Abstract
Background
At hospital admission, patients suspected of infection with influenza or respiratory syncytial virus (RSV) are placed in isolation, pending the outcome of diagnostics. In a significant number, isolated care proves unnecessary. We investigated the potential impact of molecular point‐of‐care (POC) diagnostics on patient management and in‐hospital costs.
Method
Prospective collection of data on resource utilization within the hospital from consecutive patients 18 years or older presenting at our university medical center with symptoms of respiratory tract infection from December 2016 to April 2017. A cost analysis was conducted using Markov modeling comparing the actual course of events (on the basis of routine diagnostic tests) with two hypothetical scenarios: when POC would impact time to diagnosis only (scenario 1) or on discharge from the hospital, too (scenario 2).
Results
A total of 283 patients were included, of whom 217 (76.7%) were admitted. Influenza and RSV were detected in 31% and 7% of the patients, respectively. Fifty‐four percent of patients tested negative, of which 79% were kept in isolated care waiting for test results, with a median duration of 24 hours. Median length of stay was 6.0 days. Mean total in‐hospital costs per patient were € 5243. Introducing POC would lower mean costs per patient to € 4904 (scenario 1) and € 4206 (scenario 2). At the hospital level, this would result in a total cost reduction of € 95 937 to € 293 471 in a single influenza season.
Conclusions
Introducing POC testing for patients presenting with symptoms of viral respiratory tract infection can reduce time‐to‐diagnosis, hospital stay and, thereby, in‐hospital costs.
Keywords: cost benefit, influenza virus, rapid detection, respiratory syncytial virus (RSV)
1. INTRODUCTION
Viral respiratory tract infections, especially influenza and respiratory syncytial virus (RSV), are a major cause of morbidity and mortality, causing a substantial burden on health care systems especially during annual seasonal epidemics.1, 2 When admitted to the hospital, patients with suspicion of influenza or RSV infection are placed in isolation in a single‐person room pending the outcome of diagnostic testing and are managed with extensive infection control measures (CDC infection control guidelines, 2007, last updated October 2017). If no single‐persons room is available, a multiple‐person room may be used for a single patient resulting in the loss of admission capacity. This highly impacts hospital resource utilization and healthcare costs.
Furthermore, because of the clinical uncertainty about the cause of disease (viral versus bacterial), many patients will be treated empirically with nontargeted antibiotic therapy until results of microbiological diagnostics are known, whereas only some will actually need it. Rapid detection of respiratory viral pathogens has therefore also the potential of reducing the duration of empirically started antibiotics and initiation of appropriate antiviral treatment, as an antiviral treatment for influenza should be started within 48 hours after onset of disease to be effective.
In the context of diagnostic tests, important improvements have been achieved in the last decade in terms of reducing time to conclusive diagnosis. Most recently, rapid, sensitive, and reliable point‐of‐care (POC) molecular assays have been developed, reducing the time from sample collection to result.3, 4, 5, 6, 7 From a health economic point of view, a relevant question is whether speeding up the diagnostic process incurs extra costs, and, if so, how these costs compare with any downstream savings.
The aim of this study is to assess the potential impact of a POC test for influenza and RSV on patient management and associated hospital costs.
2. MATERIAL AND METHODS
2.1. Study population
From December 2016 to April 2017, all patients aged 18 years and older with suspicion of respiratory viral infection were included, either admitted to our hospital or ambulatory. Our hospital is a tertiary referral academic center in The Netherlands. Respiratory samples were taken on the basis of the clinical judgment of the physician. Patient characteristics were collected (sex, age, underlying illness, length of hospital stay, admission to ICU, and mortality). Respiratory samples were divided in two aliquots, one for the routine laboratory molecular testing, and one for the cobas® Liat System. Aliquots for the cobas Liat System were stored in minus 80°C for retrospective testing at the end of the study.
Patients were followed up, collecting data on resource utilization within the hospital until the time of final diagnosis and discharge. Time of admission, time of collection of respiratory material, time spent in isolation for respiratory illness, time to arrival of the specimen in the laboratory, time to result of the diagnostic test, and data on antimicrobial were retrieved from our electronic patient record system and laboratory information system.
3. LABORATORY PROCEDURES
3.1. Standard methods
For routine detection of influenza virus and RSV the Diagenode assay was performed as described by Templeton et al.8 Briefly, nucleic acids were extracted from 200 µL sample using the MagNA Pure and the MagNA‐Pure LC TNA Isolation Kit (Roche Diagnostics, Almere, The Netherlands). A multiplex RT‐PCR panel assay containing 15 different viral pathogens was used (influenzavirus type A and B, RSV, coronavirus 229E, and OC43, hBoV, EV, AdV, parechovirus, PIV types 1‐4, hMPV, and RV). An internal control consisting of Phocine Herpesvirus (IC DNA control) and Equine Arthritis Virus (IC RNA control) was included in the assay. RNA was reverse‐transcribed to cDNA in a 50 µL reaction mix containing 20 µL of nucleic acid. PCRs were performed on the LightCycler 480 using LightCycler 480 Probes Master Mix (Roche Diagnostics). Cycling conditions were 95°C for 5 minutes, followed by 50 cycles of 95°C for 15 seconds and 55°C for 15 seconds and 72°C for 20 seconds.
3.2. Cobas Liat analyzer
Testing by the cobas® Liat Influenza/RSV assay was carried out according to the manufacturer's instructions, which required 200 µL of patient sample to be pipetted into the supplied assay tube and tested on the cobas® Liat analyzer.9
3.3. Model description
A Markov decision model was developed to compare costs in three scenarios: actual clinical management, compared with two hypothetical scenarios, in which clinical management would have been based on the cobas® Liat (POC). In both hypothetical scenarios, a correct diagnosis was assumed to be made within an hour upon admission. In one scenario, the impact of reduced time‐to‐diagnosis on in‐hospital resource utilization and associated costs was modeled (scenario 1), whereas the second model took into account reduced hospital stay as well (scenario 2). Patients presenting at the out‐patient clinic were distinguished from those presenting at the emergency department. A Markov model was constructed to reflect the in‐hospital routing of patients, distinguishing seven specific states (Figure 1). In each scenario, patients started at the outpatient clinic or the emergency department (state 1). They could either remain in that state, be discharged (ie, not hospitalized, state 7), or they could be hospitalized, in isolated care (state 2) or not (state 3). In both cases, their infection status was unknown. From state 2 they could either remain in that state, or progress to the state “isolated care, infectious state established/need for isolated care confirmed” (state 4), progress to the state “not isolated care, infectious state established/need for isolated care disconfirmed” (ie no influenza or RSV detected, state 5), or progress to state 6 (“not isolated care, infectious state is known, absence of isolation confirmed”). From state 3 (“hospitalized, not isolated care, infectious state unknown”), patients could remain in that state, or progress to state 6 or 4. Patients in states 4, 5 or 6 could remain in that state, or progress to state 7 (“discharge from hospital”). In addition, patients in state 4 or 5 could progress to state 6.
Figure 1.
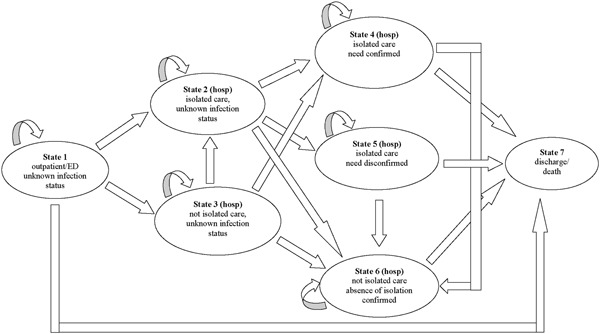
Schematic diagram of the states and the possible transitions for a specific Markov node in the Markov decision model
Cycle‐time for the Markov model was 1 hour; maximal follow up time was 200 hours. Cycle‐time dependent transition probabilities for the care‐as‐usual scenario were on the basis of observed patient routings. In both hypothetical scenarios, test results were assumed to be available within 1 hour, and clinical decisions were assumed to be made in accordance with those results.
3.4. Costs
The costs for routine and POC diagnostic tests were on the basis of estimated cost prices of the assay. Where available, standard cost prices on the basis of national guidelines were used to calculate mean costs per patient.10 Costs for admission in isolated care were assumed to be twice the costs for non‐isolated admission. This would reflect the additional protective equipment for patient and personnel, the use of a one‐person room or multiple room occupancy by a single patient, the extra workload for staff (eg need for finding capacity within own hospital or in other neighboring hospitals) and loss of productivity because of inability to admit patients because of lack of capacity. Table 1 represents an overview of the costs used in the model.
Table 1.
Costs in euros used in the model10
| Visit outpatient clinic | € 163.‐ / each time |
| Visit emergency unit | € 259.‐ / each time |
| Ward, not isolated care | € 642.‐ / day |
| Ward, isolated care | € 1284.‐ / day |
| Routine diagnostic test | € 125.‐ |
| POC molecular test | € 90.‐ |
Abbreviation: POC, point‐of‐care.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.5. Software
All analyses were performed using the TreeAge Pro software package (Version 2015, R 1.0, TreeAge Software, Inc, Williamstown, MA).
4. RESULTS
A total of 283 patients with suspected viral respiratory tract infection were included (Table 2). The majority (84.4%) of patients had a chronic underlying illness. Overall, 217 out of 283 patients (76.7%) were admitted to the hospital, mostly in isolated care (86.2%). Median time to diagnosis (ie result of the respiratory test) was 1 day. Out of 283 patients, 54% tested negative for influenza and RSV. Of those patients, 79% were initially admitted in isolated care waiting for a final test result, as were all patients with proven influenza infection. Forty patients (18.9%) were admitted to intensive care. The median length of stay in hospital was 6 days.
Table 2.
Study population
| Presenting at ER | Presenting at outpatient clinic n = 53 | |
|---|---|---|
| n = 230 | ||
| Sex, male (n, %) | 126 (54.8%) | 35 (66.0%) |
| Age, years (median, range) | 67.0 (18‐95) | 57 (19‐81) |
| Underlying illness * | 190 (82.6%) | 49 (92.5%) |
| Respiratory viruses detected | ||
| Influenza A | 77 (33.5%) | 9 (17.0%) |
| Influenza B | 1 (0.4%) | 1 (1.9%) |
| RSV | 13 (5.7%) | 6 (11.3%) |
| Negative | 128 (55.7%) | 26 (49.1%) |
| Influenza + RSV | ‐ | 1 (1.9%) |
| Other respiratory virus | 11 (4.8%) | 10 (18.9%) |
| Admission | 197 (85.7%) | 20 (37.7%) |
| ICU admission | 38 (19.2%) | 2 (10.0%) |
| Length of stay, days (median, range) | 6.0 (0‐58) | 6.0 (2‐22) |
| Isolated care | 171 (86.8%) | 16 (80.0%) |
| Time until diagnosis * , days (median, range) | 1.0 (0‐3) | 1.0 (1‐4) |
| Antiviral use | ||
| Yes | 85 (37.0%) | 7 (13.2%) |
| Duration, days (median, range) | 4.0 (0‐10) | 5.0 (1‐10) |
| Initiated but influenza negative | 41 (48.2%) | 4 (57.1%) |
| Duration if influenza negative, days (median, range) | 1.0 (0‐5) | 2.0 (1‐6) |
| Duration if influenza positive, days (median, range) | 5.0 (3‐10) * | 7.0 (5‐10) |
| Antibiotic use | ||
| Yes | 166 (72.2%) | 29 (54.7%) |
| Duration, days (median, range) | 6.0 (0‐19) | 7.0 (1‐10) |
| Duration if influenza or RSV positive, days (median, range) | 4.0 (0‐14) * | 7.0 (1‐10) |
| Duration if influenza or RSV negative, days (median, range) | 7.0 (0‐19) | 7.0 (3‐10) |
Abbreviation: RSV, respiratory syncytial virus.
hematological malignancy, COPD and other pulmonary diseases, diabetes, oncology.
time between sample collection and result of routine respiratory PCR.
P < 0.001 compared with influenza or RSV negative.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In 195 patients (68.9%), antibiotic therapy was started, with a median duration of 7 days (0‐19). Antiviral therapy (ie oseltamivir) was prescribed in 92 (32.5%) patients. In 45 (48.9%) patients, influenza diagnostic test was negative; oseltamivir was given in this group for a median duration of 1 day (0‐9). Of those who tested influenza positive (n= 86), 47 (54.7%) were treated with oseltamivir.
Retrospective testing using the cobas® Liat Influenza/RSV assay was possible in 225 patients; for 58 patients either results using the cobas® Liat assay were invalid and no material was left for re‐testing (n = 21), or no respiratory material was left after initial routine testing (n = 37). All patients who tested influenza A, influenza B or RSV positive in the routine PCR had comparable results using the cobas® Liat Influenza/RSV assay (Table 3). Four out of 115 patients who tested negative in the routine PCR turned out influenza A (n = 2) or RSV (n = 2) positive using the cobas® Liat Influenza/RSV assay. In one of these patients, routine PCR on sputum was negative for RSV, but positive on subsequent BAL material. Initial sputum was positive for RSV using the cobas® Liat Influenza/RSV assay. For the other three patients, no other respiratory material was available for further testing to distinguish between true or false positive findings using the cobas® Liat Influenza/RSV assay.
Table 3.
Comparison of cobas Liat Influenza/RSV assay and routine PCR (n = 225)
| cobas Liat | ||||||
|---|---|---|---|---|---|---|
| Influenza A | Influenza B | RSV | Influenza + RSV | negative | ||
| Routine PCR | Influenza A | 60 | ||||
| Influenza B | 1 | |||||
| RSV | 17 | |||||
| Influenza + RSV | 1 | |||||
| negative | 2 | 2 | 111 | |||
Abbreviation: RSV, respiratory syncytial virus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4.1. Modeling results
The results of the Markov modeling are presented in Figure 2. The Figure shows how probabilities that patients reside in either of the seven states change over time, starting from their presenting at the hospital till 200 hours follow up. In the scenario “care as usual,” all patients started in the state “outpatient clinic” or “emergency department” (state 1), and left this state within 10 hours. Concurrently, the probability increased that they entered the state of being hospitalized, either in isolated care or not, whereas their infectious status remained unknown (states 2 and 3). After a rapid increase during the first 10 hours, these probabilities gradually decreased and returned to zero within 60 hours of follow up. During that time, probabilities increased that patients entered states 4, 5, or 6: their infectious state had been established, and they were being hospitalized, either or not in isolated care, in accordance with their infection state. Finally, after an initial rapid increase during the first 10 hours, the probability that patients were discharged from the hospital gradually increased over time. At 200 hours follow up, there was an approximately 70% probability that patients had been discharged.
Figure 2.
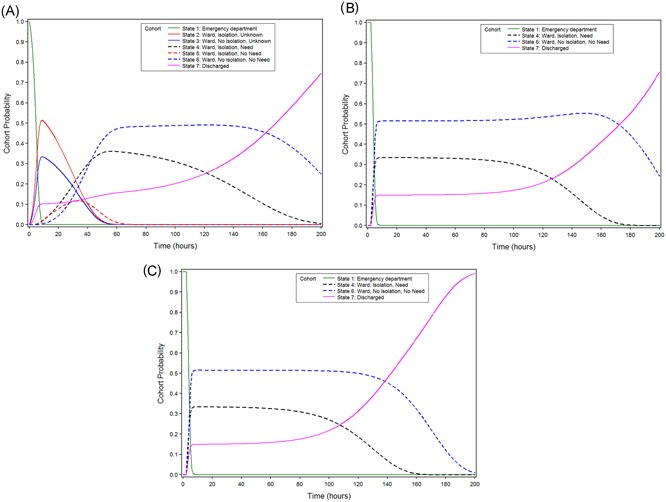
A, B, and C. Cohort probabilities, according to the model, in three scenarios for patients who enter the hospital: care as usual (A), and two hypothetical scenarios: impact on in‐hospital patient routing only (B), and impact on in‐patient routing and discharge from hospital (C)
When compared with this reference scenario, scenario 1 represents the impact of POC testing on time‐to‐diagnosis only (Figure 2B). In scenario 2, POC testing also impacts discharge from the hospital (Figure 2C). In both scenarios, all patients left the emergency department or outpatient clinic (state 1) with known infectious status. Thus, none of the patients entered states 2, 3, and 5. In scenario 2, nearly all patients had been discharged at 200 hours follow up, compared with 70% in scenario 1.
4.2. In‐hospital costs
The costs associated with in‐hospital care for patients suspected of influenza or RSV infection are presented in Table 4. Cost estimates were on the basis of the average length of stay of patients in the various model states (1‐7), combined with unit cost prices (Table 1). In the scenario “usual care,” mean costs per patient amounted to € 5243 (€ 288‐10 105). Introducing POC would lower in‐hospital costs to € 4904 (€ 253‐9604) and € 4206 (€ 253‐8267) in scenarios 1 and 2, respectively.
Table 4.
Mean duration in the various model states (hours) and associated costs (euros) per patient suspected of influenza or RSV infection, in case of care as usual and in case of reduced time‐to‐diagnosis as result of POC (scenario 1) and in case of additional impact of POC on length of stay (scenario 2). For total costs, the 90% confidence interval is presented on the basis of first‐order Monte Carlo simulation of the three models (numbers between brackets)
| Care as usual | Scenario 1 | Scenario 2 | ||||
|---|---|---|---|---|---|---|
| Duration* | Costs | Duration | Costs | Duration | Costs | |
| State 1 presentation | ‐ | 366 | ‐ | 331 | ‐ | 331 |
| State 2 ward, isolated, unknown | 11.8 | 633 | ‐ | ‐ | ‐ | ‐ |
| State 3 ward, not isolated, unknown | 8.5 | 227 | ‐ | ‐ | ‐ | ‐ |
| State 4 ward, correctly isolated | 36.0 | 1922 | 40.4 | 2163 | 35.7 | 1910 |
| State 5 ward, incorrectly isolated | 3.4 | 184 | ‐ | ‐ | ‐ | ‐ |
| State 6 ward, correctly not isolated | 72.5 | 1910 | 90.1 | 2410 | 73.5 | 1965 |
| Total | 5243 | 4904 | 4206 | |||
| (288‐10105) | (253‐9604) | (253‐8267) | ||||
Abbreviations: RSV, respiratory syncytial virus; POC, point‐of‐care.
mean, hours.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
5. DISCUSSION
Our study suggests that introducing a POC test for influenza and RSV at the entry point of adult patients in a hospital might reduce in‐hospital costs by €300‐€1000 per patient suspected of infection with influenza or RSV. These savings result from avoidance of unnecessary infection control measures and shorter length of hospital stay. Our results are consistent with recent studies describing that rapid influenza diagnostic tests reduced costs by a reduction of the time spent at the emergency department, less unnecessary patient isolation because of rapid negative results and a decrease of the number of patient admissions.11, 12, 13 Our study is the first to assess the potential impact of molecular POC tests for influenza and RSV using a model that was populated with real‐world data on the complete trajectory of a cohort of patients suspected of viral respiratory infection through the hospital, from primary contact on the emergency department or outpatient clinic to discharge.
The largest potential cost reduction attributable to the POC test was on the length of stay in the hospital, although it is not clear whether POC testing indeed reduces the length of stay. Most data regarding the effect of rapid testing for influenza and/or RSV on patient care and hospital management are on the basis of retrospective, observational studies. Observational studies have shown that POC testing contributes to a shorter length of stay, a reduction in (duration of) antibiotic use and the prescription of antiviral therapy in a timelier manner.4, 5, 14, 15, 16, 17, 18, 19 Two randomized studies showed a significant reduction in turn‐around‐time and a positive effect on the timely prescription of antiviral therapy; however, no effect was observed on the length of hospital stay and on antibiotic use.20, 21
The impact of POC respiratory virus testing on clinical decision making in patients with respiratory symptoms at the emergency department was assessed in two recent reports.13, 22 In approximately 60% of patients, physicians changed patient management on the basis of the results of the rapid test, resulting in a decreased length of stay at the emergency department, fewer ordering of additional tests and more appropriate antiviral use. Hansen et al13 also showed an effect on the decision to admit patients. As these studies were restricted to the impact of a rapid test on clinical decision making at the emergency department, the overall benefits for patient care in case of admission could not be assessed.
It should be noted that the presented study is on the basis of the current practice in a tertiary care hospital in the Netherlands. In most countries, guidelines on influenza or RSV infection control measures are comparable to the Netherlands, i.e. droplet and contact precautions in a one person‐room (CDC infection control guidelines, updated October 2017). However, to what extent these guidelines are adhered to during the annual influenza epidemics can vary locally. Also, most patients seen at our hospital have co‐morbidities, reflecting the vulnerable patient population of a tertiary care referral hospital, with a subsequent effect on the rate of admission and the need for additional antimicrobial therapy.
Using large multiplex PCR panels enables the detection of other viruses besides influenza and RSV, of which some will be associated with a need for admission to the hospital and/or infection control precautions. This could potentially impact in‐hospital costs. There is an ongoing debate about the added value of large multiplex PCR panels for respiratory pathogens compared to more specific influenza/RSV PCR especially during the influenza season, in which a substantial part of respiratory infections causing hospital admission is caused by influenza or RSV.23 In our hospital, infection control policy is mainly focused on influenza and RSV; for other respiratory viruses, a more differentiated approach is taken, implementing infection control measures only on high‐risk wards. Thus, the potential impact on in‐hospital costs of admission of patients with respiratory infections caused by other viruses than influenza and RSV during the influenza season is considered limited.
The model did not include costs of (inappropriate) antimicrobial therapy because information on the presence or absence of bacterial pathogens was lacking. Early detection of respiratory pathogens by rapid molecular tests has the potential of reducing the duration of empirically started antibiotics. This would result in a reduction in inappropriate antibiotic use, antibiotic pressure and antimicrobial resistance, which would further enhance the benefits associated with POC testing in this patient group. Moreover, available data suggest that a rapid diagnosis could lead to less additional laboratory testing and medical procedures, however, this might only be true for influenza positive patients.13, 22, 24
A key assumption underlying our model is that clinicians act upon the results of the POC test, for example by not prescribing antibiotics to patients who test positive. In practice, clinicians may decide otherwise. Thus, prospective, randomized controlled studies are needed to assess the actual impact of POC test on patient management and infection control, including the effect on patient outcome. These trials should not only focus on actual changes in patient care but also on the complex mechanisms underlying the clinical decision‐making process in the cascade of events during hospital admission and thus the impact of rapid diagnosis on the behavior and considerations regarding patient care.
In conclusion, the introduction of POC molecular diagnostic testing for influenza and RSV for patients presenting with symptoms of a viral respiratory tract infection at the hospital may lead to a more timely installment of appropriate infection control measures and a shorter duration of hospital admission, thus reducing in‐hospital costs.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The study was financially supported by an unrestrictive grant by Roche LTD. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. JRL has presented preliminary results of the study during a session organized and supported by Roche at ECCMID 2018. Other authors have nothing to disclose.
Ethical approval for the study was obtained by the medical ethical committee of the Radboud UMC (under file number 2016‐2971).
Rahamat‐Langendoen J, Groenewoud H, Kuijpers J, Melchers WJ, van der Wilt GJ. Impact of molecular point‐of‐care testing on clinical management and in‐hospital costs of patients suspected of influenza or RSV infection: a modeling study. J Med Virol. 2019;91:1408‐1414. 10.1002/jmv.25479
References
REFERENCES
- 1. Kestler M, Muñoz P, Mateos M, Adrados D, Bouza E. Respiratory syncytial virus burden among adults during flu season: an underestimated pathology. J Hosp Infect. 2018;100:463‐468. [DOI] [PubMed] [Google Scholar]
- 2. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza‐associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibson J, Schechter‐Perkins EM, Mitchell P, et al. Multi‐center evaluation of the cobas(R) Liat(R) Influenza A/B & RSV assay for rapid point of care diagnosis. J Clin Virol. 2017;95:5‐9. [DOI] [PubMed] [Google Scholar]
- 4. Nelson RE, Stockmann C, Hersh AL, et al. Economic analysis of rapid and sensitive polymerase chain reaction testing in the emergency department for influenza infections in children. Pediatr Infect Dis J. 2015;34(6):577‐582. [DOI] [PubMed] [Google Scholar]
- 5. Rappo U, Schuetz AN, Jenkins SG, et al. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. 2016;54(8):2096‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ling L, Kaplan SE, Lopez JC, Stiles J, Lu X, Tang YW. Parallel validation of three molecular devices for simultaneous detection and identification of influenza A and B and respiratory syncytial viruses. J Clin Microbiol. 2018;56:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee D, Kanwar N, Hassan F, Essmyer C, Selvarangan R. Comparison of six sample‐to‐answer influenza a/b and respiratory syncytial virus nucleic acid amplification assays using respiratory specimens from children. J Clin Microbiol. 2018;56(11):e00930‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real‐time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42(4):1564‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melchers WJG, Kuijpers J, Sickler JJ, Rahamat‐Langendoen J. Lab‐in‐a‐tube: real‐time molecular point‐of‐care diagnostics for influenza A and B using the cobas(R) Liat(R) system. J Med Virol. 2017;89(8):1382‐1386. [DOI] [PubMed] [Google Scholar]
- 10. Netherlands NHIot. Guideline for the conduct of economic evaluations in healthcare. 2015. https://www.zorginstituutnederland.nl/pakket/werkwijze+pakketbeheer/beoordeling+geneesmiddelen/economische+evaluatie
- 11. Soto M, Sampietro‐Colom L, Vilella A, et al. Economic impact of a new rapid PCR assay for detecting influenza virus in an emergency department and hospitalized patients. PLoS One. 2016;11(1):e0146620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis S, Allen AJ, O'Leary R, et al. Diagnostic accuracy and cost analysis of the Alere i Influenza A&B near‐patient test using throat swabs. J Hosp Infect. 2017;97(3):301‐309. [DOI] [PubMed] [Google Scholar]
- 13. Hansen GT, Moore J, Herding E, et al. Clinical decision making in the emergency department setting using rapid PCR: results of the CLADE study group. J Clin Virol. 2018;102:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu HY, Englund JA, Huang D, et al. Impact of rapid influenza PCR testing on hospitalization and antiviral use: a retrospective cohort study. J Med Virol. 2015;87(12):2021‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rogers BB, Shankar P, Jerris RC, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636‐641. [DOI] [PubMed] [Google Scholar]
- 16. Yee C, Suarthana E, Dendukuri N, Nicolau I, Semret M, Frenette C. Evaluating the impact of the multiplex respiratory virus panel polymerase chain reaction test on the clinical management of suspected respiratory viral infections in adult patients in a hospital setting. Am J Infect Control. 2016;44(11):1396‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the emergency department. Cochrane Database Syst Rev. 2014;9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu M, Qin X, Astion ML, et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol. 2013;139(1):118‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pettit NN, Matushek S, Charnot‐Katsikas A, et al. Comparison of turnaround time and time to oseltamivir discontinuation between two respiratory viral panel testing methodologies. J Med Microbiol. 2015;64(Pt 3):312‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point‐of‐care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open‐label, randomised controlled trial. Lancet. Respir Med. 2017;5(5):401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrews D, Chetty Y, Cooper BS, et al. Multiplex PCR point of care testing versus routine, laboratory‐based testing in the treatment of adults with respiratory tract infections: a quasi‐randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis. 2017;17(1):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogan DT, Kochar MS, Yang S, Quinn JV. Impact of rapid molecular respiratory virus testing on real‐time decision making in a pediatric emergency department. J Mol Diagn. 2017;19(3):460‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schreckenberger PC, McAdam AJ. Point‐counterpoint: large multiplex pcr panels should be first‐line tests for detection of respiratory and intestinal pathogens. J Clin Microbiol. 2015;53(10):3110‐3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Busson L, Mahadeb B, De Foor M, Vandenberg O, Hallin M. Contribution of a rapid influenza diagnostic test to manage hospitalized patients with suspected influenza. Diagn Microbiol Infect Dis. 2017;87(3):238‐242. [DOI] [PubMed] [Google Scholar]


