1‐aminoadamantanamine sulphate) is a tricyclic, symmetric amine with an antiviral activity against toga‐, myxo‐, arena‐, flavi‐ and coronaviruses [11, 12, 13, 14, –15]. The drug has been studied in detail in the influenza A virus infection and the antiviral activity was found to be related to inhibition of viral uncoating and viral budding by interaction with the viral M2 protein [16, 17, –18]. The antiviral properties of amantadine have led to clinical trials evaluating the potential therapeutic role of amantadine alone or in combination with interferon‐α in patients with chronic hepatitis C [19, 20, 21, 22, –23]. For retreatment of previous interferon‐α nonresponders with chronic hepatitis C with the combination of interferon‐α and amantadine, only data from a few uncontrolled studies with inconsistent response rates are available [24,25].
Therefore, the aims of the present study were to evaluate the therapeutic efficacy, tolerability and health‐related quality of life of interferon‐α2a (IFN‐α2a) plus amantadine sulphate in comparison with IFN‐α2a plus placebo in patients with chronic hepatitis C not responding to previous IFN‐α treatment.
PATIENTS AND METHODS
Patients
Patients with chronic hepatitis C not responding to previous IFN‐α monotherapy with a minimal total IFN‐α dose of 108 MIU and a treatment duration of at least 12 weeks were eligible for enrolment when they met all of the following inclusion criteria: (1) nonresponse to previous IFN‐α monotherapy with persistence of serum HCV‐RNA and a treatment‐free interval of at least 24 weeks; (2) elevated alanine aminotransferase (ALT) levels; (3) a positive anti‐HCV test; (4) detectable serum HCV‐RNA; (5) compensated liver disease; (6) leukocyte count ≥ 2500/μL, platelets count ≥ 70000/μL; and (7) aged between 18 and 70 years. Demographic, biochemical, serological and virological pretreatment characteristics of the 55 enrolled patients are summarized in Table 1. Exclusion criteria were coinfection with hepatitis B virus or human immunodeficiency virus types 1 and 2, concomitant autoimmune disease, clinically significant cardiovascular, metabolic, renal, haematological, rheumatological, neurological or psychiatric disease, organ grafts, systemic infections, bleeding disorders, anaphylactic reactions, a history of neoplastic disease within the last 5 years, systemic immunosuppressive treatment, average daily intake of alcohol > 50 g, drug abuse within the previous year, pregnancy and lactation period. Liver biopsy was performed in all patients 1–6 months before the initiation of the primary IFN‐α therapy. According to the study protocol, additional liver biopsies before retreatment were not required for enrolment in the present study.
Table 1.
Pretreatment demographic, biochemical, serological, molecular and histological characteristics of 55 patients receiving retreatment with IFN‐α2a with and without amantadine sulphate
Study design
In the present prospective, randomized, double‐blind, placebo‐controlled trial, which was conducted at the University Hospitals of Berlin and Frankfurt, Germany, 55 eligible patients were randomly assigned to treatment with either the combination of IFN‐α2a plus amantadine sulphate (n=26) or IFN‐α2a plus placebo (n=29). Randomization was performed with a random number generator in fixed blocks of four with a ratio of 1 : 1. All patients were treated with 6 MIU IFN‐α2a (Roferon A®, Hoffmann La‐Roche AG, Grenzach‐Wyhlen, Germany) thrice weekly subcutaneously for 24 weeks followed by 3 MIU IFN‐α2a thrice weekly subcutaneously for additional 24 weeks (Fig. 1). A daily dose of 200 mg amantadine sulphate (Infex®, Merz + Co. GmbH & Co, Frankfurt/M., Germany) or matched placebo were given orally twice daily for 48 weeks. Treatment was continued only in patients with undetectable serum HCV‐RNA (Amplicor HCV™, Roche Diagnostic Systems, Branchburg, NJ; lower detection limit: 1000 copies/mL) at treatment week 12 [27]. The follow‐up period was 24 weeks in all patients. Clinical examination, haematological and biochemical tests were performed within 2–12 weeks before treatment, at initiation of treatment, every 2 weeks for the first 8 weeks of treatment, and every 4 weeks thereafter until the end of treatment. During the follow‐up period patients were evaluated 4, 12 and 24 weeks after the end of treatment. Serum HCV‐RNA was determined in all patients quantitatively before treatment (Amplicor Monitor HCV™, version 2.0, Roche Diagnostic Systems, Branchburg, NJ) and thereafter qualitatively at treatment weeks 4, 12, 24 and 48, and at the end of the follow‐up period. Genotyping was performed by a reverse hybridization assay (Inno LiPA HCV II, Innogenetics, Gent, Belgium) [28].
Figure 1.
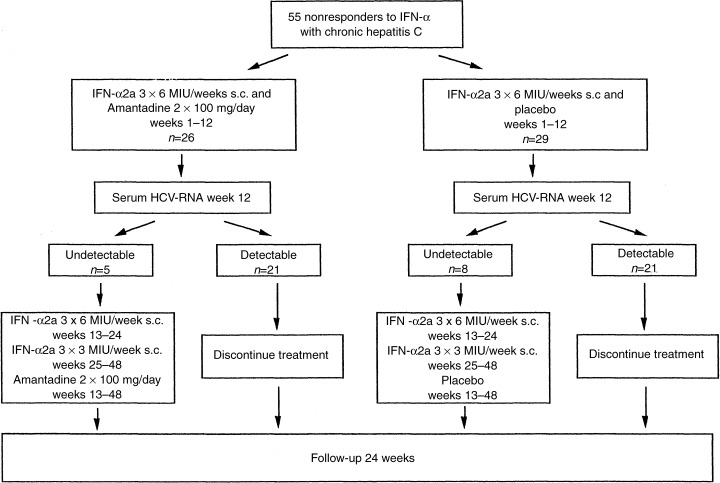
Study design.
Informed written consent was obtained from all patients before enrolment. The study was approved by the local ethics committees for medical research of the participating study centres and performed according to the Declaration of Helsinki, the German Drug Law, and the ICH guidelines of ‘Good Clinical Practice’.
Assessment of health‐related quality of live (HRQOL)
Individual emotional and psychological states were determined at baseline, at treatment week 16, and at the end of the 24 weeks follow‐up period by a German adapted and validated Profile of Mood States (POMS) scale assessing four factor scores for depression, fatigue, vigor and anger [29,30]. At the same time points quality of life was evaluated by an ‘Everyday Life’ questionnaire [31,32]. This German‐validated questionnaire, related to the SF‐36 Health Survey, assesses the following six subscales of health‐related quality‐of‐life for subjective health: body (e.g. make demands on body, concentrate on a task), mind (e.g. cope with illness, accept oneself), everyday life (e.g. solve daily problems, personal hygiene), social activity (e.g. get along with family, count on partner’s help), zest for life (e.g. enjoy life) and medical treatment (e.g. believe in success of treatment). Sum scores were determined for every patient. Missing items were replaced by the mean for the nonmissing items of the subscales. However, missing questionnaires were not replaced.
Definition of response and study endpoints
The primary efficacy endpoint of the present study was defined as a sustained virological response with undetectable serum HCV‐RNA by qualitative serum HCV‐RNA at the end of the follow‐up period. Secondary efficacy parameters included the virological response at treatment weeks 12, 24 and 48 as well as the corresponding biochemical response defined as a normalization of ALT. As an additional secondary efficacy parameter changes of HRQOL during and after treatment were evaluated.
Statistical analysis
Based on an intent‐to‐treat analysis, 55 primary IFN‐α nonresponders, who received at least one dose of the study medication, were included in the primary statistical analysis. Virological response rates of the two treatment arms were compared with a one‐tailed Fisher’s exact test on a significance level of α=10%. Corresponding biochemical response rates were analysed with a two‐tailed Mann–Whitney U‐test (α=0.05). Statistical procedures were performed using SAS procedures (version 6.12). For the evaluation of HRQOL, the differences of the sum scores between the two treatment groups at treatment weeks 16 and 48, and at the end of follow‐up were compared with a two‐tailed Wilcoxon rank sum test (α=0.05) using StatXact4.
RESULTS
Biochemical and virological response
An initial virological response with undetectable serum HCV‐RNA (< 1000 copies/mL) at treatment week 12 was achieved in five of 26 (19%) and in eight of 29 (28%) patients treated with IFN‐α2a and amantadine sulphate or placebo, respectively (Table 2). According to the protocol the antiviral therapy was discontinued at treatment week 16 in 42 patients because of detectable serum HCV‐RNA at treatment week 12. Because of breakthrough events during treatment weeks 16–48, a virological end‐of‐treatment response was found only in one of 26 (4%) patients receiving combined IFN‐α2a/amantadine sulphate treatment and in four of 29 (14%) patients on IFN‐α2a and placebo. At the end of follow‐up, a sustained virological response was observed in no patient treated with IFN‐α2a and amantadine sulphate and in two patients treated with IFN‐α2a plus placebo.
Table 2.
Virological and biochemical response rates during treatment, end‐of‐treatment (week 48), and after the end of the 24 weeks follow‐up period. Responses are defined as undetectable HCV‐RNA by RT‐PCR and normalized ALT, respectively
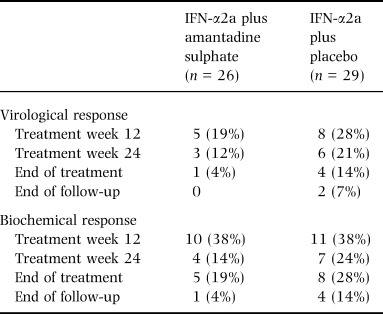
Initial biochemical responses with ALT values within the normal range at treatment week 12 were comparable in both groups (38% for each treatment arm, Table 2). A sustained biochemical response was achieved in only one of the patients treated with IFN‐α2a plus amantadine sulphate and in four of 29 patients receiving IFN‐α2a plus placebo.
Health‐related quality of life (HRQOL)
According to the study protocol antiviral treatment was discontinued at week 16 in 42 of 55 patients with detectable serum HCV‐RNA at treatment week 12. Therefore, health‐related quality of life during treatment was evaluated in all patients at treatment week 16. Assessment of health‐related quality of live during treatment revealed a deterioration of the mean of all four factor scores of the POMS scale in the patients treated with IFN‐α plus placebo and in three of the four factor scores (depression, fatigue and anger) in patients treated with combined IFN‐α/amantadine sulphate (Fig. 2a). The extent of the observed impairment of the POMS scale was less pronounced for the subscales depression and fatigue in patients treated with IFN‐α2a plus amantadine sulphate. At the end of follow‐up, a sustained improvement of the mean factor scores for depression (P=n.s.), fatigue (P=0.023), and vigor (P=0.025) in comparison with baseline levels was observed only in patients receiving combined treatment with IFN‐α2a plus amantadine sulphate.
Figure 2.
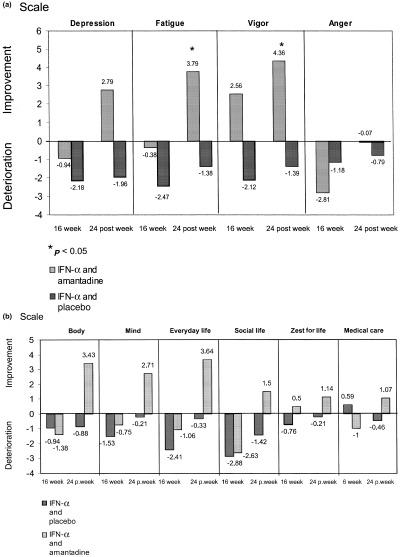
(a) Profile of Mood States scale at treatment week 16 and after the end of the 24‐week follow‐up period. (b) Everyday Life questionnaire at treatment week 16 and after the end of the 24‐week follow‐up period.
Irrespective of treatment, the evaluation of the ‘Everyday Life’ questionnaire at treatment week 16 showed an impairment in the means of most subscales in our patients compared with the corresponding pretreatment scores (Fig. 2b). At the end of the follow‐up period, a sustained improvement in the means of all subscales was observed only in patients receiving treatment with IFN‐α plus amantadine sulphate. In the group of patients treated with IFN‐α plus placebo the means of all subscales at the end of the follow‐up period was worse than the corresponding pretreatment scores.
Adverse events
Adverse events (n=269) occurred in a similar frequency in both treatment arms and were mostly related to IFN. The spectrum and frequency of the observed adverse events in relation to treatment are listed in detail in Table 3. Serious adverse events were not observed in this study. However, one patient treated with IFN‐α2a plus placebo was discontinued prematurely from antiviral treatment at week 20 due to depression.
Table 3.
Adverse events during treatment with IFN‐α2a with and without amantadine sulphate
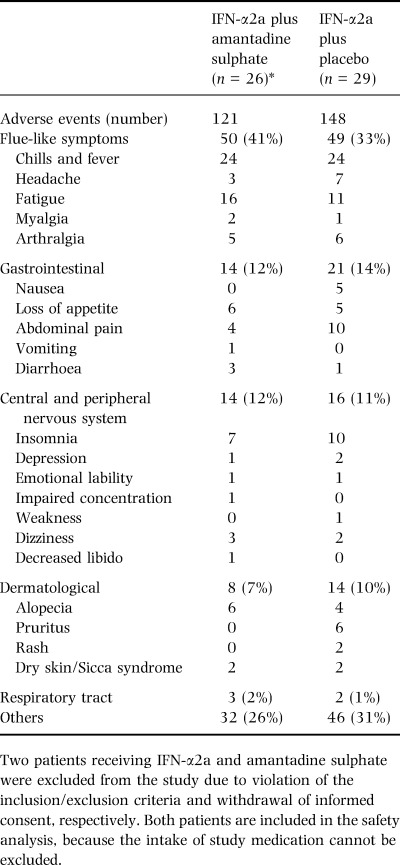
A slight decrease of mean leukocyte and thrombocyte count was found in most patients irrespective of the treatment group from baseline to treatment week 4 (5.8 ± 1.6/nL to 4.3 ± 1.4/nL, and 197 ± 77/nL to 153 ± 83/nL, respectively). At the end of the follow‐up period leukocyte and thrombocyte counts returned to baseline levels with no significant differences between the treatment groups. Hyperthyroidism developed in one patient treated with IFN‐α2a alone and in five patients receiving IFN‐α2a plus amantadine sulphate. In one patient, diabetes mellitus developed and in another patient diabetes mellitus was aggravated during treatment. Both patients received IFN‐α2a plus amantadine sulphate.
DISCUSSION
During the last decade IFN‐α therapy has been established as the standard treatment for chronic hepatitis C. Sustained viral clearance can be achieved by IFN‐α monotherapy and IFN‐α/ribavirin combination therapy in 5–20% and 38–41% of treatment naive patients, respectively [5, 6, 7, –8]. With respect to the low sustained response rates to retreatment with IFN‐α alone or in combination with ribavirin in IFN‐α nonresponder, the need for the development of alternative, effective retreatment regimens in this subgroup of patients became apparent. In a 1997 published pilot study, a beneficial therapeutic effect of six months amantadine retreatment in IFN‐α nonresponders with chronic hepatitis C was reported with a sustained biochemical and virological response in four of 22 patients (18%) after a 24 week follow‐up period [19]. The postulated antiviral effect of amantadine was supported in vitro by a dose‐dependent decrease of HCV‐RNA in the supernatant of isolated blood mononuclear cells from patients with chronic hepatitis C cultured with amantadine [33]. However, in vivo determination of hepatitis C viral load failed to show a direct synergistic antiviral effect of amantadine sulphate during combined IFN‐α/amantadine treatment in previously untreated patients with chronic hepatitis C [23].
Subsequently, mainly uncontrolled clinical trials of amantadine monotherapy in small cohorts of nonresponders with chronic hepatitis C showed conflicting results with sustained virological response rates varying from 0% to 15%. As indicated by a recently published pilot study, the combination of IFN‐α with amantadine may lead to an improvement of virological response rates in nonresponders with chronic hepatitis C [25].
The present study is the first randomized, double‐blind, placebo‐controlled trial evaluating the efficacy of retreatment with IFN‐α plus amantadine sulphate compared with IFN‐α alone in previous IFN‐α nonresponders with chronic hepatitis C. Considering the demographic, biochemical, virological and histological patients’ characteristics, significant differences between both treatment groups were not present. The sustained virological response rates were similar in both treatment groups. Thus, with respect to the results of the present controlled study, the addition of amantadine sulphate to IFN‐α does not improve the sustained virological response rates in nonresponders with chronic hepatitis C. These results confirm a larger controlled trial in 119 treatment naive patients with chronic hepatitis C which also showed no beneficial virological effect of the addition of amantadine sulphate to IFN‐α treatment in these patients [23]. In contrast, a recently published study in 60 nonresponders, treated with IFN‐α, ribavirin and amantadine or IFN‐α and amantadine, could demonstrate increased virological response rates in nonresponders with chronic hepatitis C who received amantadine [34]. However, these data have to be confirmed by larger randomized, double‐blind, placebo‐controlled trials in nonresponders with chronic hepatitis C.
Despite a similar number of adverse events in both treatment groups, additional analysis of the individual emotional and psychological state as well as the evaluation of health‐related quality of live revealed an improved outcome in patients treated with the combination of IFN‐α plus amantadine sulphate compared with those patients receiving IFN‐α alone. In the POMS scale all four subscores deteriorated during treatment with IFN‐α alone in comparison with the corresponding baseline levels and remained impaired during the follow‐up period. The combined retreatment with IFN‐α plus amantadine sulphate led to a smaller reduction of three of the four subscores, i.e. depression, vigor, and fatigue during treatment and interestingly, to a sustained improvement of the corresponding subscores in comparison with baseline levels at the end of the 24 week follow‐up period. A similar improvement during amantadine sulphate treatment has also been shown for patients with central nervous disease, including multiple sclerosis and Parkinson’s disease [35]. In the present study, analysis of the SF‐36 health‐related ‘Everyday Life’ questionnaire showed a sustained improvement of all six subscores in the group of patients treated with IFN‐α plus amantadine sulphate at the end of the follow‐up period in comparison with the corresponding baseline scores while five of six subscores remained impaired in patients treated with IFN‐α alone. A sustained improvement for the POMS scale and the SF‐36 health‐related ‘Everyday Life’ questionnaire has also been reported in a previously published study in naive patients with chronic hepatitis C receiving IFN‐α/amantadine sulphate combination therapy compared with those treated with IFN‐α alone [23].
In summary, retreatment with IFN‐α in combination with amantadine sulphate does not increase the low sustained virological response rates of IFN‐α monotherapy in primary IFN‐α nonresponders with chronic hepatitis C, but may lead to a sustained improvement of health‐related quality of life. The recently suggested improved antiviral effect of triple retreatment with IFN‐α, ribavirin and amantadine in previous IFN‐α nonresponders has to be confirmed in larger randomized, double‐blind, placebo‐controlled trials.
References
- 1. Saito I, Miyamura T, Ohbayashii A et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA 1990; 87: 6547–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alter MJ, Margolis HS, Krawczynski K et al. The natural history of community‐acquired hepatitis C in the United States. N Eng J Med 1992; 327: 1899–1905. [DOI] [PubMed] [Google Scholar]
- 3. Tong MJ, El‐Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion associated hepatitis C. N Eng J Med 1995; 332: 1899–1905. [DOI] [PubMed] [Google Scholar]
- 4. Di Bisceglie AM. Hepatitis C. Lancet 1998; 351: 351–355.DOI: 10.1016/s0140-6736(97)07361-3 [DOI] [PubMed] [Google Scholar]
- 5. Lindsay KL. Therapy of hepatitis C: overview. Hepatology 1997; 26 (Suppl. 1): 71–77. [DOI] [PubMed] [Google Scholar]
- 6. Poynard T, Leroy V, Cohard M et al. Meta‐Analysis of interferon randomized trials in the treatment of viral hepatitis C: Effects of dose and duration. Hepatology 1996; 24: 778–789. [DOI] [PubMed] [Google Scholar]
- 7. McHutchison JG, Gordon SC, Schiff ER et al. Interferon‐α2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Eng J Med 1998; 339: 1485–1492. [DOI] [PubMed] [Google Scholar]
- 8. Poynard T, Marcellin P, Lee S et al. An international randomized trial of interferon alfa‐2b and ribavirin for 48 or for 24 weeks vs interferon alfa‐2b, 48 weeks, for first line treatment of chronic hepatitis C. Lancet 1998; 352: 1426–1432.DOI: 10.1016/s0140-6736(98)07124-4 [DOI] [PubMed] [Google Scholar]
- 9. Gerken G, Teuber G, Goergen B, Meyer zum Büschenfelde KH. Interferon‐alpha retreatment in chronic hepatitis C. J Hepatol 1995; 22 (Suppl. 1): 118–121. [PubMed] [Google Scholar]
- 10. Teuber G, Berg T, Hoffmann RM et al. Retreatment with interferon‐alpha and ribavirin in primary interferon‐alpha nonreponders with chronic hepatitis C. Digestion 2000; 61: 90–97. [DOI] [PubMed] [Google Scholar]
- 11. Oxford JS & Schild GC. In vitro inhibition of rubella virus by 1‐amantanamine hydrochloride. Arch Gesamte Virusforsch 1965; 17: 313–329. [DOI] [PubMed] [Google Scholar]
- 12. Kato N & Eggers HJ. Inhibition of uncoating fowl plague virus by 1‐adamantanamine hydrochloride. Virology 1969; 37: 632–641. [DOI] [PubMed] [Google Scholar]
- 13. Pfau CJ, Trowbridge RS, Welsh RM, Staneck LD, O'Connell CM. Arenaviruses: Inhibition by amantadine hydrochloride. J Gen Virol 1972; 14: 209–211. [DOI] [PubMed] [Google Scholar]
- 14. Koff WC, Elm JL, Halstead SB. Inhibition of dengue virus replication by amantadine hydrochloride. Antimicrob Agents Chemother 1980; 18: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leibowitz JL & Reneker SJ. The effect of amantadine on mouse hepatitis virus replication. Adv Exp Med Biol 1993; 342: 117–122. [DOI] [PubMed] [Google Scholar]
- 16. Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber‐Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Eng J Med 1982; 307: 580–584. [DOI] [PubMed] [Google Scholar]
- 17. Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti‐influenza action of amantadine. EMBO J 1985; 4: 3021–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein; characterization of the amantadine block. J Virol 1993; 67: 5585–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith JP. Treatment of chronic hepatitis C with amantadine. Dig Dis Sci 1997; 42: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 20. Younossi ZM & Perillo RP. The roles of amantadine, rimantadine, ursodeoxycholic acid, and NSAIDs, alone or in combination with alpha interferons, in the treatment of chronic hepatitis C. Sem Liver Dis 1999; 19 (Suppl. 1): 95–102. [PubMed] [Google Scholar]
- 21. Tabone M, Ercole E, Zaffino C, Sallio Bruno F, Pera A, Bonino F. Amantadine hydrochloride decreases serum ALT activity without effects on serum HCV‐RNA in chronic hepatitis C patients. Ital J Gastroenterol Hepatol 1998; 30: 611–613. [PubMed] [Google Scholar]
- 22. Caronia S, Crossey M, Murray‐Lyon I et al. A pilot study of interferon plus amantadine versus interferon alone in the treatment of chronic hepatitis C. J Hepatol 1999; 30 (Suppl. 1): 255–255. [DOI] [PubMed] [Google Scholar]
- 23. Zeuzem S, Teuber G, Naumann U et al. Randomized, double‐blind, placebo‐controlled trial of interferon‐alfa2a with and without amantadine as initial treatment for chronic hepatitis C. Hepatology 2000; 32: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diago M, Garcia V, Valeros MD et al. Amantadine plus interferon in non reponders or relapsing chronic hepatitis C patients: end of treatment response. J Hepatol 1999; 30 (Suppl. 1): 139–139. [Google Scholar]
- 25. El Zayadi A, Selim O, Shawky S, Moustafa H, El‐Taweel A. A controlled study of amantadine montherapy vs. amantadine combined with interferon‐α in chronic hepatitis C patients nonresponders to interferon‐α. Hepatology 1999; 28 (Suppl. 1): 473A–473A. [Google Scholar]
- 26. Desmet VJ, Gerber MA, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994; 19: 1513–520. [PubMed] [Google Scholar]
- 27. Zeuzem S, Rüster B, Roth WK. Clinical evaluation of a new polymerase chain reaction assay (AmplicorTM HCV) for detection of hepatitis C virus. Z Gastroenterol 1994; 32: 342–347. [PubMed] [Google Scholar]
- 28. Lee JH, Roth WK, Zeuzem S. Evaluation and comparison of different hepatitis C virus genotyping and serotyping assays. J Hepatol 1997; 26: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 29. McNair DM, Lorr M, Droppleman LF. Profile of Mood States. Educational and Industrial Testing Service, San Diego., 1971.
- 30. Bullinger M, Helnisch M, Ludwig M, Geier S. Skalen zur Erfassung des Wohlbefindens. Psychometrische Analysen zum “Profile of Mood States“ (POMS) und “Psycohological general wellbeing index“ (PGWI). Z Differentielle Diagnostische Psychologie 1990; 11: 53–61. [Google Scholar]
- 31. Gandek B & Ware JE. SF‐36 Health Survey Manual and Interpretation Guide Boston: New England Medical Center, The Health Institute, 1993.
- 32. Bullinger M, Kirchberger I, Von Steinbüchel N. Der Fragebogen Alltagsleben – ein Verfahren zur Erfassung der gesundheitsbezogenen Lebensqualität. Z Med Psychologie 1993; 2: 121–131. [Google Scholar]
- 33. Martin J, Navas S, Fernández M et al. In vitro analysis of amantadine and interferon‐α‐2a on hepatitis C virus markers in cultured peripheral blood mononuclear cells from hepatitis C virus‐infected patients. Antiviral Res 1999; 42: 59–70.DOI: 10.1016/s0166-3542(99)00017-0 [DOI] [PubMed] [Google Scholar]
- 34. Brillianti S, Levantesi P, Masi L, Foli M, Bolondi L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology 2000; 32: 630–634. [DOI] [PubMed] [Google Scholar]
- 35. Krupp LB, Coyle PK, Doscher C et al. Fatigue therapy in multiple sclerosis: Results of a double‐blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 1995; 45: 1956–1961. [DOI] [PubMed] [Google Scholar]


