Abbreviations:
- BVD
bovine viral diarrhea
- CFU
colony forming units
- CT
cycles to positive threshold
- HEYM
Herrold's egg yolk medium
- JD
Johne's disease
- MAP
Mycobacterium avium ssp. paratuberculosis
- MGIT
mycobacterial growth indicator tube
- SSR
short sequence repeat
A 6‐year‐old female alpaca (case 1) was examined at the George D. Widener Hospital for Large Animals of the University of Pennsylvania for evaluation of lethargy, diarrhea, and weight loss of 3 months' duration. A diagnosis of Mycoplasma haemolamae had initially been made by the referring veterinarian based on blood smear examination findings. The animal was treated with a long‐acting form of oxytetracyline a (10 mg/kg sq q24h) for 10 days but its clinical signs failed to improve. Serial fecal floatations performed before referral showed no parasite ova. Additional treatments received before referral included ivermectin, fenbendazole, B vitamins, injectable iron, procaine penicillin, oral sulfadimethoxine, and oral electrolytes. As the diarrhea and weight loss persisted, the alpaca was referred for further evaluation. The animal was pregnant (approximately 155 days gestation) at the time of presentation. The animal was from a herd of 78 alpacas, and was pastured with a group of pregnant females and young animals. Routine vaccinations on the farm included rabies, Clostridium perfringens Types C and D, and tetanus.
On presentation, the alpaca was quiet but responsive and in poor body condition (body condition score 3/10), weighing 60 kg. Rectal temperature, heart rate, and respiratory rate were 98.2°F, 56 beats per minute, and 20 breaths per minute, respectively. The alpaca appeared well hydrated with pale mucous membranes. It had green, watery feces, and a poor appetite. Initial laboratory abnormalities included mild nonregenerative anemia (PCV, 23%; reference interval, 25–46%), hypoproteinemia (total protein, 4.2 g/dL; reference interval, 4.6–6.9 g/dL), hypoalbuminemia (albumin, 1.40 g/dL; reference interval, 2.50–4.20 g/dL), and azotemia (serum creatinine concentration, 2.28 mg/dL; reference interval, 0.6–1.80 mg/dL). Blood smear examination was negative for Mycoplasma haemolamae organisms. Feces were submitted for fecal floatation, Mycobacterium avium spp. paratuberculosis (MAP) RT‐PCR, [Link] , [Link] acid‐fast staining for Cryptosporidium, fecal occult blood, and bacterial cultures. Blood was submitted for MAP antibody detection with ELISA. d No parasite ova were observed on fecal floatation, and the acid‐fast staining was negative for Cryptosporidium organisms. The occult blood test result was negative. Fecal bacterial cultures were negative for Salmonella spp. and Clostridium spp. Fetal and abdominal ultrasound examinations were performed. The sonographic appearance of the visible gastrointestinal structures was within normal limits. Fetal sonographic evaluation detected an irregular heart rate, suggestive of fetal distress. Additional diagnostic tests performed during the hospital stay included determination of serum trace mineral concentrations (Cu, Fe, Se, and Zn), fecal coronavirus detection with electron microscopy, and bovine viral diarrhea (BVD) testing by PCR on the buffy coat. Serum trace mineral concentrations were within the reference range. Results of coronavirus and BVD tests later returned negative.
The patient remained hospitalized for 5 days while awaiting diagnostic test results. During that time, supportive care in the form of IV fluids (Normosol‐R, 1.5 mL/kg/h) and partial parenteral nutrition (1.5 mL/kg/h) were administered. Ranitidine (1.5 mg/kg IV q8h) was also administered for prevention of 3rd compartment ulceration. RT‐PCR results for MAP detection identified a strong positive result with duplicate values of 17.43 and 17.79 cycles to positive threshold (C T). Because of the animal's advanced clinical signs and potential risk to the rest of the herd, permission was obtained for euthanasia. The alpaca was euthanized by administration of a barbiturate overdose and a postmortem examination performed.
At necropsy, gross findings included emaciation, fluid intestinal contents, and markedly enlarged ileocecal lymph nodes. The uterus contained a 34‐cm crown‐to‐rump length female fetus. No macroscopic lesions were observed in the bone marrow or intestine. An impression smear of an ileocecal lymph node disclosed acid‐fast bacilli among numerous lymphocytes and macrophages (Fig 1). On histology, most of the bone marrow consisted of mature adipocytes, with occasional small foci of cellularity where all cell lines were represented. The ileocecal lymph nodes were expanded by infiltrating sheets of epithelioid macrophages, scattered multinucleate cells, and low to moderate numbers of eosinophils. The mucosa of the distal jejunum and ileum was markedly expanded by sheets of infiltrating macrophages (Fig 2). The villi were atrophied, and nodular infiltrates of macrophages surrounded the lymphatics. Sheets of macrophages also infiltrated the submucosal lymphoid tissue of the large intestine. A postmortem a diagnosis of severe chronic granulomatous enterocolitis and granulomatous lymphadenitis consistent with paratuberculosis was made. Samples of ileum, ileocecal junction, ileocecal lymph node, and feces were collected for mycobacterial culture with liquid media, and standard Herrold's Egg Yolk Medium (HEYM) according to methods described previously. 1 , 2 , 3 In order to facilitate quantification of MAP growth, 4 serial 10‐fold dilutions (1 : 10; 1 : 100, 1 : 1,000, and 1 : 10,000) were prepared from each original tissue and fecal samples. For each sample type, 100 μL of the original inoculum, and of each inoculum dilution, was added to 1 mycobacterial growth indicator tube (MGIT) e culture tube. Also, for each sample type, 100 μL of the original inoculum, and all but 2 dilutions (1 : 10 and 1 : 10,000), were added to 3 HEYM flasks. For liquid culture, time to positive was recorded in days for each inoculum, and the growth was confirmed as MAP by RT‐PCR. For solid media culture, the total number of MAP colony forming units (CFU)/g of tissue or feces was estimated based on the sample weight, dilution factor, and the average number of colonies counted per flask.
Figure 1.
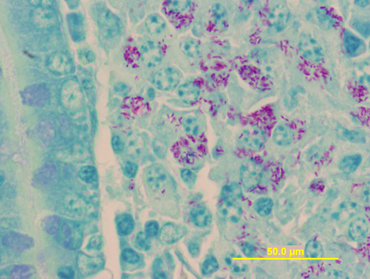
Oil immersion (100 ×) image of epithelioid macrophages stained by the Ziehl‐Neelsen (“acid‐fast” stain) method. Typical of herbivore Mycobacterium avium ssp. paratuberculosis enteric infections, the cytoplasm of many of macrophages is filled with brilliantly stained bacilli.
Figure 2.
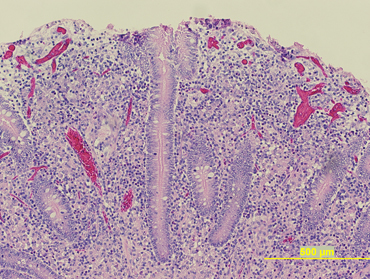
Low magnification (10 ×) view of the distal jejunum on hematoxylin and eosin reveals sheets of epitheliod macrophages occupying the submucosa. Clusters of macrophages are also within the lamina of the mucosa.
MAP was isolated from the fecal sample and all tissue samples obtained at necropsy. The estimated number of CFU/g of tissue was extremely high at 2,884,000 CFU/g, 10,500,000 CFU/g, and 1,813,000 CFU/g for ileum, ileocecal lymph node, and ileocecal junction, respectively. The estimated number of CFU/g of feces also was extremely high at 2,100,000 CFU/g. Genotyping of 1 of the isolates obtained from the alpaca was performed. The genotype was determined to be a cattle strain by IS1311 restriction endonuclease analysis and was confirmed as MAP by hsp65‐PCR‐REA and IS900 PCR. 4 The patient was positive for MAP antibody detection with a commercial ELISA test kit (0.433 optical density [OD]; cutoff value, 0.190).
After the animal's presentation to the university veterinary medical teaching hospital, individual blood and fecal samples were obtained from the remaining 77 herd animals. The individual samples were processed for MAP antibody detection with ELISA, fecal RT‐PCR, and fecal cultures on HEYM. Pooled environmental fecal samples also were obtained from each of the farm's 3 pastures (pastures A, B, and C), and analyzed by RT‐PCR. One alpaca had a positive ELISA, and 12 alpacas had positive fecal RT‐PCR (including the ELISA‐positive alpaca). None of the animals were positive on fecal culture. Of the 12 RT‐PCR‐positive animals, 5 had been pastured (pasture A) with the patient in the 8 months before testing. In addition, 4 of the 12 RT‐PCR positive animals that were <12 months of age were kept on a pasture (pasture B) neighboring that of case 1. The remaining 3 RT‐PCR positive animals had no known direct contact with the patient before referral to the hospital. Both pastures A and B were positive for MAP on RT‐PCR; pasture C was negative.
Approximately 2 months after the patient's presentation to the veterinary medical teaching hospital, 2 additional alpacas from the same herd were submitted for postmortem examination. Both animals had been pastured with case 1. Case 2 was a 10‐year‐old female alpaca that was euthanized on the farm after complications from dystocia. At necropsy, a uterine prolapse associated with a vaginal laceration was found. No histologic evidence of paratuberculosis was detected but 2 of 8 (25%) intestinal tissue samples submitted for MAP culture on HEYM were positive. Interestingly, during the whole herd testing 2 months previously, case 2 was negative on fecal RT‐PCR and ELISA. Case 3 was a 14‐year‐old female euthanized on the farm because of chronic weight loss. At necropsy, chronic peritonitis secondary to 3rd compartment ulcers was identified. Similar to case 2, no histologic evidence of paratuberculosis was detected in the intestinal tract but 18 of 28 (64%) intestinal tissue samples submitted for MAP culture on HEYM were positive. At the whole herd testing 2 months previously, case 3 was positive on fecal RT‐PCR and was negative on ELISA. At that time, individual fecal samples were obtained from the herd animals that had previously tested positive on fecal RT‐PCR. Of the 12 previously positive animals, 11 were available for follow‐up testing (case 3 had been euthanized). Nine animals were now negative. Environmental samples were not obtained at the time.
The MAP isolates obtained from all 3 cases were further genotyped with 2 short sequence repeat (SSR) segments by a technique described elsewhere. 5 Three major genotypes were identified based on the combinations of numbers of repeats within each SSR locus. All genotyped isolates from cases 1 and 2 carried 12G residues and 5 GGT repeats. This strain was identified as a cattle strain. Three isolates from case 3 were identified as a 7G, 6 GGT genotype, while 1 additional isolate from case 3 was identified as a 7G, 5 GGT genotype, both of which were identified as sheep strains.
Johne's disease (JD) or paratuberculosis previously has been reported in camelid species 6 , 7 but disease prevalence in North American herds is unknown. Clinical signs of paratuberculosis in camelids are similar to those reported in cattle and small ruminant species and may include lethargy, chronic weight loss, and dependent edema. In contrast to cattle, but similar to small ruminants, not all camelids in advanced stage of disease will develop diarrhea or changes in fecal consistency. Case 1 had diarrhea for at least 3 months before presentation, but cases 2 and 3 did not. Two llamas from a previous report showed signs of weight loss associated with diarrhea. 6 Another report on 10 alpacas described 5 animals that experienced diarrhea before death. 7 The difference in the occurrence of diarrhea between cattle and camelids (and small ruminants) may be related to the latter's intestinal tract physiology and their ability to reabsorb water. Also in contrast with cattle, camelids and small ruminants affected with paratuberculosis can present with anemia of variable severity. The pathophysiology for the development of anemia in camelids and small ruminants with JD is unclear, but could be secondary to the chronicity of the disorder, especially when the anemia is classified as normocytic and normochromic. 8 In addition, concurrent disease processes such as internal parasitism or infection with Mycoplasma haemolamae may contribute to the degree of anemia. The reported age at onset of clinical signs tends to be younger in camelids than in cattle. Three of the 10 alpacas reported before were between 11 and 14 months of age, 6 and 1 of the 2 llamas in the other report was 16 months old. 7 A possible explanation for this finding is that the incubation period for certain MAP strains is shorter in camelids than in other species, but this has not been proven.
Case 1 had been purchased 3 years before becoming ill. Information regarding the JD status of the farm of origin was not available to the authors. During 3 years on the current farm, no contact with other domestic ruminants was identified. To the farm owner's knowledge, this was the 1st case of JD in the herd since it was established several years ago. For both cases 2 and 3, the lack of histologic findings consistent with paratuberculosis in the presence of positive tissue cultures was suggestive of early infection with MAP. The absence of histologic lesions in the face of positive tissue culture has been documented in short‐term bovine experimental challenges with MAP. 9 Furthermore, we hypothesized that case 1, which was shedding very high numbers of MAP in the feces, could have been the source of infection within the herd. However, genotyping of the MAP strains isolated from all 3 alpacas described here suggests that >1 strain of MAP was present in the herd and that case 1 was not solely responsible for the presence of additional positive animals within the herd. The MAP strain identified in cases 1 and 2 was reported as a cattle strain, whereas the 2 different MAP strains isolated from case 3 were identified as a sheep strains. Isolation of bovine‐type strains in camelids affected by JD has been reported before, 6 , 7 and most strains probably can infect across ruminant species lines. 10 The practice of feeding bovine colostrum to neonatal crias is believed to be a possible source of MAP introduction within a herd. Cases 2 and 3 would have been 7 and 11 years old, respectively, when case 1 joined the herd. Traditionally, adult animals have been considered fairly resistant to infection with MAP, because the critical period for acquiring infection is during the neonatal period. 11 Age resistance could have been overcome by the pressure of infection if the environment was highly contaminated by a high‐shedding animal (case 1).
Previous reports of JD in camelids have not included quantitative culture results for MAP on either tissues or feces. In this report, the number of MAP CFU/g was obtained from tissue samples (cases 2 and 3) or from both tissue and fecal samples (case 1). Although cases 2 and 3 had small to moderate numbers of CFU in their tissues, case 1 had extremely high numbers of MAP CFU/g of tissue and feces. Similar numbers (>10,000 MAP CFU/g) have been reported in cattle. 12 These cows, shedding extremely large numbers of MAP in their feces, recently have been referred to as “super‐shedders.” 12 According to an arbitrary definition (> 10,000 MAP CFU/g feces), case 1 would fall into this category. In cattle, the presence of 1 or more high shedding cows in a herd has been considered as an important factor in the transmission and control of the disease. 12 Because high‐shedding animals can shed millions of MAP CFU daily, they represent a greater risk for the spread of the disease to herd mates. In addition, as demonstrated in this report, the presence of 1 or several animals shedding large numbers of MAP in their feces may contribute to “passive shedding” of MAP by herd mates. As MAP is deposited on pasture by the infected animals, it may be ingested by other animals and detected in their feces by RT‐PCR. Follow‐up fecal RT‐PCR testing performed on the farm described here showed a decrease in the number of animals classified as positive after removal of case 1 from the herd, suggesting that these animals were passive shedders, and passive shedding ceased once the high‐shedding animal was removed.
To the authors' knowledge, this is the 1st description of MAP high shedding in an alpaca. Detection and culling of high‐shedding animals within a herd is an important part of disease control.
Footnotes
aLiquamycin, Pfizer Animal Health, New York, NY
bVetAlert, Tetracore Inc, Rockville, MD
cSmartCycler, Cepheid, Sunnyvale, CA
dParachek, Biocor Animal Health Inc, Omaha, NE
eMGIT, Bactec, BD Diagnostic Systems, Franklin Lakes, NJ
Acknowledgment
The authors acknowledge Terry Fyock and Sue McAdams for their technical assistance.
This work was performed at the Department of Clinical Studies, New Bolton Center, School of Veterinary Medicine, University of Pennsylvania, Kennett Square, PA.
References
- 1. Shin SJ, Han JH, Manning EJB, et al Rapid and reliable method for quantification of Mycobacterium paratuberculosis by use of the BACTEC MGIT 960 system. J Clin Microbiol 2007;45:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shin SJ. Double incubation technique for cultivation of M. paratuberculosis from bovine faeces. In Proceedings 93rd US Animal Health Association Meeting, 1989, 381.
- 3. Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol 1992;30:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motiwala AS, Strother M, Theus NE, et al Rapid detection and typing of strains of Mycobacterium avium subsp. paratuberculosis from broth cultures. J Clin Microbiol 2005;43:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motiwala AS, Strother M, Amosin A, et al Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis : Evidence for limited strain diversity, strain sharing, and identification of unique targets for diagnosis. J Clin Microbiol 2003;41:2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belknap EB, Getzy DM, Johnson LW, et al Mycobacterium paratuberculosis infection in two llamas. J Am Vet Med Assoc 1994;204:1805–1808. [PubMed] [Google Scholar]
- 7. Ridge SE, Harkin JT, Badman RT, et al Johne's disease in alpacas (Lama pacos) in Australia. Aust Vet J 1995;72:150–153. [DOI] [PubMed] [Google Scholar]
- 8. Duncan JR, Prasse KW, Mahaffey EA. Erythrocytes In: Duncan JR, Prasse KW, Mahaffey EA, eds. Veterinary Laboratory Medicine, Clinical Pathology, 3rd ed Ames: Iowa State University Press; 1994:3–36. [Google Scholar]
- 9. Sweeney RW, Uzonna J, Whitlock RH, et al Tissue predilection sites and effects of dose on Mycobacterium avium subs. paratuberculosis organism recovery in a short‐term bovine experimental oral infection model. Res Vet Sci 2006;80:253–259. [DOI] [PubMed] [Google Scholar]
- 10. Stehman SM. Paratuberculosis in small ruminants, deer, and South American camelids. Vet Clin NA: Food Anim Pract 1996;12:441–455. [DOI] [PubMed] [Google Scholar]
- 11. Sweeney RW. Transmission of paratuberculosis. Vet Clin NA: Food Anim Pract 1996;12:305–312. [DOI] [PubMed] [Google Scholar]
- 12. Whitlock RH, Sweeney RW, Fyock TL. Mycobacterium avium spp paratuberculosis super‐shedders: Another factor in the control of Johne's disease. In: Proceedings 8th International Colloquium on Paratuberculosis, 2005, 164.


