Abstract
Few studies have evaluated the contribution of multiple virus and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease. This study estimated the burden of multiple viral and bacterial respiratory infections in moderate to very severe chronic obstructive pulmonary disease patients that were prospectively followed‐up during a 12‐month pilot study. Clinical data were collected monthly and sputum was collected at the time of each acute exacerbation event. Classical culture techniques for bacteria and multiplex polymerase chain reaction (PCR) and microarray detection assays were performed to identify viral and atypical bacterial pathogens in the sputum. Overall, 51 patients were included and 45 acute exacerbation events were investigated clinically and microbiologically. Among the 45 acute exacerbation events, 44% had evidence of viral infection involving human rhinovirus (HRV) and metapneumovirus (hMPV) in 20% and 18%, respectively. Intracellular bacteria were not found in sputum by PCR. Common bacterial pathogens were identified in 42% of acute exacerbation patients, most frequently Branhamella catarrhalis, Streptococcus pneumoniae and Haemophilus influenzae. Viral or virus and bacteria co‐infections were detected in 27% of acute exacerbation events (n = 12) with HRV and hMPV involved in 92% of cases. Patients with co‐infections did not present greater clinical severity scores at exacerbation and more recurrence of acute exacerbation events at 3 and 6 months than those with single infections (P > 0.4). These results suggest that HRV and hMPV may be contributors or cofactors of AECOPD. These findings indicate that viral or virus and bacterial co‐infections do not impact significantly on the clinical severity of acute exacerbation of chronic obstructive pulmonary disease and recurrence at 3 and 6 months. J. Med. Virol. 85:866–873, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: chronic obstructive pulmonary disease, exacerbation, respiratory virus, polymerase chain reaction (PCR), human rhinovirus (HRV), human metapneumovirus (hMPV)
INTRODUCTION
Acute exacerbation of chronic obstructive pulmonary disease is an important cause of morbidity and mortality. This disease is associated with worsening quality of life and decline in lung function [Seemungal et al., 1998; Donaldson et al., 2002]. Acute exacerbation of chronic obstructive pulmonary disease is triggered by bacterial or viral infection of the airways but the pathophysiological importance of these infectious agents is not fully understood [Melbye et al., 1992; Falsey et al., 1995; Dowell et al., 1996; Soler et al., 1998]. Classical bacteria are usually isolated in 40–60% of sputum sampled at the time of acute exacerbation of chronic obstructive pulmonary disease but 20–40% of patients with stable chronic obstructive pulmonary disease have also positive sputum cultures suggesting that bacteria may sometimes be innocent bystanders [Sethi et al., 2007]. The role of viruses has been largely investigated with recent reports describing viral infection in 29–44% of acute exacerbation of chronic obstructive pulmonary disease events. Using PCR techniques, the most common detected viruses in upper respiratory airway samples of acute exacerbation of chronic obstructive pulmonary disease patients were rhinoviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, coronaviruses, and adenoviruses [Mohan et al., 2010]. Viral infections can also be detected in stable chronic obstructive pulmonary disease patients suggesting that some respiratory viruses may cause persistent low‐grade infection contributing to the pathogenesis of the disease [Seemungal et al., 2001; Papi et al., 2006; Mohan et al., 2010]. Only few recent studied investigated the presence of mixed viral and classical and intracellular bacterial infections in sputum samples taken at the time of acute exacerbation event [Soler et al., 1998; Bandi et al., 2003; Papi et al., 2006; Wilkinson et al., 2006a; De Serres et al., 2009]. In the present time, the burden of multiple viral and bacterial respiratory infections as contributors of acute exacerbation of chronic obstructive pulmonary disease remains to be investigated.
In this prospective 1‐year follow‐up study, the burden of multiple viral and bacterial respiratory pathogens was assessed in moderate‐to‐severe chronic obstructive pulmonary disease patients and its relationship with the clinical severity criteria of acute exacerbation of chronic obstructive pulmonary disease and the frequency of recurrence at 3 and 6 months was assessed.
PATIENTS AND METHODS
Patients
Patients with chronic obstructive pulmonary disease were consecutively recruited from the Department of Respiratory Medicine of Reims (France) from January 2006 to December 2008. Chronic obstructive pulmonary disease was clinically defined in accordance with the Global Initiative for Chronic Obstructive Lung Disease criteria (GOLD) [Rabe et al., 2007] with post‐bronchodilator FEV1 <80% and FEV1/FVC <70%. At inclusion, all patients were stable with no acute exacerbation of chronic obstructive pulmonary disease for 4 weeks. Patients with asthma, bronchiectasis, cancer, or other respiratory diseases were excluded. The Hospital Ethics Committee approved the study (UCH Reims, Champagne Ardenne; number 2006‐05, France) and a written informed consent was obtained from the patients.
Study Design and Clinical Follow‐Up
Clinical characteristics of the patients were assessed at the time of inclusion, monthly and at the time of each acute exacerbation of chronic obstructive pulmonary disease event during 1 year. A diary card was given to each patient to determine a daily clinical score based upon self‐reported symptoms: dyspnoea, sputum production, and purulence. Symptoms were noted as 1 (as usual), 2 (worse than usual), or 3 (much worse than usual). Acute exacerbation was defined as a minor worsening (+1) of at least two symptoms or a major worsening (+2) of at least one symptom for at least 2 days [Seemungal et al., 2001; Sethi et al., 2002]. Patients remained on their regular treatment for the duration of the study. Patients were consulting monthly to record changes in symptoms; medication and lung function and they were asked to contact the study team at each acute exacerbation of chronic obstructive pulmonary disease. For each patient a physical exam was performed to assess acute exacerbation of chronic obstructive pulmonary disease severity. Arterial blood gases, chest X‐ray, and pulmonary functional tests were performed. Induced sputum was obtained prior to initiation of AECOPD treatment. One week after acute exacerbation of chronic obstructive pulmonary disease event, recovery was clinically assessed and monthly follow‐up was resumed. Pulmonary functional tests were performed using a Body box 5500 plethysmograph (Medisoft, Sorinnes, Belgium). Sputum samples collected at the time of acute exacerbation were induced with hypertonic saline at 3%, 4% then 5% concentration delivered by ultrasonic nebuliser as previously described [Papi et al., 2006].
Microbiological Assays
Bacteriological assays
Samples were homogenized as described previously [Sethi et al., 2002]. Serial dilutions were placed on blood, chocolate, and Mac Conkey agar plates. Bacterial identification was performed by standard techniques. Sputum isolates were classified as potential pathogens microorganisms or normal flora. Potential pathogens microorganisms were: Haemophilus influenzae, Moraxella catarrhalis, Streptocuccus pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and other gram‐negative rods [Sethi et al., 2002]. Colony forming units (cfu)/ml were then calculated.
Classical virological assays
Immunofluorescence assays for detection of respiratory syncytial viruses A and B, influenza viruses, parainfluenza viruses, and adenoviruses antigens were performed as described previously [Bouscambert‐Duchamp et al., 2005]. Virus isolates were typed by the standard method of virus neutralization onto cell culture for enteroviruses and by classical immunofluorescence antigen detection assays onto infected cell monolayers [Bouscambert‐Duchamp et al., 2005; Jacques et al., 2006].
Multiplex PCR assays detection of viruses and atypical bacteria
Total nucleic acid extraction (DNA/RNA) was performed using the NucliSens easyMAG instrument (BioMérieux, Lyon, France). Nucleic acids were eluted in a final volume of 50 µl and then stored at −80°C [Boom et al., 1990; Loens et al., 2007]. CLART® PneumoVir and CLART® FluAVir kits (Genomica, Madrid, Spain) were performed according to the manufacturer's instructions allowing a simultaneous detection and identification of 20 different types and subtypes of human respiratory viruses (Influenza A (seasonal A/H1N1 and A/H3N2 and new influenza A/H1N1v/2009 strains), influenza B and C, parainfluenza (PIV 1, 2, 3, 4A, 4B), respiratory syncytial virus (RSV A and RSV B), human rhinovirus (HRV), adenovirus, human enteroviruses, human bocaviruses, human coronavirus E‐229, human metapneumovirus (hMPV‐A and hMPV‐B) [Renois et al., 2010]. RT‐PCR inhibitors were not detected in the analyzed samples as demonstrated by the positive detection of the internal control [Renois et al., 2010]. Each sputum sample was also tested for the presence of intracellular bacteria using a multiplex PCR and microplate assay (Chlamylege assay, Argene, Varhiles, France) allowing the detection of Legionella pneumophila, Chlamydia pneumoniae, and Mycoplasma pneumonia [Ginevra et al., 2005].
HRV genotyping
In cases of positive HRV genomic RNA detection, the VP4 region and part of the VP2 genome region (VP4‐VP2) were amplified with specific primers as described previously [Savolainen‐Kopra et al., 2009; Wisdom et al., 2009]. To determine the rhinovirus type, the obtained consensus sequences of VP4‐VP2 were compared to all corresponding HRV sequences available in GenBank for each region by using BLAST software. Sequence alignments were performed using the Clustal W program (version 1.81). Phylogenetic and molecular evolutionary analyses were conducted using MEGA (version 4.0) software and allowed us to perform reliable HRV genotyping identification [Savolainen‐Kopra et al., 2009; Wisdom et al., 2009]. The sequences generated in the present study have been assigned to the following GenBank accession numbers: JQ918189–JQ918195.
Statistical analyses
Clinical or biological data were expressed as mean values ± standard deviation. Chi‐squared test, Mc Nemar Chi‐squared test, Fisher's exact test or Wilcoxon rank‐sums test was carried out when necessary using the SAS software, version 9.1.3 (SAS Institute, Cary, NC). A P‐value <0.05 was considered significant.
RESULTS
Patients' Characteristics
Fifty‐one chronic obstructive pulmonary disease patients were included consecutively from January 2006 to December 2008. According to GOLD classification, 10 patients (20%) were classified as GOLD II (moderate), 32 (63%) GOLD III (severe), and 9 (17%) were GOLD IV (very severe). Twenty‐five patients (49%) reported at least one acute exacerbation of chronic obstructive pulmonary disease during the 12‐month follow‐up (mean: 1.8 ± 0.8 acute exacerbation event per year; Table I). Eleven patients (22%) reported one acute exacerbation of chronic obstructive pulmonary disease, nine patients (18%) reported two acute exacerbations, four patients (7%) reported three acute exacerbations, and only one patient (2%) reported four acute exacerbations. A total of 45 acute exacerbations of chronic obstructive pulmonary disease were reported and analyzed in the study. Nine acute exacerbations events (20%) required hospitalization. One patient died from acute respiratory failure. The retrospective analysis of diary cards showed that 22 acute exacerbation events were not reported. Comparison between patients with no reported acute exacerbation of chronic obstructive pulmonary disease, one acute exacerbation of chronic obstructive pulmonary disease, and >2 acute exacerbations of chronic obstructive pulmonary disease did not show any differences, except a slight difference for age (66 ± 8 years for no acute exacerbation vs. 60 ± 8 years for >2 acute exacerbation events, P < 0.05; Table I).
Table I.
Clinical and Functional Baseline Characteristics of 51 Prospectively Studied Patients
| No reported AECOPD (n = 26) | 1 reported AECOPD (n = 11) | >2 reported AECOPD (n = 14) | |
|---|---|---|---|
| Age, year | 66 ± 8 | 60 ± 9 | 60 ± 8* |
| Male gender, % | 84 | 73 | 86 |
| Current smoker, % | 23 | 55 | 29 |
| Smoking history, pack‐year | 43 ± 20 | 57 ± 24 | 41 ± 15 |
| Chronic bronchitis, % | 54 | 82 | 79 |
| Influenza vaccination, % | 68 | 56 | 89 |
| Pneumococcus vaccination, % | 47 | 40 | 50 |
| FEV1, % predicted | 45 ± 8 | 44 ± 13 | 41 ± 16 |
| FVC, % predicted | 75 ± 14 | 69 ± 9 | 72 ± 15 |
| FEV1/FVC, % | 47 ± 9 | 49 ± 14 | 45 ± 14 |
| BMI, kg/m2 | 28 ± 9 | 27 ± 6 | 27 ± 9 |
| PaO2, mmHg | 74 ± 9 | 74 ± 6 | 74 ± 13 |
| PaCO2, mmHg | 41 ± 5 | 41 ± 5 | 42 ± 5 |
| pH | 7.41 ± 0.04 | 7.42 ± 0.02 | 7.41 ± 0.04 |
| Inhaled corticosteroid,% | 88 | 81 | 93 |
| Long‐acting β2‐agonist, % | 96 | 91 | 100 |
| Short‐acting bronchodilator, % | 50 | 91 | 71 |
| Long term oxygen therapy, % | 4 | 9 | 0 |
AECOPD, acute exacerbation of chronic obstructive pulmonary disease.
Data are presented as mean ± SD.
P < 0.05 versus no AECOPD.
Microbiological Findings in Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Sputum samples were analyzed in 45 acute exacerbations of chronic obstructive pulmonary disease (Table IIA). No pathogen was found in 16 acute exacerbations of chronic obstructive pulmonary disease (35%). Viruses were detected by PCR in 20 (44%) acute exacerbations of chronic obstructive pulmonary disease cases: one virus in eight cases (18%), two viruses in two cases (4%), and co‐infection virus and potential pathogen microorganisms in 10 cases (22%; Table IIB). HRV or hMPV represented 72% of detected viruses. As expected, viral detection rates by PCR were significantly greater than those observed by classical viral culture assays (44% vs. 7%, respectively, P < 0.001). hMPV was mainly detected in spring whereas HRV was predominant in autumn and spring (Fig. 1). In case of positive HRV detection at time exacerbation (n = 4), HRV genotyping assay on VP4/VP2 capsid viral genes was performed as previously described [Wisdom et al., 2009]. These genotyping results identified several HRV phylogenetic groups corresponding to the common geno‐groups A (serotypes 14 and 18) and B (serotype 44) and interestingly to the newly described geno‐group C (not shown).
Table II.
Microbiological Findings Obtained at Exacerbation of Chronic Obstructive Pulmonary Disease A Global Findings Obtained Using Classical Culture Techniques for Bacteria and Viruses Associated to Multiplex Polymerase Chain Reaction (PCR) Assays for the Detection of Viral and Atypical Bacterial Pathogens in Sputum Specimens
| Number of samples positive bya | ||||
|---|---|---|---|---|
| PCR microarray techniques | Culture assays | |||
| n | % | n | % | |
| Respiratory virusesb | 20 | 44 | 3 | 6 |
| HRV | 9 | 20 | 0 | 0 |
| HMPV | 8 | 18 | 0 | 0 |
| Influenza A virus | 2 | 4 | 1 | 2 |
| Influenza B virus | 1 | 2 | 0 | 0 |
| RSV | 1 | 2 | 1 | 2 |
| Parainfluenza virus | 1 | 2 | 1 | 2 |
| Respiratory bacteria | 19 | 42 | ||
| Classical PPM | ||||
| Moraxella catarrhalis | ND | ND | 6 | 13 |
| Haemophilus influenzae | ND | ND | 8 | 17 |
| Streptococcus pneumoniae | ND | ND | 3 | 7 |
| Streptococcus species | ND | ND | 1 | 2 |
| Pasteurella multocida | ND | ND | ND | 2 |
| Citrobacter freundii | ND | ND | ND | 2 |
| Haemophilus parainfluenzae | ND | ND | 1 | 2 |
| Proteus vulgaris | ND | ND | 1 | 2 |
| Atypical respiratory pathogens | ||||
| Legionella pneumophila | 0 | 0 | ||
| Mycoplasma pneumoniae | 0 | 0 | ||
| Chlamydia pneumoniae | 0 | 0 | ||
| B‐Viral and bacterial co‐infections at exacerbation of chronic obstructive pulmonary disease | ||
|---|---|---|
| Coinfections | n = 12 | |
| Pathogen 1 | Pathogen 2 | |
| HMPV | 6 | |
| Influenza A virus | 1 | |
| HRV | 1 | |
| H. influenzae | 1 | |
| M. catarrhalis | 1 | |
| S. pneumoniae | 1 | |
| H. parainfluenzae | 1 | |
| HRV | 6 | |
| HMPV | 1 | |
| H. influenzae | 4 | |
| M. catarrhalis | 1 | |
| RSV | H. influenzae | 1 |
More than one pathogen possible.
Twenty viral pathogens were detected by microarray system; only positive detected viral types were notified in this table.
Figure 1.
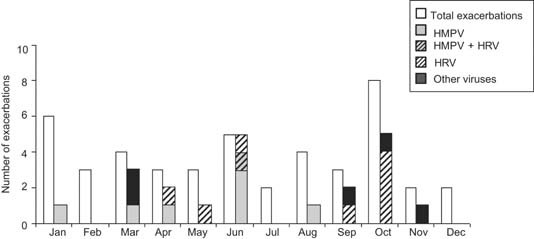
Monthly distribution of viruses detected by PCR‐microarray assays at acute exacerbation of chronic obstructive pulmonary disease.
Potential pathogen microorganisms were detected in 19 acute exacerbations of chronic obstructive pulmonary disease (42%): one in 17 cases, two in one case, and three in one case (Table IIA). M. catarrhalis, H. influenzae, or Streptococcus pneumoniae were found in 77% of potential pathogen microorganism detection. The bacterial load in positive samples was 106 cfu/ml or greater. Intracellular bacteria (Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae) were not found in sputum samples at time of acute exacerbation of chronic obstructive pulmonary disease by PCR. Viral or virus and bacteria co‐infections were detected in 27% of acute exacerbations of chronic obstructive pulmonary disease (n = 12). HRV and HMPV were involved in 11/12 co‐infections (92%; Table IIB). HRV was associated with H. influenzae in four co‐infection cases (33%).
Clinical Findings and Outcome
The influence of the viral infection and the impact of multiple infections on severity of acute exacerbation of chronic obstructive pulmonary disease were analyzed. Interestingly, viral detection by PCR at time of acute exacerbation of chronic obstructive pulmonary disease was not associated statistically with the severity of exacerbation in term of hospitalization rate and clinical score, FEV1 and PaO2 variations from baseline (P > 0.2; Table III). In addition, co‐infections cases (virus/bacteria or virus/virus) did not present greater clinical severity scores at exacerbation than those with single infections (P = 0.45; Table III). Next, we analyzed the potential statistical relationships between the frequency of acute exacerbation recurrence and multiple infections. We observed no significant statistical differences in the frequency of recurrence after acute exacerbation of chronic obstructive pulmonary disease between patients with and without multiple viral or bacterial infections at 3 months (17% vs. 33%, respectively, P = 0.46) and at 6 months (42% vs. 52%, respectively, P = 0.74; data not shown). Multiple infection rates were not significantly different in cases of frequent (>2 per year) or infrequent acute exacerbation of chronic obstructive pulmonary disease (<2 per year) (29% vs. 18% of exacerbations, respectively, P = 0.70). Finally, co‐infected patients did not present greater clinical severity scores at exacerbation and more recurrences of exacerbation at 3 and 6 months than those with single infections (P > 0.4; Table III).
Table III.
Clinical Severity Criteria of Acute Exacerbation of Chronic Obstructive Pulmonary Disease According to the Microbiological Findings
| Total (n = 45) | Virusa (n = 8) | Co‐infectiona (n = 12) | Bacteriaa (n = 9) | No pathogena (n = 16) | |
|---|---|---|---|---|---|
| Clinical score | 6.1 ± 1.2 | 6.4 ± 1.2 | 6.5 ± 1.4 | 6.3 ± 1.2 | 5.6 ± 1.2 |
| Δ clinical score | 3.1 ± 1.2 | 3.4 ± 1.2 | 3.5 ± 1.4 | 3.1 ± 1.2 | 2.6 ± 1.1 |
| Hospitalization, % | 17 | 20 | 10 | 33 | 19 |
| Δ FEV1, % predicted | 1 ± 10 | 6 ± 7 | 3 ± 7 | 0.3 ± 10 | 2 ± 12 |
| Δ PaO2 | −0.9 ± 10 | −0.7 ± 6.8 | 4 ± 11.6 | −5.7 ± 9 | −2.5 ± 10.4 |
Data are presented as mean ± SD.
Δ, difference from baseline.
No significant differences between all groups.
DISCUSSION
Several studies have measured the burden of viral or bacterial infections, but only few have assessed the importance of both pathogens and the presence of mixed viral or virus and bacteria multiple infections [Soler et al., 1998; De Serres et al., 2009]. In this prospective 1‐year follow‐up study, 51 patients were included and 45 acute exacerbation events were clinically and microbiologically investigated. Of the 45 acute exacerbation events studied, 44% had evidence of viral infections, 42% demonstrated classical bacterial pathogens, and 27% had evidence of multiple viral or virus and bacteria co‐infections (Table II). The detection rates of classical bacterial pathogens and of respiratory viruses by RT‐PCR techniques were in agreement with those previously published in recent reports [Melbye et al., 1992; Falsey et al., 1995; Dowell et al., 1996; Soler et al., 1998; De Serres et al., 2009]. However the distribution of type of respiratory viruses appeared to be different from previously published studies with identification of HRV and HMPV as the primary contributors to acute exacerbation while RSV and influenza were infrequently detected during this 1‐year pilot study. Potential pathogen microorganisms were detected in 42% of acute exacerbation events demonstrating as previously described the predominance of M. catarrhalis, Haemophilus influenza, and Streptococcus pneumoniae that represented 77% of classical bacteria. No intracellular bacteria were detected at time of acute exacerbation events in patient with chronic obstructive disease (Table II).
In the present study, HMPV was identified as one of potential primary contributors of acute exacerbation events whereas this respiratory virus has been rarely detected at time of exacerbation in previous studies ranging from 0% to 4.7% with a weighted mean prevalence of 0.7% in a recent review (Table II) [Beckham et al., 2005; Rohde et al., 2005; Mohan et al., 2010]. These discrepant results could be explained by the sensitivity of the used techniques and by the design of the study or the temporal epidemiological characteristics. HRV was previously identified as a one of the major contributor of acute exacerbation of chronic obstructive pulmonary disease and interestingly our genotyping analyses showed that the geno‐group C could trigger acute exacerbation events. Because HRV group C strains are not easily detectable by classical culture techniques; these results indicate the need to use PCR techniques to explore the relationship between virus infections and acute exacerbation of chronic obstructive pulmonary disease. In the present study, viral respiratory pathogens were detected by use of a multiplex PCR‐DNA and microarray detection assay allowing a rapid and accurate detection of conventional and newly discovered viral respiratory pathogens. Respiratory viruses were detected in 44% of acute exacerbations of chronic obstructive pulmonary disease by multiplex PCR‐DNA microarray system, a rate 6.7 higher than those obtained by classical viral detection techniques. However, this qualitative molecular approach was not able to distinguish an ongoing infection from a chronic or persistent viral shedding [Seemungal et al., 2001; Kuypers et al., 2006; De Serres et al., 2009]. Further prospective clinical studies testing the viral load levels at time of acute exacerbation versus stable state are now necessary to differentiate ongoing infection from past or beginning viral infections in acute exacerbation of chronic obstructive pulmonary disease cases.
One of the main objectives of this study was to evaluate the potential relationships between multiple bacterial and viral infections and the severity and risk of recurrence of acute exacerbation of chronic obstructive pulmonary disease. In the present study, multiple infections were not related to demographic characteristics, smoking history, lung function or inhaled treatment, in accordance with previous studies [Seemungal et al., 2001; Wilkinson et al., 2006b]. Finally, clinical outcomes of acute exacerbation of chronic obstructive pulmonary disease that were depending on multiple infections were analyzed. Mixed infections (virus–virus or virus–bacteria) were not found to be associated with the severity of acute exacerbation of chronic obstructive pulmonary disease or with an increased risk of recurrence at 3 and 6 months (P > 0.4). A recent study using PCR detection methods for nasal aspirate samples described that acute exacerbation of chronic obstructive pulmonary disease cases of mixed virus–bacterial infections do not appear to be more severe among virus‐infected patients [De Serres et al., 2009]. The present study provides additional data on the impact of mixed virus or bacterial infections onto the clinical severity and the risk of recurrence of acute exacerbation that are critical elements in chronic obstructive pulmonary disease management. However, it must be pointed out that the present monocentric study was conducted in a relatively low number of chronic obstructive pulmonary disease patients and acute exacerbation of chronic obstructive pulmonary disease events. Further multicentric studies involving larger population are needed to come to definitive conclusions regarding the clinical impact of multiple infections in acute exacerbation of chronic obstructive pulmonary disease.
The present results demonstrated chronic obstructive pulmonary disease patients infected by two respiratory viruses therefore suggesting a potential intrinsic susceptibility to particular triggers of acute exacerbation of chronic obstructive pulmonary disease [Seemungal et al., 2001]. This susceptibility could be related to polymorphism of genes encoding CCL1, a chemotactic factor for leukocytes [Takabatake et al., 2006], mannose binding lectin (MBL2) [Yang et al., 2003] or surfactant protein B [Foreman et al., 2008], altering host defense factors against pathogens. Recently, an experimental human model of HRV infection clearly showed the role of HRV as triggering factor in acute exacerbation of chronic obstructive pulmonary disease [Mallia et al., 2011]. A deficient induction of type III interferon‐λ by HRV in asthmatic primary bronchial cells and alveolar macrophages was correlated with the severity of HRV related asthma exacerbation [Contoli et al., 2006]. The hypothesis of a lower immunological response against viral infections in acute exacerbation of chronic obstructive pulmonary disease remains to be investigated in further clinical prospective studies.
In conclusion, these results suggest that HRV and hMPV may be contributors or cofactors of acute exacerbation of chronic obstructive pulmonary disease. These findings indicate that viral or virus and bacterial co‐infections do not impact significantly on the clinical severity of acute exacerbation of chronic obstructive pulmonary disease and recurrence at 3 and 6 months.
Conflict of interest: None.
REFERENCES
- Bandi V, Jakubowycz M, Kinyon C, Mason EO, Atmar RL, Greenberg SB, Murphy TF. 2003. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non‐typeable Haemophilus influenzae. FEMS Immunol Med Microbiol 37:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, Atmar RL. 2005. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 5:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol CJ, Salimans MM, Jansen CL, Wertheim‐van Dillen PM, van der Noordaa J. 1990. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouscambert‐Duchamp M, Lina B, Trompette A, Moret H, Motte J, Andréoletti L. 2005. Detection of human metapneumovirus RNA sequences in nasopharyngeal aspirates of young French children with acute bronchiolitis by real‐time reverse transcriptase PCR and phylogenetic analysis. J Clin Microbiol 43:1411–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contoli M, Message SD, Laza‐Stanca V, Edwards MR, Wark PAB, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis‐Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. 2006. Role of deficient type III interferon‐lambda production in asthma exacerbations. Nat Med 12:1023–1026. [DOI] [PubMed] [Google Scholar]
- De Serres G, Lampron N, La Forge J, Rouleau I, Bourbeau J, Weiss K, Barret B, Boivin G. 2009. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol 46:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. 2002. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell SF, Anderson LJ, Gary HE, Jr. , Erdman DD, Plouffe JF, File TM, Jr , Marston BJ, Breiman RF. 1996. Respiratory syncytial virus is an important cause of community‐acquired lower respiratory infection among hospitalized adults. J Infect Dis 174:456–462. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Cunningham CK, Barker WH, Kouides RW, Yuen JB, Menegus M, Weiner LB, Bonville CA, Betts RF. 1995. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 172:389–394. [DOI] [PubMed] [Google Scholar]
- Foreman MG, DeMeo DL, Hersh CP, Carey VJ, Fan VS, Reilly JJ, Shapiro SD, Silverman EK. 2008. Polymorphic variation in surfactant protein B is associated with COPD exacerbations. Eur Respir J 32:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginevra C, Barranger C, Ros A, Mory O, Stephan J‐L, Freymuth F, Joannès M, Pozzetto B, Grattard F. 2005. Development and evaluation of Chlamylege, a new commercial test allowing simultaneous detection and identification of Legionella, Chlamydophila pneumoniae, and Mycoplasma pneumoniae in clinical respiratory specimens by multiplex PCR. J Clin Microbiol 43:3247–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques J, Bouscambert‐Duchamp M, Moret H, Carquin J, Brodard V, Lina B, Motte J, Andréoletti L. 2006. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J Clin Virol 35:463–466. [DOI] [PubMed] [Google Scholar]
- Kuypers J, Wright N, Ferrenberg J, Huang M‐L, Cent A, Corey L, Morrow R. 2006. Comparison of real‐time PCR assays with fluorescent‐antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 44:2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loens K, Bergs K, Ursi D, Goossens H, Leven M. 2007. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J Clin Microbiol 45:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza‐Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. 2011. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbye H, Berdal BP, Straume B, Russell H, Vorland L, Thacker WL. 1992. Pneumonia—A clinical or radiographic diagnosis? Etiology and clinical features of lower respiratory tract infection in adults in general practice. Scand J Infect Dis 24:647–655. [DOI] [PubMed] [Google Scholar]
- Mohan A, Chandra S, Agarwal D, Guleria R, Broor S, Gaur B, Pandey RM. 2010. Prevalence of viral infection detected by PCR and RT‐PCR in patients with acute exacerbation of COPD: A systematic review. Respirology 15:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. 2006. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173:1114–1121. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez‐Roisin R, van Weel C, Zielinski J. 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176:532–555. [DOI] [PubMed] [Google Scholar]
- Renois F, Talmud D, Huguenin A, Moutte L, Strady C, Cousson J, Leveque N, Andreoletti L. 2010. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza‐like illnesses by use of reverse transcription‐PCR DNA microarray systems. J Clin Microbiol 48:3836–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde G, Borg I, Arinir U, Kronsbein J, Rausse R, Bauer TT, Bufe A, Schultze‐Werninghaus G. 2005. Relevance of human metapneumovirus in exacerbations of COPD. Respir Res 6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen‐Kopra C, Blomqvist S, Smura T, Roivainen M, Hovi T, Kiang D, Kalra I, Yagi S, Louie JK, Boushey H, Boothby J, Schnurr DP. 2009. 5' Noncoding region alone does not unequivocally determine genetic type of human rhinovirus strains. J Clin Microbiol 47:1278–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal T, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. 1998. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157:1418–1422. [DOI] [PubMed] [Google Scholar]
- Seemungal T, Harper‐Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. 2001. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164:1618–1623. [DOI] [PubMed] [Google Scholar]
- Sethi S, Evans N, Grant BJB, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347:465–471. [DOI] [PubMed] [Google Scholar]
- Sethi S, Sethi R, Eschberger K, Lobbins P, Cai X, Grant BJB, Murphy TF. 2007. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176:356–361. [DOI] [PubMed] [Google Scholar]
- Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El‐Ebiary M, Hernandez C, Rodriguez‐Roisin R., 1998. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 157:1498–1505. [DOI] [PubMed] [Google Scholar]
- Takabatake N, Shibata Y, Abe S, Wada T, Machiya J, Igarashi A, Tokairin Y, Ji G, Sato H, Sata M, Takeishi Y, Emi M, Muramatsu M, Kubota I. 2006. A single nucleotide polymorphism in the CCL1 gene predicts acute exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174:875–885. [DOI] [PubMed] [Google Scholar]
- Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. 2006a. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 129:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TM, Donaldson GC, Johnston SL, Openshaw PJM, Wedzicha JA. 2006b. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173:871–876. [DOI] [PubMed] [Google Scholar]
- Wisdom A, Leitch EC, Gaunt E, Harvala H, Simmonds P. 2009. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: Comprehensive VP4‐VP2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol 47:3958–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IA, Seeney SL, Wolter JM, Anders EM, McCormack JG, Tunnicliffe AM, Rabnott GC, Shaw JG, Dent AG, Kim ST, Zimmerman PV, Fong KM. 2003. Mannose‐binding lectin gene polymorphism predicts hospital admissions for COPD infections. Genes Immunol 4:269–274. [DOI] [PubMed] [Google Scholar]


