Abstract
Human rhinovirus (HRV) is a causative agent of acute respiratory tract infections. This study analyzed the prevalence and clinical characteristics of three HRV groups (HRV‐A, ‐B, and ‐C) among 1,165 children aged 14 years or younger who were hospitalized with acute lower respiratory tract infection in China. PCR or reverse transcription‐PCR was performed to detect 14 respiratory viruses in nasopharyngeal aspirates collected from September 2007 to August 2008 in Changsha, China. HRV was detected in 202 (17.3%) of the 1,165 children; 25.3% of the HRV‐positive children were 13–36 months of age (χ2 = 22.803, P = 0.000). HRV was detected year round and peaked between September and December. Fifty‐three percent of the HRV‐positive samples were also positive for other respiratory viruses; respiratory syncytial virus (RSV) was the most common secondary virus. Phylogenetic analysis using the VP4/VP2 region grouped the HRV‐positive strains as follows: 101 HRV‐A (50.0%), 21 HRV‐B (10.4%), and 80 HRV‐C (39.6%). HRV‐A infections occurred predominantly in spring and autumn, and the peak prevalence of HRV‐C was in early winter and late autumn. HRV‐B infections were less common in spring (χ2 = 31.914, P = 0.000). No significant difference in clinical severity or presentation was found between patients with HRV single infection and HRV co‐detections. Furthermore, the clinical characterizations did not differ among the three HRV species. These results suggest that HRV‐C is an important viral agent along with HRV‐A and HRV‐B and that among hospitalized children with acute lower respiratory tract infection in China, the three HRV genotypes have similar clinical characteristics. J. Med. Virol. 86:1983–1989, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: human rhinovirus, acute lower respiratory tract infections, child, China
INTRODUCTION
Acute respiratory tract infection is a major cause of death for children worldwide [Williams et al., 2002]. HRV is one of the most important viral agents of acute respiratory tract infections and has been frequently detected in upper and lower respiratory tract infections using modern molecular detection methods. Previous studies suggest that HRV is associated with an extensive range of human respiratory disorders including the common cold, viral bronchiolitis, exacerbations of asthma, and chronic obstructive pulmonary disease [Gern et al., 1997; Rakes et al., 1999; Seemungal et al., 2000; Johnston et al., 2005; Jackson et al., 2008;]. A recent study also showed that HRV was the most prevalent agent associated with severe bronchiolitis in a population of preterm infants [Miller et al., 2012].
HRV is a small non‐enveloped single‐stranded RNA virus that is now classified within the genus Enterovirus belonging to the family Picornaviridae. Classical HRV consists of more than 100 distinct serotypes. Until recently, on the basis of gene sequence analysis, HRV was classified into three species, HRV‐A, HRV‐B, and the newly designated HRV‐C [Lau et al., 2007; McErlean et al., 2007; Bizzintino et al., 2011]. Recent studies have suggested that the illness severity differs among HRV species [Khetsuriani et al., 2008; Calvo et al., 2010] and that HRV‐C could be associated with more severe clinical illnesses, including wheezing, lower respiratory infections and asthmatic exacerbations, than HRV‐A and HRV‐B [Lau et al., 2007; Khetsuriani et al., 2008; Han et al., 2009; Lau et al., 2009; Linsuwanon et al., 2009; Miller et al., 2009; Piralla et al., 2009; Bizzintino et al., 2011]. However, some reports have indicated that HRV‐C has been detected in healthy individuals without any acute respiratory symptoms [Calvo et al., 2010] and that there is no difference between the clinical presentations of patients infected with the different HRV species [Iwane et al., 2011]. Therefore, the clinical significance of HRV‐C remains controversial and needs to be addressed further.
In the present study, 1,165 children aged 14 years or younger with acute lower respiratory tract infection were included and screened for the three HRV species and other respiratory viruses to explore the impact and the epidemiological and clinical characteristics of HRV‐C infections in children with acute lower respiratory tract infection in Changsha, China.
MATERIALS AND METHODS
Patients and Clinical Specimens
Nasopharyngeal aspirates samples were collected from children hospitalized for acute lower respiratory tract infection at The People's Hospital of Hunan province, Changsha, China, during 2 days of each week between September 2007 and August 2008. All enrolled hospitalized patients were 14 years of age or younger and had symptoms of acute lower respiratory tract infection on admission. All nasopharyngeal aspirates were collected within 1–3 days of admission. Demographic data and details of the clinical findings were recorded. Informed consent was obtained from the parents of all children who provided specimens. The study protocol was approved by the hospital ethics committee. All nasopharyngeal aspirates were collected and transported immediately to the laboratory at the National Institute for Viral Disease Control and Prevention, China CDC, and stored at –80°C until further analysis.
Nucleic Acid Extraction
Total nucleic acids (DNA and RNA) were extracted from 140 µl of each nasopharyngeal aspirates using the QIAamp viral DNA and the QIAamp viral RNA Mini Kits (Qiagen, Shanghai, China) according to the manufacturer's instructions.
Molecular Detection of HRV
A primer pair targeting a 549‐bp fragment between the VP4/VP2 region and the 5′‐non‐coding region was used to amplify HRV. P1: 5′‐GGG ACC AAC TAC TTT GGG TGT CCG TGT‐3′ and P2: 5′‐GCA TCI GGY ARY TTC CAC CAC CAN CC‐3′, as described previously [Savolainen et al., 2002]. The cycling conditions for the PCR were 94°C for 8 min, followed by 35 cycles at 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s, with a final extension at 72°C for 8 min.
Detection of Other Respiratory Viruses
RSV, human metapneumovirus (HMPV), influenza virus (IFVA, IFVB), parainfluenza virus (PIV types 1–3), and human coronaviruses (229E, OC43, NL63, and HKU1) were screened using a standard reverse transcription‐PCR technique [Vabret et al., 2001; Bastien et al., 2005; Bellau‐Pujol et al., 2005; Vabret et al., 2006]. In addition, adenovirus (AdV) and human bocavirus (HBoV) were screened using PCR methods [Hierholzer et al., 1993; Allander et al., 2005].
Nucleotide Sequence Analysis
All PCR products were purified using the QIAquick PCR purification kit (Qiagen, Shanghai, China) and sequenced by SinoGenoMax (Beijing, China). All positive sequences were determined and analyzed using the DNASTAR software package. Phylogenetic analysis was performed with Mega version 3.1 by using 1,000 bootstrapped replicates and the neighbor‐joining algorithm with coxsackievirus (M17711) as the outgroup.
Statistical Analysis
The statistical significance of the differences among variables of groups was evaluated using the Chi‐squared test, Fisher's exact test, independent sample t‐test, Kruskal–Wallis H‐test or ANOVA. All analyses were performed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL).
RESULTS
Patient Characteristics
The age of 1,165 enrolled children ranged from 1 day to 156 months, with a median age of 15.4 months. Most of the specimens (1,124/1,165, 96.5%) were collected from patients under 60 months old, and the male to female ratio was 1.9–1 (763:402).
Detection of HRV and Other Viral Agents
At least one respiratory virus was detected in 871 of the 1,165 samples, and 202 (17.3%) were positive for HRV by PCR. HRV accounted for 23.2% of the total viral agents detected. Other respiratory viruses were detected in 107 of the 202 (53.0%) children who were HRV positive, including 51 with RSV (25.2%), 26 with HBoV (12.9%), 17 with PIV3 (8.4%), and some other viruses. RSV was the most common additional respiratory virus detected, accounting for 51 of the 107 (47.7%) co‐detections. Among the patients with co‐detections, 41.1% (83/202) were positive for HRV plus one additional viral agent and 11.9% (24/202) were positive for HRV plus two or three additional viruses; of these 11.9%, 18 patients were positive for three and six patients were positive for four viral agents. No significant difference was found between the patients with HRV single infection and HRV co‐detections in their epidemiological characteristics (age, gender, or duration of hospital stay), clinical presentations (cough, wheezing, fever, crackles, rhonchus, supplemental oxygen, underlying illness) or seasonal distribution (P > 0.05, Table I).
Table I.
Demographic Data and Clinical Symptoms in Children With HRV or HRV‐C Single Infections Compared With Co‐Detections
| HRV single infection (n = 95), no. (%) | HRV co‐detections (n = 107), no. (%) | P‐value | HRV‐C single infection (n = 38), no. (%) | HRV‐C co‐detections (n = 42), no. (%) | P‐value | |
|---|---|---|---|---|---|---|
| Male gender | 64 (67.4) | 68 (63.6) | 0.569 | 26 (68.4) | 30 (71.4) | 0.769 |
| Age in months | 0.171 | a | a | 0.554 | ||
| ≤12 | 40 (42.1) | 57 (53.3) | 15 (39.5) | 22 (52.4) | ||
| 12.1 to ≤36 | 48 (50.5) | 40 (37.4) | 20 (52.6) | 18 (42.8) | ||
| >36 | 7 (7.4) | 10 (9.3) | 3 (7.9) | 2 (4.8) | ||
| Median age in monthsb | 17.1 | 14.6 | 0.307 | 17.0 | 12.7 | 0.157 |
| Average duration of Hospitalization in daysb | 8.0 | 8.6 | 0.423 | 8.2 | 8.6 | 0.603 |
| Underlying diseasea | 6 (6.3) | 2 (1.9) | 0.151 | 2 (5.3) | 0 (0) | 0.222 |
| Symptoms and signs | ||||||
| Cougha | 92 (96.8) | 103 (96.3) | 1.000 | 38 (100) | 40 (95.2) | 0.495 |
| Wheezing | 49 (51.6) | 63 (58.9) | 0.297 | 24 (63.2) | 25 (59.5) | 0.739 |
| Fever | 26 (27.4) | 29 (27.1) | 0.966 | 11 (28.9) | 13 (31.0) | 0.845 |
| Rhonchus | 52 (54.7) | 59 (55.1) | 0.954 | 26 (68.4) | 24 (57.1) | 0.298 |
| Crackles | 75 (78.9) | 93 (86.9) | 0.131 | 31 (81.6) | 36 (85.7) | 0.617 |
| Gastrointestinal symptoms | 9 (9.5) | 17 (15.9) | 0.174 | 1 (2.6) | 6 (14.3) | 0.065 |
| Supplemental oxygen | 18 (18.9) | 13 (12.1) | 0.181 | 7 (18.4) | 4 (9.5) | 0.249 |
| Seasonal distribute | 0.206 | a | a | 0.885 | ||
| Spring | 29 (30.5) | 32 (29.9) | 9 (23.7) | 11 (26.2) | ||
| Summer | 9 (9.5) | 16 (15.0) | 2 (5.3) | 3 (7.1) | ||
| Autumn | 40 (42.1) | 32 (29.9) | 14 (36.8) | 12 (28.6) | ||
| Winter | 17 (17.9) | 27 (25.2) | 13 (34.2) | 16 (38.1) | ||
Fisher's exact test.
Independent samples t‐test.
All the other: Chi‐squared test. Seasonal distribution: Spring (March–May), Summer (June–August), Autumn (September–November), Winter (December–February).
Epidemiology of HRV Infection
Seventeen point three percent (132/763) of the males and 17.4% (70/402) of the females had HRV detected in this study. Of those with HRV, 65.3% (132/202) were male and 34.7% female. The male to female ratio was not significantly different between the patients with and without HRV infections (χ2 = 0.002, P = 0.961). The ages of the 202 HRV‐positive children ranged from 1 day to 156 months (mean age ± SD, 15.8 ± 17.8 months) and 91.6% (185/202) were ≤36 months of age. Children 13–36 months of age had the highest infection rate (25.3%). The HRV infection rates were significantly different between the age groups (χ2 = 22.803, P = 0.000; Fig. 1). HRV could be detected throughout the year; however, the majority of the cases occurred between September and December of 2007 and in April of 2008. The number of positive specimens peaked in November 2007 (36.40%; Fig. 2).
Figure 1.
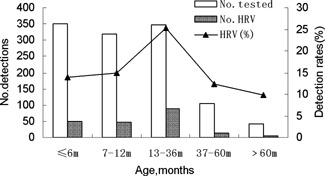
Age distribution of HRV in children with ALRTIs during a 1‐year study period.
Figure 2.
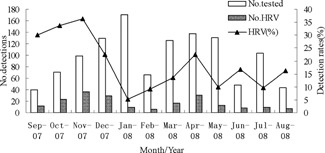
Seasonal distribution of HRV in children with ALRTIs, September 2007 to October 2008.
Clinical Characteristics of HRV in Children
Clinical symptom information was available for all of the HRV‐positive subjects. The main clinical diagnoses of patients who were HRV‐positive included bronchitis (5, 2.5%), bronchiolitis (44, 21.8%), acute asthmatic bronchopneumonia (16, 7.9%), and pneumonia (137, 67.8%). The most common symptom was cough, which occurred in 195 patients (96.5%). Other clinical presentations included wheezing (n = 112, 55.4%), fever (n = 55, 27.2%), and gastrointestinal symptoms (n = 26, 12.9%). Crackles and rhonchus were common pulmonary symptoms in children with HRV infection. The duration of the hospital stays ranged from 1 to 56 days (mean ± SD, 8.3 ± 4.8 days).
Phylogenetic Analysis of HRV
Among the 202 HRV strains, phylogenetic analysis of the viral protein VP4/VP2 coding regions indicated that 101 (50.0%) were classified as genetic group A, 21 (10.4%) as genetic group B, and 80 (39.6%) as a separate cluster, HRV‐C. The nucleotide and deduced amino acid sequences of the VP4/VP2 gene of 188 HRV specimens were compared with those of HRV strains available at the GenBank site. Outgroup rooting was used for phylogeny with coxsackievirus (M17711). The GenBank accession numbers of the previously published sequences are as follows: HRV‐A (DQ473509), HRV‐A (DQ473507), HRV‐97 (AY040242), HRV‐B (EU081787), HRV‐C (EF186077), and HRV‐C (EF582386; Fig. 3).
Figure 3.
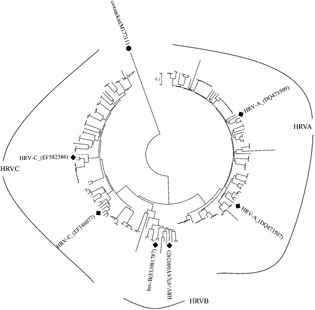
Phylogenetic analysis of the VP4/VP2 region of the 188 HRV strains from nasopharyngeal aspirates. Phylogenetic trees were constructed by the neighbor‐joining method using MEGA3.1 and coxsackievirus (M17711) is indicated as the outgroup.The viral sequences in black solid rhombus were generated from GenBank; other reference sequences were obtained from the present study.
Epidemiological and Clinical Characteristics of Three HRV Genotypes
The HRV‐A infections occurred predominantly in the spring and autumn (April 2008, 24 samples; October and November 2007, 14 and 15 samples, respectively), and the peak prevalence of HRV‐C was in the early winter and late autumn (December and November, 20 and 16 samples, respectively). HRV‐B infections rarely occurred in the spring. A significant difference was observed between the seasonal prevalence of HRV‐A, HRV‐B and HRV‐C (χ2 = 31.914, P = 0.000). No significant differences were observed between HRV‐A, HRV‐B and HRV‐C with regard to the epidemiological characteristics (age, gender, or duration of hospital stay), clinical diagnoses (bronchitis, bronchiolitis, acute asthmatic bronchopneumonia, and pneumonia), or other clinical presentations (cough, wheezing, fever, crackles, rhonchus, supplemental oxygen, underlying illness, and co‐detection) (P > 0.05, Table II). Additionally, no significant differences were found between the epidemiological characteristics or clinical presentations of the patients with HRV‐C single infection and patients with HRV‐C co‐detections (P > 0.05, Table I).
Table II.
Clinical Characteristics of Patients Infected With Different HRV Species From September 2007 Through August 2008
| HRV‐A (n = 101), no. (%) | HRV‐B (n = 21), no. (%) | HRV‐C (n = 80), no. (%) | χ2 | P‐value | |
|---|---|---|---|---|---|
| Male gender | 62 (61.4) | 14 (66.7) | 56 (70.0) | 1.481 | 0.477 |
| Age in months | 5.436 | 0.245 | |||
| ≤12 | 53 (52.5) | 7 (33.3) | 37 (46.2) | ||
| 12.1 to ≤36 | 40 (39.6) | 10 (47.6) | 38 (47.5) | ||
| >36 | 8 (7.9) | 4 (19.1) | 5 (6.3) | ||
| Median age in monthsa | 15.1 | 23.3 | 14.7 | 1.901a | 0.387 |
| Average duration of hospitalization in daysb | 8.4 | 7.7 | 6.7 | 0.186b | 0.830 |
| Coinfection | 52 (51.5) | 13 (61.9) | 42 (52.5) | 0.769 | 0.681 |
| Underlying diseasec | 5 (5.0) | 1 (4.8) | 2 (2.5) | 0.991 | 0.572 |
| Symptoms and signs | |||||
| Coughc | 98 (97.0) | 19 (90.5) | 78 (97.5) | 2.583c | 0.253 |
| Wheezing | 51 (50.5) | 12 (57.1) | 49 (61.3) | 2.118 | 0.347 |
| Fever | 26 (25.7) | 5 (23.8) | 24 (30.0) | 0.547 | 0.761 |
| Rhonchus | 49 (48.5) | 12 (57.1) | 50 (62.5) | 3.573 | 0.168 |
| Crackles | 83 (82.2) | 18 (85.7) | 67 (83.8) | 0.187 | 0.911 |
| Gastrointestinal symptoms | 17 (16.8) | 2 (9.5) | 7 (8.8) | 2.834 | 0.242 |
| Supplemental oxygen | 14 (13.9) | 6 (28.6) | 11 (13.8) | 3.156 | 0.206 |
| Diagnosisc | 11.286c | 0.056 | |||
| Bronchitis | 4 (4.0) | 1 (4.8) | 0 (0) | ||
| Bronchopneumonia | 18 (17.8) | 8 (38.1) | 18 (22.5) | ||
| Pneumonia | 73 (72.3) | 9 (42.9) | 55 (68.7) | ||
| Acute asthmatic | |||||
| Bronchopneumonia | 6 (5.9) | 3 (14.3) | 7 (8.8) | ||
| Seasonal distribute | 31.914 | 0.000 | |||
| Spring | 40 (39.6) | 1 (4.8) | 20 (25.0) | ||
| Summer | 13 (12.9) | 7 (33.3) | 5 (6.3) | ||
| Autumn | 36 (35.6) | 10 (47.6) | 26 (32.5) | ||
| Winter | 12 (11.9) | 3 (14.3) | 29 (36.2) | ||
Kruskal–Wallis H‐test.
ANOVA.
Fisher's exact test, All the other: Chi‐square test; Seasonal distribution: Spring (March–May), Summer (June–August), Autumn (September–November), Winter (December–February).
DISCUSSION
In the present study, HRV was detected in 202 out of 1,165 (17.3%) nasopharyngeal aspirates collected from The People's Hospital of Hunan province from September 2007 to August 2008. A similar detection rate was reported in Lanzhou, China (13.1%) [Jin et al., 2009]. However, the study rate is lower than 30% detection rate reported in a study of hospitalized pediatric patients diagnosed with acute lower respiratory illness in Thailand [Linsuwanon et al., 2009] and is also lower than the 29.7% detection rate found in a study of hospitalized children <18 years of age in Hong Kong [Lau et al., 2009]. The present study indicates that HRV‐A (50.0%) and HRV‐C (39.6%) may be more prevalent than HRV‐B (10.4%). Similar results have been reported in Hong Kong, Italy, Thailand, and Jordan, where the percentages of HRV‐C were 43.8%, 41.1%, 58%, and 26%, respectively [Lau et al., 2009; Linsuwanon et al., 2009; Miller et al., 2009; Piralla et al., 2009]. This shows that HRV‐C has been found in patients from various countries and plays an important role in respiratory tract infections worldwide.
The HRV‐positive ratio was highest (33.3%) in children <12 months of age in the Hong Kong study [Lau et al., 2009]. Similarly, 64% of the infected children were younger than 6 months in a study in Jordan [Miller et al., 2009]. In addition, in a study in Thailand, most HRV‐positive specimens were from children 6 to 23 months of age [Linsuwanon et al., 2009]. In a 21‐year study, HRV was more frequently detected in younger children and infants than in older children [Linder et al., 2013]. A recent case‐control study found that both the HRV‐A and HRV‐C detection rates were significantly higher in young children hospitalized for acute respiratory illnesses than in asymptomatic controls [Iwane et al., 2011]. In the present study, 91.6% (185/202) of the HRV‐positive individuals were ≤36 months old, and the highest infection rate was observed in children 13–36 months old (25.3%). Parents of younger children may take children to the doctor more often than parents of older children. In addition, only children hospitalized with acute lower respiratory tract infection were included in the study. Further studies are necessary to determine why age is associated with HRV infections.
In some studies that included clinical specimens collected throughout the year, HRV‐C appears to show seasonal patterns of infection. In Hong Kong, a subtropical city, infections caused by HRV, including HRV‐C, occur throughout the year, although a higher incidence has been observed during the fall and winter months for all three HRV species [Lau et al., 2007, 2009]. One study suggested that HRV, including HRV‐C, could be found throughout the year but that it predominates during the rainy season in the pediatric population in Thailand [Linsuwanon et al., 2009]. In Kenya, there was no clear seasonal pattern of occurrence for any species [Onyango et al., 2012], while the number of HRV‐C infections peaked in early winter (December) and late spring (April) in Lanzhou, China [Jin et al., 2009]. A recent 21‐year study suggested that HRV‐C was found most commonly during the winter months [Linder et al., 2013]. In this study, HRV was detected throughout the year and peaked in the winter and spring, and the HRV‐C prevalence peaked in early winter and late autumn. These data suggest that HRV may follow different epidemiological patterns in different regions and that different genotypes may present in different seasons.
Other respiratory viruses were co‐detected frequently in the HRV‐positive patients. In a recent study involving patients from Thailand, other respiratory viruses were co‐detected in approximately 38% of the HRV‐positive patients; of these patients, 40% were co‐infected with HRV‐C and 36% were infected with HRV/RSV [Linsuwanon et al., 2009]. RSV and HRV were the viruses identified most frequently in mixed infections in infants hospitalized with bronchiolitis [Richard et al., 2008]. Of those with HRV in the present study, 53.0% were co‐detected with other respiratory viruses and 41.1% with HRV‐C. RSV (47.7%) was the most common additional respiratory virus detected in the HRV‐positive patients. These findings were in agreement with a report from Thailand. Children with HRV mono‐infection versus HRV co‐detection with other viruses had similar clinical courses, comparable to those in some US studies [Iwane et al., 2011; Miller et al., 2011]. One study suggested co‐infections were not associated with a particular HRV species or with severity [Lauinger et al., 2013]. No significant differences in the clinical severity were found between patients with HRV single infection and those with HRV co‐detections. No significant differences in median age, gender, symptomatology (fever, wheezing, cyanosis, and crackles), supplemental oxygen, and time hospitalized were observed between children infected with HRV‐C only and those with HRV‐C co‐detections.
Previous studies have suggested that HRV‐C might play a role in severe clinical disease. HRV‐C was present in the majority of children with acute asthma and was associated with more severe asthma [Bizzintino et al., 2011]. In another study by Miller et al. [2009], children with HRV‐C were more likely to require supplemental oxygen than those with HRV‐A. Wheezing episodes were also more common among individuals with HRV‐C and HRV‐A infection than among those with HRV‐B infection [Lau et al., 2009]. It was found that HRV‐C was associated with respiratory infections with few symptoms and could trigger apparently life‐threatening events in young infants [Calvo et al., 2009]. Another study suggested that HRV‐C was associated with more severe disease in children <3 years of age [Lauinger et al., 2013]. However, some reports found no differences in the clinical characteristics among hospitalized enrolled patients positive for HRV‐A, HRV‐B, or HRV‐C, including wheezing [Fry et al., 2011; Iwane et al., 2011]. In the present study, no significant differences were observed in clinical symptoms, signs, and clinical diagnoses including wheezing, supplemental oxygen and acute asthmatic bronchopneumonia among the three HRV genotypes. With the absence of a control group in both the current and previous studies, it is difficult to evaluate the exact role that HRV‐C plays in acute lower respiratory tract infection.
The present study showed that HRV‐C is an important viral agent, along with HRV‐A and HRV‐B, in children with acute lower respiratory tract infection in China. HRV was detected throughout the year and peaked in the winter and spring. The majority of HRV‐positive individuals were ≤36 months old, and HRV‐C was mainly epidemic during early winter and late autumn. The results do not support those of previous studies, which showed differences in the clinical symptoms, signs, and clinical diagnoses among the three HRV genotypes. Additional studies with healthy controls are needed to completely define the epidemiological and clinical characteristics and genetic characterization of HRV‐C with HRV‐A and HRV‐B.
Conflicts of interest: none.
REFERENCES
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 102:12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien N, Anderson K, Hart L, Van Caeseele P, Brandt K, Milley D, Hatchette T, Weiss EC, Li Y. 2005. Human coronavirus NL63 infection in Canada. J Infect Dis 191:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellau‐Pujol S, Vabret A, Legrand L, Dina J, Gouarin S, Petitjean‐Lecherbonnier J, Pozzetto B, Ginevra C, Freymuth F. 2005. Development of three multiplex RT‐PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 126:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, McMinn PC, Goldblatt J, Gern JE, Le Souëf PN. 2011. Association between human rhinovirus c and severity of acute asthma in children. Eur Respir J 37:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C, Garcia ML, Pozo F, Reyes N, Pérez‐Breña P, Casas I. 2009. Role of rhinovirus C in apparently life‐threatening events in infants, Spain. Emerg Infect Dis. 15:1506–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C, Casas I, Garcia‐Garcia ML, Pozo F, Reyes N, Cruz N, García‐Cuenllas L, Pérez‐Breña P. 2010. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J. 29:717–720. [DOI] [PubMed] [Google Scholar]
- Fry AM, Lu X, Olsen SJ, Chittaganpitch M, Sawatwong P, Chantra S, Baggett HC, Erdman D. 2011. Human rhinovirus infections in rural Thailand: Epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE 6:e17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. 1997. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med 155:1159–11161. [DOI] [PubMed] [Google Scholar]
- Han TH, Chung JY, Hwang ES, Koo JW. 2009. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch Virol 154:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer JC, Halonen PE, Dahlen PO, Bingham PG, McDonough MM. 1993. Detection of adenovirus in clinical specimens by polymerase chain reaction and liquid‐phase hybridization quantitated by time‐resolved fluorometry. J Clin Microbiol 31:1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, Griffin MR, Staat MA, Anderson LJ, Williams JV, Weinberg GA, Ali A, Szilagyi PG, Zhu Y, Erdman DD. 2011. Human Rhinovirus Species Associated With Hospitalizations for Acute Respiratory Illness in Young US Children. J Infect Dis 204:1702–1710. [DOI] [PubMed] [Google Scholar]
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson‐Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF Jr. 2008. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med 178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Yuan XH, Xie ZP, Gao HC, Song JR, Zhang RF, Xu ZQ, Zheng LS, Hou YD, Duan ZJ. 2009. Prevalence and clinical characterization of a newly identified human rhinovirus c species in children with acute respiratory tract infections. J Clin Microbiol 47:2895–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, Roy M, Waserman S, Sears MR. 2005. The september epidemic of asthma exacerbations in children: A search for etiology. J Allergy Clin Immunol 115:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetsuriani N, Lu X, Teague WG, Kazerouni N, Anderson LJ, Erdman DD. 2008. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis 14:1793–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected hrv species, hrv‐c, associated with acute respiratory illness in children. J Clin Microbiol 45:3655–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Yip CC, Lin AW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2009. Clinical and molecular epidemiology of human rhinovirus c in children and adults in hong kong reveals a possible distinct human rhinovirus c subgroup. J Infect Dis 200:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauinger IL, Bible JM, Halligan EP, Bangalore H, Tosas O, Aarons EJ, MacMahon E, Tong CY. 2013. Patient characteristics and severity of human rhinovirus infections in children. J Clin Virol 58:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JE, Kraft DC, Mohamed Y, Lu Z, Heil L, Tollefson S, Saville BR, Wright PF, Williams JV, Miller EK. 2013. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol 131:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, Poovorawan Y. 2009. High prevalence of human rhinovirus c infection in Thai children with acute lower respiratory tract disease. J Infect 59:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. 2007. Characterisation of a newly identified human rhinovirus, hrv‐qpm, discovered in infants with bronchiolitis. J Clin Virol 39:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Khuri‐Bulos N, Williams JV, Shehabi AA, Faouri S, Al Jundi I, Chen Q, Heil L, Mohamed Y, Morin LL, Ali A, Halasa NB. 2009. Human rhinovirus c associated with wheezing in hospitalised children in the middle east. J Clin Virol 46:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Williams JV, Gebretsadik T, Carroll KN, Dupont WD, Mohamed YA, Morin LL, Heil L, Minton PA, Woodward K, Liu Z, Hartert TV. 2011. Host and viral factors associated with severity of human rhinovirus‐associated infant respiratory tract illness. J Allergy Clin Immunol 127:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Bugna J, Libster R, Shepherd BE, Scalzo PM, Acosta PL, Hijano D, Reynoso N, Batalle JP, Coviello S, Klein MI, Bauer G, Benitez A, Kleeberger SR, Polack FP. 2012. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics 129:e60–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango CO, Welch SR, Munywoki PK, Agoti CN, Bett A, Ngama M, Myers R, Cane PA, Nokes DJ. 2012. Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J Med Virol 84:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piralla A, Rovida F, Campanini G, Rognoni V, Marchi A, Locatelli F, Gerna G. 2009. Clinical severity and molecular typing of human rhinovirus c strains during a fall outbreak affecting hospitalized patients. J Clin Virol 45:311–317. [DOI] [PubMed] [Google Scholar]
- Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts‐Mills TA, Heymann PW. 1999. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 159:785–790. [DOI] [PubMed] [Google Scholar]
- Richard N, Komurian‐Pradel F, Javouhey E, Perret M, Rajoharison A, Bagnaud A, Billaud G, Vernet G, Lina B, Floret D, Paranhos‐Baccalà G. 2008. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J 27:213–217. [DOI] [PubMed] [Google Scholar]
- Savolainen C, Mulders MN, Hovi T. 2002. Phylogenetic analysis of rhinovirus isolates collected during successive epidemic seasons. Virus Res 85:41–46. [DOI] [PubMed] [Google Scholar]
- Seemungal TA, Harper‐Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. 2000. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 16:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. 2001. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods 97:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A, Dina J, Gouarin S, Petitjean J, Corbet S, Freymuth F. 2006. Detection of the new human coronavirus HK U1: A report of 6 cases. Clin Infect Dis 42:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. 2002. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2:25–32. [DOI] [PubMed] [Google Scholar]


