Abstract
This study aimed to assess the clinical characteristics and T‐helper 1 (Th1)/Th2 profile of human rhinovirus (HRV) infection in children with bronchiolitis and pneumonia, compared with the respiratory syncytial virus (RSV). In September 2013 to August 2014, 335 nasopharyngeal aspirates from children below 14 with bronchiolitis and pneumonia were screened for HRV and 13 other respiratory viruses by PCR or reverse transcription PCR. Interferon (IFN)‐γ, interleukin (IL)‐2, IL‐4, IL‐6, IL‐10, and tumor necrosis factor (TNF)‐α were detected by multiplex enzyme‐linked immunosorbent assay. HRVs were found in 66 cases (19.7%), including 35 bronchiolitis and 31 pneumonia cases. Compared with the RSV alone group, children with pneumonia had more frequent wheezing episodes in HRV (P a = .001) and HRV + non‐RSV (P b = .002) groups, and fever in the HRV (P f = .004) and HRV + RSV (P g = .005) groups. Among patients with bronchiolitis, cases with HRV alone were more likely to present in winter than those with RSV alone (P i = .010) and HRV + non‐RSV (P j = .014), and less numerous in summer compared with HRV + non‐RSV (P h = .005). Children with HRV alone were more susceptible to have a history of eczema than RSV alone among bronchiolitis (P c < .001) and pneumonia (P e = .033) cases. HRV bronchiolitis cases had increased IL‐4/IFN‐γ and decreased TNF‐α/IL‐10 ratios, compared with HRV pneumonia counterparts. HRV is a major non‐RSV pathogen causing hospitalization in children with bronchiolitis and pneumonia and induces an imbalanced Th1/Th2 response in bronchiolitis. Compared with RSV infection, HRV bronchiolitis and pneumonia differ significantly regarding wheezing episodes, susceptibility to eczema, fever occurrence, and seasonal prevalence.
Keywords: human rhinovirus, infection, Th1/Th2 immune responses
Highlight
HRV is a major non‐RSV pathogen causing hospitalization in children with bronchiolitis and pneumonia and induces an imbalanced Th1/Th2 response in bronchiolitis. Compared with RSV infection, HRV bronchiolitis and pneumonia differ significantly regarding wheezing episodes, susceptibility to eczema, fever occurrence and seasonal prevalence.
Abbreviations
- HRV‐B
human rhinovirus bronchiolitis
- HRV‐P
human rhinovirus pneumonia
- RSV‐B
respiratory syncytial virus bronchiolitis
- RSV‐P
respiratory syncytial virus pneumonia
1. INTRODUCTION
Human rhinovirus (HRV) is the most commonly detected respiratory virus in children, and more than 90% of individuals under 2 years of age have experienced at least one episode of HRV infection.1 Previous findings show that the rates of HRV causing bronchiolitis and viral CAP in hospitalized children reach to 25% and 50%, respectively, revealing that HRV is a common etiologic agent of these two diseases.2, 3, 4 However, HRV coinfects with other viruses and bacteria frequently, especially respiratory syncytial virus (RSV), and the clinical features and disease severity of HRV infection differ among studies.5, 6, 7
HRV belongs to the Enterovirus genus (Picornaviridae family) and is genetically diverse. Based on phylogenetic sequence criteria, HRVs are classified into three species, including HRV‐A, HRV‐bronchiolitis (HRV‐B), and HRV‐C,8 and more than 150 distinct serotypes according to surface proteins.9 The prevalence and disease severity of bronchiolitis and pneumonia induced by HRV‐A and HRV‐C are higher than those involving HRV‐B. A previous study demonstrated that HRV‐B exhibits lower and slower replication and less cytopathic effects.10
Exposure of airway epithelial cells to HRV leads to the release of proinflammatory products, which are related to pathogenic mechanisms and help initiate antiviral responses. The T‐helper 1 (Th1)/Th2 imbalance plays an important role in immune responses to viral infections and asthma development. Previous findings indicate that rhinovirus or RSV bronchiolitis early in life is associated with an increased risk of asthma.11, 12 Wheezing induced by HRV in young children is an important predictive factor of recurrent wheezing.13, 14 Therefore, understanding clinical and associated immunological features of HRV would help prevent and treat HRV respiratory diseases. In this study, we aimed to assess the epidemiological and clinical characteristics of HRV infection, determining the Th1‐ and Th2‐related cytokine profiles of HRV infection in children with bronchiolitis and pneumonia.
2. MATERIALS AND METHODS
2.1. Study subjects
From 1 September 2013 to 30 August 2014, a total of 335 hospitalized children under 14 years of age diagnosed with bronchiolitis or pneumonia in Nanjing Children's Hospital were enrolled in this study. Inclusion criteria were: first clinical diagnosis of bronchiolitis or pneumonia during the disease course; case requiring hospitalization. Patients were enrolled within 24 hours of admission, showing no underlying diseases, including asthma, congenital heart disease, immunodeficiency disease, and bronchopulmonary dysplasia. Pneumonia was defined as acute respiratory infection with pulmonary infiltrates on chest X‐ray diagnosed by a radiologist. Bronchiolitis was diagnosed by the presence of wheezing combined with tachypnea, atelectasis, or peribronchial thickening on chest X‐ray in children aged ≤2 years. Ten healthy children without a history of recurrent wheezing and no respiratory infection symptoms within 2 weeks in the children's health clinics were recruited as a control group for cytokine detection. Demographic data, clinical findings, and medical test results were collected with standardized forms. Informed consent was obtained from the parents of enrolled children. The study protocol was approved by the hospital's ethics committee.
2.2. Specimen collection
Nasopharyngeal aspirates (NPAs) were collected from inpatients the day of admission alongside healthy children in the children's health clinics. Then, samples were transferred into virus transport medium and stored at −80℃ until further processing.
2.3. Detection of HRV and other respiratory viruses
DNA and RNA were extracted from 0.2 mL of each NPA with QIAamp viral DNA Minikit and QIAamp viral RNA Minikit (Qiagen, Nanjing, China), respectively. The primers used to amplify a 549‐base pair fragment of HRV (forward: 5′‐GGGACCAACTACTTTGGGTGTCCGTGT‐3′ and reverse: 5′‐GCATCIGGYARYTTCCACCACCANCC‐3′) were described previously.15 RSV, human metapneumovirus (hMPV), adenovirus (ADV), influenza viruses (IFVA and IFVB), parainfluenza virus type 1 to 4 (PIV1 to 4, respectively), human bocavirus (HBOV), coronavirus (NL63, HKU1, and OC43) were detected by reverse transcription polymerase chain reaction (RT‐PCR) or PCR as previously described.15, 16 All laboratory tests were performed at the State Key Laboratory of Children's Hospital of Nanjing Medical University. All positive PCR products were sequenced by beijing genomics institution (BGI) (Shanghai, China).
2.4. Cytokines detection
Next, NPA specimens from patients infected with either HRV or RSV alone and healthy children were selected for cytokine detection. There were five groups, including the control (10 healthy children), RSV‐bronchiolitis (RSV‐B; 10 patients with bronchiolitis infected with RSV alone), RSV‐pneumonia (RSV‐P; 11 patients with pneumonia infected with RSV alone), HRV‐B (9 HRV single infection patients with bronchiolitis) and HRV‐pneumonia (HRV‐P; 13 HRV single infection patients with pneumonia) groups. Interferon (IFN)‐γ, interleukin (IL)‐2, IL‐4, IL‐6, IL‐10, and tumor necrosis factor (TNF)‐α were detected with Human Th1/Th2/Th17 Cytokine Kit (BD, Nanjing, China), following the manufacturer's instructions for Cytometric Bead Array.
2.5. Statistical analysis
Statistical analysis was performed with SPSS, version 19.0. Demographic and clinical parameters were compared among the HRV alone, RSV alone, HRV + RSV, and HRV + non‐RSV groups. The χ 2 test was applied for categorical variables. The Student t test or nonparametric the Kruskal‐Wallis test was used for continuous variables, as appropriate. Comparisons of cytokines concentration between the groups were performed using the nonparametric the Mann‐Whitney U test. Two‐tailed P < .05 was considered statistically significant.
3. RESULTS
3.1. Detection of HRV and other viruses
Of the 335 inpatients, there were 257 males, indicating a male‐female ratio of 3.3:1. Among all patients, there were 171 bronchiolitis and 164 pneumonia cases. A total of 223 (66.6%) cases had at least one respiratory virus, and cases with single virus detection were 158 (47.2%). HRV and RSV were found in 66 (19.7%) and 166, (49.6%) cases, respectively. Other viral pathogens detected included PIV (n = 73 cases or 21.8%, including 46 PIV3, 15 PIV4, 9 PIV1, and 3 PIV2), ADV (n = 70 cases, 20.9%), HMPV (n = 22 cases, 6.7%), HBOV (n = 19 cases, 5.7%), coronavirus (n = 12 cases or 3.6%, including 2 NL63 and 10 HKU1) and IFV (n = 8 cases or 2.4%, including 3 IFVA and 5 IFVB). Phylogenetic analysis of the coding regions of the viral proteins VP4‐VP2 in HRV infected patients revealed that 29 (44.0%), 1 (1.5%), and 36 (54.5%) cases had genotype A, B, C viruses, respectively. Among the 171 bronchiolitis cases, RSV was found in 94 (55.0%), followed by HRV which was detected in 35 patients (20.5%; 14 HRV‐A, 2 HRV‐B, and 19 HRV‐C). In the 164 cases of pneumonia, RSV was found in 72 (43.9%) and HRV in 31 (18.9%, including 13 HRV‐A, 1 HRV‐B, and 17 HRV‐C). The incidence rates of HRV, HRV‐A, HRV‐B, and HRV‐C in the bronchiolitis and pneumonia groups were similar (P > .05). However, RSV was more likely associated with bronchiolitis than pneumonia (P = .043).
3.2. Other copathogens detected in HRV positive cases
There were 33 (50.0%) coinfection cases involving HRV, including 18 RSV, 4 ADV, 2 PIV3, 2 hMPV, 3 ADV + PIV3, 1 ADV + PIV1, 2 RSV + ADV, and 1 PIV4 + HBOV cases. Next, 335 LRTIs cases were assessed by sputum bacteria culture. A total of 9.1% (6/66) of HRV cases were coinfected with bacteria, including three with Haemophilus influenza, two with Klebsiella pneumonia, and one with Streptococcus pneumonia.
3.3. Seasonal distributions of HRV and RSV
The prevalence cycles of HRV and RSV associated bronchiolitis and pneumonia are presented in Figure 1. HRV cases occurred from October 2013 to May 2014, with double peaks in December 2013 and May 2014. Meanwhile, RSV circulated throughout the year and was prevalent from November 2013 to April 2014. HRV‐A was detected from November 2013 to May 2014, with no obvious seasonal distribution. HRV‐C was prevalent in October and December 2013 as well as in May 2014. Other viruses were prevalent mainly in summer and autumn. It should be noted that 91.2% (156/171) of bronchiolitis and 82.9% (136/164) of pneumonia cases were detected in winter (November to January) and spring (February to April) months, which accorded with the epidemic seasons of HRV and RSV.
Figure 1.
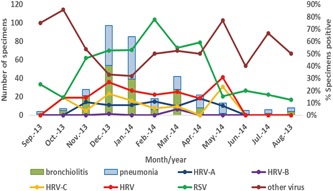
Seasonal distributions of HRV, RSV, and other viruses. HRV, human rhinovirus; RSV, respiratory syncytial virus
3.4. Comparison of HRV with RSV infections in bronchiolitis and pneumonia
Since HRV coinfects with other viruses frequently, with RSV being the most common copathogen, we evaluated the clinical characteristics of children with HRV and RSV single infections, as well as HRV cases coinfected with or without RSV (Table 1). Mean ages and age distributions of patients with bronchiolitis and pneumonia were similar among various viral groups (P > .05). Furthermore, children with HRV infection had more frequent wheezing episodes compared with those with RSV (P a = .001) and HRV + non‐RSV (P b = .002) infections among pneumonia cases, but comparable with other groups among bronchiolitis cases (P = .138). Children with a history of eczema, who did not present during the current illness were more likely to be infected with HRV than RSV (P c = .000) and HRV + non‐RSV (P d = .003) among bronchiolitis cases. Similar results were found in children with pneumonia (P e = .033). Median length of hospital stay (LOS) values in HRV alone cases with bronchiolitis and pneumonia, respectively, were identical, that is, 8.5 days, and similar with those of other groups. LOS more than seven was not different in bronchiolitis and pneumonia cases among HRV alone and other viral groups (P > .05). No significant differences were found in terms of supplemental oxygen, tachypnea, and white blood count among the HRV and the other three groups (P > .05). Children with pneumonia in the HRV group were more likely to have fewer than those of the RSV (P f = .004) and HRV + RSV (P g = .005) groups. HRV associated pneumonia was less likely to occur in summer months than HRV + non‐RSV (P h = .005), while HRV associated bronchiolitis was more likely to occur in winter months than RSV (P i = .01) and HRV + non‐RSV (P j = .014).
Table 1.
Demographic data and clinical profiles of children diagnosed with bronchiolitis or pneumonia associated with HRV and RSV
| Bronchiolitis | Pneumonia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRV | RSV | HRV + RSV | HRV + non‐RSV | HRV | RSV | HRV + RSV | HRV + non‐RSV | |||||
| Group 1 | Group 2 | Group 3 | Group 4 | Statistic | P value | Group 5 | Group 6 | Group 7 | Group 8 | Statistic | P value | |
| (N = 16) | (N = 49) | (N = 10) | (N = 8) | (N = 13) | (N = 35) | (N = 10) | (N = 9) | |||||
| Male; n (%) | 15 (93.8) | 42 (85.7) | 7 (70.0) | 7 (87.5) | 2.67 | .445 | 9 (69.2) | 27 (27.1) | 6 (60.0) | 6 (66.7) | 1.311 | .727 |
| Age (mean ± SD) | 8.5 ± 11.6 | 6.2 ± 6.5 | 13.6 ± 21.3 | 8.6 ± 5.9 | 1.52 | .928 | 14.3 ± 19.6 | 10.5 ± 13.7 | 12.6 ± 20.2 | 11.3 ± 14.7 | 1.98 | .577 |
| <6 m; n (%) | 6 (37.5) | 31 (63.3) | 6 (60.0) | 4 (50.0) | 3.41 | .333 | 2 (15.4) | 18 (51.4) | 4 (40.0) | 3 (33.3) | 5.258 | .154 |
| 6‐24 m; n (%) | 8 (50.0) | 17 (34.7) | 2 (20.0) | 4 (50.0) | 7 (53.8) | 13 (37.1) | 5 (50) | 6 (66.7) | ||||
| >24 m; n (%) | 2 (12.5) | 1 (2.0) | 2 (20.0) | 0 (0.0) | 4 (30.8) | 4 (11.4) | 1 (10.0) | 0 (0.0) | ||||
| History of wheezing episodes; n (%) | 5 (31.3) | 9 (18.4) | 5 (50.5) | 1 (12.5) | 5.517 | .138 | 10 (79.6) | 8 (22.9) a | 4 (40.0) | 1 (11.1) b | 14.58 | .002 |
| History of eczema; n (%) | 12 (75) | 11(22.4) c | 5 (50.0) | 1 (12.5) d | 17.34 | .001 | 5 (38.5) | 4 (22.9) e | 5 (50.0) | 1 (11.1) | 8.062 | .045 |
| MLOS (day); n (%) | 8.5 (2‐14) | 7 (2‐22) | 7 (5‐10) | 8 (4‐16) | 2.50 | .476 | 8.5 (7‐10) | 9 (2‐26) | 8 (4‐17) | 7.5 (6‐19) | 1.147 | .766 |
| LOS > 7 days; n (%) | 5 (31.3) | 30 (61.2) | 4 (40.0) | 5 (62.5) | 5.403 | .145 | 5 (38.5) | 23 (65.7) | 7 (70.0) | 5 (55.6) | 3.434 | .329 |
| Supplemental oxygen; n (%) | 4 (25.0) | 7 (14.3) | 2 (20.0) | 0 | 3.855 | .278 | 4 (30.8) | 12 (34.3) | 3 (30.3) | 1 (11.1) | 2.173 | .544 |
| Clinical manifestation | ||||||||||||
| Tachypnea; n (%) | 4 (25) | 13 (26.5) | 3 (30.0) | 1 (12.5) | 0.965 | .812 | 2 (15.4) | 13 (37.1) | 3 (30.0) | 0 | 6.11 | .106 |
| Fever | 6 (37.5) | 19 (38.8) | 4 (40.0) | 1 (12.5) | 2.508 | .474 | 12 (92.3) | 16 (45.7) f | 4 (40.0) g | 6 (66.7) | 11.44 | .01 |
| T > 38.5℃; n (%) | 4 (25.0) | 17 (34.7) | 4 (40.0) | 1 (12.5) | 2.453 | .484 | 6 (46.2) | 7 (20.0) | 1 (10.0) | 3 (33.3) | 4.965 | .174 |
| WBC counts (mean ± SD, 10^9) | 8.9 ± 3.5 | 11.2 ± 4.6 | 10.0 ± 4.1 | 9.0 ± 2.2 | 1.014 | .392 | 14.5 ± 5.1 | 10.3 ± 4.2 | 9.8 ± 3.2 | 12.0 ± 4.1 | 2.296 | .087 |
| Detection season; n (%) | ||||||||||||
| Spring mo (2, 3, 4) | 1 (6.3) | 12 (24.5) | 2 (20) | 3 (37.5) | 4.177 | .234 | 5 (38.5) | 11 (31.4) | 3 (30.0) | 2 (22.2) | 0.675 | .879 |
| Summer mo (5, 6, 7) | 0 | 2 (4.1) | 0 | 0 | 2.142 | .543 | 0 | 0 | 0 | 3 (33.3) h | 13.94 | .005 |
| Autumn mo (8, 9, 10) | 0 | 4 (8.2) | 1 (10.0) | 1 (12.5) | 2.842 | .417 | 1 (7.7) | 4 (11.4) | 0 | 0 | 3.642 | .303 |
| Winter mo (11, 12, 1) | 15 (93.8) | 31 (63.3) i | 7 (70.0) | 4 (50.0) j | 7.972 | .047 | 7 (53.8) | 20 (57.1) | 7 (70.0) | 4 (50.0) | 0.918 | .821 |
Note: Tachypnea: age 0~12 mo, respiratory rate ≥ 60 times per minute, 1~5‐y old, respiratory rate ≥40 times per minute. Comparisons between the groups (HRV vs RSV, HRV vs HRV + RSV, HRV vs HRV + non‐RSV) were performed using the Bonferroni test to correct α. α ≤ .017 was considered significant.
Abbreviations: HRV, human rhinovirus; LOS, length of stay in hospital; MLOS, median length of stay in hospital; RSV, respiratory syncytial virus; SD, standard deviation; WBC, white blood cell.
The bold values in Table 1 means that the difference between the two sets of data compared is statistically significant.
χ 2 = 11.822, P a = .001, group 5 vs group 6.
χ 2 = 9.214, P b = .002, group 5 vs group 8.
χ 2 = 14.568, P c = .000, group 1 vs group 2.
χ 2 = 9.081, P d = .003, group 1 vs group 4.
χ 2 = 4.547, P e = .033, group 5 vs group 6.
χ 2 = 8.467, P f = .004, group 5 vs group 6.
χ 2 = 7.756, P g = .005, group 5 vs group 7.
χ 2 = 13.343, P h = .005, group 5 vs group 8.
χ 2 = 6.628, P i = .010, group 1 vs group 2.
χ 2 = 5.992, P j = .014, group 1 vs group 4.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.5. Cytokine levels in HRV and RSV bronchiolitis
To assess the main Th1 and Th2 cytokines induced by HRV and RSV in children with bronchiolitis or pneumonia, we detected IL‐2, IL‐4, IL‐6, IL‐10, IFN‐γ, and TNF‐α in NPAs with Cytometric Bead Array. Median ages in the five groups were: control, 20 (6‐46) months; RSV‐B, 8.9 (6‐19) months; RSV‐P, 16 (1‐56) months; HRV‐B, 8 (1‐21) months; HRV‐P, 15 (1.8‐75) months. No significant differences were found in age (P = .331), sex (P = .478), and histories of wheezing (P = .912) and eczema (P = .059) among various groups. Compared with control values, IL‐2, IL‐4, IL‐6, IL‐10, IFN‐γ, and TNF‐α levels were significantly elevated in the HRV‐B, HRV‐P, RSV‐B, and RSV‐P groups (P < .001) (Figure 2A and 2B). Patients with pneumonia had higher TNF‐α levels than those with bronchiolitis both in the HRV (P < .001) and RSV (P = .04) groups. There were no significant differences in other cytokines between bronchiolitis and pneumonia cases among patients with HRV or RSV. To examine the balance between type 1 and 2 cytokine responses, and to correct for dilution variation among samples, IL‐4/IFN‐γ, IL‐6/IL‐2, and TNF‐α/IL‐10 ratios were assessed. As shown in Figure 3, IL‐4/IFN‐γ ratios were markedly higher in children with bronchiolitis compared with those suffering from pneumonia, both in the HRV (HRV‐B vs HRV‐P: P < .001) and RSV (RSV‐B vs RSV‐P: P < .001) groups, as well as in the control group (HRV‐B vs control, P = .039; RSV‐B vs control, P = .001). Meanwhile, TNF‐α/IL‐10 ratios were lower in children with bronchiolitis compared with those suffering from pneumonia, both in the HRV (HRV‐P vs HRV‐B: P = .001) and RSV (RSV‐P vs RSV‐B: P = .022) groups. Compared with control values, TNF‐α/IL‐10 ratios were higher in the HRV‐P group (P = .009) but not significantly different in the RSV‐P (P = .991). IL‐6/IL‐2 ratios were increased significantly in the HRV (HRV‐B vs control, P = .002; HRV‐P vs control, P < .001) and RSV (RSV‐B vs control, P < .001; RSV‐P vs control, P < .001) groups. Whereas IL‐6/IL‐2 ratios were not different between bronchiolitis and pneumonia cases in the HRV and RSV groups.
Figure 2.
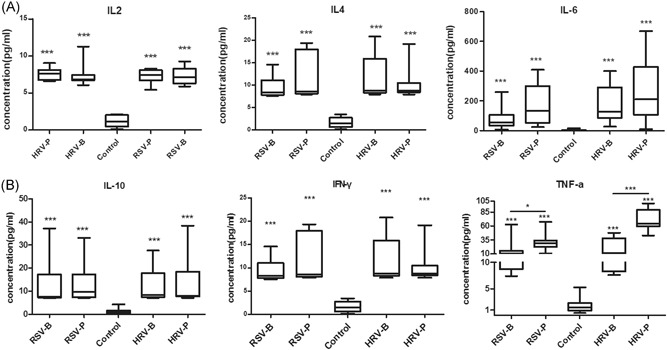
(A and B). Elevated cytokine levels in nasopharyngeal aspirates from HRV and RSV induced bronchiolitis or pneumonia compared with controls. HRV, human rhinovirus; RSV, respiratory syncytial virus
Figure 3.
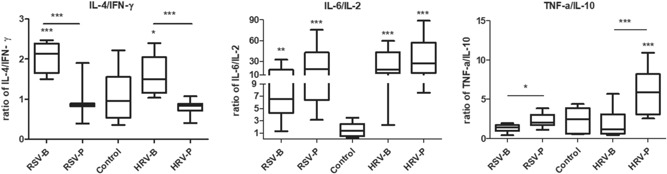
IL‐4/IFN‐γ, IL‐6/IL‐2, and TNF‐a/IL‐10 ratios in nasopharyngeal aspirates from HRV and RSV induced bronchiolitis or pneumonia and the controls. Medians are represented by horizontal bars. Note: Data in Figures 2 and 3 were analyzed by the Mann‐Whitney U test or the Kruskal‐Wallis test; *P ≤ .05; **P < .01; ***P < .001. RSV‐B: bronchiolitis cases with RSV alone, RSV‐P: pneumonia cases with RSV alone, HRV‐B: bronchiolitis cases with HRV alone, HRV‐P: pneumonia cases with HRV alone. HRV‐B, human rhinovirus bronchiolitis; IL, interleukin; RSV‐B, respiratory syncytial virus bronchiolitis; RSV‐P: respiratory syncytial virus pneumonia; TNF, tumor necrosis factor
4. DISCUSSION
This study revealed that HRV was the main virus associated with hospitalization in children with bronchiolitis and pneumonia. The clinical characteristics of these two diseases caused by HRV were similar to those of RSV, except for wheezing episodes, frequency of history of eczema, fever occurrence, and seasonal prevalence. Like RSV, HRV bronchiolitis presented an imbalanced Th1/Th2 immune response in the airway mucosa.
In the present study, HRV was found in 19.5% of children with lower respiratory infection (LRIs), and detection rates were similar in bronchiolitis (20.5%) and pneumonia (18.9%) cases. The results revealed that HRV is not only a major viral etiology of pneumonia but also the second leading cause of bronchiolitis after RSV, corroborating previous studies.2, 3, 17, 18 Unlike RSV, HRV showed similar incidence rates between the bronchiolitis and pneumonia cases. Many prospective long‐term studies have shown that RSV‐induced bronchiolitis is associated with later development of asthma.19, 20 However, a recent cohort study suggested that infants wheezing as a result of HRV infection may be at greater risk of subsequent asthma compared with RSV, PIV, and influenza virus‐infected counterparts.21 Therefore, HRV is not only a cause of lower respiratory tract illness but also represents a significant pathogen associated with wheezing, and routine detection of rhinovirus should be performed in hospitalized children with lower respiratory tract infections, especially wheezing. Furthermore, HRV‐A and HRV‐C infection cases were much numerous than HRV‐B cases in both pneumonia and bronchiolitis groups. Previous research indicated that HRV‐A and HRV‐C infections were more prevalent than HRV‐B18, 22 and caused more severe respiratory illnesses.22, 23 Besides, Nakagome et al10 infected bronchial epithelial cells under air‐liquid interface conditions with rhinovirus and found that HRV‐B has slower replication and lower cellular cytotoxicity compared with HRV‐A and HRV‐C. This might explain why HRV‐B is detected at a lower frequency and causes a milder disease. Since single infections with different species had small sample sizes, we did not compare clinical characteristics among the HRV‐A, HRV‐B, and HRV‐C groups.
Previous data have revealed that HRVs circulate year‐round and prevail in fall and winter.18, 24, 25 The seasonal distributions of HRVs vary according to the geographic area as well as the resulting disease. This study showed that HRV‐induced pneumonia and bronchiolitis were prevalent in winter and spring. HRV‐C incidence peaked in winter and late spring months, while HRV‐A was detected mainly during the autumn and spring months, in general agreement with findings by others.5, 9 Previous studies reported that the seasonal distributions of HRVs are consistent with asthma occurrence in hospitalized children,26 and indicated that HRV infection is more likely to cause severe illness in winter than other seasons.23 This study demonstrated that the epidemic seasons of HRV were predominantly in winter and spring months, which accorded with the episodes of most bronchiolitis and pneumonia cases. Therefore, rhinovirus is an important pathogen causing bronchiolitis and pneumonia in children in winter and spring.
In the present study, mean ages and age distributions were similar among the HRV alone and RSV alone groups, which is consistent with previous studies27, 28, but in contrast with the finding that patients with HRV‐induced lower respiratory tract infection (LRTI) tend to be older than RSV cases.29 This may be related to different study designs. Patients with HRV single infection presented more often a history of eczema than the RSV and HRV + non‐RSV groups, which accorded with findings in a multicenter study.6 Furthermore, the COAST study evaluated the relationship between HRV illness and allergic sensitization and showed that allergic sensitization may precede rhinovirus associated wheezing but not RSV infection.30 This may be partly explained by that more and more children develop allergies with age, which makes them more sensitive to HRV infection.
In this research, HRV was the second most frequent virus detected in bronchiolitis cases. HRV single infection patients with pneumonia were more likely to have a history of wheezing compared with the RSV single infection and HRV + non‐RSV infection. An Italian cohort study showed that the rate of recurrent wheezing is higher after hospitalization with HRV bronchiolitis than with other respiratory viruses during a 1‐year follow‐up period.31 Moreover, a study performed in the United States demonstrated that HRV wheezing in the first 3 years of life is a much stronger risk factor for asthma development at the age of 6 years compared with RSV associated wheezing or aeroallergen sensitization.32 Taken together, HRV infection in early life is a major risk factor for recurrent wheezing disease and asthma development. Compared with children infected with RSV alone, cases with HRV alone and HRV coinfection had similar LOS values. Meanwhile, supplemental oxygen and tachypnea were not different among the HRV single infection and the other three groups. Our findings corroborated studies performed in Netherland and Spain,27, 33 but were in contrast with previous reports showing that hospital duration in children with HRV single infection is shorter7 or longer than 28 that of children with RSV infection. Patients with HRV single infection had a higher frequency of fever and slightly elevated leucocyte amounts in HRV‐infected patients with pneumonia compared with the RSV and HRV + RSV groups, similar to findings by O. Adams.17 This might be associated with large amounts of IL‐8 released during HRV infection; IL‐8 is known as a neutrophil chemotactic factor and induces chemotaxis in neutrophils and other granulocytes.34 Of note, pneumonia cases associated with HRV + non‐RSV infection frequently presented in summer, which may be due to viruses other than HRV and RSV. HRV single infection‐induced bronchiolitis more likely in winter than the other three infection groups, indicating that rhinovirus is an important pathogen that causes bronchiolitis in winter.
Although previous reports have shown virus detection and cytokine profiles in virus‐induced acute wheezing,35, 36 we assessed Th1 and Th2‐type cytokines secreted by patients with bronchiolitis and pneumonia caused by HRV and RSV. We found that both Th1‐type (IFN‐γ, IL‐2, and TNF‐α) and Th2‐type (IL‐4, IL‐6, and IL‐10) cytokines significantly increased in HRV or RSV infected patients with bronchiolitis and pneumonia. Previous studies demonstrated that HRV infection causes a massive upregulation of inflammatory mediators in the host,37 and Th2‐type cytokines may reflect a chronic inflammation of the lower respiratory tract.38 Th2‐type cytokines such as IL‐4, IL‐5, IL‐10, and IL‐13 favor HRV infection by increasing the expression of ICAM‐1.34 A research in the UK demonstrated that the IL‐4/IFN‐γ ratio in infants with acute bronchiolitis elevated in nasal lavage fluid sample on both days 1 to 2 and days 5 to 7 of disease compared with infants suffering from upper respiratory tract infection alone.39 Moreover, a recent study showed that the majority of HRV‐infected infants display a high Th2 response, while the majority of RSV‐infected infants show a Type‐1 response.40 In the current study, Th2/Th1 ratios were higher in HRV and RSV associated bronchiolitis compared with pneumonia and control cases, reflected by increased IL‐4/IFN‐γ and decreased TNF‐α/IL‐10 ratios, which suggested that excessive Th2 or deficient Th1 response may be associated with the pathogenesis of HRV bronchiolitis. The reason why IL‐6/IL‐2 ratios were not significantly different between bronchiolitis and pneumonia cases in both HRV and RSV groups is difficult to explain. However, it may be explained by that IL‐6 represents a potent mediator of cellular communication, is crucial for the regulation of innate and adaptive inflammatory responses,41 and is positively associated with illness severity in RSV and HRV associated lower respiratory tract infection.42, 43 Taken together, an imbalanced Th1/Th2 response plays an important role in the pathogenesis of HRV bronchiolitis and may be one of the reasons for asthma development.
This study has potential limitations. First, we did not test concurrent healthy controls to determine the prevalence of asymptomatic rhinovirus shedding. Another limitation is the low number of subjects in the virus groups and cytokine detection assays since the cases recruited had single virus infections and similar backgrounds.
In conclusion, this study showed that HRV is the second most frequent virus causing childhood bronchiolitis in southeast China and induces Th1/Th2 response imbalance in children with bronchiolitis. HRV and RSV induce bronchiolitis and pneumonia; although having similar clinical characteristics, HRV induced bronchiolitis is more frequent in winter compared with RSV, and cases are more likely to present atopic dermatitis. Meanwhile, HRV pneumonia patients are more likely to have recurrent wheezing and fever than RSV cases. Further studies should consider the pathogenic mechanism of HRV induced wheezing and asthma development.
ACKNOWLEDGMENT
This work was supported by the Major National Science And Technology Projects (81672020) All work was performed in Jiangsu Provincial Key Laboratory of Medicine, Nanjing University.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Yuan X‐h, Li Y‐m, Shen Y‐y, Yang J, Jin Y. Clinical and Th1/Th2 immune response features of hospitalized children with human rhinovirus infection. J Med Virol. 2020;92:26–33. 10.1002/jmv.25587
References
REFERENCES
- 1. Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol. 2002;66(2):263‐268. [DOI] [PubMed] [Google Scholar]
- 2. García‐García ML, Calvo C, Pozo F, Villadangos PA, Pérez‐Breña P, Casas I. Spectrum of respiratory viruses in children with community‐acquired pneumonia. Pediatr Infect Dis J. 2012;31(8):808‐813. [DOI] [PubMed] [Google Scholar]
- 3. Rudi JM, Molina F, Díaz R, et al. The role of rhinovirus in children hospitalized for acute respiratory disease, Santa Fe, Argentina. J Med Virol. 2015;87(12):2027‐2032. [DOI] [PubMed] [Google Scholar]
- 4. Meissner HC. Viral bronchiolitis in children. N Engl J Med. 2016;374(1):62‐72. [DOI] [PubMed] [Google Scholar]
- 5. Morikawa S, Kohdera U, Hosaka T, et al. Seasonal variations of respiratory viruses and etiology of human rhinovirus infection in children. J Clin Virol. 2015;73:14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jartti T, Aakula M, Mansbach JM, et al. Hospital length‐of‐stay is associated with rhinovirus etiology of bronchiolitis. Pediatr Infect Dis J. 2014;33(8):829‐834. [DOI] [PubMed] [Google Scholar]
- 8. Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase‐chain‐reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza‐like illness in New York State during 2004‐2005. J Infect Dis. 2006;194(10):1398‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richter J, Nikolaou E, Panayiotou C, Tryfonos C, Koliou M, Christodoulou C. Molecular epidemiology of rhinoviruses in Cyprus over three consecutive seasons. Epidemiol Infect. 2015;143(9):1876‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakagome K, Bochkov YA, Ashraf S, et al. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134(2):332‐341.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Çalışkan M, Bochkov YA, Kreiner‐Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood‐onset asthma. N Engl J Med. 2013;368(15):1398‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner S, Custovic A, Ghazal P, et al. Pulmonary epithelial barrier and immunological functions at birth and in early life ‐ key determinants of the development of asthma? A description of the protocol for the breathing together study. Wellcome Open Res. 2018;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119(3):570‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemanske RF Jr., Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571‐577. [DOI] [PubMed] [Google Scholar]
- 15. Jin Y, Yuan XH, Xie ZP, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol. 2009;47(9):2895‐2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasman H, Pachucki CT, Unal A, et al. Aetiology of influenza‐like illness in adults includes parainfluenzavirus type 4. J Med Microbiol. 2009;58(Pt 4):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams O, Weis J, Jasinska K, Vogel M, Tenenbaum T. Comparison of human metapneumovirus, respiratory syncytial virus and rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol. 2015;87(2):275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tran DN, Trinh QD, Pham NTK, et al. Human rhinovirus infections in hospitalized children: clinical, epidemiological, and virological features. Epidemiol Infect. 2016;144(2):346‐354. [DOI] [PubMed] [Google Scholar]
- 19. Henderson J, Hilliard TN, Sherriff A, Stalker D, Shammari NA, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386‐392. [DOI] [PubMed] [Google Scholar]
- 20. Ruotsalainen M, Hyvärinen MK, Piippo‐Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48(7):633‐639. [DOI] [PubMed] [Google Scholar]
- 21. Gern JE. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84(15):7418‐7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin EK, Kuypers J, Chu HY, et al. Molecular epidemiology of human rhinovirus infections in the pediatric emergency department. J Clin Virol. 2015;62:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee WM, Lemanske RF Jr., Evans MD, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanner H, Boxall E, Osman H. Respiratory viral infections during the 2009‐2010 winter season in Central England, UK: incidence and patterns of multiple virus coinfections. Eur J Clin Microbiol Infect Dis. 2012;31(11):3001‐3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linder JE, Kraft DC, Mohamed Y, et al. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131(1):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller EK, Lu X, Erdman DD, et al. Rhinovirus‐associated hospitalizations in young children. J Infect Dis. 2007;195(6):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Leeuwen JC, Goossens LK, Hendrix RMGR, Van Der Palen J, Lusthusz A, Thio BJ. Equal virulence of rhinovirus and respiratory syncytial virus in infants hospitalized for lower respiratory tract infection. Pediatr Infect Dis J. 2012;31(1):84‐86. [DOI] [PubMed] [Google Scholar]
- 28. Janahi I, Abdulkayoum A, Almeshwesh F, Alkuwari M, Al Hammadi A, Alameri M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect Dis. 2017;17(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mansbach JM, Clark S, Teach SJ, et al. Children hospitalized with rhinovirus bronchiolitis have asthma‐like characteristics. J Pediatr. 2016;172:202‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson DJ, Evans MD, Gangnon RE, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185(3):281‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Midulla F, Pierangeli A, Cangiano G, et al. Rhinovirus bronchiolitis and recurrent wheezing: 1‐year follow‐up. Eur Respir J. 2012;39(2):396‐402. [DOI] [PubMed] [Google Scholar]
- 32. Jackson D. J., Gangnon R. E., Evans M. D., et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. American journal of respiratory and critical care medicine. 2008;178(7):667‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvo C, Pozo F, García‐García M, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three‐year prospective study. Acta Paediatr. 2010;99(6):883‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J. 2015;45(3):774‐789. [DOI] [PubMed] [Google Scholar]
- 35. Kato M, Tsukagoshi H, Yoshizumi M, et al. Different cytokine profile and eosinophil activation are involved in rhinovirus‐ and RS virus‐induced acute exacerbation of childhood wheezing. Pediatr Allergy Immunol. 2011;22(1 Pt 2):e87‐e94. [DOI] [PubMed] [Google Scholar]
- 36. Roh DE, Park S‐H, Choi HJ, Kim YH. Comparison of cytokine expression profiles in infants with a rhinovirus induced lower respiratory tract infection with or without wheezing: a comparison with respiratory syncytial virus. Korean J Pediatr. 2017;60(9):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atkinson SK, Sadofsky LR, Morice AH. How does rhinovirus cause the common cold cough? BMJ Open Respir Res. 2016;3(1), e000118 10.1136/bmjresp-2015-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Message SD, Laza‐Stanca V, Mallia P, et al. Rhinovirus‐induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL‐10 production. Proc Natl Acad Sci USA. 2008;105(36):13562‐13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168(6):633‐639. [DOI] [PubMed] [Google Scholar]
- 40. Fedele G, Schiavoni I, Nenna R, et al. Analysis of the immune response in infants hospitalized with viral bronchiolitis shows different Th1/Th2 profiles associated with respiratory syncytial virus and human rhinovirus. Pediatr Allergy Immunol. 2018;29:555‐557. [DOI] [PubMed] [Google Scholar]
- 41. Jones SA. Directing transition from innate to acquired immunity: defining a role for IL‐6. J Immunol. 2005;175(6):3463‐3468. [DOI] [PubMed] [Google Scholar]
- 42. Tabarani CM, Bonville CA, Suryadevara M, et al. Novel inflammatory markers, clinical risk factors, and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J. 2013;32(12):e437‐e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Díaz PV, Valdivia G, Gaggero AA, et al. Proinflammatory cytokines in nasopharyngeal aspirate from hospitalized children with respiratory syncytial virus infection with or without rhinovirus bronchiolitis, and use of the cytokines as predictors of illness severity. Medicine. 2015;94(39), e1512 10.1097/MD.0000000000001512 [DOI] [PMC free article] [PubMed] [Google Scholar]


