Abstract
Background
Th17 cytokines are associated with modulation of inflammation and may be beneficial in clearing influenza infection in experimental models. The Th17 cytokine profile was evaluated in a pilot study of respiratory virus infections.
Methods
Consecutive patients with symptoms of respiratory tract infection visiting the emergency department of a tertiary care hospital during the winter influenza season of 2014 to 2015 were evaluated. CLART PneumoVir kit, (GENOMICA, Madrid, Spain) was used for viral detection of all known respiratory viruses. Th17 cytokine profile was evaluated with the MILLIPLEX MAP Human TH17 Magnetic Bead Panel (Millipore Corp., Billerica, MA). Correlation of the TH17 profile with viral detection was performed with univariate and multivariate analysis.
Results
Seventy‐six patients were evaluated (median age 56 years, 51.3% female); a respiratory virus was identified in 60 (78.9%) patients; 45% had confirmed influenza. Influenza A (H3N2) correlated with higher levels of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interleukin 1β (IL‐1β), IL‐17A, IL‐17E, IL‐17F, IL‐21, IL‐22, and IL‐23 (P < 0.05 by analysis of variance [ANOVA]) compared with respiratory syncytial virus (RSV). Parainfluenza virus (PIV) similarly had higher levels of GM‐CSF, IL‐1b, IL‐17A, IL‐22 compared with those detected in RSV, influenza B and any other virus infection ( P < 0.05; ANOVA). Increasing age (β‐coefficient = 1.11, 95% CI, 1.04‐1.2, P < 0.01) as well as IL‐17A levels (β‐coefficient = 1.03, 95% CI, 1.001‐1.05, P = 0.04) predicted hospital admission.
Conclusion
Main Th17 cell effector cytokines were upregulated in laboratory‐confirmed A(H3N2) influenza and PIV. Excessive amounts of Th17 cytokines may be implicated in the pathogenesis and immune control of acute influenza and PIV infection in humans and may predict the severity of disease.
Keywords: IL‐17, influenza, RSV, Th17
1. INTRODUCTION
A significant interplay between innate and adaptive immunity controls the response to respiratory viral infections.1 Influenza upon infecting a human cell is recognized by pattern recognition receptors; this leads to the mobilization of the innate immune system and the secretion of chemokines and cytokine.1, 2, 3 Through a sequence of events neighboring innate immune cells activate transforming growth factor‐β from its latent form and result in further secretion of chemokines and interleukins from parenchymal and inflammatory cells.2 In the initial phase of the immune response, a crucial role is contributed by proinflammatory cytokines, members of the interleukin 1 (IL‐1) superfamily, like cytokines IL‐1β and IL‐18 by infected macrophages and dendritic cells (DCs). The activation of the pyrin domain‐containing protein 3 (NLRP3) inflammasome triggers this cytokine production. In addition, type I and type III interferons are secreted to block virus replication by infected as well as by plasmatocytoid DCs. At the same time, B cells secrete antibodies to mediate the adaptive immune response.2 The end result is either host resistance or tolerance promoting tissue repair.2
This cascade of cytokine secretion is responsible for several of the clinical symptoms and signs associated with the influenza syndrome. Besides the clinical syndrome, the duration and extent of this inflammatory response may lead to further pathology at the local tissue level. Although not clearly associated with viral infections, Th17 cells may play an important role in maintaining the mucosal barrier and contributing to pathogen clearance at mucosal surfaces. There is limited available knowledge of the role of the interleukin‐17 pathway in the response of the human host to a respiratory virus infection. In experimental models, Th17 cytokines have been proposed to be associated with modulation of inflammation and may be beneficial in clearance of influenza infection.4 However, others have suggested a potential role in increasing disease related severity in animal models.5 Limited data have suggested an association of an exaggerated systemic Th1 and Th17 response in severe disease with respiratory involvement in patients suffering from pandemic influenza caused by the AH1N1pdm09 strain.6
In the current study, we evaluated the Th17 cytokine profile in a pilot study of respiratory virus infection where molecular testing was used for virus identification.
2. METHODS
Consecutive patients with symptoms of respiratory tract infection visiting the emergency department (ER) of a Tertiary Care Hospital were evaluated during the 2014 to 2015 winter season. Pharyngeal swab specimens were taken from 76 consecutive patients (outpatients) who presented to the ER, with symptoms of respiratory tract infection (headache or malaise accompanied by either cough, sore throat, or nasal congestion with or without fever). Samples were collected on every call day of the hospital (every 4 days) during the period of 24 October 2014 to 2 April 2015. Basic clinical and epidemiological characteristics were collected through a structured case report form. A nasopharyngeal swab was stored in Thin Prep CytoL solution (Cytyc Corporation, Malborough, MA) and was immediately sent to the laboratory for analysis. The CLART PneumoVir kit, (GENOMICA, Spain) was used for viral detection of all known respiratory viruses according to the manufacturer’s protocol. Briefly, viral RNA was extracted from 400 μL of Thin Prep CytoLyt (Cytyc Corporation, Boxborough, MA) solution using the MagMAX Viral RNA Isolation Kit (Thermo Fisher, Foster City, CA) and eluted in 50 μL elution buffer. The Thin Prep CytoL solution, from each clinical sample, was centrifuged at 2000g for 5 minutes and 20 to 40 μL of the pellet was placed in Thin Prep PreservCyt vials before the molecular testing for the respiratory viruses. The CLART PneumoVir kit was used (GENOMICA) that is capable of detecting and characterizing the presence of the 17 most frequent types of human viruses causing respiratory infections, in the most common clinical samples. Viruses analyzed include: influenza virus A, B, and C; PIV 1, 2, 3, and 4 (subtypes A and B); respiratory syncytial virus type A (RSV‐A); RSV‐B; rhinovirus; human metapneumovirus (hMPV; subtypes A and B); enterovirus (echovirus); adenovirus; coronavirus; and bocavirus. The molecular procedure was followed according to the manufacturer’s instructions. Briefly, 200 μL of Thin Prep PreservCyt solution were used for viral DNA/RNA extraction. Virus amplification was performed via two reverse transcriptase multiplex polymerase chain reactions, and the detection/visualization was performed based on the low‐density microarrays. Negative controls were included in each experiment. The point of care test mariPOC (ArcDia International Oy Ltd, Turku, Finland) an automated, multianalyte antigen test was used in conjunction with our clinical arrays system. The second test was used because it provided the results within 2 hours of testing and thus assisted in management decisions regarding the discharge of the patient from the emergency department; in addition examined the presence of Streptococcus pneumoniae antigen in conjunction. Positive results by any of the two methods used were considered for the analysis. In five cases (three influenza, one hMPV, and one bocavirus infections there was a positive‐ point‐of‐care (POC) test with the negative array test.
Th17 cytokine profile was evaluated with the MILLIPLEX MAP Human TH17 Magnetic Bead Panel a kit used for the simultaneous quantification of the following cytokines known to be involved in the Th17 pathway7: GM‐CSF, IL‐1β, IL‐6, IL‐17A, IL‐17F, IL‐17E/IL‐25, IL‐21, IL‐22, and IL‐23. These cytokines were chosen because they are involved in the IL‐17 cascade. Correlation of the TH17 profile with viral detection and clinical characteristics (eg, age, sex, presence of comorbidities like chronic obstructive pulmonary disease, cardiovascular disease, diabetes or malignancy, history of vaccination, more severe disease requiring hospitalization) as well as laboratory parameters (eg, C‐reactive protein levels, white blood cell counts [WBCs]) of the participating subjects was performed with univariate and multivariate analysis. Cytokine levels were further examined from six healthy volunteers with no evidence of respiratory infection.
Comorbidities and virus positivity were correlated using Fisher’s exact test. Cytokine levels were correlated with the detection of any virus positivity as well as the detection of specific viruses (eg, influenza AH1N1pdm09, influenza B) with nonparametric tests (independent sample t test, Kruskal‐Wallis). We also performed analysis of variance (ANOVA) to compare cytokine levels between the groups of no virus identification, A(H3N2) influenza positive, A(H1N1)pdm09 influenza positive, RSV positive, PIV positive, and any other virus positive (least significant ifference i.e. LSD and Games‐Howell post hoc tests are reported as appropriate according to the Levene’s homogeneity of variance testing and Welch testing of equality of means). Mixed infections were analyzed separately and not added in the individual virus (contributing to the mixed infection) numbers. Further, we looked for cytokine interrelations using Spearman’s correlation coefficient. Correlation between symptoms and virus detection was performed with univariate analysis. We separately examined influenza AH3N2, AH1N1pdm09, influenza B, RSV, and PIV infections compared with other or no virus detection. Multivariate analysis examined factors associated with more severe disease and need for hospitalization using logistic regression. More specifically any factor with a statistical significance of P ≤ 0.1 was included in the multivariate analyses. In final models effects of potential confounding variables in the association with IL‐17 levels (eg, WBC, the presence of comorbidities, fever, fever height, and other clinical and laboratory parameters) as well as their interaction terms were included in the statistical models. All statistical tests were two‐tailed. Data analysis was performed using the SPSS program (version 25.0; SPSS, Chicago, IL).
Written informed consent was obtained from all the participating patients, while the study was approved by the Institutional Review Board of the Hospital.
3. RESULTS
Seventy‐six patients were evaluated with a median age 56 years, (interquartile range [IQR] 39‐78 years); 48.7% were female. A respiratory virus was identified in 60 (78.9%) patients (Table 1). Thirty‐four (45%) patients had confirmed influenza infection (seven mixed with another virus and more specifically three with RSV, two with adenovirus, one with PIV, and one with rhinovirus), 13 (17.1%) PIV infection (one mixed infection with influenza A), and nine (11.8%) had RSV infection (three mixed with influenza B). Overall eight (10.5%) patients had a mixed viral infection. Regarding other viruses, in four (5.3%) patients hMPV was detected, four (5.3%) patients had rhinovirus, two patients (2.6%) had adenovirus, and one patient (1.3%) had bocavirus infection. Patients diagnosed with RSV infection were on average older than patients diagnosed with any influenza or any other virus infection (P < 0.05; ANOVA; Table 2). Influenza patients (both types A and B) had more fever compared to patients with no virus detection or patients with RSV or PIV infections (P < 0.05; ANOVA). RSV‐infected patients when admitted stayed longer in the hospital compared with all other types of viruses (P ≤ 0.01 for all comparisons; ANOVA).
Table 1.
Demographics of the study population
| Any virus, n (%) | Influenza total, n (%) | RSV, n(%) | PIV, n(%) | Mixed virus, (%) | |
|---|---|---|---|---|---|
| Variables, n (%), Total n = 76 | n = 60/76 (78.9) | n = 34/76 (44.7) | n = 9/76 (11.8) | n = 13/76 (17.1) | n = 8/76 (10.5) |
| Age, 18‐40 y, n = 21/76 (27.6) | 17/60 (28.3) | 10/34 (29.4) | 0/9 (0) | 3/13 (23.1) | 2/8 (25.0) |
| Age, 41‐65 y, n = 28/76 (36.8) | 22/60 (36.7) | 14/34 (41.2) | 2/9 (22.2) | 5/13b (38.5) | 2/8 (25.0) |
| Age, >65 y, n = 27/76 (35.5) | 21/60 (35.0) | 10/34 (29.4) | 7/9 (77.8) | 5/13 (38.5) | 4/8 (50.0) |
| Female sex, n = 37/76 (48.7) | 31/60 (51.7) | 18/34 (52.9) | 5/9 (55.6) | 4/13 (30.8) | 4/8 (50) |
| COPD, n = 34/76 (44.7) | 28/60 (46.7) | 15/34 (44.1) | 6/9 (66.7) | 5/13 (38.5) | 4/8 (50.0) |
| CVD, n = 21/76 (27.6) | 19/60 (31.7) | 11/34 (32.4) | 3/9 (33.3) | 3/13 (23.1) | 4/8 (50.0) |
| Diabetes, n = 17/76 (22.4) | 14/60 (23.3) | 8/34 (23.5) | 3/9 (33.3) | 2/13 (15.4) | 2/8 (25.0) |
| Malignancy, n = 4/76 (5.3) | 4/60 (6.7) | 1/34 (2.9) | 0/9 (0) | 2/13 (15.4) | 0/8 (0) |
| ESRD, n = 2/76 (2.6) | 0/60 (0)* | 0/34 (0) | 0/9 (0) | 0/13 (0) | 0/8 (0) |
| Allergy, n = 8 (10.5) | 7/60 (11.7) | 4/34 (11.8) | 2/9 (22.2) | 0/13 (0) | 1/8 (12.5) |
| Smoking, n = 36/76 (47.4) | 27/60 (45.0) | 14/34 (41.2) | 6/9 (66.7) | 8/13 (61.5) | 5/8 (62.5) |
| Influenza vaccine, n = 25/76 (32.9) | 21/60 (35.0) | 10/34 (29.4) | 5/9 (55.6) | 4/13 (30.8) | 3/8 (37.5) |
| Hospitalization, n = 37/76 (48.7) | 31/60 (51.7) | 17/34 (50.0) | 8/9 (88.8)* | 5/13 (38.5) | 5/8 (62.5) |
| Days of hospitalization, mean ± SE | 5.8 ± 1.4 | 4.4 ± 1.5 | 20.1 ± 7.1 | 3.3 ± 1.3 | 10 ± 5.6 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESRD, end‐stage renal disease.
Statistical significance for comparisons between groups with positive results and the rest of the group are flagged with an asterisk.
Statistically significant associations (P < 0.05) for comparison with the rest of the study group are flagged.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Laboratory and cytokine data per specific virus infection for patients in the study
| Virus | No virus (+), n = 12/76 (15.8%) | AH3N2 (+) only, n = 10/76 (13.2%) | AH1N1pdm09 (+) only, n = 4/76 (5.3%) | Influenza B (+) only, n = 11/76 (14.5) | RSV (+) only, n = 6/76 (7.9) | PIV (+) total, n = 11/76 (14.5) | Any other virus (+), n = 8/76 (10.5) | Mixed virus (+), n = 8/76 (10.5) | Streptococcus pneumoniae, n = 6/76 (7.9) | Healthy volunteers, n = 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| WBC, K/mm3 | 8.9 ± 1.2 | 7.3 ± 0.9 | 7.5 ± 0.9 | 7.1 ± 0.98 | 9.5 ± 1.3 | 11.6 ± 1.2* | 7.1 ± 0.94 | 9.4 ± 1.5 | 12.4 ± 1.9* | 6.9 ± 0.8 |
| PMNs, K/mm3 | 6.9 ± 1.2 | 5.6 ± 0.9 | 5.4 ± 1 | 4.9 ± 0.9 | 7.5 ± 1.1 | 9.3 ± 1.2* | 6.8 ± 1.1 | 7.5 ± 1.3 | 10.8 ± 1.9* | 5.1 ± 0.5 |
| LYMPH, K/mm3 | 1.4 ± 0.02 | 0.9 ± 0.1* | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.07 | 1.4 ± 0.2 | 1 ± 0.1 | 0.9 ± 0.1* | 1.1 ± 0.07 |
| MONO, K/mm3 | 0.6 ± 0.08 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.09 | 0.8 ± 0.2 | 1 ± 0.02 | 0.5 ± 0.09 | 0.8 ± 0.2 | 0.8 ± 0.03 | 0.4 ± 0.1 |
| CRP, mg/L | 80.6 ± 26.4 | 37.7 ± 5.5* | 27.9 ± 7.4 | 52.6 ± 23.9 | 40.5 ± 17.9 | 157 ± 72.7 | 36.4 ± 15.2 | 34 ± 8 | 153 ± 47* | 3.4 ± 0.5 |
| GM‐CSF, pg/ml | 1442 ± 252.8 | 2434.7 ± 400* | 1956.8 ± 558.1 | 1158.7 ± 2.87.3 | 488.2 ± 228.9* | 2194.2 ± 323.5* | 1132 ± 433.3 | 1739.3 ± 75.8 | 1154 ± 425.9 | 63.7 ± 156.1 |
| IL‐1β, pg/ml | 63.8 ± 6.6 | 85.8 ± 12* | 80.3 ± 16.8 | 49.1 ± 7.6* | 37.3 ± 6.2* | 79.5 ± 9.4 | 50.9 ± 13.4 | 71.6 ± 17 | 60.7 ± 12.6 | 12.4 ± 8.5 |
| IL‐6, pg/ml | 120.7 ± 39.5 | 187.8 ± 84.9 | 87.3 ± 20.4 | 86.6 ± 21.8 | 54.9 ± 8.6 | 106.4 ± 14.2 | 60.5 ± 17.6 | 99.6 ± 17.8 | 229.3 ± 93.5 | 4.2 ± 7.1 |
| IL‐17A, pg/ml | 76.4 ± 8.2 | 121.6 ± 18* | 100.5 ± 29.2 | 65.4 ± 12.4 | 47 ± 10.4* | 109.7 ± 13.9 | 60.6 ± 17.4 | 98.1 ± 26.1 | 72.8 ± 16.9 | 32.2 ± 39.3 |
| IL‐17E/1L‐25, pg/ml | 1340.9 ± 210 | 2677.1 ± 678.3* | 1843.5 ± 680.4 | 1403.2 ± 342.6 | 609.1 ± 107* | 1986 ± 332 | 908.5 ± 314.1 | 1959.7 ± 564.8 | 1707.4 ± 462.3 | a |
| IL‐17F, pg/ml | 57.3 ± 8.6 | 104.8 ± 16* | 70.9 ± 25 | 58.8 ± 13.8 | 25.9 ± 6.5* | 73.6 ± 10.7 | 36.4 ± 13.7* | 78 ± 59.7 | 77.4 ± 18 | 11.6 ± 28.4 |
| IL‐21, pg/ml | 166.5 ± 17.6 | 242.1 ± 30.3* | 200.6 ± 50.7 | 130.3 ± 21.1* | 99.8 ± 15.9* | 199.1 ± 23.7 | 127.8 ± 33.6 | 200.3 ± 46.3 | 152.1 ± 36 | 39.7 ± 48.3 |
| IL‐22, pg/ml | 643.8 ± 92.2 | 1102.9 ± 160.4* | 796.7 ± 241.9 | 630.7 ± 130.9 | 321.8 ± 64.3* | 844.9 ± 119.5 | 450.6 ± 138.8 | 820.9 ± 219.7 | 828.9 ± 215.4 | a |
| IL‐23, pg/ml | 14111.2 ± 1428.3 | 20360.9 ± 2893.1* | 17269.4 ± 4267.8 | 14123.6 ± 2475.2 | 8889 ± 1230.6* | 18190. ± 2301 | 11077.7 ± 2468.9 | 16636.9 ± 3721.5 | 15220.8 ± 3157 | a |
Statistical significance for comparison with the rest of the group of infections with a respiratory virus is flagged.
P < 0.05 for comparison with the rest of the group. Due to mixed infections numbers in any other virus category overlap.
No measurable levels were obtained for the IL17E, IL‐22 and IL‐23 cytokines in healthy volunteers.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Laboratory and cytokine data per specific virus infection for patients in the study are depicted in Table 2. Data are additionally shown for six healthy volunteers that had significantly lower cytokine values from all the rest of the group (P < 0.01 for all comparisons; Table 2). Elevated C‐reactive protein levels values were noted for most of the patients seen (Table 2; normal values less than 6 mg/L). The highest C‐reactive protein levels values were seen with PIV detection that reached statistical significance when compared with the levels seen with all other viruses including all types of influenza, RSV, and any other virus detection (P ≤ 0.01 for all comparisons; ANOVA). PIV was additionally associated with higher WBCs and polymorphonuclear cell counts (PMNs) compared with no virus detection, A(H3N2) influenza, A(H1N1)pdm9, and influenza B (P < 0.05; ANOVA; Table 2; Figure 1). Influenza B had the lowest WBCs overall and had lower WBCs and PMNs compared with RSV or any other virus detection (P < 0.05; ANOVA; Table 2; Figure 1).
Figure 1.
Mean cytokine values for TH17 pathway cytokines are shown for laboratory parameters and each cytokine (1a‐1l) per type of viral infection vs no infection. All cytokine levels are measured in pg/ml. Asterisks (*) and circles depict outlier values (within 2 and 3 SE of mean). PIV, parainfluenza virus; RSV, respiratory syncytial virus
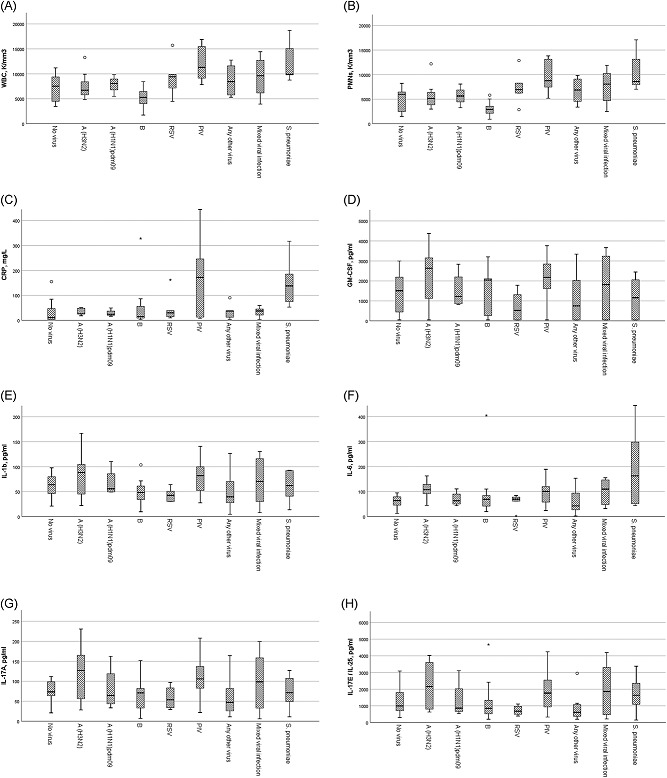
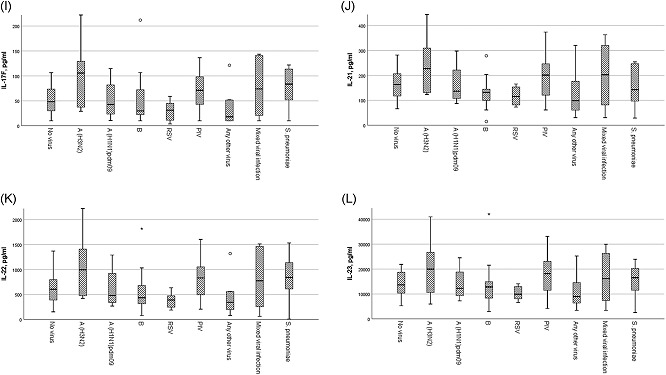
Any virus detection (n = 60/76 patients [78.9%]) or influenza detection (overall as a group, n = 34/76 [44.7%]) was not associated with Th17‐related cytokines. Examining specific viruses, influenza A (H3N2) was associated with higher IL‐17A, IL‐17E/IL‐25, IL‐17F, IL‐21, and IL‐22 levels compared with people presenting with URI symptoms but no respiratory virus detection (P < 0.05; ANOVA with post hoc testing) while IL‐23 (P = 0.09) levels approached statistical significance. Furthermore, influenza A(H3N2) infection was associated with higher levels of GM‐CSF, IL‐1b, IL‐17A, IL‐17E/IL‐25, IL‐17F, IL‐21, IL‐22, and IL‐23 compared with RSV infection or any other virus infection (all P < 0.05; ANOVA with post hoc testing; Table 2; Figure 1). In addition influenza A(H3N2) had higher levels of several Th17 cytokines like IL‐17A, IL‐17F, IL‐21, and IL‐22 compared with B influenza (all P < 0.05; ANOVA with post hoc testing; Figure 1).
PIV similarly had higher levels of GM‐CSF, IL‐1b, IL‐17A, and IL‐22 compared with those detected in RSV, influenza B, and any other virus infection (P < 0.05; ANOVA with post hoc testing; Table 2; Figure 1).
Increasing age correlated positively with hematological parameters including WBCs and PMNs (Spearman rho = 0.34 and 0.4, respectively; P < 0.01), lengthier hospitalization (Spearman rho = 0.6; P < 0.001), and negatively with fever, IL‐17A and IL‐17E levels (Spearman rho = −0.3, −0.25, and −0.22, respectively; P < 0.01); IL‐17A highly correlated with all other IL‐17 cytokines (Spearman rho = 0.73‐0.95; P < 0.001).
We did not identify a significant association of the IL‐17 cascade with specific clinical parameters like the presence of fever, the height of fever (eg, >38.3°C), wheezing, myalgias, and the presence of a dry or a productive cough. Presence of nasal symptoms showed a statistically significant negative correlation with all studied cytokines except for IL‐6 (P < 0.05 for all comparison‐independent samples t test).
A logistic regression model adjusting for age, the presence of any comorbidity, and an interaction term for WBCs, CRP levels, and IL‐17A levels found that age (β‐coefficient = 1.11, 95% CI, 1.04‐1.2, P < 0.01) as well as IL‐17A levels (β‐coefficient = 1.03, 95% CI, 1.001‐1.05, P = 0.04) predicted severity of disease and hospital admission correctly in 86.1% of cases.
4. DISCUSSION
Increased levels of main Th17 cell effector cytokines were identified in laboratory‐confirmed A (H3N2) influenza compared with other types of influenza, RSV, and other respiratory viruses in this pilot study. These excessive amounts of Th17 cytokines may be implicated in the pathogenesis and immune control of acute A(H3N2) influenza infection. Similar observations in PIV infection may indicate a role of the Th17 pathway in parainfluenza infections.
It is unclear how IL‐17 is involved in the immune response to influenza or PIV infections. A crucial role for IL‐17‐mediated inflammation has been found for microbial clearance8; on the other hand, uncontrolled signaling has been associated with autoimmune disease and even cancer progression.8 Specific family members have discrete roles in human immunity eg, IL‐17F is mainly involved in mucosal host defense mechanisms, whereas IL‐17E (IL‐25) is an amplifier of Th2 immune responses.9, 10, 11 Over the last few years very few studies have examined the role of the Th17 pathway in human influenza infections,12 and none to our knowledge in humans with the respiratory infection, and in comparison with other viruses affecting the respiratory tract. Recent literature suggests that Th17 adaptive immunity may be involved in the clearance of influenza viruses from the respiratory tract.
The immune system of patients suffering from influenza responds with the release of several proinflammatory cytokines.12, 13, 14 Among this cascade of cytokines,15 IL‐6 appears to have a central role in symptom generation and local inflammatory effects.16, 17, 18, 19 The clinical relevance of this cytokine storm is that it recruits neutrophils, monocytes, and macrophages to the sites of infection,15 and may be associated with more severe disease and outcomes that appear to depend on the pathogenicity of the virus and the host response. The role of IL‐17 in this process has not been clearly delineated yet although its importance is increasingly recognized.4, 6, 9, 20, 21, 22, 23
Some studies have not shown a positive association with only mild increases in IL‐17 levels.19 Two studies on patients severely affected by the 2009 pandemic virus, higher IL‐17A levels were noted in milder cases, a finding consistent with enhanced‐viral clearance.6, 22 In one study, there was a marked suppression of the adaptive Th1/Th17 immunity A(HN1)pdm09 influenza compared with the seasonal type A and type B influenza.12 In another study of pediatric infections with the novel pandemic virus A(H1N1)pdm09 higher levels of interferon‐α and IL‐6 were seen in patients with the pandemic infection compared to controls with pneumonia attributed to other causes; moreover higher IL‐6 correlated with disease severity.24 Nevertheless, in that study, IFN‐γ and IL‐17 did not differ between mildly and severely affected patients.24 An increase of IL‐17 in patients suffering from diffuse alveolar damage was seen in one experimental lung immunopathology autopsy study of patients affected by the pandemic A (H1N1) virus.25 In patients with pandemic influenza in Beijing, elevated levels of IL‐17, TH17 mediators, and IL‐17 responsive cytokines were detected; the same authors performed A(H1N1)pdm09 influenza experiments in mice showing milder disease in IL‐17 deficient animals or in animals treated with IL‐17 inhibitors.5 On the other hand downregulation of T‐cell responses in severe influenza may lead to Th17 functional impairment, delay in viral clearance, sustained proinflammatory response, and further dysregulation of the adaptive immunity; various factors affect this interplay such as virus pathogenicity and host factors.12
Regarding our RSV observations, limited literature suggests a role for IL‐17 cytokines in RSV pathogenicity.26, 27, 28, 29, 30 In one infant study, IL‐17A and IL‐23 levels were associated with a reduction in clinical symptoms of respiratory distress.31 Lower levels of IL‐17 cytokines in patients with RSV in our study might have contributed to an inability to properly control the host inflammatory response leading to a more serious disease pattern and sicker patients with RSV that were hospitalized longer.
Regarding the association of clinical symptoms with the cytokine profile, we did not observe any significant association except for the negative correlation with the presence of nasal symptoms, probably indicating the presence of other than influenza viruses. Nevertheless, AH3N2 or PIV positive subjects with nasal symptoms did not differ as far as their cytokine profile with those without symptoms suggesting that the observed effects on cytokines were independent of local symptomatology. It is possible that our low sample size did not permit us to find any other significant cytokine effects related to clinical parameters.
Strengths of our study include the first in the literature testing of several Th17 family cytokines across a spectrum of viral infections. We further showed a clear increase in the levels of several of the cytokines belonging to this family in association with AH3N2 influenza and PIV infections. Findings from our study can assist in developing and testing hypotheses regarding the use of this family of cytokine as a therapeutic target in influenza or PIV respiratory infections or about specific cytokine use as a biomarker regarding the severity of disease in certain viral infections.
Limitations of our study include its pilot nature and the small amount of observations; for example, the fact that we could not verify ‐as seen by others‐ a stronger—compared with other viruses—inflammatory response in the A(H1N1)pdm09 influenza virus infection, may be due to a limited number of observations in patients affected by the pandemic virus. Furthermore, we did not study the dynamic evolution of these markers over the course of the viral infection; thus our study is just a snapshot of mild to moderately ill patients showing up in the emergency department of a tertiary care center. It is possible that we have missed patients with milder pictures that quickly cleared the virus from their systems (like some of the patients with no virus detection in our study) thus missing the full spectrum of Th17 response in respiratory viral infections. Sampling from other sites, for example nasal sampling or BAL (in severe cases), would assist in better understanding the difference/synergy between local compared with systemic cytokine effects. In addition, although we carefully recorded and analyzed data on variables potentially adversely affecting an effective immune response we could not account for residual confounding in this study. We can only hypothesize about the mechanism behind the difference in the cytokine profile observed between influenza A and B in our effort. Influenza B infection remains less studied than influenza A both in humans and animals despite the fact that it is associated with a significant number of respiratory infection cases during winter.32 Contrary to our findings, in a study of 72 patients comparing cytokine levels in type A vs type B influenza, IL‐17A (the hallmark cytokine of the TH17 cell subset) along with IL‐6 levels were significantly higher in type B influenza.33 A less virulent circulating B strain could explain our findings. In an animal study that used an immunosuppressed‐mouse model of influenza B infection and a cytokine panel that included IL‐17, there was no evidence of an important contribution of Th17 in the pathogenicity.34 Moreover different degrees of immunopathology was caused by two different influenza B viruses; the Victoria lineage virus was more virulent and was associated with greater morbidity and 100% lethality in these experiments.34 Interestingly, during the period of our study the main influenza B strain across Europe and in Greece belonged to the Yamagata lineage.35 Due to the small sample of this study, this difference will have to be verified in other studies that would specifically examine different lineages of the virus. Finally, the role of Th17 in the effects of influenza on other systems was not assessed in this effort. For example, in a mouse model of respiratory influenza a significant Th17 response in the intestine that was associated with immune intestinal injury and development of diarrhea was noted; furthermore neutralizing IL‐17A reduced intestinal injury.36 Intestinal microbiota changes and their relation to influenza or other virus‐mediated cytokine changes, which is an interesting field for further human research; it could not be assessed in the current effort.36
In conclusion, we described IL‐17 family cytokine level associations with respiratory viral infections confirmed by a molecular method in a pilot study. A large‐scale study of patients with respiratory viral infections would further assist in concurring and understanding the observed upregulation of main Th17 cell effector cytokines in relation to specific types of respiratory viruses. This association and especially the one observed for influenza AH3N2 and PIV is novel and merits consideration even if the finding comes from a small pilot. Future verification of our study results as well as further exploitation of this cytokine pathway for diagnostic, therapeutic, and prognostic purpose that may greatly assist in the management of common but potentially serious viral respiratory infections is necessary.
Antalis E, Spathis A, Kottaridi C, et al. Th17 serum cytokines in relation to laboratory‐confirmed respiratory viral infection: A pilot study. J Med Virol. 2019;91:963‐971. 10.1002/jmv.25406
Note: Dedicated to the loving memory of an outstanding scientist and teacher, Petros Karakitsos, MD, PhD, Professor of Cytology at the University of Athens Medical School, who contributed to the planning and execution of this study until his untimely demise of 26 June 2017.
References
REFERENCES
- 1. Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nature Immunol. 2006;7(5):517‐523. [DOI] [PubMed] [Google Scholar]
- 4. Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17‐mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186(3):1666‐1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C, Yang P, Sun Y, et al. IL‐17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22(3):528‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bermejo‐Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13(6):R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amatya N, Garg AV, Gaffen SL. IL‐17 signaling: The Yin and the Yang. Trends Immunol. 2017;38(5):310‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miossec P, Korn T, Kuchroo VK. Interleukin‐17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888‐898. [DOI] [PubMed] [Google Scholar]
- 10. Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Sem Immunol. 2007;19(6):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang YH, Angkasekwinai P, Lu N, et al. IL‐25 augments type 2 immune responses by enhancing the expansion and functions of TSLP‐DC‐activated Th2 memory cells. J Exp Med. 2007;204(8):1837‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee N, Wong CK, Chan PKS, et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLOS One. 2011;6(10):e26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282(13):1240‐1246. [DOI] [PubMed] [Google Scholar]
- 15. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu W, Booth JL, Duggan ES, et al. Innate immune response to H3N2 and H1N1 influenza virus infection in a human lung organ culture model. Virology. 2010;396(2):178‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL‐6 induction. Virology. 2008;376(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 18. Nakajima N, Van Tin N, Sato Y, et al. Pathological study of archival lung tissues from five fatal cases of avian H5N1 influenza in Vietnam. Mod Pathol. 2013;26(3):357‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arankalle VA, Lole KS, Arya RP, et al. Role of host immune response and viral load in the differential outcome of pandemic H1N1 (2009) influenza virus infection in Indian patients. PLOS One. 2010;5(10):e13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crowe CR, Chen K, Pociask DA, et al. Critical role of IL‐17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301‐5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamada H, Garcia‐Hernandez ML, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182(6):3469‐3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. To KKW, Hung IFN, Li IWS, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50(6):850‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang TJ, Zhang JY, Li WG, et al. Preferential loss of Th17 cells is associated with CD4 T cell activation in patients with 2009 pandemic H1N1 swine‐origin influenza A infection. Clin Immunol. 2010;137(3):303‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YH, Kim JE, Hyun MC. Cytokine response in pediatric patients with pandemic influenza H1N1 2009 virus infection and pneumonia: comparison with pediatric pneumonia without H1N1 2009 infection. Pediatr Pulmonol. 2011;46(12):1233‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buttignol M, Pires‐Neto RC, Rossi e silva RC, Albino MB, Dolhnikoff M, Mauad T. Airway and parenchyma immune cells in influenza A(H1N1)pdm09 viral and non‐viral diffuse alveolar damage. Respir Res. 2017;18(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukherjee S, Lindell DM, Berlin AA, et al. IL‐17‐induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179(1):248‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoppelenburg AJ, de Roock S, Hennus MP, Bont L, Boes M. Elevated Th17 response in infants undergoing respiratory viral infection. Am J Pathol. 2014;184(5):1274‐1279. [DOI] [PubMed] [Google Scholar]
- 28. Petersen BC, Dolgachev V, Rasky A, Lukacs NW. IL‐17E (IL‐25) and IL‐17RB promote respiratory syncytial virus‐induced pulmonary disease. J Leukoc Biol. 2014;95(5):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang G, Zhou KF, Lu ZH. Interleukin‐17 enhances the removal of respiratory syncytial virus in mice by promoting neutrophil migration and reducing interferon‐gamma expression. Genet Mol Res. 2016;15(1 [DOI] [PubMed] [Google Scholar]
- 30. Mebratu YA, Tesfaigzi Y. IL‐17 plays a role in respiratory syncytial virus‐induced lung inflammation and emphysema in elastase and LPS‐injured mice. 2018;58, 717‐726 (6).. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christiaansen AF, Syed MA, Ten Eyck PP, et al. Altered Treg and cytokine responses in RSV‐infected infants. Pediatr Res. 2016;80(5):702‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43‐e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bian JR, Nie W, Zang YS, Fang Z, Xiu QY, Xu XX. Clinical aspects and cytokine response in adults with seasonal influenza infection. Int J Clin Exp Med. 2014;7(12):5593‐5602. [PMC free article] [PubMed] [Google Scholar]
- 34. Marathe BM, Mostafa HH, Vogel P, et al. A pharmacologically immunosuppressed mouse model for assessing influenza B virus pathogenicity and oseltamivir treatment. Antiviral Res. 2017;148:20‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broberg E, Snacken R, Adlhoch C, et al. Start of the 2014/15 influenza season in Europe: drifted influenza A(H3N2) viruses circulate as dominant subtype. Euro Surveill. 2015;20(4):pii21023. [DOI] [PubMed] [Google Scholar]
- 36. Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota‐mediated Th17 cell‐dependent inflammation. J Exp Med. 2014;211(12):2397‐2410. [DOI] [PMC free article] [PubMed] [Google Scholar]


