Abstract
Background
The quantitative effect of strong electrolytes, unmeasured anions (UAs), p CO 2, and plasma protein concentrations in determining plasma pH and bicarbonate (HCO 3 −) can be demonstrated using the physicochemical approach. Demeanor of calves with diarrhea is associated with acidemia, dehydration, and hyper‐d‐lactatemia.
Hypothesis
Unmeasured anions are a major factor influencing changes in plasma pH and HCO 3 − of calves with diarrhea and UAs and strong UAs, estimated by anion gap (AG) and strong ion gap (SIG), respectively, are more strongly associated with alteration of demeanor compared to other acid–base variables.
Animals
A total of 264 calves with diarrhea from two data sets (DS1 and DS2).
Methods
Retrospective study. Forward stepwise regression was performed to determine the relationship between measured pH or HCO 3 −, and physicochemical variables. A two‐way ANOVA was performed to investigate the association between acid–base variables and attitude (bright, obtunded, and stuporous), posture (standing, sternal or lateral recumbency), and strength of suckling reflex (strong, weak, or absent).
Results
Increased strong UAs estimated by SIG was the most important contributor to changes in measured pH and HCO 3 − (DS1: r 2 66 and 59%, DS2: 39 and 42%, P < .0001). SIG and AG were correlated to deteriorating calf demeanor for all three clinical scoring categories: attitude, posture, and suckle reflex (P < .0001).
Conclusion and Clinical Relevance
Elevated concentrations of strong UAs were the primary cause of acidemia and had an important influence on the demeanor of calves with diarrhea. These findings emphasize the importance of the calculation of UAs when evaluating acid–base abnormalities in calves.
Keywords: Anion gap, d‐lactate, Henderson–Hasselbalch, l‐lactate, Strong ion difference, Strong ion gap
Abbreviations
- A−
total net negative charge of blood proteins
- AG
anion gap
- Atot
total plasma concentration of nonvolatile weak acids
- BE(ecf)
base excess extracellular fluid
- d‐lac−
d‐lactate
- HCO3−
bicarbonate
- H–H
Henderson–Hasselbalch approach
- l‐lac−
l‐lactate
- mmol
millimoles
- pCO2
partial carbon dioxide pressure
- SID
strong ion difference
- SIG
strong ion gap
- TP
total protein
- UAs
unmeasured anions
Neonatal diarrhea is a major cause of illness and death in calves less than 1 month of age.1 Diarrhea can lead to death from dehydration, acidemia, hyperkalemia, and impaired cardiovascular and renal function, regardless of the causative pathogens or pathophysiologic mechanisms.2, 3, 4 Historically the most commonly stated causes of metabolic acidosis in diarrheic calves were fecal bicarbonate loss, electrolyte and water loss, and l‐lactic acidosis.5, 6 More recently, acidemia in sick calves, with or without diarrhea, was primarily attributed to hyponatremia accompanied by normochloremia or hyperchloremia, increased strong unmeasured anions particularly d‐lactate ([d‐lac−]), and to a lesser extent increased plasma protein concentrations.7
Two approaches have been used to evaluate acid‐base imbalances, the Henderson–Hasselbalch approach (H–H)8 and more recently the physicochemical approach.9, 10 The H–H centers on plasma bicarbonate ([HCO3 −]) and extracellular base excess (BE(ecf)) concentrations as indicators of the metabolic components of acid–base balance. This approach is clinically useful for assessing acid–base disorders; but it is more descriptive than mechanistic and ignores the importance of relative strong electrolyte and protein concentrations in the determination of acid–base balance.11, 12 The quantitative physicochemical approach emphasizes the importance of strong electrolytes (Na+, K+, Cl−), strong unmeasured anions (including l‐lactate− [l‐lac−] and d‐lactate [d‐lac−]), pCO2, and the total plasma protein concentration (Atot) in determining plasma pH and [HCO3 −].9 This approach is mechanistic and provides additional information regarding the pathophysiology of acid–base imbalances.
Altered demeanor is associated with the degree of hypoglycemia, metabolic acidosis, hypothermia and dehydration. Hypoglycemia is a concurrent disease in calves with acute severe diarrhea.13, 14 Hypoglycemia is associated with alteration of demeanor such as impaired concentration, focal neurological deficits, confusion, drowsiness, coma, seizure, and neuronal death in different species.13, 14
Assessment of the degree of metabolic acidosis on the basis of clinical signs has been investigated by several authors.3, 5, 15, 16, 17, 18 A calf's demeanor is influenced by the severity of metabolic acidosis, hypothermia and degree of dehydration. Furthermore, dehydration correlates with blood [l‐lac−] concentration.19 There is an association between BE(ecf) and [HCO3 −] and changes in the posture, behavior, and suckling reflex of calves with diarrhea.15, 20 Increased serum concentration of [d‐lac−] occurs in calves with diarrhea with or without signs of dehydration.21, 22, 23 The variations in behavior and posture of diarrheic calves can be better explained by an increase in serum [d‐lac−] rather than by a decrease in BE(ecf).24 In support of this, alterations in behavior and posture, impairment of the palpebral reflex, somnolence, and staggering gait occurs after intravenous administration of sodium [d‐lac−] or isotonic d‐l‐lactate to healthy calves25; whereas experimentally induced severe, uncomplicated, hyperchloremic metabolic acidosis does not result in changes in posture or mental status.26 These studies indicate that metabolic acidosis without an appreciable increase in [d‐lac−] had only a minor influence on posture and behavior.18 The routine measurement of plasma [d‐lac−] is not currently a common practice in veterinary medicine. Clinicians assume that other frequently calculated acid–base parameters such as anion gap (AG) are sufficient for predicting changes in the concentration of the unmeasured anions. A useful clinical application of the physicochemical approach is calculation of the strong ion gap (SIG). SIG represents the difference between unmeasured strong ion cation concentration and unmeasured strong ion anion concentration in plasma or serum. SIG treats total plasma protein and phosphate concentration as variables, whereas AG assumes they are constants.11 Both AG and SIG were intended to identify the presence of increased concentrations of unmeasured anions such as d‐ and l‐lactate and uremic acids.27
We hypothesized that increased plasma concentration of unmeasured anions are the major independent variable influencing changes in measured plasma pH and calculated plasma HCO3 − and that UAs and strong UAs, estimated by AG and SIG, respectively, are more strongly associated with alteration of demeanor in calves with diarrhea compared to other acid–base variables. The objectives of this study were (1) to determine and characterize the mechanism of acid–base disorders in calves with diarrhea using the physiochemical approach and (2) to investigate the correlation between the degree of metabolic acidosis, using parameters from the H–H and physicochemical approaches, and alteration in calf demeanor in a population of calves with naturally occurring diarrhea.
Materials and Methods
Study Population
The analysis was based on 2 data sets (DS1 and DS2). DS1 was derived from 171 calves ≤28 days of age that were admitted to the Atlantic Veterinary College Teaching Hospital (AVC‐TH) with a primary complaint of diarrhea between 1989 and 1993, and that had venous blood gas and biochemical profile values determined upon admission.28 DS2 consisted of data extracted from the medical records of 83 calves ≤28 days of age, admitted to the AVC‐TH between January 2000 and December 2010, with a diagnosis of neonatal diarrhea, and that had blood gas analyses and biochemistry profiles performed upon admission.
Data Collection
Medical records were systematically reviewed and the following signalment information was recorded: breed, sex, and age in weeks (week 1: calves ≤7 days old, week 2: calves 8–14 days old, etc.). Recorded physical examination findings were as follows: attitude (bright, obtunded, or stuporous), posture (standing, sternal or lateral recumbence), suckle reflex (strong, weak, or absent), heart rate (HR) as beats/minute (bpm), respiratory rate (RR) as breaths/minute (rpm), injection of the sclera, rectal temperature (T) in degrees Celsius (C), and state of dehydration estimated based on clinical signs by the attended clinician (5–6%, mild dehydration; 7–9%, moderate dehydration; >10%, severe dehydration). The following clinicopathologic data were extracted: p vCO2 (mmHg), pH, [HCO3 −] (mmol/L), BE(ecf) (mmol/L), [Na+] (mmol/L), [K+] (mmol/L), [Cl−] (mmol/L), [TP] (g/L), and creatinine concentration (μmol/L). An antemortem diagnosis of septicemia in a diarrheic calf was based on any one or more the following criteria28: (1) positive blood culture; (2) culture of the same bacterial agent from ≥2 body fluids; and (3) culture of bacterial agent from a single joint in a calf with joint effusion involving multiple joints. A postmortem diagnosis of septicemia was based on the presence of one or more of the following criteria: (1) morphologic changes such as multiple disseminated abscesses of similar size, purulent vasculitis and intravascular identification of bacteria, or fibrin in multiple body cavities; (2) bacterial isolation from heart blood; or (3) recovery of the same bacterial organism from ≥2 body tissues (excluding intestine). The pathogens causing diarrhea were also recorded. Duration of hospitalization was based on either the number of days until the calf was discharged from the hospital or on the number of days until death. Survival was defined as discharge from the hospital.
Measurement Techniques
A radiometer ABL‐11 blood gas machine with autocalibrating systems and a portable IRMA true point blood gas analyzer2 were used for blood gas and pH analysis for DS1 and DS2, respectively. Serum biochemical profile and electrolytes analyses were performed using a Coulter Dacos analyzer3 and a Copas 6000 C501 automated multianalyzer4 system for DS1 and DS2, respectively. Total protein concentration was measured using refractometry.
Calculations
The H–H's equation was used to calculate [HCO3 −] (mmol/L) and BE(ecf) (mmol/L) from the measured values for venous pH and pCO2, and assigned values for S of 0.0306 mmol/L/mmHg and pK1 of 6.099 mmol/L and for S of 0.0307 mmol/L/mmHg and pK1′ of 6.095 for DS1 and DS2, respectively. Therefore, [HCO3 −] and BE(ecf) were calculated as:
| (1) |
| (2) |
BE(ecf) assumes a fixed hemoglobin concentration of 50 g/L.4
Determination of the strong ion difference (SID) requires identification and accurate measurement of all strong ions in plasma, including ideally the measurement of unmeasured strong anions. SID has two components: measured SID, which was calculated from the measured plasma concentrations of 3 strong ions (Na+, K+, Cl−) as9:
| (3) |
and unmeasured SID (SIDum) calculated as:
| (4) |
using assigned values for S of 0.0307 mmol/L/mmHg and pK1′ of 6.120.7
A useful clinical application of the simplified SID approach is the calculation of strong ion gap (SIG), which provides an estimate for the difference between the ion charge of plasma nonvolatile buffers and unmeasured strong anion concentration.7 Rearrangement of the Equation (4) and SIG can be substituted for SIDum as7:
| (5) |
Anion gap (AG) was calculated as29:
| (6) |
Total plasma concentration of weak acids (Atot) was calculated as:
| (7) |
where total protein is in grams per liter.7
Total negative charge of the plasma proteins A− (mmol/L) were calculated as:
| (8) |
where pKa (7.08) is the effective dissociation constant of bovine plasma weak acids.7
Definitions
The definition of acid–base disorders by using the H–H and physicochemical approaches was presented in the Table 1.7, 30
Table 1.
Definition of acid–base disorders using Henderson–Hasselbalch (H–H) and physicochemical (SID) approach.
| Approach | Type of Disorder | Parameter7, 27 | Acidosis | Alkalosis |
|---|---|---|---|---|
| H–H | Respiratory | p vCO2 [34–45 mmHg] | ↑pvCO2 | ↓p vCO2 |
| Metabolic | HCO3 − [20–30 mmol/L] | ↓HCO3 − | ↑HCO3 − | |
| AG <20 mmol/L | ↑AG | N/A | ||
| SID | Respiratory | p vCO2 [34–45 mmHg] | ↑p vCO2 | ↓p vCO2 |
| Metabolic | SID3 [38–44 mmol/L] | ↓SID3 | ↑SID3 | |
| Atot [19–25 mmol/L] | ↑Atot | ↓Atot | ||
| SIG [3 to −3 mmol/L] | ↓SIG | N/A |
Modified from Constable.11 p vCO2, partial carbon dioxide pressure; HCO3 −, bicarbonate; AG, anion gap; SID3, strong ion difference measured; SIG, strong ion gap; Atot, total plasma concentration of nonvolatile weak acids.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Statistical Analyses
Data were presented as a mean and SD. Non‐normal distributed data were presented as a median and quartiles 25 and 75%. Normality of the data was tested by the Kolmogorov–Smirnov test. Forward stepwise regression (P < .05 to enter and exit) was performed to determine the relationship between measured plasma pH and SID3, SIG, pCO2, and Atot for DS1 and DS2, and to determine the relationship between calculated plasma HCO3 − and SID3, SIG, pCO2, and Atot for DS1 and DS2. The assumptions of the forward stepwise regression procedure were evaluated by examining the residual plots. The results of the regression were confirmed using backward elimination. To evaluate the association between the acid–base variables and alteration of calf demeanor, both DS1 and DS2 were compiled as a single data set. Before combining them, the 2 data sets were analyzed separately and residual standard deviation of the variables of interest was compared and was found to be similar. A two‐way ANOVA was performed to investigate the association between acid–base variables from the H–H (pCO2, BE(ecf), [HCO3 −] and AG) and the physicochemical approach (pCO2, SID3, SIG and Atot), and attitude, posture, and strength of the suckle reflex. The additive effect of DS1 and DS2 also was investigated in the ANOVA test but it did not have statistical significance (P > .05). Bonferroni correction for pairwise comparison was performed when indicated. The appropriateness of the results of the ANOVA test was evaluated by the examination of residual plots. A P‐value <.05 was considered significant. Statistical analyses were performed using statistical software.5
Results
Study Population
Of the 264 calves, 51% were female, and 49% were male. Breed distribution was similar to that for the general hospital population, which included both beef and dairy calves in both data sets. The median age of calves was 8 days (range 1–28 days). Cases were presented during all 12 months of the year, with the highest numbers seen between January and April. A final etiologic diagnosis of the diarrhea was achieved in 137 (52%) calves. Enterotoxigenic Esherichia coli was the causative agent of diarrhea in 9% (n = 11) of calves less than 5 days of age. Coronavirus was detected in 29% (n = 38) of calves and a total of 26% (n = 34) of calves tested positive for Cryptosporidium spp. One calf tested positive for Salmonella spp, whereas 2% (n = 7) were positive for Eimeria spp. Forty‐six (33%) calves with diarrhea tested positive for more than one pathogen (Rotavirus and Coronavirus n = 15, Rotavirus and Cryptosporidium spp. n = 9, and Coronavirus and Cryptosporidium spp. n = 22).
Physical Examination Findings, Acid–Base, and Electrolyte Abnormalities and Outcome
All calves had diarrhea at the time of hospitalization. Median rectal temperature was 38.5°C (Q25–Q75, 37.1–39.3°C), median HR was 120 bpm (Q25–Q75,100–136 bpm), median RR was 32 rpm (Q25–Q75, 24–48 rpm). Eight percent (n = 20) of calves were considered to have normal hydration status, 26% (n = 69) calves had 5–6% dehydration, 33% (n = 87) had 7–9% of dehydration, and 33% (n = 88) calves had dehydration >10%. Evaluation of the posture of the calves revealed that 33% (n = 88) were standing, 36% (n = 92) were in sternal recumbence, and 31% (n = 84) were in lateral recumbence. Attitude assessment showed that 17% (n = 44) of the calves were bright, 64% (n = 169) were obtunded, and 19% (n = 51) were stuporous. Evaluation of the suckle reflex showed that 23% (n = 62) of calves had a strong suckle reflex, 42% (n = 110) had a weak suckle reflex, and 35% (n = 92) had an absent suckle reflex. The values for the venous blood gas analysis, plasma concentration of electrolytes and plasma proteins, and H–H and physicochemical variables of the 264 calves with diarrhea are presented in Tables 2 and 3. On admission 12% (n = 31) calves had clinical evidence of pneumonia, 7% (n = 18) of arthritis, 1% (n = 3) of meningitis, and 21% (n = 55) of omphalophlebitis, and 33% (n = 86) of the calves were considered to be septicemic based on the previously described criteria. Seventy percent of the calves (n = 184) were discharged, whereas 30% (n = 80) died or were euthanized because of poor prognosis or economic constraints. Out of 80 calves that did not survive, 69% (n = 55) were septic and 31% (n = 25) were nonseptic.
Table 2.
Admission plasma concentration of electrolytes, total plasma protein, albumin and creatinine of 264 calves with neonatal diarrhea.
| Parameter (reference range) | Low (%) | Normal (%) | High (%) |
|---|---|---|---|
| Sodium [132–152 mmol/L]31 | 39 (15) | 206 (78) | 19 (7) |
| Potassium [3.9–5.8 mmol/L]31 | 24 (9) | 178 (68) | 62 (23) |
| Chloride [95–110 mmol/L]31 | 45 (17) | 180 (68) | 39 (15) |
| Total protein [57–81 g/dL]31 | 36 (14) | 205 (77) | 23 (9) |
| Albumin [21–35 g/dL]31 | 7 (3) | 251(95) | 6 (2) |
| Creatinine [67–195 μmol/L]31 | N/A | 174 (61) | 90 (39) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Admission values of 264 calves with neonatal diarrhea with and without sepsis obtained on admission.
| Parameter | Calves (n = 264) | Reference Range7, 27 |
|---|---|---|
| H–H model | ||
| pH | 7.15 (7.05–7.29) | 7.35–7.50 |
| pCO2 (mmHg) | 50.3 (40.42–58.8) | 34–45 |
| HCO3 − (mmol/L) | 18 (11.3–25.8) | 20–30 |
| BE(ecf) (mmol/L) | −10.2 (−18.9 to 0.3) | −2.5 to 2.5 |
| AG (mmol/L) | 22.5 (16.8–27.9) | 14–20 |
| SID model | ||
| SID3 (mmol/L) | 41.2 (36.8–46.5) | 38–44 |
| SIG (mmol/L) | −10.8 (−16.8 to −3.11) | −3 to 3 |
| Atot (mmol/L) | 21.9 (19.2–24.6) | 13–25 |
Data presented as median (25th and 75th quartiles). H–H, Henderson–Hasselbalch approach; SID, physicochemical approach; HCO3 −, bicarbonate; pCO2, partial carbon dioxide pressure; BE(ecf), base excess extracellular fluid; AG, anion gap; SID3, strong ion difference; SIG, strong ion gap; Atot, total plasma concentration of nonvolatile weak acids.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Determination of Acid–Base Disorders in Calves with Diarrhea Using Henderson–Hasselbalch and Physicochemical Approaches
Analysis of acid–base balance using the H–H approach (HCO3 −) detected acid–base alterations in 95% (n = 251) of the calves included in this study; acidosis was present in all (n = 251) of these calves. Eighteen percent (n = 46) and 82% (n = 205) of the calves, had a single or mixed acid–base disorder, respectively (Fig 1).
Figure 1.
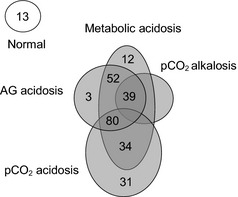
Interpretation of acid–base disorders on admission for each calf using Henderson‐Hasselbalch approach. AG, anion gap; p CO 2, partial carbon dioxide pressure.
Physicochemical analysis of acid–base balance revealed acid–base disorders in 98% (n = 259) of the calves included in this study; acidemia was present in 100% (259) of these calves. Strong ion gap acidosis was the most common disorder and was detected in 80% (n = 206) of the calves included in this study. The interpretation of the acid–base disorders on admission of each calf by the physicochemical approach is presented in Figure 2.
Figure 2.
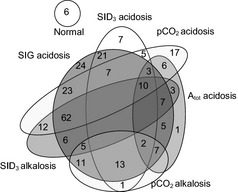
Interpretation of acid–base disorders on admission for each calf using the physicochemical approach. SIG strong ion gap; p CO 2 partial carbon dioxide pressure; SID, strong ion difference; Atot, total concentration of plasma proteins.
Determination of the Mechanism of Acid–Base Abnormalities in Calves with Diarrhea Using the Physicochemical Approach
The results of the forward stepwise regressions with venous pH and calculated HCO3 − as the dependent variables and venous SID3, SIG, pCO2, and Atot values as independent variables are presented in Tables 4 and 5. The regression analysis revealed that an increased unmeasured strong anions concentration (SIG) was the most important contributor to the changes in the measured pH and calculated plasma HCO3 −. A decrease in SID3 concentration was the next most significant contributor to the changes in the measured pH and calculated plasma HCO3 −. Changes in Atot and pCO2 had a minor but significant contribution to the changes in measured pH and calculated plasma HCO3 −.
Table 4.
Results of the forward stepwise regression of measured pH as dependent variable versus jugular venous values of the physicochemical variables.
| Order of entry into regression model dS1 | Variable | Partial R 2 | Model R 2 |
|---|---|---|---|
| 1 | SIG | 0.66 | 0.66 |
| 2 | SID3 | 0.11 | 0.77 |
| 3 | pCO2 | 0.09 | 0.86 |
| 4 | Atot | 0.05 | 0.91 |
| Order of entry into regression model DS2 | |||
| 1 | SIG | 0.39 | 0.39 |
| 2 | SID3 | 0.25 | 0.64 |
| 3 | pCO2 | 0.17 | 0.81 |
| 4 | Atot | 0.12 | 0.93 |
DS1, data set 1; DS2, data set 2; pCO2, partial carbon dioxide pressure; SID3, strong ion difference; SIG, strong ion gap; Atot, total plasma concentration of nonvolatile weak acids. P < .0001 for all the variable's coeffficients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 5.
Results of the forward stepwise regression of calculated HCO 3 − as dependent variable versus jugular venous values of the physicochemical variables.
| Order of entry into regression model dS1 | Variable | Partial R 2 | Model R 2 |
|---|---|---|---|
| 1 | SIG | 0.59 | 0.59 |
| 2 | SID3 | 0.34 | 0.93 |
| 3 | Atot | 0.04 | 0.97 |
| 4 | pCO2 | 0.01 | 0.98 |
| Order of entry into regression model DS2 | |||
| 1 | SIG | 0.42 | 0.42 |
| 2 | SID3 | 0.48 | 0.90 |
| 3 | Atot | 0.06 | 0.96 |
| 4 | pCO2 | 0.02 | 0.98 |
DS1, data set 1; DS2, data set 2; HCO3 −, bicarbonate; pCO2, partial carbon dioxide pressure; SID3, strong ion difference; SIG, strong ion gap; Atot, total plasma concentration of nonvolatile weak acids. P < .0001 for all the variables' coeffficients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Association of Acid–Base Variables with Severity of Depression in Calves with Diarrhea
Plasma concentrations of SIG and AG were significantly different between calves at all 3 clinical scoring categories for attitude, posture, and suckle reflex (P < .0001) (Table 5). Plasma [BE(efc)] and [HCO3 −] did not differ significantly between lethargic and stuporous calves, calves in sternal versus lateral recumbency, calves with a weak versus strong suckle reflex, or calves with weak versus absent suckle reflex. SID3, Atot, and pCO2 did not differ significantly among the different clinical scoring categories for attitude, suckle reflex, and posture (Table 6).
Table 6.
Comparison of acid–base variables values for calves with normal, moderate, and severe changes on posture, attitude, and suckle reflex.
| Clinical Variable | Attitude | Suckle Reflex | Posture | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acid–Base Variable | Bright (n = 44) | Obtunded (n = 169) | Stuporous (n = 51) | Strong (n = 62) | Weak (n = 110) | Absent (n = 92) | Standing (n = 88) | Sternal (n = 92) | Lateral (n = 8) |
| H–H | |||||||||
| HCO3 − | 24.7a | 22.3a | 16.9b | 21.4a | 19.3ab | 17b | 21.7a | 18.4b | 16.8b |
| pCO2 | 51.3a | 48.3a | 53.2a | 49.2a | 48.9a | 49.3a | 50.9a | 48a | 51.6a |
| BE(ecf) | −1.9a | −10.5b | −11.4b | −5.9a | −8.6ab | −12.1b | −5.7a | −10b | −12.3b |
| AG | 18a | 22.8b | 26.3c | 19.1a | 22.4b | 25.5c | 19a | 23b | 26.2c |
| SID | |||||||||
| SID3 | 42.7a | 41a | 43a | 40.6a | 41.8a | 42.4a | 40.8a | 41.5a | 42.2a |
| SIG | −5.3a | −10.8b | −15.8c | −6.3a | −10.5b | −14.3c | −6.2a | −11.2b | −15.2c |
| Atot | 22.2a | 22.3a | 22a | 22.3a | 21.8a | 22.5a | 22.1a | 22.3a | 21.9a |
Data presented as mean values. Different letters within a row indicated a statistically significant difference (P < .001). HCO3 −, bicarbonate; pCO2, partial carbon dioxide pressure; BE(ecf), base excess extracellular fluid; AG, anion gap; SID3, strong ion difference measured; SIG, strong ion gap; Atot, total plasma concentration of nonvolatile weak acids.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
Use of the Henderson–Hasselbalch and physicochemical approach to evaluate acid–base status indicated that acidemia was the most common acid–base disorder in the calves with diarrhea included in this study. Acidemia was predominant because of an increase in the strong UAs concentration (increased SIG) and changes in the concentration of the measured strong ion concentration (SID3). Increase in the total plasma protein concentration resulting in weak acids acidosis only had a minor contribution to the acidemia in this group of calves. Stewart's major contribution to the understanding of clinical acid–base physiology was his proposal that plasma [H+] is determined by 3 independent factors: pCO2, [SID], and Atot −.9 SID has 2 components: measured SID and unmeasured SID (SIDum).7 The simplified strong ion approach quantifies the unmeasured strong ions by calculating the SIG from values of plasma or serum concentration of strong anions and strong cations, the total protein or albumin concentration, and experimentally determined values of Atot and pKa in calf plasma, and can be used interchangeably for SIDum.7, 10, 32 Theoretically, [SID] can be altered in several ways to produce metabolic acidosis, such as a reduction in [Na+], an increase in [Cl−], endogenous production of strong anions (l‐lactic acidosis), absorption of strong anions (d‐lactic acidosis), and impaired excretion of unmeasured anions (uremic acids).7, 33, 34 Plasma electrolyte disorders including hyponatremia, in the presence of normochloremia or hyperchloremia, were observed in some of the calves in these data sets and were the likely cause of the decreased of the SID3. This finding confirms the important role of hyponatremia, attributed to excessive loss of sodium into the gastrointestinal tract and decreased milk intake because of anorexia,6, 35, 36 in producing acidemia and strong ion acidosis in calves with diarrhea.7
In this retrospective study in calves with diarrhea the major contributor to the changes in plasma pH and HCO3 − was an increase in unmeasured anions. Combined the SIG and SID3 accounted for 77 and 93% of the changes in the measured venous pH and calculated HCO3 − in calves of DS1, respectively, whereas SIG and SID3 accounted for 64 and 93% of the changes in the measured venous pH and calculated HCO3 − in calves of DS2, respectively. Similar results were reported previously in sick calves in which SIDum and SID3 accounted for 58–75% of the changes of the calculated pH; it was presumed that [d‐lac−] was the predominant unmeasured anion.7 Hyper‐d‐lactatemia occurs frequently in diarrheic calves.21, 22, 23[d‐lac−] acid is a major contributor to anion gap acidosis, accounting for approximately 64% of the total increase in organic acids in plasma.21 Malabsorption of nutrients in the small intestine of patients with short bowel syndrome and subsequent fermentation by colonic bacteria result in d‐lactic acidosis in humans.37 d‐lactate concentrations are significantly higher in rumen content, feces, serum, and urine in diarrheic calves with acidemia as compared to normal calves.22, 38 Our findings support the clinically useful application of SID theory in the calculation of the SIG to quantify the unmeasured strong anion concentration such as [d‐lac−] and [l‐lac−] in calves with diarrhea, and the calculation of SID3 to quantify the effects of [Na+] and [Cl−] on acid–base status.39
Strong ion gap may also be reduced by an increase in the strong anions such as l‐lactate. There is a strong evidence that neonatal diarrhea predisposes calves to septicemia.3, 28, 40, 41, 42 One of the most important consequences of the septicemia is the compromise of organ perfusion because of the arterial hypotension. Furthermore, endotoxin impairs oxygen extraction by tissues.43 As a result, anaerobic glycolysis, combined with direct inhibition of pyruvate dehydrogenase by endotoxin, generates severe l‐lactic acidosis.44, 45 Contradictory results regarding the contribution of the concentration of [l‐lac−] to the acidemia in diarrheic calves have been reported. In some studies, the contribution of [l‐lac−] to the metabolic acidosis in calves with diarrhea has been attributed to the severity of the dehydration and the age of the calf,19, 22 whereas other studies have shown that [l‐lac−] was a minor contributor the acidemia in diarrheic or severely dehydrated calves.22, 26, 46 The results of this study revealed that a high proportion of the calves were dehydrated and had strong ion metabolic acidosis, and that one‐third were considered to be septicemic, 70% of which did not survive. These findings suggest that some of the calves were in a progressive or irreversible phase of hypovolemic or septic shock or both where hypotension and hypoperfusion develop, leading to an increase in plasma [l‐lac−], which could have contributed in part to the increased concentration of the unmeasured strong ions detected by the calculation of the SIG.47 l‐lactate concentrations were not measured in the calves included in this study.
Based on our results, we speculate that the causes for the strong ion acidosis in this group of calves include (1) increased loss of fluid with high [Na+] and low [Cl−] through the feces; (2) increased plasma [l‐lac−] caused by dehydration and septicemia; and (3) absorption of [d‐lac−] from the gastrointestinal tract. These findings emphasize the importance of identifying the presence of strong UAs by calculating SIG when applying the physicochemical approach. Additionally, this study highlights the role of strong ions in the acid–base status of calves with diarrhea, and indicates that replacement fluid therapy in animals with diarrhea should be aimed at resolving volume deficits, improving peripheral perfusion, and correcting [Na+] and [Cl−] imbalances.48, 49
This retrospective study revealed that clinical scores for posture, attitude, and suckle reflex were influenced primarily by the magnitude of the increased plasma concentration of unmeasured strong anions (increased SIG and AG). Early studies demonstrated that demeanor and posture in calves with diarrhea were influenced by the degree of metabolic acidosis,16 and that base deficit had the strongest correlation with alteration of demeanor,16, 24 behavior,24 and suckle reflex.20, 24 Conversely, a recent study failed to demonstrate that severe, uncomplicated, hyperchloremic acidosis was associated with the demeanor abnormalities accompanying neonatal calves with diarrhea.25 Calves with naturally acquired diarrhea have variations in behavior, posture, and palpebral reflex, but not in the suckle reflex, that could be better explained by increases in serum [d‐lac−] than by decreases in base excess.24 Both natural and experimentally induced hyper‐d‐lactatemia in calves produced an impaired palpebral reflex, somnolence, and a staggering gait, but no impairment of the suckling reflex.18, 50 It has been suggested than metabolic acidosis without appreciable increase in [d‐lac−] has only a minor influence on posture and behavior of calves with diarrhea51; however, posture and especially demeanor normalized after correction of the acidemia with sodium bicarbonate despite a persistent increase in [d‐lac−] concentration in calves with diarrhea and metabolic acidosis.51 Moreover, in 1 study of calves with naturally acquired diarrhea and strong ion acidosis that were treated with intravenous solution of sodium, behavior was normalized despite of the persistent increases in [d‐lac−] above 3 mmol/L.30 In this retrospective study SID3 and pCO2 did not correlate with changes in attitude, suckle reflex and posture; additionally, Atot was not significantly correlated with attitude and suckle response although it did correlate with posture. This supports the hypothesis that unmeasured strong anions including [d‐lac−], [l‐lac−], and uremic anions can be the major cause for deteriorating demeanor parameters, and suggest that fluid volume replacement to re‐establish tissue perfusion and diuresis with polyionic crystalloid alkalinizing solution is crucial for the treatment of calves with diarrhea.
There were several limitations to our findings, most notably the retrospective design, as well as the selected population being referred to the VTH, which would tend to be biased toward sicker patients. Secondly, the data were collected from 2 different retrospective data sets, which can increase the limitations and inaccuracies of the retrospective studies. A further validation of these results through a prospective study is warranted. Finally, 1 potential limitation of this study was the use of 2 different blood gas analyzers with 2 different values of S and pK1′ for calculation of plasma HCO3 − from pCO2 and pH values.52 Assigned values for S and pK1′ for DS1 were 0.0306 mmol/L/mmHg and 6.099 mmol/L, respectively; assigned values for S and pK1′ for DS2 were 0.0307 mmol/L/mmHg and 6.095 mmol/L, respectively. However, the rearrangement of the H–H equation as pH = [(−log10K1 − log10(S)] + log10 (HCO3 −/pCO2) permitted the combination of the 2 constants into one, expressed as [pK1 − log10(S)]. Substituting S and pK1′ for the assigned values for each blood gas resulted in [6.099 + 1.514 = 7.613] for DS1 and [6.095 + 1.513 = 7.608] for DS2; these values differ by 0.066%. A difference of 0.066% between DS1 and DS2 for the calculation of plasma concentration of HCO3 − seemed to be of little importance for the purposes of this study.
In conclusion, the results of this study revealed that UAs and strong UAs concentrations calculated using AG and SIG, respectively, were the primary causes of acidemia in calves with diarrhea. They also had a greater influence on clinical scores of attitude, suckle reflex, and posture, as compared to BE(ecf) and [HCO3 −]. These findings emphasize the importance of the calculation of the strong ion gap or anion gap when evaluating acid–base abnormalities in diarrheic calves.
Acknowledgments
The authors acknowledge Henrik Stryhn for his assistance in the statistical analysis.
Conflict of Interest: Authors disclose no conflict of interest.
Presented in part as an oral presentation at the 2013 ACVIM Forum, Seattle, Washington
Footnotes
Radiometer medical APs, Bronshoj, Denmark
ITC, Edison, NJ
Coulter Electronics Inc, Hialeah, FL
Roche Diagnostics, Indianapolis, IN
Minitab Software, Phyladelphia, PA
References
- 1. McClure JT. Oral fluid therapy for treatment of neonatal diarrhoea in calves. Vet J 2001;162:87–89. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Available at: http://www.who.int/mediacentre/factsheets/fs330/en/ind ex.html. Accessed October 19, 2012.
- 3. Grove‐White DH. Diagnosis and treatment of metabolic acidosis in calves: A field study. Vet Rec 1993;133:499–501. [DOI] [PubMed] [Google Scholar]
- 4. Kasari TR. Metabolic acidosis in diarrheic calves: The importance of alkalinizing agents in therapy. Vet Clinics North Am Food Anim Pract 1999;6:629–643. [DOI] [PubMed] [Google Scholar]
- 5. Kasari TR, Naylor JM. Clinical evaluation of sodium bicarbonate, sodium L‐lactate, and sodium acetate for the treatment of acidosis in diarrheic calves. J Am Vet Med Assoc 1985;187:392–397. [PubMed] [Google Scholar]
- 6. Bertchtold J. Treatment of calf diarrhea: Intravenous fluid therapy. Vet Clin North Am Food Anim Pract 2009;25:73–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Constable PD, Staempfli HR, Navetat H, et al. Use of strong on approach to determine mechanism of acid‐base abnormalities in sick calves with or without diarrhea. J Vet Intern Med 2005;19:581–589. [DOI] [PubMed] [Google Scholar]
- 8. Hasselbalch KA. Die Berechnung der Wassersroffzahl des Blutes ous der freien und gebunden Kohlensaure desselben, und die Sauerstoffbindung des Blutes als Funktion der Wasserstoffzahl. Biochemische Zeitschrift 1917;78:112–144. [Google Scholar]
- 9. Stewart PA. Modern quantitative acid‐base chemistry. Can J Physiol Pharmacol 1983;61:1444–1446. [DOI] [PubMed] [Google Scholar]
- 10. Constable PD. A simplified strong ion model for acid‐base equilibria: Application to horse plasma. J Appl Physiol 1997;83:297–311. [DOI] [PubMed] [Google Scholar]
- 11. Constable PD. Clinical assessment of acid‐base status: Comparison of the Henderson‐Hasselbalch and strong ion approaches. Vet Clin Path 2000;29:115–128. [DOI] [PubMed] [Google Scholar]
- 12. Staempfli HR, Carlson GP. How to use the routine serum biochemical profile to understand and interpret acid‐base disorders in the horse. Proceedings of the annual convention of the AAEP; November 2001. San Diego, CA;2001: 259–261. [Google Scholar]
- 13. Malouf R, Brust JC. Hypoglycemia: Causes, neurological manifestations and outcome. Ann Neurol 1985;17:421–430. [DOI] [PubMed] [Google Scholar]
- 14. Hollis AR, Furr MO, Magdesian KG, et al. Blood glucose concentrations in critically ill neonatal foals. J Vet Intern Med 2008;22:1223–1227. [DOI] [PubMed] [Google Scholar]
- 15. Kasari JM, Naylor JM. Further studies on the clinical features and clinicopathological findings of a syndrome of metabolic acidosis with minimal dehydration in neonatal calves. Can J Vet Res 1986;50:502–508. [PMC free article] [PubMed] [Google Scholar]
- 16. Naylor JM. A retrospective study of the relationship between clinical signs and severity of acidosis in diarrheic calves. Can Vet J 1989;30:577–580. [PMC free article] [PubMed] [Google Scholar]
- 17. Bellino C, Arnaudo F, Biolatti C, et al. Development of a diagnostic diagram for rapid field assessment of acidosis severity in diarrheic calves. J Am Vet Med Assoc 2011;240: 312–316. [DOI] [PubMed] [Google Scholar]
- 18. Treftz FM, Lorch A, Feist M. Metabolic acidosis in neonatal calf diarrhea—clinical findings and theoretical assessment of a simple treatment protocol. J Vet Intern Med 2012; 26:162–170. [DOI] [PubMed] [Google Scholar]
- 19. Naylor JM. Severity and nature of acidosis in diarrheic calves over and under one week of age. Can Vet J 1987;28:168–173. [PMC free article] [PubMed] [Google Scholar]
- 20. Geishauser T, Thunker B. Metaboliche azidose bei neugeborenen Kalbern mit Durchfall – Abschatzung and saugreflex oder stehvermogen. Prakt Tierarzt 1997;78:600–605. [Google Scholar]
- 21. Omole O, Nappert G, Naylor J, et al. Both L‐ and D‐lactate contribute to metabolic acidosis in diarrheic calves. J Nutr 2001;131:2128–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ewaschuk JB, Naylor JM, Palmer R, et al. D‐lactate production and excretion in diarrheic calves. J Vet Intern Med 2004;18:744–747. [DOI] [PubMed] [Google Scholar]
- 23. Lorenz I. Influence of D‐lactate on metabolic acidosis and on prognosis in neonatal calves with diarrhea. J Vet Med A 2004;51:425–428. [DOI] [PubMed] [Google Scholar]
- 24. Lorenz I. Investigation on the influence of serum D‐lactate levels on clinical signs in calves with metabolic acidosis. Vet J 2004;168:323–327. [DOI] [PubMed] [Google Scholar]
- 25. Gentile A, Lorenz I, Sconza S, et al. Experimentally induced systemic hyperchloremic acidosis in calves. J Vet Intern Med 2008;22:190–195. [DOI] [PubMed] [Google Scholar]
- 26. Ewasshuck JB, Naylor JM, Zelio GA. Anion gap correlates with serum D‐lactate and DL‐lactate concentration in diarrheic neonatal calves. J Vet Intern Med 2003;17:940–942. [DOI] [PubMed] [Google Scholar]
- 27. Constable PD. Calculation of variables describing plasma nonvolatile weak acids for use in the strong ion approach to acid‐base balance in cattle. Am J Vet Res 2002; 63:482–489. [DOI] [PubMed] [Google Scholar]
- 28. Lofstetd J, Dohoo IR, Duizer G. Model to predict septicemia in diarrheic calves. J Vet Intern Med 1999;13:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emmet M, Narins RG. Clinical use of anion gap. Medicine 1977;56:38–54. [PubMed] [Google Scholar]
- 30. Mueller KR, Gentile A, Klee W, et al. Importance of the effective strong ion difference of an intravenous solution in the treatment of diarrheic calves with naturally acquired academia and strong ion (metabolic) acidosis. J Vet Intern Med 2012;26:674–683. [DOI] [PubMed] [Google Scholar]
- 31. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Reference laboratory values. Appendix 2 In: Radostits OM, Gay CC, Hinchcliff KW, Constable PD, eds. Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed Philadelphia, PA: W.B. Saunders Company; 2007: 2047–2050. [Google Scholar]
- 32. Constable PD, Hinchcliff KW, Muir WW 3rd. Comparison of anion gap and strong ion gap as predictors of unmeasured strong ion concentration in plasma and serum from horses. Am J Vet Res 1998;59:881–887. [PubMed] [Google Scholar]
- 33. Fencl V, Rossing T. Acid‐base disorders in critical care medicine. Ann Rev Med 1989;40:17–29. [DOI] [PubMed] [Google Scholar]
- 34. Jones N. A quantitative physicochemical approach to acid‐base physiology. Clin Biochem 1990;23:189–195. [DOI] [PubMed] [Google Scholar]
- 35. Lewis LD, Phillips RW. Pathophysiologic changes due to coronavirus‐induced diarrhea in the calf. J Am Vet Med Assoc 1978;173:636–642. [PubMed] [Google Scholar]
- 36. Constable PD, Walker PG, Morin DE, et al. Clinical and laboratory assessment of hydration status of neonatal calves with diarrhea. J Am Vet Med Assoc 1998;212:991–996. [PubMed] [Google Scholar]
- 37. Lord L, Schaffner R, DeCross A, et al. Management of the patient with short bowel syndrome. AACN Clin ISS 2000;11:604–606. [DOI] [PubMed] [Google Scholar]
- 38. Gentile A, Sconza S, Lorenz I, et al. D‐lactic acidosis in calves as a consequence of experimentally induced ruminal acidosis. J Vet Med A 2004;51:64–70. [DOI] [PubMed] [Google Scholar]
- 39. Staempfli HR, Constable PD. Increased strong ion gap in dairy calves with diarrhea is likely due to d‐lactate. J Vet Intern Med 2012;26:739. [Google Scholar]
- 40. Fecteau G, Van Metre DC, Pare J, et al. Bacteriological culture of ill neonatal calves. Can Vet J 1997;38:95–100. [PMC free article] [PubMed] [Google Scholar]
- 41. Fecteau G, Pare J, Van Metre DC, et al. Use of a clinical sepsis score from predicting bacteremia in neonatal dairy calves on a calf rearing farm. Can Vet J 1997;38:101–104. [PMC free article] [PubMed] [Google Scholar]
- 42. Aldridge BM, Garry FB, Adams R. Neonatal septicemia in calves: 25 cases (1985‐1990). J Am Vet Med Assoc 1993;203:1324–1329. [PubMed] [Google Scholar]
- 43. Ince C. The microcirculation is the motor of the sepsis. Crit Care 2005;9:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porta F, Takala J, Weiikert C, et al. Effects of prolonged endotoxemia on liver, skeletal muscle and kidney mitochondrial function. Crit Care 2006;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yelich RM, Witek‐Janusec L. Glucose, lactate, insulin, and somatostatin responses to endotoxin in developing rats. Shock 1994;2:438–444. [DOI] [PubMed] [Google Scholar]
- 46. Constable PD, Streeter RN, Koenig GJ, et al. Determinants and utility of the anion gap in predicting hyperlactatemia in cattle. J Vet Intern Med 1997;11:71–79. [DOI] [PubMed] [Google Scholar]
- 47. Fecteau G, Smith BP, George LW. Septicemia and meningitis in the newborn calf. Vet Clin North Am Food Anim 2009;25:195–208. [DOI] [PubMed] [Google Scholar]
- 48. Naylor J, Dunkel B. The treatment of diarrhoea in adult horses. Equine Vet Educ 2009;21:494–504. [Google Scholar]
- 49. Gomez DE, Arroyo LG, Staempfli HR, et al. Physicochemical interpretation of acid‐base abnormalities in 54 horses with acute severe colitis and diarrhea. J Vet Intern Med 2013;27:548–553. [DOI] [PubMed] [Google Scholar]
- 50. Lorenz I, Gentile A, Klee W. Investigation of D‐lactate metabolism and clinical signs of D‐lactatemia in calves. Vet Rec 2005;156:412–415. [DOI] [PubMed] [Google Scholar]
- 51. Lorenz I, Vogt S. Investigation on the association of D‐lactate blood concentration with the outcome of therapy of acidosis, and with posture and demeanour in young calves with diarrhea. J Vet Med A Physiol Pathol Clin Med 2006;53:490–494. [DOI] [PubMed] [Google Scholar]
- 52. Natanelson S, Nobel D. Effect of the variation of pK′ of the Henderson‐Hasselbalch equation on values obtained for total CO2 calculated from pCO2 and pH values. Clin Chem 1977;23:767–769. [PubMed] [Google Scholar]


