Abstract
Objectives: To evaluate prevalence of enteric viruses in healthy dogs and to compare it with prevalences in dogs with acute haemorrhagic diarrhoea.
Methods: Faecal samples were collected from 200 healthy dogs and examined by electron microscopy for presence of viral particles. Data were compared with viral prevalences that had been determined retrospectively by electron microscopy for 936 dogs with acute haemorrhagic diarrhoea.
Results: There were significantly more negative faecal samples among the healthy dogs (82·0 per cent) compared with 55·8 per cent in dogs with acute haemorrhagic diarrhoea (P<0·001). With a prevalence of 17·5 per cent, significantly more healthy dogs were shedding coronavirus compared with 11·6 per cent in dogs with acute haemorrhagic diarrhoea (P=0·034). Parvovirus was only detected in one healthy dog (0·5 per cent), thus with a prevalence that was significantly lower than 16·0 per cent detected in the dogs with acute haemorrhagic diarrhoea (P<0·001). Paramyxovirus was not found in any of the healthy dogs but was found in 9·3 per cent of dogs with acute haemorrhagic diarrhoea (P<0·001).
Clinical Significance: Results suggest that shedding of parvovirus and paramyxovirus is strongly associated with acute haemorrhagic diarrhoea. However, coronavirus seems to be even more prevalent among healthy dogs, raising the need for further studies to investigate the strain‐associated pathogenicity of this virus.
Introduction
Infections with enteric viruses are thought to play a significant role as an aetiological agent of acute enteritis in dogs. Among the viral species commonly detected in dogs with diarrhoea are canine parvoviruses (CPVs), canine coronaviruses (CCoVs) and paramyxoviruses (PVs). Rotaviruses, caliciviruses and not further classified virus particles have also been described (Hammond and Timoney 1991, 1985, 1995, 1983, 2001, 2005, 1995). Prevalence of enteric viruses in dogs with diarrhoea seems to depend on age of dogs investigated, as well as on vaccination status of affected dogs; however, environmental factors are also thought to play an important role. High prevalences of CPV and CCoV were detected in faecal samples of dogs with diarrhoea from animal shelters, indicating the influence of hygiene and infection pressure (Stann and others 2005, 1984, 1993). Furthermore, geographic differences may play a role in prevalence of enteric viruses in canine gastrointestinal disease.
However, pathogenicity of some enteric viral species is a matter of discussion because viral agents were also identified in faecal samples of healthy dogs. While shedding of CPV seems to be associated with clinical signs of gastroenteritis, CCoV can be detected in healthy dogs without signs of gastrointestinal disease (Tennant and others 1994, 2005, 1993). Antibody prevalence against CCoV is high in dogs from animal shelters and breeding colonies in different geographic regions (Tennant and others 2001b, 1993, 2004). However, there are studies suggesting a significant role for CCoV in acute diarrhoea in dogs (Tennant and others 1993, 2004).
The present study was conducted to evaluate the prevalence of enteric viruses in healthy dogs and to compare the data with prevalences in samples obtained from dogs with acute haemorrhagic diarrhoea (AHD) to gain more information about incidence and importance of enteric viruses in the dog population in Southern Germany.
Materials and methods
Faecal samples were collected prospectively from 200 healthy privately owned dogs. Dogs belonged to students and employees or their families and friends (most of them living in Southern Germany) of the Veterinary Teaching Hospital in Munich, Germany, or presented to the hospital’s health service for routine vaccination. Dogs were included in the study if they were clinically healthy and free of gastrointestinal problems (no history of vomiting, diarrhoea or anorexia) in the four weeks before sampling. Currently, all dogs were on vaccination according to the owners, but no detailed information was obtained about time point and components of the last vaccination. Information about deworming history and feeding management was not available. All dogs were housed indoors and were kept as pet dogs. Detailed information about patient data and number of dogs sampled per household is displayed in Table 1.
Table 1.
Information about breed, sex, age and housing in healthy dogs (n=200)
| Parameter | Result |
|---|---|
| Breed* | 103 (51·5%) mixed breed dogs |
| 17 (8·5%) huskies† | |
| 9 (4·9%) dachshunds | |
| 8 (4·0%) Australian shepherds | |
| 7 (3·5%) Rottweilers | |
| 6 (3·0%) golden retrievers | |
| 6 (3·0%) Greyhounds | |
| 5 (2·5%) beagles | |
| 4 (2·0%) German shepherd dogs | |
| Sex | 101 (50·5%) female or female/spayed |
| 99 (49·5%) male or male/neutered | |
| Age | Mean age 5·2 years; median age four years (range one month to 19 years) |
| Number of dogs sampled per household‡ | 158 single dogs |
| 5×2 dogs | |
| 2×3 dogs | |
| 1×4 dogs | |
| 1×5 dogs | |
| 1×17 dogs† |
Breed only listed if accounts for ≥2 per cent of study population
All Huskies derived from the same household from a private breeding colony
Number of dogs sampled per household did not necessarily equal absolute number of dogs per household
For the group of dogs with AHD, medical records of patients examined at the University of Munich Veterinary Teaching Hospital between 1991 and 2001 were searched, and records of dogs that had presented with an acute onset of bloody diarrhoea of less than 48 hours duration were reviewed. Of these, dogs in which a faecal examination for virus detection had been performed by electron microscopy (EM) were eligible for inclusion in the study. Details regarding patient data are displayed in Table 2.
Table 2.
Information about breed, sex and age in dogs with AHD (n=936)
| Parameter | Result |
|---|---|
| Breed* | 220 (23·5%) mixed breed dogs |
| 108 (11·5%) dachshunds | |
| 86 (9·2%) Yorkshire terriers | |
| 81 (8·7%) German shepherd dogs | |
| 41 (4·4%) poodles | |
| 29 (3·1%) cocker spaniels | |
| 24 (2·4%) golden retrievers | |
| 24 (2·4%) schnauzers | |
| Sex | 421 (44·9%) female or female/spayed |
| 515 (55·1%) male or male/neutered | |
| Age | Mean age 6·0 years; median age five years (range two months to 17 years) |
AHD Acute haemorrhagic diarrhoea
Breed only listed if accounts for ≥2 per cent of study population
Samples were transferred to the Institute for Medical Microbiology, Infectious and Epidemic Diseases and examined by EM (EM 10; Carl‐Zeiss). For this method, faecal material was diluted 1:5 in phosphate buffered saline and centrifuged at low speed. The supernatant was applied on copper grids and negative stained with tungstic acid.
To obtain data on dogs with AHD, medical records of dogs examined at the Veterinary Teaching Hospital within a period of 10 years were searched, and records of dogs presented with AHD were reviewed. In the years 1991 to 2001, 936 dogs in which a faecal examination for virus detection had been performed by (EM) were eligible for inclusion in the study.
Viral prevalences obtained for the 200 healthy dogs were compared statistically with the prevalences determined for the 936 dogs with AHD. The parameter age was compared between the dogs shedding CCoV and the virus‐negative dogs in the healthy dog population; and age was also compared between the groups of dogs shedding different viruses in the group with AHD. Furthermore, age comparison was performed between healthy dogs and dogs with AHD with a virus‐negative status and between healthy dogs and dogs with AHD shedding CCoV, respectively. Gender distribution was compared between healthy dogs and dogs with AHD. Statistical comparison was performed by use of commercially available statistical software (GraphPad software, Instat 3, version 3.06; GraphPad software Inc., 2003). Viral prevalences were compared with Fischer’s exact test (two‐sided P value), and relative risks were calculated. Sex distribution was compared with Fischer’s exact test. Age comparison was performed with the Mann‐Whitney test (two‐sided P value) because values were not normally distributed. Values of P<0·05 were considered significant.
Results
Viruses were detected in 36 of 200 (18 per cent) faecal samples from healthy dogs and in 414 of 936 (44·2 per cent) samples from dogs with AHD (Fig 1). There were significantly more negative samples among the healthy dogs compared with dogs with AHD (P<0·001; relative risk: 2·99). Significantly, more healthy dogs were shedding CCoV, with a prevalence of 17·5 per cent, compared with 11·6 per cent in dogs with AHD (P=0·034; relative risk: 1·46). CPV was only detected in one healthy dog, representing a prevalence of 0·5 per cent, which proved to be significantly lower than the prevalence of 16·0 per cent detected in the group with AHD (P<0·001; relative risk: 0·031). PV was found in 9·3 per cent of dogs with AHD and in none of the healthy dogs (P<0·001; relative risk: indeterminate). None of the healthy dogs was shedding more than one viral species in a single faecal sample. In the group of dogs with AHD, more than one enteric virus was present in 6·7 per cent of the samples; 4·2 per cent of the faecal samples showed CCoV and PV, 1·2 per cent CPV and CCoV, 0·9 per cent PV and CPV and 0·4 per cent PV, CPV and CCoV.
Figure 1.
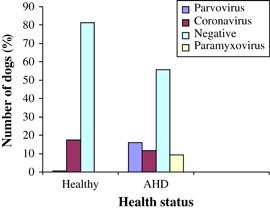
Comparison of viral prevalences between healthy dogs and dogs with acute haemorrhagic diarrhoea (AHD)
Comparison of gender distribution between the group of healthy dogs and dogs with AHD did not show a significant difference between groups (P=0·1601).
Mean age of the healthy dogs shedding CCoV was 2·9 years; mean age of the virus‐negative healthy dogs was 5·2 years (details displayed in Table 3). CCoV‐positive healthy dogs were significantly younger than virus‐negative healthy dogs (P=0·002). Mean age of the patients with AHD shedding CCoV was 4·9 years; mean age of the virus‐negative dogs with AHD was 5·5 years. There was no significant difference between CCoV‐positive and virus‐negative dogs with AHD for the parameter age (P=0·222). When age distribution was compared between groups of dogs with AHD, dogs with CPV infection were significantly younger than dogs infected with CCoV (P<0·001), PV (P<0·001) or virus‐negative (P<0·001).
Table 3.
Comparison of mean age of healthy dogs and dogs with AHD infected with different virus species
| Virus species | Healthy dogs | Dogs with AHD * | ||
|---|---|---|---|---|
| Mean age † | Age range † | Mean age † | Age range † | |
| Virus‐negative | 5·2 (n=164) | 0·1‐19·0 | 5·5 (n=522) | 0·2‐17·0 |
| CCoV | 2·9 (n=35) | 0·1‐11·0 | 4·9 (n=109) | 0·3‐16·0 |
| CPV | 1 (n=1) | – | 1·8 (n=156) | 0·2‐14·0 |
| PV | – | – | 5·9 (n=87) | 0·3‐15·0 |
AHD Acute haemorrhagic diarrhoea, CCoV Canine coronavirus, CPV Canine parvoviruses, PV Paramyxovirus
Dogs shedding more than one virus species were excluded from statistical comparison
Age in years
Comparison of the parameter age for virus‐negative healthy dogs and virus‐negative dogs with AHD showed no statistical significance (P=0·6754). Furthermore, no statistical significant difference was detected when age was compared between healthy dogs shedding CCoV and dogs with AHD shedding CCoV (P=0·0579).
Discussion
While several studies have investigated the role of enteric viruses in dogs with gastrointestinal disease, little information is available about their prevalence in healthy dogs. And because enteric viruses can be detected in a large number of dogs with diarrhoea, this raises the question as to if these viral species actually are the aetiological pathogens causing the gastrointestinal disease? Or if they are an incidental finding of non‐pathogenic organisms simply being shed by animals with diarrhoea as a result of another aetiology. Therefore, the aim of the present study was to further investigate this question by evaluating privately owned pet dogs in Southern Germany and to compare viral prevalences in healthy dogs and in dogs with AHD in the same geographic region.
In this study, CPV was detected in 16·6 per cent of dogs with AHD and in only one healthy dog. These data clearly suggest that shedding of CPV is associated with clinical disease. This assumption is underlined by the results of other studies in which CPV could not be detected in the faeces of healthy dogs (Stann and others 2003, 2005, 1984). The only dog shedding CPV in this study was a one‐year‐old male hunting dog that had been adopted from a shelter six weeks earlier and since then had not displayed any gastrointestinal signs. A possible explanation could be a recent natural CPV infection before adoption because some naturally infected dogs have been shown to shed virus for up to 46 days after occurrence of first clinical signs (Decaro and others 2005). Also, the dog could have ingested CPV contaminated faecal material leading to enteric passage of the virus without systemic infection. This could, for example, happen if the dog is protected by parvovirus vaccination against development of clinical signs but not against infection, thus leading to virus shedding in an asymptomatic animal, as described for puppies shedding CPV despite presence of maternal antibody titres considered sufficiently high for protection (Elia and others 2005). The dog was fully vaccinated according to the owner.
While this study confirms the role of CPV as a primary enteric pathogen, assessment of the role of CCoV in canine diarrhoea seems to be more difficult. CCoV was detected in 17·5 per cent of healthy dogs and only in 11·6 per cent of dogs with AHD, thus questioning the role of CCoV as a primary intestinal pathogen in dogs. The prevalence of CCoV in dogs with AHD is comparable with older German studies, which examined the faecal samples from dogs with diarrhoea by EM, but so far no information is available about the prevalence of this virus in healthy dogs in Southern Germany. Studies performed in Europe and northern America showed high antibody prevalences of CCoV in up to 59 per cent of healthy dogs from animal shelters (Tennant and others 2005, 1993). A study performed by Tennant and others (1993) failed to detect CCoV in the faeces of healthy pet dogs in England.
There are several possible explanations for the high number of healthy dogs shedding CCoV in this study. CCoV belongs to one of three groups of the genus Coronavirus and is genetically closely related to feline coronavirus (FCoV) and transmissible gastroenteritis virus of swine. Because of the high error frequencies of the RNA polymerase, coronaviruses are prone to rapidly develop point mutations in coding and non‐coding sequences, thus resulting in proliferation of different strains, serotypes and subtypes of the virus (Dolja and Carrington 1992). At least two distinct genetic clusters of CCoV can be identified in naturally infected dogs. Type I CCoV is classified as being genetically closely related to FCoV and type II as representing the “typical” CCoV (Pratelli and others 2003), with both genotypes frequently being harboured by one dog (Pratelli and others 2004). Because of the broad genetic variability and fast rate of recombination, even specific strains seem to develop within several weeks in the dog population of animal shelters (Benetka and others 2006). Thus, dogs can be continuously re‐infected with new virus variants exhibiting a broad range of antigenic and genetic variability, potentially evading the host’s immune response. This might be an explanation why, as is known for FCoV in cats, dogs are also suspected to become persistently infected with CCoV. Dogs were found to shed CCoV for up to 156 days after natural infection, and the virus strains involved underwent several mutations during that period (Pratelli and others 2002). A persistent infection could therefore also explain the shedding of CCoV in the healthy dogs in this study. Follow‐up faecal examinations and genetic sequencing would be interesting options in these patients to assess duration of shedding and strains involved. Because of the wide genetic variation of CCoV, different strains are also thought to have different characteristics concerning pathogenicity and virulence factors, possibly being responsible for variation of clinical signs associated with CCoV infection. Some strains seem to cause severe clinical signs of AHD predominantly in very young dogs (Pratelli and others 2006, 2005, 2003), especially in cases of dual infection with other viral species 1999, 2001). Although healthy dogs shedding CCoV were shown to be significantly younger than virus‐negative dogs in this study, this difference in age could not be demonstrated for the patients with AHD. Therefore, it could be assumed that healthy young dogs are more likely to serve as hosts and carriers for CCoV, but other factors are probably necessary to cause clinical disease related to CCoV infection.
No molecular analysis and differentiation of CCoV strains were performed in this study, but it would be an interesting option for future studies to sequence and compare viral strains found in dogs with and without clinical signs. Another factor that could not be investigated in this study was the immune system of the dogs. In a recent study investigating host immune response after oral or parenteral inoculation of CCoV field or vaccine strains, Decaro and others (2004b) found significantly higher CCoV‐specific faecal immunoglobulin A (IgA) antibodies in dogs orally infected with CCoV live‐vaccine and in naturally infected dogs. Further studies are needed to investigate if CCoV‐specific faecal IgA as part of the immune response correlates with grade of protection.
Although the prevalence of PV in the dogs with AHD in this study was higher than that reported in previous studies (Biermann and others 1991, 2001), no PV could be detected in the group of healthy dogs. Little information is available about the role of PV in healthy dogs, but data from this study suggest a strong association of PV infection with clinical disease. Canine distemper virus (CDV) is an important PV, and while earlier studies described respiratory and neurological signs in combination with enteritis in dogs infected with CDV, results from this study suggest that CDV might be involved in the aetiology of AHD without affecting other organ systems (Decaro and others 2004a). Further studies are warranted to investigate the role of this viral species in canine enteritis as the EM did not distinguish between different members of the paramyxoviridae family.
In this study, EM was used as the diagnostic technique for viral detection. One of the advantages of this diagnostic tool is the possibility to detect different viral species in one faecal sample in one diagnostic procedure and to evaluate the samples for concomitant infection with more than one virus. The disadvantages of this method are an implied relatively high cost, lack of quantitative results and a lower sensitivity and specificity compared with PCR assays (Schunck and others 2000, 1995). New PCR methods have been proven to be up to 4×104 times more sensitive than EM and virus isolation for detection of CCoV, thus being able to detect virus also in the faeces of low‐grade shedding animals below 106 particles per gram of unprocessed faeces, which is considered the detection limit for EM (Naylor and others 2001a). Because of the lower sensitivity of EM, the prevalence of enteric viruses might have been underestimated in this study, and at the same time, the prevalence of virus‐negative animals might have been overestimated. In future studies investigating enteric viruses in the Southern German dog population, strain differentiation, especially concerning CCoV, would be desirable to obtain more information about its strain‐associated pathogenicity and clinical characteristics.
Acknowledgements
The authors would like to thank all dog owners for participation and sample collection for the study. Special thanks go to Dr David Morgan and Dr Alex German for their helpful comments on the manuscript.
References
- Benetka, V. , Kolodziejek, J. , Walk, K. , Rennhofer, M. & Möstl, K . (2006) M gene analysis of atypical strains of feline and canine coronavirus circulating in an Austrian animal shelter. Veterinary Record 159, 170‐174 [DOI] [PubMed] [Google Scholar]
- Biermann, U. , Schmitt, K. & Krauss, H . (1991) Electron microscopic virus diagnosis in dogs, cats, calves, swine, and foals in the year 1989. Berliner Münchner Tierärztliche Wochenschrift 104, 117‐119 [PubMed] [Google Scholar]
- Buonavoglia, C. , Decaro, N. , Martella, V. , Elia, G. , Campolo, M. , Desario, C. , Castagnaro, M. & Tempesta, M . (2006) Canine coronavirus highly pathogenic for dogs. Emerging Infectious Diseases 12, 492‐494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Camero, M. , Greco, G. , Zizzo, N. , Tinelli, A. , Campolo, M. , Pratelli, A. & Buonavoglia, C . (2004a) Canine distemper and related diseases: report of a severe outbreak in a kennel. New Microbiology 27, 177‐181 [PubMed] [Google Scholar]
- Decaro, N. , Pratelli, A. , Tinelli, A. , Martella, V. , Camero, M. , Buonavoglia, D. , Tempesta, M. , Caroli, A. M. & Buonavoglia, C . (2004b) Fecal immunoglobulin A antibodies on dogs infected or vaccinated with canine coronavirus. Clinical and Vaccine Immunology 11, 102‐105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Desario, C. , Campolo, M. , Elia, G. , Martella, V. , Ricci, D. , Lorusso, E. & Buonavoglia, C . (2005) Clinical and virological findings in pups naturally infected by canine parvovirus type 2 Glu‐426 mutant. Journal of Veterinary Diagnostic Investigation 17, 133‐138 [DOI] [PubMed] [Google Scholar]
- Dolja, V. V. & Carrington, J. C. (1992) Evolution of positive‐strand RNA viruses. Seminars in Virology 3, 315‐326 [Google Scholar]
- Elia, G. , Cavalli, A. , Cirone, F. , Lorusso, E. , Camero, M. , Buonavoglia, D. & Tempesta, M . (2005) Antibody levels and protection to canine parvovirus type 2. Journal of Veterinary Medicine 52, 320‐322 [DOI] [PubMed] [Google Scholar]
- Evermann, J. F. , McKeirnan, A. J. , Smith, A. W. , Skilling, D. E. & Ott, R. L. (1985) Isolation and identification of caliciviruses from dogs with enteric infections. American Journal of Veterinary Research 46, 218‐220 [PubMed] [Google Scholar]
- Evermann, J. F. , Abbott, J. R. & Han, S . (2005) Canine coronavirus‐associated puppy mortality without evidence of concurrent canine parvovirus infection. Journal of Veterinary Diagnostic Investigation 17, 610‐614 [DOI] [PubMed] [Google Scholar]
- Finlaison, D. S. (1995) Faecal viruses of dogs – an electron microscope study. Veterinary Microbiology 46, 295‐305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, T. & Lappin, M. R. (2003) Prevalence of enteric pathogens in dogs of north‐central Colorado. Journal of the American Animal Hospital Association 39, 52‐56 [DOI] [PubMed] [Google Scholar]
- Hammond, M. M. & Timoney, P. J. (1983) An electron microscopic study of viruses associated with canine gastroenteritis. Cornell Veterinarian 73, 82‐97 [PubMed] [Google Scholar]
- Mochizuki, M. , Hashimoto, M. & Ishida, T . (2001) Recent epidemiological status of canine viral enteric infections and Giardia infection in Japan. Journal of Veterinary Medical Science 63, 573‐575 [DOI] [PubMed] [Google Scholar]
- Möstl, K. , Buxbaum, A. & Odörfer, G . (1994) Verbreitung und Bedeutung von Coronavirusinfektionen in heimischen Hundepopulationen. Wiener Tierärztliche Monatsschrift 84, 355‐361 [Google Scholar]
- Naylor, M. J. , Harrison, G. A. , Monckton, R. P. , McOrist S., Lehrbach P. R. & Deane, E. M. (2001a) Identification of canine coronavirus strains from feces by S gene nested PCR and molecular characterization of a new Australian isolate. Journal of Clinical Microbiology 39, 1036‐1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor, M. J. , Monckton, R. P. , Lehrbach, P. R. & Deane, E. M. (2001b) Canine coronavirus in Australian dogs. Australian Veterinary Journal 79, 116‐119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Tempesta, M. , Roperto, F. P. , Sagazio, P. , Carmichael, L. & Buonavoglia, C . (1999) Fatal coronavirus infection in puppies following canine parvovirus 2b infection. Journal of Veterinary Diagnostic Investigation 11, 550‐553 [DOI] [PubMed] [Google Scholar]
- Pratelli, A. , Buonavoglia, D. , Martella, V. , Tempesta, M. , Lavazza, A. & Buonavoglia, C . (2000) Diagnosis of canine coronavirus infection using nested‐PCR. Journal of Virological Methods 84, 91‐94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Martella, V. , Elia, G. , Tempesta, M. , Guarda, F. , Capucchio, M. T. , Carmichael, L. E. & Buonavoglia, C . (2001) Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. Journal of Veterinary Medicine B 48, 385‐392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Elia, G. , Martella, V. , Tinelli, A. , Decaro, N. , Marsilio, F. , Buonavoglia, D. , Tempesta, M. & Buonavoglia, C . (2002) M gene evolution of canine coronavirus in naturally infected dogs. Veterinary Record 151, 758‐761 [PubMed] [Google Scholar]
- Pratelli, A. , Martella, V. , Decaro, N. , Tinelli, A. , Camero, M. , Cirone, F. , Elia, G. , Cavalli, A. , Corrente, M. , Greco, G. , Buonavoglia, D. , Gentile, M. , Tempesta, M. & Buonavoglia, C . (2003) Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. Journal of Virological Methods 110, 9‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli, A. , Decaro, N. , Tinelli, A. , Martella, V. , Elia, G. , Tempesta, M. , Cirone, F. & Buonavoglia, C . (2004) Two genotypes of canine coronavirus simultaneously detected in the fecal samples of dogs with diarrhea. Journal of Clinical Microbiology 42, 1797‐1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunck, B. , Kraft, W. & Truyen, U . (1995) A simple touch‐down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in faeces. Journal of Virological Methods 55, 427‐433 [DOI] [PubMed] [Google Scholar]
- Sokolow, S. H. , Rand, C. , Marks, S. L. , Drazenovich, N. L. , Kather, E. J. & Foley, J. E. (2005) Epidemiologic evaluation of diarrhea in dogs in an animal shelter. American Journal of Veterinary Research 66, 1018‐1024 [DOI] [PubMed] [Google Scholar]
- Stann, S. E. , DiGiacomo, R. F. , Giddens, W. E. Jr & Evermann, J. F. (1984) Clinical and pathologic features of parvoviral diarrhea in pound‐source dogs. Journal of the American Veterinary Medical Association 185, 651‐655 [PubMed] [Google Scholar]
- Tennant, B. J. , Gaskell, R. M. , Jones, R. C. & Gaskell, C. J. (1993) Studies on the epizootiology of canine coronavirus. Veterinary Record 132, 7‐11 [DOI] [PubMed] [Google Scholar]
- Vieler, E. & Herbst, W . (1995) Electron microscopic demonstration of viruses in faeces of dogs with diarrhea. Tierarztliche Praxis 23, 66‐69 [PubMed] [Google Scholar]
- Yesilbag, K. , Yilmaz, Z. , Torun, S. & Pratelli, A . (2004) Canine coronavirus infection in the Turkish dog population. Journal of Veterinary Medicine B 51, 353‐355 [DOI] [PMC free article] [PubMed] [Google Scholar]


