Short abstract
Review on EMMPRIN in numerous immunological/inflammatory disease conditions and its complex roles in T cell biology.
Keywords: T cell activation, proliferation, migration, invasion, adhesion
Abstract
EMMPRIN (CD147), originally described as an inducer of the expression of MMPs, has gained attention in its involvement in various immunologic diseases, such that anti‐EMMPRIN antibodies are considered as potential therapeutic medications. Given that MMPs are involved in the pathogenesis of various disease states, it is relevant that targeting an upstream inducer would make for an effective therapeutic strategy. Additionally, EMMPRIN is now appreciated to have multiple roles apart from MMP induction, including in cellular functions, such as migration, adhesion, invasion, energy metabolism, as well as T cell activation and proliferation. Here, we review what is known about EMMPRIN in numerous immunologic/inflammatory disease conditions with a particular focus on its complex roles in T cell biology.
Abbreviations
- −/−
deficient
- ASCT2
alanine‐serine‐cysteine transporter 2
- aTreg
activated regulatory T cell
- BBB
blood brain barrier
- C
constant
- CIA
collagen‐induced arthritis
- CyPA/B
cyclophilin A/B
- DN
double negative
- DP
double positive
- EAE
experimental autoimmune encephalomyelitis
- EC
extracellular
- ECM
extracellular matrix
- EMMPRIN
extracellular matrix metalloproteinase inducer
- FLS
fibroblast‐like synoviocytes
- FoxP3
forkhead box P3
- GVHD
graft‐versus‐host disease
- HCC
hepatocellular carcinoma
- HIF‐1α
hypoxia‐inducible factor 1α
- HSPG
heparin sulfate proteoglycan
- I
intermediate
- LAT1
l‐type amino acid transporter 1
- Lck
lymphocyte‐specific protein tyrosine kinase
- MCT
monocarboxylate transporters
- MMP
matrix metalloproteinase
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MT1/2
membrane type 1/2
- RA
rheumatoid arthritis
- rTreg
resting regulatory T cell
- siRNA
small interfering RNA
- SLE
systemic lupus erythematosus
- SP
single positive
- TM
transmembrane
- Treg
regulatory T cell
- V
variable
- VEGF
vascular endothelial growth factor
Introduction
EMMPRIN is a cell‐surface‐glycosylated TM protein that belongs to the Ig superfamily. It was first discovered in a lung cancer cell line, where it stimulated the production of collagenase (MMP‐1) in adjacent fibroblastic cells [1]; thus, it was first designated as a tumor cell‐derived collagenase stimulatory factor [1, 2]. Other names now include CD147, basigin, and HAb18G. Although studied most extensively in tumor biology, the role of EMMPRIN in normal cell function and other disease states has been investigated. EMMPRIN levels are up‐regulated and/or involved in diseases, including lung‐injury models, ischemic myocardial injury, atherosclerosis, cancer, SLE, GVHD, RA, MS, and pathogenic invasion, among other insults. In this review, we introduce the biochemistry of EMMPRIN, evaluate its multiple functions in T cells, and discuss its roles in immunologic disorders.
EMMPRIN STRUCTURE AND BINDING PARTNERS
EMMPRIN is a 269 aa TM protein with a nonglycosylated molecular weight of ∼27 kDa and a glycosylated molecular weight with an approximate range of 43–66 kDa [3]. Glycosylation patterns may vary in different organs or cell types [3, 4–5] and may be a reason for its multiple physiologic roles. The structure of EMMPRIN consists of a heavily glycosylated EC domain, a TM domain, and a short cytoplasmic tail ( Fig. 1 ). There are 4 isoforms of EMMPRIN that differ only in its EC domain [6, 7]. The EC domain of the most prominent isoform, basigin‐2 and here forward called EMMPRIN, is comprised of 2 Ig‐like loops (EC1 and EC2) held together by disulfide bonds (Fig. 1). Sequence analyses and the crystal structure of EMMPRIN demonstrated that the EC1, the more N‐terminal portion, structurally resembles the β‐strand pattern of the C region of Igs, particularly the C2‐set fold, a subclass that has some structural features of the V region of Igs in addition to the C region. EC2, the C‐terminal loop, is slightly longer and showed strong homology to the V region of Igs, thus termed as a shortened V‐set fold or an I‐set [8, 9]. EMMPRIN is unique among Igs, as its EC2 domain has a high degree of homology to the V region of Igs as well as the MHCII β chain that contains a C region. For this reason, it is suggested that the EMMPRIN Ig domain may be a primordial form of the Ig superfamily [9, 10]. The C2–V/I arrangement of the EC portion of EMMPRIN is also distinctive among Igs, as most follow a V/I–C arrangement and undergo V–V or I–I dimerization [8]. Instead, the EC domain of EMMPRIN appears to undergo C2–C2 or C2–V/I dimerization based on the crystal structure [8]. As inferred, the Ig superfamily, which encompass the most diverse group of receptors known, contains domains that share evolutionary homology and are identified to be associated with functions, such as adhesion, migration, cell recognition, and T cell activation [11, 12], which EMMPRIN clearly exemplifies (as mentioned below). The complexity of EMMPRIN, in terms of its various glycosylation patterns, and types of homo/heterophilic interactions between domains all contribute to its multifunctional nature. A rare isoform also exists, expressed only in the retina, comprised of 3 Ig‐like loops and named basigin‐1 [13]. Other rare and less understood variants include basigin‐3 and ‐4, both comprising only 1 Ig‐like loop in which the EC domain of basigin‐4 is slightly extended from that of basigin‐3 [6]. Interestingly, basigin‐3 was found to act as an inhibitor of EMMPRIN (basigin‐2) likely by hetero‐oligomerization [7]. This was demonstrated in HCC cells, where in contrast to basigin‐2, basigin‐3 overexpression inhibited cellular proliferation, MMP induction, and invasion [7]. A highly conserved glutamic acid residue and leucine zipper are found in the TM domain and hypothesized to interact with a number of yet‐unidentified proteins in the plasma membrane. Although the cytoplasmic tail is short (only ∼40 aa), this does not rule out the involvement of EMMPRIN in the initiation of various signaling pathways, even as a coreceptor. The TM and cytoplasmic domains are highly conserved among species [14], thus emphasizing a critical, functional role in this region of EMMPRIN. A 22 kDa soluble form of EMMPRIN can be shed from the cell surface by MMP‐14, cleaving off the first Ig‐like domain [15]. In addition, full‐length EMMPRIN can be released by cells via microvesicle shedding, an efficient vehicle to elicit communication between cells [16, 17]. It is reasoned that the outward budding of EMMPRIN‐containing microvesicles from the plasma membrane of tumor cells shortly after breaks apart, giving rise to membrane fragment‐associated EMMPRIN in the EC space; it is this soluble form (not the microvesicle‐associated form) that primarily induced MMP activities in stromal cells [16]. Since then, studies have described the involvement of EMMPRIN‐containing microvesicles in aspects, such as angiogenesis [18] and the migration and invasion of cancer cells [19, 20].
Figure 1.
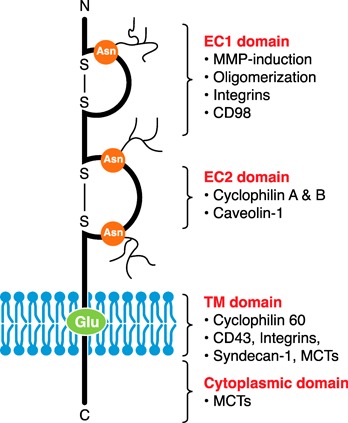
EMMPRIN structure. EMMPRIN is a single TM protein consisting of 3 N‐linked glycosylated sites at asparagine residues (Asn) in the EC region, a TM containing a highly conserved glutamic acid residue (Glu), and a short cytoplasmic tail. The EC region can be broken into 2 Ig‐like loops (EC1 and EC2), held together by disulfide bonds. Each domain of EMMPRIN interacts with different proteins, thus, having different functional capabilities. See text for details. C, C‐terminal; N, N‐terminal; S‐S, disulfide bond.
Each domain of EMMPRIN has been identified to interact with different proteins and correspond to particular functions, such that mAbs toward EMMPRIN may alter 1 function without affecting another. Importantly, N‐linked glycosylation of asparagine residues and homophilic interactions is significant for the function and protein interactions of EMMPRIN [3, 4, 8]; this was demonstrated when purified, deglycosylated EMMPRIN failed to induce MMP‐1 and MMP‐2 activity in tumor cells [5]. The EC1 domain of EMMPRIN is important in MMP induction (MMP‐1, ‐2, ‐3, and ‐9, as well as MT1‐MMP and MT2‐MMP), oligomerization, the binding of integrins, and likely CD98 (a single TM glycoprotein involved in adhesion and amino acid transport; Fig. 1) [14, 21]. The EC2 domain has been described to bind cyclophilins (important in chemotaxis and adhesion) [14] and caveolin‐1 (a negative regulator that binds EMMPRIN at its low glycosylated form, preventing its glycosylation and clustering at the cell surface; Fig. 1) [4]. The TM domain binds cyclophilin 60 (serving to chaperone EMMPRIN up to the cell surface) [22] and likely involves interactions with CD43 (an adhesion molecule) [23], syndecan‐1 (a HSPG involved in adhesion) [24], and β1 integrins [14] (Fig. 1). Lastly, both the TM and the cytoplasmic domain of EMMPRIN interact with MCTs [25], such as MCT1 or MCT4 (both widely expressed) or MCT3 (predominantly expressed in the retinal pigment epithelium). EMMPRIN shuttles MCTs to the plasma membrane, where it plays essential metabolic roles through the transport of monocarboxylates, such as pyruvate and lactate [2, 14] (Fig. 1). Other EMMPRIN‐interacting proteins include glycoprotein VI (involved in platelet rolling) [26], annexin II (implicated in cytoskeleton rearrangement) [27], shrew1 (influencing cell invasiveness) [28], and the γ‐secretase complex (implicated in Alzheimer's disease in the production of amyloid β peptides) [29].
EMMPRIN KNOCKOUT MICE AND EXPRESSION
To understand EMMPRIN function, EMMPRIN knockout mice had been generated. Although no gross abnormalities were observed histologically, these mice exhibited neurologic deficits associated with decreased sensitivity to irritating odors [30] and light as a result of blindness [31, 32–33], hypersensitivity to electric foot shock, as well as deficits in learning and memory [34]. These abnormalities appeared to correlate with the subregions of the mouse brain shown to express EMMPRIN [35]. No abnormalities were found at the BBB in the mutant mice, even though EMMPRIN is expressed on endothelial vessels in the CNS [30, 34]. Furthermore, EMMPRIN appears to be involved in early embryogenesis and reproduction, as EMMPRIN knockout mice were rarely born, and those that did survive were small, weak, and sterile, in which half died within 1 month as a result of interstitial pneumonia [36].
EMMPRIN is expressed on a wide variety of cell types at varying levels, including hematopoietic, epithelial, endothelial, and tumor cells. Expression profiling of EMMPRIN at the transcript level showed that in humans, EMMPRIN is highly expressed in the heart and skeletal muscle compared with other tested organs, likely demonstrating its importance in metabolism [7]. The kidney also has high expression levels, as does the testis correlating to its involvement in spermatogenesis [7]. Of the hematopoietic cells, EMMPRIN is expressed on all leukocytes (monocytes, granulocytes, and lymphocytes) [37], erythrocytes [38], and platelets [39]. EMMPRIN is up‐regulated on activated T cells and also shown recently to serve as a marker for aTregs (CD25+EMMPRIN+) versus rTreg or naive Treg (CD25+EMMPRIN−) in the human system [40, 41]. Furthermore, the labeling with FoxP3, a well‐known intracellular Treg marker, and EMMPRIN enables the identification of 3 distinct populations: 1) EMMPRINlowFoxP3+ cells representing rTregs; 2) EMMPRINmediumFoxP3+ cells, representing Tregs that can produce cytokines (reviewed in ref. [41]), including IL‐2, IFN‐γ, TNF‐α, and IL‐17; and 3) EMMPRINhighFoxP3+ cells, representing aTregs that do not secrete high levels of cytokines, demonstrate highly suppressive activity, and exhibit demethylation in the Treg‐specific demethylated region of the FOXP3 gene, a region whose demethylation status correlates to stabilized FoxP3 expression and suppressive activity [40]. It is unknown if EMMPRIN plays a role in immune homeostasis, but it may serve as a useful marker for Treg subsets when evaluating disease progression.
EMMPRIN FUNCTION IN T CELLS
EMMPRIN, first characterized as an MMP inducer, is now known to have multiple other functions in different cell types, including T cells [42]. Evidence supports a functional role for EMMPRIN at many different stages of T cell activity, such as T cell development, activation, proliferation, migration, adhesion, and invasion ( Fig. 2 ); these are steps important in the pathogenesis of various diseases. Interestingly, EMMPRIN exemplifies positive/inducing actions and negative/regulatory actions in T cells. Here, we review the details of what is known about the function of EMMPRIN in T cell biology.
Figure 2.
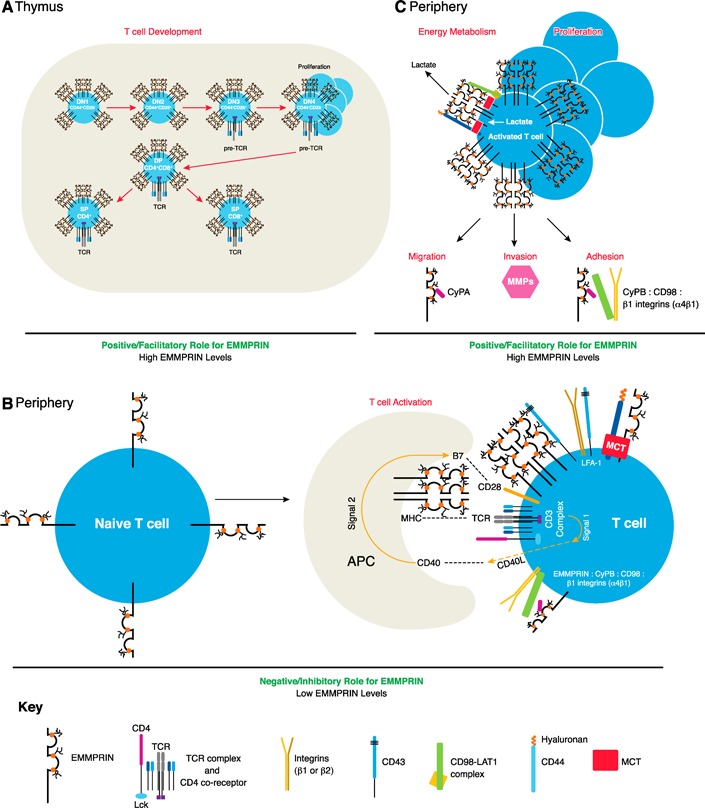
The role of EMMPRIN in T cell biology. EMMPRIN is involved in T cell development, activation, proliferation, migration, invasion, adhesion, and energy metabolism. It is highly expressed in thymocytes during T cell development, playing a role in the transition phase from DN3 to DN4, where a proliferative burst occurs (A). In the periphery, EMMPRIN expression levels are low and localize to the immune synapse upon TCR‐mediated T cell activation. TCR‐MHC engagement leads to the up‐regulation of CD40 ligand (CD40L; Signal 1), allowing for B7‐CD28 costimulation (Signal 2). APCs also express EMMPRIN, where it may act as an alternate costimulator to provide “Signal 2” for T cell activation. EMMPRIN interacts with a number of molecules regulating adhesion (see Fig. 3), which could play a role in immune synapse formation (B). Once T cells are activated, EMMPRIN levels are up‐regulated and involved in a number of actions (C; see text for further details). MHC, major histocompatibility complex.
EMMPRIN in T cell development
The thymus is the primary site of T cell ontogeny, where T lymphocyte precursors develop to yield mature CD4+ or CD8+ T cells. Upon entry into the thymus, the T cell precursors are classified as DN thymocytes, in which CD4+ and CD8+ surface markers are not expressed. The first stage of development can be separated into 4 DN phases, distinguished by CD25 and CD44 expression (Fig. 2A). Here, the TCR‐β chain is tested for functionality, and a dramatic expansion of functional pre‐TCRs ensues, marking the end of the DN phase. The second stage of T cell development includes the DP thymocytes, which are positive for CD4 and CD8 surface markers, have undergone gene rearrangements for the TCR‐α chain and express complete TCR:CD3 complexes. Selection processes then occur leading to the 3rd stage: positive selection of CD4+ or CD8+ T cells to yield SP thymocytes and negative selection, which causes the elimination of potential autoreactive T cells by apoptosis [43] (Fig. 2A).
EMMPRIN is highly expressed on the surface of all thymocytes (Fig. 2A) and shown to influence their maturation [44, 45]. Whereas all thymocytes express EMMPRIN, the mean fluorescence intensity was higher on cells that cycled more, particularly during the DN3–DN4 transition, where TCR‐β‐selected cells undergo a proliferative burst [45]. The presence of an anti‐EMMPRIN antibody (clone RL73.2) halted murine thymocyte development by largely reducing levels of the DN4 (CD44−CD25−) stage, as well as the DP (CD4+CD8+) and CD4+ SP populations [45]. These results were corroborated by the newly generated EMMPRIN‐floxed mice, where EMMPRIN was conditionally ablated in T cells by use of the Lck‐cre promoter [46]. The conditional deletion of EMMPRIN in Lck‐cre‐expressing T cells, similar to the addition of anti‐EMMPRIN antibodies, resulted in a decrease in DP (CD4+CD8+; not a result of enhanced apoptosis) and CD4+ SP cells. A significant drop in thymus weight and cell number was also apparent. Although the DN phase as a whole showed no alterations in EMMPRIN−/− thymocytes, the 4 DN stages were not assessed separately to analyze if there were any differences at the DN4 stage [46]. As the cycling ability of thymocytes was suggested to correlate with EMMPRIN‐expression levels, it is reasonable to assume that EMMPRIN plays a role in the dramatic expansion of the DN4 phase. The rapid proliferation of thymocytes likely undergoes glycolysis to meet energy demands, thus leading to the buildup of toxic intracellular lactate levels [47]. In this way, it is possible that EMMPRIN is needed as a chaperone to shuttle MCTs up to the surface to export the toxic lactate build‐up out of the cells. The absence of EMMPRIN would hinder this export, possibly leading to cell death or alternate metabolic pathways.
EMMPRIN in T cell activation and proliferation
Evidence illustrates that EMMPRIN plays a role in T cell activation and proliferation; however, it seems to display different functional responses at particular phases of T cell activity. As EMMPRIN levels are high in thymocytes (Fig. 2A), its levels in the periphery of resting T cells are low (Fig. 2B) [44] and become up‐regulated upon T cell activation (Fig. 2C) [44, 48, 49]. It was demonstrated that EMMPRIN negatively regulates/inhibits T cell proliferation in a MLR, where splenocytes from EMMPRIN−/− animals exhibited an enhanced, proliferative response compared with wild‐type splenocytes when cocultured with irradiated BALB/c splenocytes [30]. A similar response was observed in anti‐CD3/CD28‐stimulated, EMMPRIN−/− T cells from Lck‐Cre;EMMPRIN flox/flox mice, which also revealed elevated CD69 levels, an early T cell activation marker [46]. At the resting state, spleen weight and proportions of CD8 and CD4 expression levels were no different between EMMPRIN−/− T cells and their controls [46]. In the human system, knockdown of EMMPRIN by use of siRNA in primary cells also increased T cell proliferation (without any changes in CD25 expression) upon activation with PHA, a surface receptor cross‐linker [50]. These results demonstrate EMMPRIN having a negative/inhibitory effect on T cell activation.
In contrast, numerous studies that use antibodies against EMMPRIN (e.g., clone MEM‐M6/6, 5A12, or clone 10) have shown that these antibodies suppress T cell proliferation in human PBMCs [48, 51, 52, 53–54]. It is suggested that anti‐EMMPRIN antibodies may be acting as agonists, mimicking ligand engagement [51, 52]. Interestingly, EMMPRIN−/− T cells or anti‐EMMPRIN antibodies only altered the proliferation of T cells when stimulated with anti‐CD3/CD28 and not with PMA/ionomycin (a stimulant that bypasses the need for surface receptors). This implies that EMMPRIN is involved at the very early stages of T cell activation involving TCRs or costimulators [46, 51]. Minutes after TCR‐dependent activation, EMMPRIN aggregates to immune synapses (Fig. 2B), an area formed upon T cell activation that is densely packed with receptors and signaling molecules pivotal in initiating T cell activation. EMMPRIN colocalized with immune synapse markers, including CD4, CD8, CD48, and GM1 [51, 52], whereas the presence of anti‐EMMPRIN antibodies (both clone MEM‐M6/6 and 5A12) altered the formation of these immune synapses [51, 52]. Administration of the MEM‐M6/6 clone upon TCR‐mediated activation displaced the T cell coreceptors CD48 and CD59 from lipid rafts, leading to reduced expression of the T cell activation marker CD25 and hence, reduced T cell proliferation. Transcripts for IFN‐γ, IL‐15, IL‐4, and IL‐10, among other cytokines (12 hours after activation), were also diminished. However, not all microdomains were affected by this antibody, as the distribution pattern of Lck did not change nor did tyrosine phosphorylation levels (5 minutes after activation), as well as IL‐2 levels (24 hours after activation) [51]. Another EMMPRIN antibody, 5A12 clone (which docks to the EC1 domain of EMMPRIN), had similar and contrasting effects from that of the MEM‐M6/6 clone (which recognizes the EC2 domain) upon TCR‐mediated activation of human T cells. The 5A12 clone demonstrated reduced CD25 and IL‐4 protein levels; however, the 5A12 clone also decreased tyrosine phosphorylation (2 hours after activation), EC calcium influx, and IL‐2 protein levels, in addition to increased IFN‐γ protein levels (72 hours after activation) [52]. The differences in antibody responses could be a result of differences in experimental methods (e.g., the time analyzed after T cell activation) or perhaps a result of the different functional domains/epitopes of EMMPRIN that the antibodies recognize and to which they bind ( Table 1 ). If these antibodies act as agonists, this suggests that the inhibitory effect of EMMPRIN upon TCR‐dependent activation works to trigger the dissociation of certain molecules from microdomains to help shut down T cell responses. The mechanism behind the involvement of EMMPRIN in T cell activation still needs to be defined clearly.
Table 1.
EMMPRIN antibodies
| Species | EMMPRIN mAb | Binding domain | Effect on function | Reference |
|---|---|---|---|---|
| Human | MEM‐M6/6 (IgG1) | EC2 | •Altered immune synapse formation upon TCR‐mediated activation of T cells | [48], [51], [55], [56] |
| •Decreased proliferation of PBMCs and purified T cells | ||||
| •Decreased CD25, cytokine expression upon TCR‐mediated activation of T cells (no effect on tyrosine phosphorylation and IL‐2 levels) | ||||
| •Prevented pathogenic invasion (meningitis, malaria) | ||||
| 5A12 (IgG1) | EC1 | •Altered immune synapse formation upon TCR‐mediated activation of T cells | [52] | |
| •Decreased proliferation of purified T cells | ||||
| •Reduced CD25, IL‐4, tyrosine phosphorylation, calcium influx, IL‐2 levels; increased IFN‐γ | ||||
| MEM‐M6/1 (IgG1) | EC1 | •Reduced tyrosine phosphorylation levels in TCR‐stimulated T cells from SLE patients (not healthy patients) | [23], [51], [57] | |
| •Did not affect MMP activity | ||||
| •Did not inhibit T cell proliferation | ||||
| •Did not induce cell aggregation | ||||
| HI197 (IgG1) | ? | •Decreased TCR‐mediated proliferation of T cells when pre‐exposed to U937 myeloid cells before coculture | [58] | |
| MEM‐M6/8 (IgG1) | EC1 | •Induced cell aggregation and adhesion in Jurkat T cells | [23] | |
| HAb18 (IgG1) | EC1 | •Did not inhibit proliferation of purified T cells | [52], [59], [60] | |
| •Induced cell aggregation of Jurkat cells | ||||
| •Decreased CyPA‐mediated migration of Jurkat cells | ||||
| •Prevented MMP activity of fibroblasts in coculture | ||||
| M6‐1F3 (IgM) | Not EC1 nor EC2 | •Induced cell aggregation in U937 cells | [54] | |
| •Did not inhibit TCR‐mediated lymphocyte proliferation | ||||
| M6‐1B9 (IgG3) | EC1 | •Did not induce cell aggregation in U937 cells | [54] | |
| •Inhibited TCR‐mediated lymphocyte proliferation | ||||
| •Inhibited CD25 expression, IL‐2 production | ||||
| UM‐8D6 (IgG1) | EC1 | •Inhibited MMP‐9 secretion | [49], [58], [61] | |
| •Decreased T cell proliferation | ||||
| •Decreased CyPA‐mediated migration | ||||
| Mouse | RL73.2 (IgG2a) | ? | •Altered murine thymocyte development | [38], [45], [62], [61], [63], [64], [65] |
| •Decreased CyPA‐mediated migration | ||||
| •Inhibited CIA, lung inflammation, EAE, infarct size | ||||
| •Did not inhibit proliferation of MOG‐primed T cells | ||||
| •Did not inhibit the release of MMP‐9 | ||||
| Human and mouse | Clone 10 (IgM) | EC1 | •Inhibited EAE | [53], [66] |
| •Inhibited MMP‐9 secretion | ||||
| •Inhibited T cell proliferation | ||||
| •Reduced α4 integrin levels | ||||
| •Decreased T cell adhesion to endothelial cells |
mAbs toward EMMPRIN demonstrate additional differences. In the mouse, the anti‐EMMPRIN mAb (RL73.2 clone) was able to inhibit the migration of T cells and monocytes toward CyPA and reduce disease severity of mouse models of RA and lung inflammation (see section below) [61, 62–63]; however, the antibody was unable to inhibit the proliferation of MOG‐primed T cells [64] or inhibit the release of MMP‐9 in a coculture of fibroblasts and mouse macrophages. In contrast, the anti‐EMMPRIN clone UM‐8D6 was able to inhibit MMP‐9 secretion by 90% [61]. A novel anti‐EMMPRIN antibody, clone 10, suppressed EAE disease activity and inhibited T cell proliferation, as well as MMP‐9 production [53]. It was also observed that EMMPRIN mAbs, which induced cell aggregation (e.g., M6‐1F3) of U937 cells, were not able to inhibit cell proliferation of human T cells and vice versa (e.g., M6‐1B9) [54]. A summary of the different antibodies discussed in this review and their affects on the activities of EMMPRIN are outlined (Table 1).
Evidence also suggests that EMMPRIN may act as an alternate costimulatory molecule. T cell activation and proliferation require an initiation event (Signal 1, provided by TCR engagement) and a costimulatory event (Signal 2; Fig. 2B). Studies suggest that EMMPRIN expression on APCs, rather than on T cells, may be what is important in allowing for T cells to proliferate [58, 67, 68]. This was demonstrated when “Signal 1” provided by anti‐CD3 stimulation did not proliferate T cells when mixed with irradiated Signal 2‐supplying U937 cells, prepulsed with an anti‐EMMPRIN antibody (clone HI197). U937 cells are a human monoblastoid cell line that lacks B7 (Fig. 2B); thus, they depend on alternate costimulatory pathways to provide Signal 2 [58]. This result was confirmed by identifying EMMPRIN as one of the candidate genes involved in Chinese hamster ovary [67] or dendritic cell [68]‐mediated costimulation of T cells. This implicates that EMMPRIN on APCs, and not T cells, may be acting as an important costimulatory molecule for T cell activation. Yet, studies have also demonstrated proliferative inhibition by anti‐EMMPRIN antibodies (by use of clone 5A12 and MEM‐M6/6) on purified T cells, as well as PBMCs [51, 52]. This implies that EMMPRIN expression on APCs as well as T cells has important implications for T cell activation.
Once a T cell is activated, and EMMPRIN levels are elevated, EMMPRIN evolves to have positive/inducing T cell actions. Actions, such as migration, MMP induction, adhesion, and energy metabolism (activities discussed in the sections below), are reduced in the absence of EMMPRIN and even upon the administration of certain antibodies against EMMPRIN (Fig. 2C).
EMMPRIN in T cell migration
EMMPRIN plays a role in the recruitment of cells into inflamed tissue. EMMPRIN has been identified as the main receptor for EC CyPA, a chemotactic factor for leukocytes (Fig. 2C) [69]. Cyclophilins are abundant intracellular proteins known for their binding to the immunosuppressive drug, cyclosporine A; however, it has been reported that cyclophilins are released into the EC space of inflamed tissue, such as the synovial fluid of patients with RA [70], sepsis [71], and vascular smooth muscle cell disease [72]. The importance of CyPA‐EMMPRIN interactions in recruiting leukocytes (neutrophils, eosinophils, monocytes, and T cells) and mediating inflammatory diseases has been demonstrated by blocking studies that use an anti‐EMMPRIN antibody (RL73.2 clone) and/or the nonimmunosuppressive cyclosporine A analog in mouse models of acute lung inflammation [62], asthmatic inflammation [63], and RA (via CIA) [61]. EMMPRIN‐dependent migration of activated T cells was also demonstrated in the human system when anti‐EMMPRIN antibodies (UM‐8D6) inhibited CyPA‐mediated migration and not migration stimulated by RANTES [49]. In addition to CyPA, EMMPRIN is a receptor for CyPB [73], which induces chemotaxis of T cells in vitro comparable to RANTES [63]. Thus, therapeutic targeting of cyclophilin‐EMMPRIN interactions in inflammatory diseases may be a future possibility.
EMMPRIN in T cell invasion
MMP induction is required for T cells to invade through the basement membrane and drive disease pathogenesis [74]; thus, ways to inhibit MMP production are critical. It was shown that the MMP‐9 produced from a PBMC culture upon T cell activation was suppressed by the administration of an EMMPRIN mAb (clone 10) [53]. In patients with T cell lymphomas, EMMPRIN was found highly expressed on peripheral blood T cells compared with healthy controls, and biopsies of skin invasions demonstrated that infiltrating T cell lymphomas were in regions of MMP‐2+ fibroblasts [75]. Moreover, an activity‐blocking peptide against EMMPRIN (corresponding to the EC1 domain) inhibited the production of MMP‐2 in an experiment by use of transformed lymphocytes (MT2) cocultured with EMMPRIN‐negative fibroblasts [75]. Another group also demonstrated increased MMP expression by human T cell lines in the presence of fibronectin [76]. Thus, T cells with high levels of EMMPRIN may facilitate invasion via MMP induction through contact with fibroblasts or other substrates (Fig. 2C).
EMMPRIN in T cell adhesion
A number of adhesion molecules associate with EMMPRIN on the cell surface, important for cell–cell contact for events, such as antigen presentation, or to enter sites of inflammation. In erythrocytes, EMMPRIN appears to function as an adhesion molecule, as administration of F(ab′)2 fragments of the anti‐EMMPRIN antibody (clone RL73.2) inhibited the migration of murine erythrocytes out of the spleen and into the circulation causing anemia [38]. In platelets, EMMPRIN interactions were shown to be involved in their rolling [39]. Likewise, in Jurkat cells, a T‐lymphoma cell line, siRNA‐mediated knockdown of EMMPRIN reduced their adhesion to fibronectin [77]. It is noteworthy that EMMPRIN oligomerization is important in increasing the avidity of EMMPRIN interactions with other molecules and with itself for its various functional purposes ( Fig. 3A ) [3, 5, 66, 75]. Coimmunoprecipitation and immunofluorescence experiments demonstrated the interaction of EMMPRIN (likely in a lateral fashion) with α3β1 and α6β1 integrins but not α2β1 or α5β1 integrins in the membrane at sites of cell–cell contact in cell lines [78] (Fig. 3B). CyPB, an EMMPRIN ligand, along with glycosaminoglycans, was shown to play a role in the adhesion of human PBMCs (mainly of the CD4+ CD45RO+ phenotype) to the ECM [79]. Furthermore, syndecan‐1, a HSPG present on the cell surface of T cells, also associates with EMMPRIN (likely in the TM region) and is involved in adhesion to fibronectin via CyPB‐induced MAPK activation [24] (Fig. 3C). Another report identified EMMPRIN in a complex with CD98, β1 integrins, and CyPB in a human promonocytic leukemia THP‐1 cell line that mediated cell adhesion to fibronectin [81] (Fig. 3D). The binding of CyPB is thought to catalyze peptidyl‐prolyl cis‐trans isomerase activity in EMMPRIN, triggering intracellular signaling events that induce cell aggregation or integrin‐mediated adhesion through a PI3K‐protein kinase Cδ‐MAPK pathway. It is proposed that CD98 can regulate β1 integrin affinity and was reported to coimmunoprecipitate with α4β1 integrins in PBMCs [81]. Hyaluronan‐CD44‐MCT‐EMMPRIN complexes were also shown to exist in human breast adenocarcinoma cell lines [83] (Fig. 3G). Besides its metabolic roles, in T cells, the best established function of CD44 is its adhesive capabilities [86]. In addition, the presence of a mAb toward EMMPRIN (clone 10) on activated human PBMCs was reported to reduce α4 integrin and NF‐κB signaling, leaving Akt and MAPK signaling unaffected, and clone 10 decreased T cell adhesion to endothelial cells [66].
Figure 3.
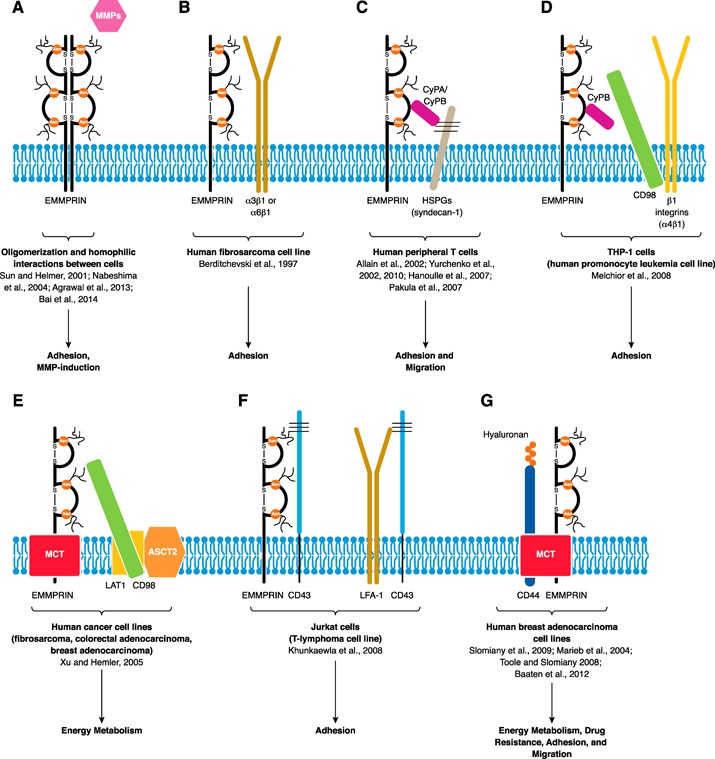
EMMPRIN interactions. EMMPRIN can interact with a wide range of binding partners, such as itself forming oligomers (increasing its avidity for other interacting partners) and/or through homotypic interactions between cells, allowing for MMP induction (A). EMMPRIN interacts with β1 integrins (B). EC cyclophilin (CyPA/B) may bind and isomerize EMMPRIN in an HSPG‐dependent fashion, likely triggering intracellular signaling events, allowing for migration and/or integrin‐mediated adhesion. Upon T cell activation, increased EMMPRIN oligomerization allows CyPA to bind without the need for HSPGs. CyPB associates by interacting with the heparin sulfates on syndecan‐1 and the EC regions of EMMPRIN (C). EMMPRIN also interacts with CD98 (likely via the EC region of EMMPRIN) and β1 integrins (D). Interestingly, a MCT‐EMMPRIN‐CD98‐LAT1‐ASCT2 supercomplex exists, demonstrating that CD98 is involved in regulating EMMPRIN‐mediated adhesion and energy metabolism (E). Competitive binding of EMMPRIN for CD43 with LFA‐1 affects the adhesive capability of T cells (F). Lastly, a hyaluronan‐CD44‐MCT‐EMMPRIN supercomplex is shown, important in energy metabolism and drug resistance; in T cells, hyaluronan‐CD44 is important in adhesion and migration (G).
The addition of various EMMPRIN mAbs alone to the U937 monocytic cell line would initiate homotypic cell aggregation via LFA‐1, a β2 integrin [54, 87]. In the human Jurkat T cell leukemia cell line, EMMPRIN mAbs (MEM‐M6/8 and/or HAb18) also induced homotypic aggregation [23, 59] and adhesion to the LFA‐1 ligand, ICAM‐1; this adhesion was also regulated by the presence of CD43 [23]. All 3 adhesion molecules, CD18 (representing LFA‐1), CD43, and EMMPRIN, colocalized together at sites of cell–cell contact; however, 2 distinct CD43 complexes with EMMPRIN or LFA‐1 existed based on coimmunoprecipitation experiments [23] (Fig. 3F). CD43 is a sialoglycoprotein that is highly glycosylated containing a net negative charge; thus, it likely is able to repulse cell contacts, which is important for cell migration, but can still permit cell contacts in the first place. It is probable that CD43 interactions with EMMPRIN or LFA‐1 may inhibit their adhesive capabilities. Moreover, it was shown recently that in the presence of an anti‐EMMPRIN antibody (HAb18), alone, in Jurkat cells or CD4+ primary cells, cell aggregation increased with time, whereas CyPA‐induced chemotaxis decreased; these results imply a functional trade‐off for EMMPRIN [59]. Interestingly, knocking down EMMPRIN in Jurkat cells by RNA interference also induced cell aggregation and decreased levels of CD98 [59]. This is surprising, as EMMPRIN and CD98 are thought to mediate adhesion. Clearly, EMMPRIN and CD98 expression levels are linked. It is speculated that EMMPRIN may inhibit cell aggregation and that the addition of an anti‐EMMPRIN antibody may work to inhibit EMMPRIN in carrying out this particular negative role (thus acting as an antagonist instead of an agonist) [59]. Even though EMMPRIN is shown to be involved in adhesion (as demonstrated in the paragraphs above), different adhesion complexes likely exist, and treatment with EMMPRIN mAbs may cause rearrangement of these adhesion complexes.
EMMPRIN seems to influence the adhesive ability of 2 prominent T cell integrins, α4β1 as well as LFA‐1, which are important in antigen presentation and the adhesion of T cells to endothelial cells upon entry into sites of inflammation (Fig. 2B and C). Distinct complexes of adhesion molecules may have a positive or negative role in T cell adhesion. Clarity is still needed to understand the role of EMMPRIN in cellular adhesion and what its influences are.
EMMPRIN in energy metabolism
The rapid rate of T cell proliferation requires cells to undergo glycolysis (instead of oxidative phosphorylation) to meet their fast‐paced energy demands [47]. Although glycolysis generates energy faster than oxidative phosphorylation, it is a less efficient approach and leads to the accumulation of lactic acid byproducts. In cancer, this altered energy metabolism is known as the Warburg effect, an effect that EMMPRIN appears to regulate. As mentioned previously, EMMPRIN is an important player in alleviating the toxic build‐up of lactic acid in cancers by shuttling lactate transporters—MCTs—up to the plasma membrane [14, 25, 88]. The silencing of EMMPRIN in pancreatic or breast cancer cells inhibited MCT1 and MCT4 expression, reduced their growth, and decreased their glycolytic rate and lactate efflux [83, 89]. An MCT‐EMMPRIN‐CD98‐LAT1 supercomplex with a neutral amino acid transporter (ASCT2) was shown to exist in human fibrosarcoma, colorectal adenocarcinoma, and breast adenocarcinoma cell lines, demonstrating importance in cellular metabolism [82] (Fig. 3E). Moreover, hyaluronan‐CD44‐MCT‐EMMPRIN complexes exist in human breast adenocarcinoma cell lines [83] (Fig. 3G). That being said, lactate transport is also important in lymphocyte activation [47], where inhibition of MCT1 (an interacting partner of EMMPRIN) during T cell activation inhibited T cell proliferation and lactate efflux [90]. Thus, EMMPRIN may play a role in alleviating toxic lactic acid byproducts during the rapid proliferation of T cells (Fig. 2C), an area requiring investigation.
IMMUNOLOGIC DISEASES KNOWN TO INVOLVE EMMPRIN
Cancer/lymphoma
EMMPRIN is up‐regulated in a number of different cancers, in which its levels correlate with a poor prognosis in cancer patients [21, 91, 92, 93–94]. MMPs have been identified as potential therapeutic targets in cancers for a long time [95, 96–97]; thus, EMMPRIN, an MMP‐inducer, is becoming recognized as a suitable candidate for therapeutic intervention. EMMPRIN has been shown to promote multiple stages of tumor progression, such as tumor cell proliferation, invasiveness, and metastasis, via MMP induction [98, 99]; angiogenesis by stimulating VEGF production via the PI3K pathway [100, 101]; anchorage‐independent growth [84]; chemoresistance [85]; and lactate efflux (see section above on energy metabolism) [2, 82, 83, 89]. With all of these positive roles for EMMRIN in tumor growth and biology, more and more groups are exploring and understanding the benefit of the use of anti‐EMMPRIN antibodies as a potential tumor therapy, including HCC (HAb18 and Licartin) [102] and head and neck cancer (CNTO 3899 clone) [103].
In relation to T cell biology, glycosylated EMMPRIN is highly expressed in Jurkat cells compared with normal human PBMCs [77, 104]. The silencing of EMMPRIN in Jurkat cells decreased its proliferation, migration, and adhesion to fibronectin; this emphasizes the importance of EMMPRIN in the survival and growth of cancer cells. As noted before, the absence of EMMPRIN in primary T cells led to their hyperproliferation [30, 46, 50]. The contradictory results can be explained by the proliferation of cancer cells acting through a mechanism not dependent on TCR‐mediated signaling events, as is the case in primary T cells. When Jurkat cells were cocultured with superantigen‐loaded Raji B cells, thus subjected to conjugate formation, EMMPRIN aggregated at T cell immune synapses. When Jurkat cells were also stimulated with anti‐CD3 antibodies to initiate TCR‐dependent signaling events, contrary to TCR‐independent mechanisms [77], EMMPRIN overexpression inhibited T cell activity, as demonstrated by NFAT inhibition (an essential transcription factor in T cell activation), whereas EMMPRIN knockdown promoted NFAT signaling activity (events umpired by the intracellular domain of EMMPRIN) [104]. The inhibitory effect of EMMPRIN on TCR‐mediated activation in Jurkat cells occurred downstream of the Vav1 (a guanine nucleotide exchange factor for Ras‐related C3 botulinum toxin substrate 1) signaling pathway and upstream of the JNK and serine/threonine‐protein kinase 1 pathway; ERK signaling remained unaffected. Besides signaling, functional activities, such as proliferation, however, was not assessed [104]. This supports that TCR‐mediated activation brings out a regulatory/inhibitory effect of EMMPRIN at the early stages of T cell activity. However, in TCR‐independent activation/proliferation, as found in cancer cells (such as lymphomas) or in activated autoimmune T cells (such as in SLE), EMMPRIN (highly up‐regulated compared with resting primary T cells) may act in a positive or stimulatory manner for activities, such as adhesion, invasion, migration, or aiding, in the Warburg effect.
SLE
SLE is a systemic autoimmune disease associated with abnormal T and B cell functions leading to the dysfunction of a number of organs [105]. It is reported that T cells from PBMCs of SLE patients are hyperactivated and secrete enhanced levels of MMP‐9 compared with healthy controls [106]. It was later shown that the enhanced, secreted MMP‐9 activity correlated with enhanced EMMPRIN expression on CD3+ T cells in these patients [57]. The application of the anti‐EMMPRIN antibody (clone MEM‐M6/1) reduced tyrosine phosphorylation levels in anti‐CD3/anti‐CD28‐stimulated T cells from SLE patients, although this did not affect MMP activity [57]. It is possible that the administration of other anti‐EMMPRIN antibody clones could reduce MMP activity. All in all, the dampening of T cell responses with EMMPRIN mAbs could be a useful therapeutic strategy in SLE; however, further studies are required to validate this approach.
GVHD
When an individual requires the transplantation of stem cells, bone marrow cells, or other forms of tissue grafts, therapies to suppress the immune system are taken to prevent the transplanted donor cells from attacking the recipient, a complication known as GVHD. Despite these immunotherapies (with steroids usually being the first‐line treatment), acute GVHD still persists in a fair percentage of cases and many die, frequently of infections, thus the need for alternate secondary therapies [107, 108]. A phase 1/2 clinical trial that uses ABX‐CBL (also known as gavilimomab), an IgM mAb that recognizes EMMPRIN, for the treatment of steroid‐resistant acute GVHD had shown encouraging results [109]. ABX‐CBL recognizes EMMPRIN, up‐regulated on activated T and B cells, as well as on resting and activated monocytes and dendritic cells, and depletes these cell populations though complement‐mediated mechanisms. Resting lymphocytes that express low levels of EMMPRIN were shown to remain unaffected in vitro [109]. It was reported that patients did experience myalgia, but this was manageable with narcotics. An additional phase 2/3 multicenter‐randomized clinical trial of ABX‐CBL was completed for steroid‐resistant acute GVHD; this again showed that ABX‐CBL was well tolerated with a similar response rate as before but did not gain Food and Drug Administration approval, as its response was not improved compared with antithymocyte globulin, an alternate, second‐line therapy [110]. Indeed, ABX‐CBL did show activity and good tolerance, thus has potential as a future treatment for other inflammatory diseases or in combination with other treatments.
RA
As EMMPRIN can induce MMP production, factors that can lead to cartilage and bone destruction [111], its role was investigated in RA. EMMPRIN is up‐regulated in the rheumatoid synovial membrane [112], where its expression was found to correlate to MMP‐1 and MMP‐3 production in the synovial tissue [113]. Indeed, EMMPRIN is involved in the pathogenesis of RA, as administration of the anti‐EMMPRIN antibody (RL73.2 clone) in its animal model suppressed the development of CIA by reducing joint inflammation by >75% [61].
In the peripheral blood of RA patients, monocytes/macrophages showed increased expression of EMMPRIN compared with healthy controls and a further increase in the synovial fluid. Anti‐EMMPRIN antibodies and EMMPRIN antagonistic peptides prevented the CyPA‐mediated chemotaxis of mononuclear cells, as well as MMP‐9 and MMP‐2 production and invasive properties of human fibroblasts cocultured with monocytes [114]. FLS from RA patients also possessed high EMMPRIN expression levels, where EMMPRIN antagonistic peptides inhibited their production and activation of MMPs, as well as their invasive potential in a coculture with human monocytic THP‐1 cells [115]. This demonstrates a potential therapeutic role for anti‐EMMPRIN antibodies in preventing RA progression.
The elevated expression of EMMPRIN found on neutrophils in the peripheral blood of RA patients compared with controls was also shown to play a role in neutrophil chemotaxis via CyPA. Anti‐EMMPRIN antibodies (HAb18) prevented MMP production and activation and invasive capabilities of RA FLS, when cocultured with RA neutrophils [60]. In addition to MMP induction, this same group demonstrated the involvement of EMMPRIN in the induction of VEGF and HIF‐1α in human RA FLS, thus contributing to angiogenesis and disease progression. Anti‐EMMPRIN antibody treatment of a severe combined immunodeficiency mouse model of RA decreased expression of VEGF and HIF‐1α, improving outcomes [116, 117]. With its involvement in MMP induction and angiogenesis in RA, EMMPRIN mAbs seem like a promising therapy. It is even reported to have stronger antiangiogenic effects than infliximab, an agent approved for RA treatment [116].
MS
MS is an immune‐mediated disorder of the CNS characterized by the generation of autoreactive T cells, demyelination, and axonal degeneration, where the standard disease‐modifying treatments are immunomodulators [118]. EMMPRIN was found up‐regulated in leukocytes in active lesions in MS and its animal model, EAE. EMMPRIN colocalized with T cells, macrophages, and astrocytes in inflammatory perivascular cuffs of EAE and MS, where its up‐regulation was associated with increased MMP activity [66]. Furthermore, administration of anti‐EMMPRIN antibodies (RL73.2 clone or clone 10) reduced the severity of EAE [53, 64], emphasizing its role in MS pathogenesis. Anti‐EMMPRIN antibody treatments reduced CNS infiltrates, the number of perivascular cuffs, and MMP activity [64]. In vitro, treatments reduced the adhesion and migration of leukocytes across the BBB endothelial cell [66], as well as T cell neurotoxicity, demonstrating reduced granzyme B levels compared with the isotype control [53]. As EMMPRIN is involved in all critical stages of MS disease, including T cell activation and proliferation; chemotaxis of leukocytes, adhesion, and migration across the BBB; MMP induction, which is essential to enter into the brain parenchyma; and neurotoxicity, its multifunctional nature would make for an excellent therapeutic target.
Atherosclerosis and acute myocardial infarction
Atherosclerosis is an inflammatory disease of the vascular wall that may lead to acute myocardial infarction (diminished blood supply to the heart) or stroke (diminished blood supply to the brain) as a result of the build‐up and/or rupture of plaques inside arteries. Plaques consist of an accumulation of inflammatory cells and subsequent production of MMPs, which may lead to plaque breakdown and surrounding cell death, thereby worsening the disease progress [119, 120, 121–122]. EMMPRIN expression in patients with acute myocardial infarction was found up‐regulated on circulating monocytes and correlated with monocytic MT1‐MMP levels and MMP‐9 plasma activity [123]. As platelets and monocytes play a pathogenic role in atherosclerosis, it was demonstrated that EMMPRIN–EMMPRIN interactions in a coculture of platelets with monocytes induced the secretion of MMPs, proinflammatory (IL‐6 and TNF‐α), and anti‐inflammatory (IL‐10) cytokines in monocytes via an NF‐κB‐mediated pathway [39]. In addition, a mouse model of myocardial infarction demonstrated that EMMPRIN+/− mice, mice treated with an anti‐EMMPRIN antibody (RL73.2 clone), or CyPA−/− mice exhibited a reduced infarct size compared with controls; this is likely a consequence of impaired CyPA‐mediated recruitment and adhesion of monocytes and neutrophils to the injured site [65]. This suggests that EMMPRIN plays a role in leukocyte recruitment and MMP induction in cardiovascular disease, and thus, the targeting of cyclophilin‐EMMPRIN interactions may be of a therapeutic benefit. However, a balance between harmful inflammatory monocytes and beneficial reparative monocytes involved in MMP‐dependent remodeling and the development of scar tissue must be considered [124].
Lung interstitial fibrosis
Lung interstitial fibrosis is a chronic lung disease, as a result of excessive accumulation of the ECM, resulting in scarring and thickening of the lung. Its cause is unknown and has very few effective therapies [125]. EMMPRIN expression was found increased in murine and human lung fibrosis in alveolar macrophages and epithelial cells [126, 127]. Treatment with anti‐EMMPRIN mAbs (HAb18) in a mouse model of lung interstitial fibrosis reduced clinical symptoms and decreased M1 macrophage and Th17 infiltration [128]. It was also discovered that M1 macrophages induced Th17 differentiation in an EMMPRIN‐dependent manner [128]. The cellular mechanism behind this is currently unknown and may involve cytokine regulation. The understanding of whether EMMPRIN is involved in the regulation of Th17 cells in other inflammatory diseases, such as MS or RA, would be of significance.
Pathogenic invasion
EMMPRIN was identified recently as a receptor for the invasion of bacterial, parasitic, and viral strains. Meningitis is an infection that leads to inflammation of the membranes surrounding the CNS and can cause associated vascular dysfunction. EMMPRIN expression on peripheral and brain endothelial cells was identified as an important receptor for the initial invasion of blood‐borne meningococci (particularly the meningococcal type IV pilus components PilE and PilV) [55]. The expression of EMMPRIN on erythrocytes also mediated the erythrocyte invasion of Plasmodium falciparum, a malaria parasite [56]. Interference that uses soluble EMMPRIN, knockdown, or anti‐EMMPRIN antibodies (MEM‐M6/6) successfully prevented pathogenic colonization [55, 56]. Furthermore, EMMPRIN was also discovered to play a role in the host entry of CyPA‐associated HIV1 [129] and CyPA‐bound severe acute respiratory syndrome coronavirus [130], in addition to a role in viral oncogenesis [131].
CONCLUDING REMARKS
Anti‐EMMPRIN antibodies showed efficacy in treatment of GVHD, tumorigenesis, models of RA, MS, acute myocardial infarction, and lung inflammation, disease states involving various inflammatory mediators. These results highlight the important but still uncertain roles of dysregulated EMMPRIN on immune cells in disease pathogenesis. EMMPRIN could also be regarded as a potential diagnostic marker for early‐stage disease or for disease monitoring [40, 41, 91, 132, 133, 134, 135–136]. T cell‐mediated inflammation relies on a cascade of events, including its development, activation, proliferation, migration, and adhesion; all of these steps have been shown to be influenced by EMMPRIN in complex ways ( Table 2 ). Administration of most mAbs dampens these T cell functional responses (Tables 1 and 2), in which different EMMPRIN antibodies may target specific T cell activities, likely depending on its recognized epitope (e.g., antibodies that inhibited T cell proliferation did not induce cell aggregation and vice versa; Table 1) [54]. This targeted approach will likely result in less toxic side‐effects when used as a therapy, but it requires an improved understanding of EMMPRIN biology in relation to the epitope that the various antibodies recognize for therapeutic implications.
Table 2.
Summary of EMMPRIN studies and relevance to T cells
| Relevance | Manipulation | Observation |
|---|---|---|
| Thymus Positive/facilitatory role for EMMPRIN High EMMPRIN levels | ||
| T cell development | EMMPRIN mAb (RL73.2) on thymocytes [45] | Decreased DN4, DP, CD4+ SP populations |
| Lck‐cre; EMMPRIN flox/flox thymocytes [46] | Decreased DP, CD4+ SP populations (DN4 not assessed) | |
| Periphery Negative/inhibitory role for EMMPRIN Low EMMPRIN levels | ||
| T cell activation | TCR‐mediated activation of human T cells [51, 52] | Identified EMMPRIN at immune synapses |
| EMMPRIN mAb (MEM‐M6/6, 5A12) on human T cells upon TCR‐mediated activation [51, 52] | Altered immune synapse formation | |
| CD48 and CD59 displaced from lipid rafts | ||
| Decreased CD25, tyrosine phosphorylation, cytokines | ||
| Proliferation | EMMPRIN−/− splenocytes in a MLR [30] | Increased proliferation |
| Lck‐cre; EMMPRIN flox/flox T cells upon anti‐CD3/CD28 stimulation [46] | Increased proliferation | |
| EMMPRIN siRNA KD in human T cells and PHA activation [50] | Increased proliferation | |
| EMMPRIN mAb (MEM‐M6/6, 5A12, clone 10, M6‐1B9, UM‐8D6, HI197) on human PBMCs and/or purified T cells upon TCR‐mediated activation [48, 51, 52, 53–54, 58] | Decreased proliferation | |
| Periphery Positive/facilitatory role for EMMPRIN High EMMPRIN levels | ||
| Migration | EMMPRIN mAb (UM‐8D6) on human T cells [49] | Decreased CyPA‐mediated migration |
| EMMPRIN mAb (RL73.2) in the mouse system [61, 62–63] | Decreased CyPA‐mediated migration | |
| EMMPRIN KD or EMMPRIN mAb (HAb18) in/on Jurkat cells [59, 77] | Decreased transendothelial migration and CyPA‐mediated migration | |
| Invasion | EMMPRIN mAb (UM‐8D6, clone 10) in cocultures of mouse mϕ or fibroblasts with human T cells [53, 61] | Inhibited MMP‐9 secretion |
| EMMPRIN activity‐blocking peptide in cocultures of transformed human T cells and fibroblasts [75] | Decreased MMP‐2 stimulation | |
| Adhesion | Immunoprecipitation and co‐localization experiments in cell lines [78] | Identified EMMPRIN to interact with β1 integrins |
| EMMPRIN mAb (HIM6) on human PBMCs and THP‐1 cells [24, 79, 81] | Reduced CyPB‐induced P‐ERK and adhesion to fibronectin | |
| Identified EMMPRIN‐CyPB‐CD98‐β1 integrin complexes | ||
| EMMPRIN mAb (clone 10) upon TCR‐mediated activation of human PBMCs [66] | Decreased α4 integrin levels | |
| Decreased adhesion to endothelial cells | ||
| EMMPRIN mAb (MEM‐M6/8, HAb18) on Jurkat cells [23, 59] | Induced homotypic cell aggregation and adhesion to a LFA‐1 ligand | |
| Identified CD43‐EMMPRIN complexes | ||
| EMMPRIN KD in Jurkat cells [59, 77] | Reduced adhesion to ECM fibronectin | |
| Induced homotypic cell aggregation, decreased CD98 expression | ||
| Energy metabolism | EMMPRIN siRNA KD in human cell lines [25, 82, 83, 89] | Decreased MCT1/4 expression and efflux of toxic lactic acid byproducts |
| Identified MCT‐EMMPRIN complexes | ||
| MCT1 inhibition in human PBMCs [90] | Decreased T cell proliferation and lactate efflux | |
| Proliferation | EMMPRIN siRNA KD in Jurkat cells or other cell lines [77, 89] | Decreased proliferation |
A positive/facilitatory role for EMMPRIN exists when EMMPRIN expression levels are high and a negative/inhibitory role when EMMPRIN expression levels are low. KD, Knockdown; mϕ, macrophages; P‐ERK, phosphorylated ERK.
It appears that EMMPRIN mAbs can behave as agonists (have stimulatory roles) for some functions and antagonists (have inhibitory or function‐blocking roles) for other functions. For example, knocking out EMMPRIN in primary T cells led to increased proliferation upon TCR‐mediated activation (Table 2), implying that EMMPRIN functions to shut down these responses. On the other hand, the application of EMMPRIN mAbs decreased TCR‐mediated proliferation (Table 2); this indicates that these antibodies work in an agonistic fashion for that particular task. Alternatively, the reduction of EMMPRIN expression by RNA interference in cancer cells or the application of EMMPRIN mAbs led to a reduction in migration, adhesion, energy metabolism, and proliferation (via TCR‐independent mechanisms) yet an increase in cell aggregation. This likely demonstrates that EMMPRIN works to promote the migration, adhesion, and energy metabolism of cells and TCR‐independent proliferation of cancer cells but functions to impede cell aggregation. In these circumstances, EMMPRIN mAbs appear to have function‐blocking attributes. How EMMPRIN is involved in inhibiting cell aggregation or inducing adhesion, migration, and proliferation of cancer cells is not clearly understood.
It is also demonstrated that mAbs that recognize the EC1 (e.g., 5A12) or the EC2 (e.g., MEM‐M6/6) domain have similar effects in dampening down T cell proliferation (Table 1). Interestingly, MEM‐M6/1 (which also recognizes the EC1 domain of EMMPRIN) seems to behave as a functionally inactive antibody; however, it does have an effect on T cells of diseased patients with SLE (Table 1). Thus, antibody affinity also plays a role in functionality. Differences in antibody responses could be a result of a number of reasons. For example, as mentioned before, disparity in epitope recognition, antibody affinity, and the density of EMMPRIN on the cell surface could all influence antibody functionality. How the structure of EMMPRIN may be oriented upon binding with various interacting partners may also have an influence; there is a flexible linker between the EC1 and EC2 domain of EMMPRIN, allowing for conformational changes [8].
The observation that EMMPRIN seems to exhibit functional duality in T cells is not clearly understood. As already described, it acts as a regulatory molecule upon the initial stages of TCR‐mediated activation and proliferation and likely for cell aggregation via a TCR‐independent mechanism; however, once T cells are activated or during T cell development when EMMPRIN expression levels are high, positive/facilitatory roles are displayed for activities, such as chemotaxis, MMP induction, adhesion, and likely, the shuttling of lactate in proliferating cells. One reconciling factor for the positive or negative effects of EMMPRIN on T cells appears to be its levels on these leukocytes; when levels of EMMPRIN are high, such as in thymocytes or on T cells in the periphery during disease states, EMMPRIN facilitates several T cell phenotypes (Table 2). In contrast, when EMMPRIN levels are low, such as on circulating T cells in the periphery in normal homeostasis, EMMPRIN appears to have an inhibitory role on T cell activation and proliferation; an inhibitory role for EMMPRIN is consistent when activated under TCR‐mediated mechanisms.
In conclusion, clarity on how EMMPRIN expression levels are controlled, what signaling pathways EMMPRIN activates, its binding partners, and an understanding of how it carries out its functions is necessary. The understanding of the importance of EMMPRIN on APCs versus T cells in initiating TCR‐mediated cell activation also requires further clarification. EMMPRIN could be acting through an unidentified coreceptor or be a novel costimulator for T cell activation. Many more lessons are to be learned in understanding the involvement of EMMPRIN in T cell activities.
AUTHORSHIP
J.N.H. wrote the first draft of this review with frequent discussions and collaboration with D.K.K., who also provided editorial help. V.W.Y. is the senior author of this review, who provided overall supervision and edited the final manuscript for submission.
DISCLOSURES
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The research into EMMPRIN in the authors’ laboratory is funded by an operating grant from the Canadian Institutes of Health Research. J.N.H. is supported by a postdoctoral fellowship from the Multiple Sclerosis Society of Canada. D.K.K. is supported by a postdoctoral fellowship from the University of Calgary's Eyes High Postdoctoral Fellowship program and from the Multiple Sclerosis Society of Canada.
REFERENCES
- 1. Kataoka, H. , DeCastro, R. , Zucker, S. , Biswas, C. (1993) Tumor cell‐derived collagenase‐stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72‐kDa gelatinase. Cancer Res. 53, 3154–3158. [PubMed] [Google Scholar]
- 2. Yan, L. , Zucker, S. , Toole, B.P. (2005) Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb. Haemost. 93, 199–204. [DOI] [PubMed] [Google Scholar]
- 3. Bai, Y. , Huang, W. , Ma, L.T. , Jiang, J.L. , Chen, Z.N. (2014) Importance of N‐glycosylation on CD147 for its biological functions. Int. J. Mol. Sci. 15, 6356–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang, W. , Chang, S.B. , Hemler, M.E. (2004) Links between CD147 function, glycosylation, and caveolin‐1. Mol. Biol. Cell 15, 4043–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun, J. , Hemler, M.E. (2001) Regulation of MMP‐1 and MMP‐2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 61, 2276–2281. [PubMed] [Google Scholar]
- 6. Belton, Jr., R.J. , Chen, L. , Mesquita, F.S. , Nowak, R.A. (2008) Basigin‐2 is a cell surface receptor for soluble basigin ligand. J. Biol. Chem. 283, 17805–17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liao, C.G. , Kong, L.M. , Song, F. , Xing, J.L. , Wang, L.X. , Sun, Z.J. , Tang, H. , Yao, H. , Zhang, Y. , Wang, L. , Wang, Y. , Yang, X.M. , Li, Y. , Chen, Z.N. (2011) Characterization of basigin isoforms and the inhibitory function of basigin‐3 in human hepatocellular carcinoma proliferation and invasion. Mol. Cell. Biol. 31, 2591–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu, X.L. , Hu, T. , Du, J.M. , Ding, J.P. , Yang, X.M. , Zhang, J. , Yang, B. , Shen, X. , Zhang, Z. , Zhong, W.D. , Wen, N. , Jiang, H. , Zhu, P. , Chen, Z.N. (2008) Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J. Biol. Chem. 283, 18056–18065. [DOI] [PubMed] [Google Scholar]
- 9. Miyauchi, T. , Kanekura, T. , Yamaoka, A. , Ozawa, M. , Miyazawa, S. , Muramatsu, T. (1990) Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta‐chain of major histocompatibility complex class II antigen. J. Biochem. 107, 316–323. [DOI] [PubMed] [Google Scholar]
- 10. Muramatsu, T. , Miyauchi, T. (2003) Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol. Histopathol. 18, 981–987. [DOI] [PubMed] [Google Scholar]
- 11. Buck, C.A. (1992) Immunoglobulin superfamily: structure, function and relationship to other receptor molecules. Semin. Cell Biol. 3, 179–188. [DOI] [PubMed] [Google Scholar]
- 12. Williams, A.F. , Barclay, A.N. (1988) The immunoglobulin superfamily—domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405. [DOI] [PubMed] [Google Scholar]
- 13. Hanna, S.M. , Kirk, P. , Holt, O.J. , Puklavec, M.J. , Brown, M.H. , Barclay, A.N. (2003) A novel form of the membrane protein CD147 that contains an extra Ig‐like domain and interacts homophilically. BMC Biochem. 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yurchenko, V. , Constant, S. , Eisenmesser, E. , Bukrinsky, M. (2010) Cyclophilin‐CD147 interactions: a new target for anti‐inflammatory therapeutics. Clin. Exp. Immunol. 160, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egawa, N. , Koshikawa, N. , Tomari, T. , Nabeshima, K. , Isobe, T. , Seiki, M. (2006) Membrane type 1 matrix metalloproteinase (MT1‐MMP/MMP‐14) cleaves and releases a 22‐kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J. Biol. Chem. 281, 37576–37585. [DOI] [PubMed] [Google Scholar]
- 16. Sidhu, S.S. , Mengistab, A.T. , Tauscher, A.N. , LaVail, J. , Basbaum, C. (2004) The microvesicle as a vehicle for EMMPRIN in tumor‐stromal interactions. Oncogene 23, 956–963. [DOI] [PubMed] [Google Scholar]
- 17. Muralidharan‐Chari, V. , Clancy, J.W. , Sedgwick, A. , D'Souza‐Schorey, C. (2010) Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci. 123, 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Millimaggi, D. , Mari, M. , D'Ascenzo, S. , Carosa, E. , Jannini, E.A. , Zucker, S. , Carta, G. , Pavan, A. , Dolo, V. (2007) Tumor vesicle‐associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 9, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang, W. , Zhao, P. , Xu, X.L. , Cai, L. , Song, Z.S. , Cao, D.Y. , Tao, K.S. , Zhou, W.P. , Chen, Z.N. , Dou, K.F. (2013) Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147‐harboring microvesicles from tumor cells. PLoS ONE 8, e67268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menck, K. , Scharf, C. , Bleckmann, A. , Dyck, L. , Rost, U. , Wenzel, D. , Dhople, V.M. , Siam, L. , Pukrop, T. , Binder, C. , Klemm, F. (2014) Tumor‐derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J. Mol. Cell Biol. 7, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nabeshima, K. , Iwasaki, H. , Koga, K. , Hojo, H. , Suzumiya, J. , Kikuchi, M. (2006) EMMPRIN (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 56, 359–367. [DOI] [PubMed] [Google Scholar]
- 22. Pushkarsky, T. , Yurchenko, V. , Vanpouille, C. , Brichacek, B. , Vaisman, I. , Hatakeyama, S. , Nakayama, K.I. , Sherry, B. , Bukrinsky, M.I. (2005) Cell surface expression of CD147/EMMPRIN is regulated bycyclophilin 60. J. Biol. Chem. 280, 27866–27871. [DOI] [PubMed] [Google Scholar]
- 23. Khunkaewla, P. , Schiller, H.B. , Paster, W. , Leksa, V. , Cermak, L. , Andera, L. , Horejsí, V. , Stockinger, H. (2008) LFA‐1‐mediated leukocyte adhesion regulated by interaction of CD43 with LFA‐1 and CD147. Mol. Immunol. 45, 1703–1711. [DOI] [PubMed] [Google Scholar]
- 24. Pakula, R. , Melchior, A. , Denys, A. , Vanpouille, C. , Mazurier, J. , Allain, F. (2007) Syndecan‐1/CD147 association is essential for cyclophilin B‐induced activation of p44/42 mitogen‐activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology 17, 492–503. [DOI] [PubMed] [Google Scholar]
- 25. Kirk, P. , Wilson, M.C. , Heddle, C. , Brown, M.H. , Barclay, A.N. , Halestrap, A.P. (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seizer, P. , Borst, O. , Langer, H.F. , Bültmann, A. , Münch, G. , Herouy, Y. , Stellos, K. , Kramer, B. , Bigalke, B. , Büchele, B. , Bachem, M.G. , Vestweber, D. , Simmet, T. , Gawaz, M. , May, A.E. (2009) EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI‐EMMPRIN interaction. Thromb. Haemost. 101, 682–686. [DOI] [PubMed] [Google Scholar]
- 27. Zhao, P. , Zhang, W. , Wang, S.J. , Yu, X.L. , Tang, J. , Huang, W. , Li, Y. , Cui, H.Y. , Guo, Y.S. , Tavernier, J. , Zhang, S.H. , Jiang, J.L. , Chen, Z.N. (2011) HAb18G/CD147 promotes cell motilityby regulating annexin II‐activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology 54, 2012–2024. [DOI] [PubMed] [Google Scholar]
- 28. Schreiner, A. , Ruonala, M. , Jakob, V. , Suthaus, J. , Boles, E. , Wouters, F. , Starzinski‐Powitz, A. (2007) Junction protein shrew‐1 influences cell invasion and interacts with invasion‐promoting protein CD147. Mol. Biol. Cell 18, 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou, S. , Zhou, H. , Walian, P.J. , Jap, B.K. (2006) The discovery and role of CD147 as a subunit of gamma‐secretase complex. Drug News Perspect. 19, 133–138. [DOI] [PubMed] [Google Scholar]
- 30. Igakura, T. , Kadomatsu, K. , Taguchi, O. , Muramatsu, H. , Kaname, T. , Miyauchi, T. , Yamamura, K. , Arimura, K. , Muramatsu, T. (1996) Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood‐brain barrier. Biochem. Biophys. Res. Commun. 224, 33–36. [DOI] [PubMed] [Google Scholar]
- 31. Fadool, J.M. , Linser, P.J. (1993) 5A11 Antigen is a cell recognition molecule which is involved in neuronal‐glial interactions in avian neural retina. Dev. Dyn. 196, 252–262. [DOI] [PubMed] [Google Scholar]
- 32. Hori, K. , Katayama, N. , Kachi, S. , Kondo, M. , Kadomatsu, K. , Usukura, J. , Muramatsu, T. , Mori, S. , Miyake, Y. (2000) Retinal dysfunction in basigin deficiency. Invest. Ophthalmol. Vis. Sci. 41, 3128–3133. [PubMed] [Google Scholar]
- 33. Ochrietor, J.D. , Linser, P.J. (2004) 5A11/Basigin gene products are necessary for proper maturation and function of the retina. Dev. Neurosci. 26, 380–387. [DOI] [PubMed] [Google Scholar]
- 34. Naruhashi, K. , Kadomatsu, K. , Igakura, T. , Fan, Q.W. , Kuno, N. , Muramatsu, H. , Miyauchi, T. , Hasegawa, T. , Itoh, A. , Muramatsu, T. , Nabeshima, T. (1997) Abnormalities of sensory and memory functions in mice lacking Bsg gene. Biochem. Biophys. Res. Commun. 236, 733–737. [DOI] [PubMed] [Google Scholar]
- 35. Fan, Q.W. , Yuasa, S. , Kuno, N. , Senda, T. , Kobayashi, M. , Muramatsu, T. , Kadomatsu, K. (1998) Expression of basigin, a member of the immunoglobulin superfamily, in the mouse central nervous system. Neurosci. Res. 30, 53–63. [DOI] [PubMed] [Google Scholar]
- 36. Igakura, T. , Kadomatsu, K. , Kaname, T. , Muramatsu, H. , Fan, Q.W. , Miyauchi, T. , Toyama, Y. , Kuno, N. , Yuasa, S. , Takahashi, M. , Senda, T. , Taguchi, O. , Yamamura, K. , Arimura, K. , Muramatsu, T. (1998) A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri‐implantation development and spermatogenesis. Dev. Biol. 194, 152–165. [DOI] [PubMed] [Google Scholar]
- 37. Weidle, U.H. , Scheuer, W. , Eggle, D. , Klostermann, S. , Stockinger, H. (2010) Cancer‐related issues of CD147. Cancer Genomics Proteomics 7, 157–169. [PubMed] [Google Scholar]
- 38. Coste, I. , Gauchat, J.F. , Wilson, A. , Izui, S. , Jeannin, P. , Delneste, Y. , MacDonald, H.R. , Bonnefoy, J.Y. , Renno, T. (2001) Unavailability of CD147 leads to selective erythrocyte trapping in the spleen. Blood 97, 3984–3988. [DOI] [PubMed] [Google Scholar]
- 39. Schmidt, R. , Bültmann, A. , Fischel, S. , Gillitzer, A. , Cullen, P. , Walch, A. , Jost, P. , Ungerer, M. , Tolley, N.D. , Lindemann, S. , Gawaz, M. , Schömig, A. , May, A.E. (2008) Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB‐dependent inflammation in monocytes. Circ. Res. 102, 302–309. [DOI] [PubMed] [Google Scholar]
- 40. Solstad, T. , Bains, S.J. , Landskron, J. , Aandahl, E.M. , Thiede, B. , Taskén, K. , Torgersen, K.M. (2011) CD147 (basigin/EMMPRIN) identifies FoxP3+CD45RO+CTLA4+‐activated human regulatory T cells. Blood 118, 5141–5151. [DOI] [PubMed] [Google Scholar]
- 41. Landskron, J. , Taskén, K. (2013) CD147 in regulatory T cells. Cell. Immunol. 282, 17–20. [DOI] [PubMed] [Google Scholar]
- 42. Agrawal, S.M. , Yong, V.W. (2011) The manyfaces of EMMPRIN—roles in neuroinflammation. Biochim. Biophys. Acta 1812, 213–219. [DOI] [PubMed] [Google Scholar]
- 43. Germain, R.N. (2002) T‐cell development and the CD4‐CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322. [DOI] [PubMed] [Google Scholar]
- 44. Kirsch, A.H. , Diaz, Jr., L.A. , Bonish, B. , Antony, P.A. , Fox, D.A. (1997) The pattern of expression of CD147/neurothelin during human T‐cell ontogeny as defined by the monoclonal antibody 8D6. Tissue Antigens 50, 147–152. [DOI] [PubMed] [Google Scholar]
- 45. Renno, T. , Wilson, A. , Dunkel, C. , Coste, I. , Maisnier‐Patin, K. , Benoit de Coignac, A. , Aubry, J.P. , Lees, R.K. , Bonnefoy, J.Y. , MacDonald, H.R. , Gauchat, J.F. (2002) A role for CD147 in thymic development. J. Immunol. 168, 4946–4950. [DOI] [PubMed] [Google Scholar]
- 46. Yao, H. , Teng, Y. , Sun, Q. , Xu, J. , Chen, Y.T. , Hou, N. , Cheng, X. , Yang, X. , Chen, Z.N. (2013) Important functional roles of basigin in thymocyte development and T cell activation. Int. J. Biol. Sci. 10, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bröer S. (2005) Lactate transportation is required for lymphocyte activation. Nat. Chem. Biol. 1, 356–357. [DOI] [PubMed] [Google Scholar]
- 48. Koch, C. , Staffler, G. , Hüttinger, R. , Hilgert, I. , Prager, E. , Cerný, J. , Steinlein, P. , Majdic, O. , Horejsí, V. , Stockinger, H. (1999) T cell activation‐associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 11, 777–786. [DOI] [PubMed] [Google Scholar]
- 49. Damsker, J.M. , Bukrinsky, M.I. , Constant, S.L. (2007) Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J. Leukoc. Biol. 82, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biegler, B. , Kasinrerk, W. (2012) Reduction of CD147 surface expression on primary T cells leads to enhanced cell proliferation. Asian Pac. J. Allergy Immunol. 30, 259–267. [PubMed] [Google Scholar]
- 51. Staffler, G. , Szekeres, A. , Schütz, G.J. , Saemann, M.D. , Prager, E. , Zeyda, M. , Drbal, K. , Zlabinger, G.J. , Stulnig, T.M. , Stockinger, H. (2003) Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J. Immunol. 171, 1707–1714. [DOI] [PubMed] [Google Scholar]
- 52. Hu, J. , Dang, N. , Yao, H. , Li, Y. , Zhang, H. , Yang, X. , Xu, J. , Bian, H. , Xing, J. , Zhu, P. , Chen, Z. (2010) Involvement of HAb18G/CD147 in T cell activation and immunological synapse formation. J. Cell. Mol. Med. 14, 2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agrawal, S.M. , Silva, C. , Wang, J. , Tong, J.P. , Yong, V.W. (2012) A novel anti‐EMMPRIN function‐blocking antibody reduces T cell proliferation and neurotoxicity: relevance to multiple sclerosis. J. Neuroinflammation 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chiampanichayakul, S. , Peng‐in, P. , Khunkaewla, P. , Stockinger, H. , Kasinrerk, W. (2006) CD147 contains different bioactive epitopes involving the regulation of cell adhesion and lymphocyte activation. Immunobiology 211, 167–178. [DOI] [PubMed] [Google Scholar]
- 55. Bernard, S.C. , Simpson, N. , Join‐Lambert, O. , Federici, C. , Laran‐Chich, M.P. , Maïssa, N. , Bouzinba‐Segard, H. , Morand, P.C. , Chretien, F. , Taouji, S. , Chevet, E. , Janel, S. , Lafont, F. , Coureuil, M. , Segura, A. , Niedergang, F. , Marullo, S. , Couraud, P.O. , Nassif, X. , Bourdoulous, S. (2014) Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crosnier, C. , Bustamante, L.Y. , Bartholdson, S.J. , Bei, A.K. , Theron, M. , Uchikawa, M. , Mboup, S. , Ndir, O. , Kwiatkowski, D.P. , Duraisingh, M.T. , Rayner, J.C. , Wright, G.J. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum . Nature 480, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pistol, G. , Matache, C. , Calugaru, A. , Stavaru, C. , Tanaseanu, S. , Ionescu, R. , Dumitrache, S. , Stefanescu, M. (2007) Roles of CD147 on T lymphocytes activation and MMP‐9 secretion in systemic lupus erythematosus. J. Cell. Mol. Med. 11, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stonehouse, T.J. , Woodhead, V.E. , Herridge, P.S. , Ashrafian, H. , George, M. , Chain, B.M. , Katz, D.R. (1999) Molecular characterization of U937‐dependent T‐cell co‐stimulation. Immunology 96, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo, N. , Zhang, K. , Lv, M. , Miao, J. , Chen, Z. , Zhu, P. (2015) CD147 and CD98 complex‐mediated homotypic aggregation attenuates the CypA‐induced chemotactic effect on Jurkat T cells. Mol. Immunol. 63, 253–263. [DOI] [PubMed] [Google Scholar]
- 60. Wang, C.H. , Dai, J.Y. , Wang, L. , Jia, J.F. , Zheng, Z.H. , Ding, J. , Chen, Z.N. , Zhu, P. (2011) Expression of CD147 (EMMPRIN) on neutrophils in rheumatoid arthritis enhances chemotaxis, matrix metalloproteinase production and invasiveness of synoviocytes. J. Cell. Mol. Med. 15, 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Damsker, J.M. , Okwumabua, I. , Pushkarsky, T. , Arora, K. , Bukrinsky, M.I. , Constant, S.L. (2009) Targeting the chemotactic function of CD147 reduces collagen‐induced arthritis. Immunology 126, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arora, K. , Gwinn, W.M. , Bower, M.A. , Watson, A. , Okwumabua, I. , MacDonald, H.R. , Bukrinsky, M.I. , Constant, S.L. (2005) Extracellular cyclophilins contribute to the regulation of inflammatory responses. J. Immunol. 175, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gwinn, W.M. , Damsker, J.M. , Falahati, R. , Okwumabua, I. , Kelly‐Welch, A. , Keegan, A.D. , Vanpouille, C. , Lee, J.J. , Dent, L.A. , Leitenberg, D. , Bukrinsky, M.I. , Constant, S.L. (2006) Novel approach to inhibit asthma‐mediated lung inflammation using anti‐CD147 intervention. J. Immunol. 177, 4870–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agrawal, S.M. , Silva, C. , Tourtellotte, W.W. , Yong, V.W. (2011) EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. 31, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seizer, P. , Ochmann, C. , Schonberger, T. , Zach, S. , Rose, M. , Borst, O. , Klingel, K. , Kandolf, R. , MacDonald, H.R. , Nowak, R.A. , Engelhardt, S. , Lang, F. , Gawaz, M. , May, A.E. (2011) Disrupting the EMMPRIN (CD147)‐cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arterioscler. Thromb. Vasc. Biol. 31, 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agrawal, S.M. , Williamson, J. , Sharma, R. , Kebir, H. , Patel, K. , Prat, A. , Yong, V.W. (2013) Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain 136, 1760–1777. [DOI] [PubMed] [Google Scholar]
- 67. Gaglia, J.L. , Mattoo, A. , Greenfield, E.A. , Freeman, G.J. , Kuchroo, V.K. (2001) Characterization of endogenous Chinese hamster ovary cell surface molecules that mediate T cell costimulation. Cell. Immunol. 213, 83–93. [DOI] [PubMed] [Google Scholar]
- 68. Woodhead, V.E. , Stonehouse, T.J. , Binks, M.H. , Speidel, K. , Fox, D.A. , Gaya, A. , Hardie, D. , Henniker, A.J. , Horejsi, V. , Sagawa, K. , Skubitz, K.M. , Taskov, H. , Todd III, R.F. , van Agthoven, A. , Katz, D.R. , Chain, B.M. (2000) Novel molecular mechanisms of dendritic cell‐induced T cell activation. Int. Immunol. 12, 1051–1061. [DOI] [PubMed] [Google Scholar]
- 69. Yurchenko, V. , Zybarth, G. , O'Connor, M. , Dai, W.W. , Franchin, G. , Hao, T. , Guo, H. , Hung, H.C. , Toole, B. , Gallay, P. , Sherry, B. , Bukrinsky, M. (2002) Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277, 22959–22965. [DOI] [PubMed] [Google Scholar]
- 70. Billich, A. , Winkler, G. , Aschauer, H. , Rot, A. , Peichl, P. (1997) Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J. Exp. Med. 185, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tegeder, I. , Schumacher, A. , John, S. , Geiger, H. , Geisslinger, G. , Bang, H. , Brune, K. (1997) Elevated serum cyclophilin levels in patients with severe sepsis. J. Clin. Immunol. 17, 380–386. [DOI] [PubMed] [Google Scholar]
- 72. Jin, Z.G. , Melaragno, M.G. , Liao, D.F. , Yan, C. , Haendeler, J. , Suh, Y.A. , Lambeth, J.D. , Berk, B.C. (2000) Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ. Res. 87, 789–796. [DOI] [PubMed] [Google Scholar]
- 73. Yurchenko, V. , O'Connor, M. , Dai, W.W. , Guo, H. , Toole, B. , Sherry, B. , Bukrinsky, M. (2001) CD147 is a signaling receptor for cyclophilin B. Biochem. Biophys. Res. Commun. 288, 786–788. [DOI] [PubMed] [Google Scholar]
- 74. Goetzl, E.J. , Banda, M.J. , Leppert, D. (1996) Matrix metalloproteinases in immunity. J. Immunol. 156, 1–4. [PubMed] [Google Scholar]
- 75. Nabeshima, K. , Suzumiya, J. , Nagano, M. , Ohshima, K. , Toole, B.P. , Tamura, K. , Iwasaki, H. , Kikuchi, M. (2004) EMMPRIN, a cell surface inducer of matrix metalloproteinases (MMPs), is expressed in T‐cell lymphomas. J. Pathol. 202, 341–351. [DOI] [PubMed] [Google Scholar]
- 76. Esparza, J. , Vilardell, C. , Calvo, J. , Juan, M. , Vives, J. , Urbano‐Márquez, A. , Yagüe, J. , Cid, M.C. (1999) Fibronectin upregulates gelatinase B (MMP‐9) and induces coordinated expression of gelatinase A (MMP‐2) and its activator MT1‐MMP (MMP‐14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood 94, 2754–2766. [PubMed] [Google Scholar]
- 77. Chen, X. , Su, J. , Chang, J. , Kanekura, T. , Li, J. , Kuang, Y.H. , Peng, S. , Yang, F. , Lu, H. , Zhang, J.L. (2008) Inhibition of CD147 gene expression via RNA interference reduces tumor cell proliferation, activation, adhesion, and migration activity in the human Jurkat T‐lymphoma cell line. Cancer Invest. 26, 689–697. [DOI] [PubMed] [Google Scholar]
- 78. Berditchevski, F. , Chang, S. , Bodorova, J. , Hemler, M.E. (1997) Generation of monoclonal antibodies to integrin‐associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 272, 29174–29180. [DOI] [PubMed] [Google Scholar]
- 79. Allain, F. , Vanpouille, C. , Carpentier, M. , Slomianny, M.C. , Durieux, S. , Spik, G. (2002) Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin‐mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc. Natl. Acad. Sci. USA 99, 2714–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hanoulle, X. , Melchior, A. , Sibille, N. , Parent, B. , Denys, A. , Wieruszeski, J.M. , Horvath, D. , Allain, F. , Lippens, G. , Landrieu, I. (2007) Structural and functional characterisation of the interaction between cyclophilin B and a heparin derived oligosaccharide. J. Biol. Chem. 282, 34148–134158 [DOI] [PubMed] [Google Scholar]
- 81. Melchior, A. , Denys, A. , Deligny, A. , Mazurier, J. , Allain, F. (2008) Cyclophilin B induces integrin‐mediated cell adhesion by a mechanism involving CD98‐dependent activation of protein kinase C‐delta and p44/42 mitogen‐activated protein kinases. Exp. Cell Res. 314, 616–628. [DOI] [PubMed] [Google Scholar]
- 82. Xu, D. , Hemler, M.E. (2005) Metabolic activation‐related CD147‐CD98 complex. Mol. Cell. Proteomics 4, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Slomiany, M.G. , Grass, G.D. , Robertson, A.D. , Yang, X.Y. , Maria, B.L. , Beeson, C. , Toole, B.P. (2009) Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 69, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Marieb, E.A. , Zoltan‐Jones, A. , Li, R. , Misra, S. , Ghatak, S. , Cao, J. , Zucker, S. , Toole, B.P. (2004) EMMPRIN promotes anchorage‐independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 64, 1229–1232. [DOI] [PubMed] [Google Scholar]
- 85. Toole, B.P. , Slomiany, M.G. (2008) Hyaluronan, CD44 and EMMPRIN: partners in cancer cell chemoresistance. Drug Resist. Updat. 11, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baaten, B.J. , Tinoco, R. , Chen, A.T. , Bradley, L.M. (2012) Regulation of antigen‐experienced T cells: lessons from the quintessential memory marker CD44. Front. Immunol. 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kasinrerk, W. , Tokrasinwit, N. , Phunpae, P. (1999) CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA‐1/ICAM‐1 pathway. Immunology 96, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Philp, N.J. , Ochrietor, J.D. , Rudoy, C. , Muramatsu, T. , Linser, P.J. (2003) Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin‐null mouse. Invest. Ophthalmol. Vis. Sci. 44, 1305–1311. [DOI] [PubMed] [Google Scholar]
- 89. Schneiderhan, W. , Scheler, M. , Holzmann, K.H. , Marx, M. , Gschwend, J.E. , Bucholz, M. , Gress, T.M. , Seufferlein, T. , Adler, G. , Oswald, F. (2009) CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut 58, 1391–1398. [DOI] [PubMed] [Google Scholar]
- 90. Murray, C.M. , Hutchinson, R. , Bantick, J.R. , Belfield, G.P. , Benjamin, A.D. , Brazma, D. , Bundick, R.V. , Cook, I.D. , Craggs, R.I. , Edwards, S. , Evans, L.R. , Harrison, R. , Holness, E. , Jackson, A.P. , Jackson, C.G. , Kingston, L.P. , Perry, M.W. , Ross, A.R. , Rugman, P.A. , Sidhu, S.S. , Sullivan, M. , Taylor‐Fishwick, D.A. , Walker, P.C. , Whitehead, Y.M. , Wilkinson, D.J. , Wright, A. , Donald, D.K. (2005) Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1, 371–376. [DOI] [PubMed] [Google Scholar]
- 91. Xiong, L. , Edwards III, C.K. , Zhou, L. (2014) The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 15, 17411–17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tian, L. , Zhang, Y. , Chen, Y. , Cai, M. , Dong, H. , Xiong, L. (2013) EMMPRIN is an independent negative prognostic factor for patients with astrocytic glioma. PLoS ONE 8, e58069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhao, S.H. , Wang, Y. , Wen, L. , Zhai, Z.B. , Ai, Z.H. , Yao, N.L. , Wang, L. , Liu, W.C. , Chen, B.L. , Li, Y. , Yang, H. (2013) Basigin‐2 is the predominant basigin isoform that promotes tumor cell migration and invasion and correlates with poor prognosis in epithelial ovarian cancer. J. Transl. Med. 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stenzinger, A. , Wittschieber, D. , von Winterfeld, M. , Goeppert, B. , Kamphues, C. , Weichert, W. , Dietel, M. , Rabien, A. , Klauschen, F. (2012) High extracellular matrix metalloproteinase inducer/CD147 expression is strongly and independently associated with poor prognosis in colorectal cancer. Hum. Pathol. 43, 1471–1481. [DOI] [PubMed] [Google Scholar]
- 95. Zucker, S. , Cao, J. (2009) Selective matrix metalloproteinase (MMP) inhibitors in cancer therapy: ready for prime time? Cancer Biol. Ther. 8, 2371–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Overall, C.M. , Kleifeld, O. (2006) Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti‐targets for cancer therapy. Nat. Rev. Cancer 6, 227–239. [DOI] [PubMed] [Google Scholar]
- 97. Gialeli, C. , Theocharis, A.D. , Karamanos, N.K. (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 278, 16–27. [DOI] [PubMed] [Google Scholar]
- 98. Chen, X. , Lin, J. , Kanekura, T. , Su, J. , Lin, W. , Xie, H. , Wu, Y. , Li, J. , Chen, M. , Chang, J. (2006) A small interfering CD147‐targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 66, 11323–11330. [DOI] [PubMed] [Google Scholar]
- 99. Kanekura, T. , Chen, X. , Kanzaki, T. (2002) Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int. J. Cancer 99, 520–528. [DOI] [PubMed] [Google Scholar]
- 100. Tang, Y. , Nakada, M.T. , Kesavan, P. , McCabe, F. , Millar, H. , Rafferty, P. , Bugelski, P. , Yan, L. (2005) Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 65, 3193–3199. [DOI] [PubMed] [Google Scholar]
- 101. Tang, Y. , Nakada, M.T. , Rafferty, P. , Laraio, J. , McCabe, F.L. , Millar, H. , Cunningham, M. , Snyder, L.A. , Bugelski, P. , Yan, L. (2006) Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K‐Akt signaling pathway. Mol. Cancer Res. 4, 371–377. [DOI] [PubMed] [Google Scholar]
- 102. Xu, J. , Xu, H.Y. , Zhang, Q. , Song, F. , Jiang, J.L. , Yang, X.M. , Mi, L. , Wen, N. , Tian, R. , Wang, L. , Yao, H. , Feng, Q. , Zhang, Y. , Xing, J.L. , Zhu, P. , Chen, Z.N. (2007) HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol. Cancer Res. 5, 605–614. [DOI] [PubMed] [Google Scholar]
- 103. Dean, N.R. , Newman, J.R. , Helman, E.E. , Zhang, W. , Safavy, S. , Weeks, D.M. , Cunningham, M. , Snyder, L.A. , Tang, Y. , Yan, L. , McNally, L.R. , Buchsbaum, D.J. , Rosenthal, E.L. (2009) Anti‐EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin. Cancer Res. 15, 4058–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ruiz, S. , Castro‐Castro, A. , Bustelo, X.R. (2008) CD147 inhibits the nuclear factor of activated T‐cells by impairing Vav1 and Rac1 downstream signaling. J. Biol. Chem. 283, 5554–5566. [DOI] [PubMed] [Google Scholar]
- 105. Mak, A. , Kow, N.Y. (2014) The pathology of T cells in systemic lupus erythematosus. J. Immunol. Res. 2014, 419029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Matache, C. , Stefanescu, M. , Dragomir, C. , Tanaseanu, S. , Onu, A. , Ofiteru, A. , Szegli, G. (2003) Matrix metalloproteinase‐9 and its natural inhibitor TIMP‐1 expressed or secreted by peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J. Autoimmun. 20, 323–331. [DOI] [PubMed] [Google Scholar]
- 107. Sung, A.D. , Chao, N.J. (2013) Concise review: acute graft‐versus‐host disease: immunobiology, prevention, and treatment. Stem Cells Transl. Med. 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sung, A.D. , Chao, N.J. (2013) Acute graft‐versus‐host disease: are we close to bringing the bench to the bedside? Best Pract. Res. Clin. Haematol. 26, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Deeg, H.J. , Blazar, B.R. , Bolwell, B.J. , Long, G.D. , Schuening, F. , Cunningham, J. , Rifkin, R.M. , Abhyankar, S. , Briggs, A.D. , Burt, R. , Lipani, J. , Roskos, L.K. , White, J.M. , Havrilla, N. , Schwab, G. , Heslop, H.E. (2001) Treatment of steroid‐refractory acute graft‐versus‐host disease with anti‐CD147 monoclonal antibody ABX‐CBL. Blood 98, 2052–2058. [DOI] [PubMed] [Google Scholar]
- 110. Macmillan, M.L. , Couriel, D. , Weisdorf, D.J. , Schwab, G. , Havrilla, N. , Fleming, T.R. , Huang, S. , Roskos, L. , Slavin, S. , Shadduck, R.K. , Dipersio, J. , Territo, M. , Pavletic, S. , Linker, C. , Heslop, H.E. , Deeg, H.J. , Blazar, B.R. (2007) A phase 2/3 multicenter randomized clinical trial of ABX‐CBL versus ATG as secondary therapy for steroid‐resistant acute graft‐versus‐host disease. Blood 109, 2657–2662. [DOI] [PubMed] [Google Scholar]
- 111. Burrage, P.S. , Mix, K.S. , Brinckerhoff, C.E. (2006) Matrix metalloproteinases: role in arthritis. Front. Biosci. 11, 529–543. [DOI] [PubMed] [Google Scholar]
- 112. Konttinen, Y.T. , Li, T.F. , Mandelin, J. , Liljestrom, M. , Sorsa, T. , Santavirta, S. , Virtanen, I. (2000) Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 43, 275–280. [DOI] [PubMed] [Google Scholar]
- 113. Tomita, T. , Nakase, T. , Kaneko, M. , Shi, K. , Takahi, K. , Ochi, T. , Yoshikawa, H. (2002) Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 46, 373–378. [DOI] [PubMed] [Google Scholar]
- 114. Zhu, P. , Ding, J. , Zhou, J. , Dong, W.J. , Fan, C.M. , Chen, Z.N. (2005) Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res. Ther. 7, R1023‐R1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu, P. , Lu, N. , Shi, Z.G. , Zhou, J. , Wu, Z.B. , Yang, Y. , Ding, J. , Chen, Z.N. (2006) CD147 overexpression on synoviocytes in rheumatoid arthritis enhances matrix metalloproteinase production and invasiveness of synoviocytes. Arthritis Res. Ther. 8, R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang, C.H. , Yao, H. , Chen, L.N. , Jia, J.F. , Wang, L. , Dai, J.Y. , Zheng, Z.H. , Chen, Z.N. , Zhu, P. (2012) CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia‐inducible transcription factor 1α‐mediated pathway in rheumatoid arthritis. Arthritis Rheum. 64, 1818–1827. [DOI] [PubMed] [Google Scholar]
- 117. Jia, J. , Wang, C. , Shi, Z. , Zhao, J. , Jia, Y. , Zhao‐Hui, Z. , Li, X. , Chen, Z. , Zhu, P. (2009) Inhibitory effect of CD147/HAb18 monoclonal antibody on cartilage erosion and synovitis in the SCID mouse model for rheumatoid arthritis. Rheumatology (Oxford) 48, 721–726. [DOI] [PubMed] [Google Scholar]
- 118. Weiner, H.L. (2004) Multiple sclerosis is an inflammatory T‐cell‐mediated autoimmune disease. Arch. Neurol. 61, 1613–1615. [DOI] [PubMed] [Google Scholar]
- 119. Lusis, A.J. (2000) Atherosclerosis. Nature 407, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shah, P.K. , Galis, Z.S. (2001) Matrix metalloproteinase hypothesis of plaque rupture: players keep piling up but questions remain. Circulation 104, 1878–1880. [PubMed] [Google Scholar]
- 121. Hansson, G.K. , Robertson, A.K. , Soderberg‐Naucler, C. (2006) Inflammation and atherosclerosis. Annu. Rev. Pathol. 1, 297–329. [DOI] [PubMed] [Google Scholar]
- 122. Tuttolomondo, A. , Di Raimondo, D. , Pecoraro, R. , Arnao, V. , Pinto, A. , Licata, G. (2012) Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 18, 4266–4288. [DOI] [PubMed] [Google Scholar]
- 123. Schmidt, R. , Bültmann, A. , Ungerer, M. , Joghetaei, N. , Bülbül, O. , Thieme, S. , Chavakis, T. , Toole, B.P. , Gawaz, M. , Schomig, A. , May, A.E. (2006) Extracellular matrix metalloproteinase inducer regulates matrix metalloproteinase activity in cardiovascular cells: implications in acute myocardial infarction. Circulation 113, 834–841. [DOI] [PubMed] [Google Scholar]
- 124. Nahrendorf, M. , Pittet, M.J. , Swirski, F.K. (2010) Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wick, G. , Grundtman, C. , Mayerl, C. , Wimpissinger, T.F. , Feichtinger, J. , Zelger, B. , Sgonc, R. , Wolfram, D. (2013) The immunology of fibrosis. Annu. Rev. Immunol. 31, 107–135. [DOI] [PubMed] [Google Scholar]
- 126. Betsuyaku, T. , Kadomatsu, K. , Griffin, G.L. , Muramatsu, T. , Senior, R.M. (2003) Increased basigin in bleomycin‐induced lung injury. Am. J. Respir. Cell Mol. Biol. 28, 600–606. [DOI] [PubMed] [Google Scholar]
- 127. Guillot, S. , Delaval, P. , Brinchault, G. , Caulet‐Maugendre, S. , Depince, A. , Lena, H. , Delatour, B. , Lagente, V. , Martin‐Chouly, C. (2006) Increased extracellular matrix metalloproteinase inducer (EMMPRIN) expression in pulmonary fibrosis. Exp. Lung Res. 32, 81–97. [DOI] [PubMed] [Google Scholar]
- 128. Geng, J.J. , Zhang, K. , Chen, L.N. , Miao, J.L. , Yao, M. , Ren, Y. , Fu, Z.G. , Chen, Z.N. , Zhu, P. (2014) Enhancement of CD147 on M1 macrophages induces differentiation of Th17 cells in the lung interstitial fibrosis. Biochim. Biophys. Acta 1842, 1770–1782. [DOI] [PubMed] [Google Scholar]
- 129. Pushkarsky, T. , Zybarth, G. , Dubrovsky, L. , Yurchenko, V. , Tang, H. , Guo, H. , Toole, B. , Sherry, B. , Bukrinsky, M. (2001) CD147 facilitates HIV‐1 infection by interacting with virus‐associated cyclophilin A. Proc. Natl. Acad. Sci. USA 98, 6360–6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chen, Z. , Mi, L. , Xu, J. , Yu, J. , Wang, X. , Jiang, J. , Xing, J. , Shang, P. , Qian, A. , Li, Y. , Shaw, P.X. , Wang, J. , Duan, S. , Ding, J. , Fan, C. , Zhang, Y. , Yang, Y. , Yu, X. , Feng, Q. , Li, B. , Yao, X. , Zhang, Z. , Li, L. , Xue, X. , Zhu, P. (2005) Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 191, 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dai, L. , Bai, L. , Lu, Y. , Xu, Z. , Reiss, K. , Del Valle, L. , Kaleeba, J. , Toole, B.P. , Parsons, C. , Qin, Z. (2013) EMMPRIN and KSHV: new partners in viral cancer pathogenesis. Cancer Lett. 337, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Seizer, P. , Geisler, T. , Bigalke, B. , Schneider, M. , Klingel, K. , Kandolf, R. , Stellos, K. , Schreieck, J. , Gawaz, M. , May, A.E. (2013) EMMPRIN and its ligand cyclophilin A as novel diagnostic markers in inflammatory cardiomyopathy. Int. J. Cardiol. 163, 299–304. [DOI] [PubMed] [Google Scholar]
- 133. Maeda‐Hori, M. , Kosugi, T. , Kojima, H. , Sato, W. , Inaba, S. , Maeda, K. , Nagaya, H. , Sato, Y. , Ishimoto, T. , Ozaki, T. , Tsuboi, N. , Muro, Y. , Yuzawa, Y. , Imai, E. , Johnson, R.J. , Matsuo, S. , Kadomatsu, K. , Maruyama, S. (2014) Plasma CD147 reflects histological features in patients with lupus nephritis. Lupus 23, 342–352. [DOI] [PubMed] [Google Scholar]
- 134. Iłzecka, J. (2011) EMMPRIN levels in serum of patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 124, 424–428. [DOI] [PubMed] [Google Scholar]
- 135. Moonsom, S. , Tayapiwatana, C. , Wongkham, S. , Kongtawelert, P. , Kasinrerk, W. (2010) A competitive ELISA for quantifying serum CD147: reduction of soluble CD147 levels in cancer patient sera. Hybridoma (Larchmt) 29, 45–52. [DOI] [PubMed] [Google Scholar]
- 136. Yanaba, K. , Asano, Y. , Tada, Y. , Sugaya, M. , Kadono, T. , Hamaguchi, Y. , Sato, S. (2012) Increased serum soluble CD147 levels in patients with systemic sclerosis: association with scleroderma renal crisis. Clin. Rheumatol. 31, 835–839. [DOI] [PubMed] [Google Scholar]


