Abstract
Objective
To prospectively evaluate the incidence and impact of viral respiratory infection in the institutionalized elderly during a winter season.
Design
Prospective descriptive study, without intervention.
Method
Patients with respiratory illnesses were evaluated by a directed history and physical examination. Nasopharyngeal secretions for viral culture were obtained, and acute and convalescent serum samples were obtained for analysis. Serologic evidence of infection with respiratory syncytial virus (RSV) and parainfluenza were determined by enzyme immunoassay (EIA), and influenza by hemagglutination‐inhibition assay and EIA.
Setting
A 591‐bed nursing home.
Participants
Residents with signs or symptoms of acute respiratory illness (nasal congestion, pharyngitis, cough, wheezing, or respiratory difficulty) were eligible for study.
Results
A viral etiology was documented in 62 out of 149 illnesses (42%). RSV was the most common virus associated with illness; it was documented in 27% of respiratory illnesses, followed by rhinovirus (9%), parainfluenza (6%), and influenza (1%). RSV was associated with significantly more severe disease when compared with rhinovirus. Clustering of specific viral infections occurred, suggesting nosocomial transmission.
Conclusions
Viruses are an important cause of acute respiratory infections in the institutionalized elderly during the winter months.
Acute respiratory tract infections are a major cause of acute morbidity in the United States. The frequency of respiratory infections is high in young children and appears to decline with advancing age. 1 However, the impact on the elderly host may be of greater consequence due to an aging respiratory tract, a declining immune system, and multiple medical problems. 2 Residents of long‐term‐care facilities represent a debilitated subgroup of the elderly population and thus are particularly prone to excess morbidity with respiratory infections. However, with the exception of influenza, comprehensive studies on viral respiratory infections in the institutionalized elderly are lacking. This is despite the fact that each year many residents of nursing homes become ill with respiratory infections not proven to be influenza, either by culture or serology. The purpose of this study was to identify all common respiratory viruses which cause symptomatic disease during the winter months in nursing home patients, by utilizing both viral cultures and serology, and to assess their clinical impact.
METHODS
Facility The study population resided in a 591 ‐bed nursing home in Rochester, NY, consisting of a nine‐floor skilled nursing facility and a connecting 19‐floor health‐related facility for more independent residents. Each floor of the skilled nursing facility had a separate dining area, whereas residents of the health‐related facility ate in a single large dining hall. Medical care for residents was provided by three full‐time staff physicians. Infection control policies at the facility included quarantine of floors with ≥3 documented cases of influenza and amantadine prophylaxis for 2 weeks for residents of such floors. No specific isolation procedures were implemented for non‐influenza respiratory illnesses. No attempt was made to influence either individual patient care or existing infection control policy during this study.
Subjects Between December 11, 1989 and March 13, 1990, the head nurse on each floor identified residents who had signs or symptoms of acute respiratory illness including nasal congestion, pharyngitis, cough, wheezing, or respiratory difficulty with or without fever. An institutionwide illness log was created and reviewed by a study nurse daily from Monday through Friday. Each subject listed in the illness log was then evaluated by a directed history and physical examination. A global severity of illness score was assigned by the study nurse, using an analog scoring system ranging from 0 to 10. 3 The analog score consisted of a horizontal line with one end representing mildest illness and the other end the most severely ill. This score represented the study nurses' overall assessment of the severity of an illness based on both the patient's symptoms and objective findings. In a separate analysis the analog scores were found to have a high correlation with objective indices of disease (Falsey, J Med Virol, in press). Arterial oxygen saturation (SaO2) was measured percutaneously, and a nasopharyngeal swab for virus culture and serum were obtained on the initial visit. Chest roentgenograms were obtained and antimicrobials prescribed at the discretion of the patient's staff physician. Each patient was reevaluated daily for 3 days and convalescent sera obtained 4–8 weeks after the acute illness.
Viral Cultures Nasopharyngeal secretions were inoculated onto cell culture within 4 hours. Each sample was placed on HEp‐2, Madin‐Darby Canine Kidney (MDCK), and human foreskin fibroblast (HFF) cell lines. All terminal HEp‐2 cultures were examined for RSV by a direct immunofluorescent antibody test (Genetic Systems, Seattle, WA). Any cell line demonstrating CPE was examined by IFA for parainfluenza virus (PIV) 1,2,3, adenovirus, influenza A and B, and herpes simplex virus (HSV). RSV isolates were subgrouped A or B with group specific monoclonal antibodies. 4 Rhinovirus isolates were confirmed by acid lability testing.
SEROLOGY
Paired sera were analyzed for evidence of recent infection with RSV, influenza, and parainfluenza viruses. Serum IgG levels to the fusion (F) and attachment (G) glycoproteins of A and B RSV subgroups (designated Ga and Gb) were determined for all sera by enzyme immunoassay (EIA) as previously reported. 5 Serologic evidence of RSV infection was defined as a ≥4‐fold rise in IgG to any of the RSV antigens. Sera was also tested for evidence of influence infection by hemaglutination‐inhibition assay (HAI) and hemagglutinin‐specific EIA. 6 Serum IgG levels to parainfluenza virus, types 2 (PIV‐2) and 3 (PIV‐3), were determined by enzyme immunoassay using virus infected Vero cells as antigen. Briefly, Vero cells in 96‐well microtiter plates were infected with PIV‐2 or PIV‐3 (wild isolates) and when CPE was detectable, the cells were fixed in 80% acetone/PBS. Paired sera were incubated in duplicate serial 2‐fold dilutions in the wells for 1 hour at 37°C. Plates were washed and then incubated with horseradish peroxidase conjugated goat anti‐human IgG for 1 hour. Substrate was added and optical density was read on a Dynatech spectrophotometer. Control wells with uninfected Vero cells were used to define background absorption which was substrated from the values of the antigen plates. Because heterologous titer rises between PIV types 1 and 3 are common and serologic differentiation of infecting type is not possible, specimens were screened using PIV‐2 and PIV‐3 antigens. Evidence of parainfluenza infection was defined as a ≥4‐fold rise in IgG to either PIV antigen.
RESULTS
During the 3‐month study period, 177 respiratory illnesses were identified in 164 residents. Of these, 133 residents who experienced 149 illnesses agreed to be studied. Eighty‐four percent of the study patients were female, and the mean age was 88. Most had underlying medical conditions, with neurologic (77%), cardiac (47%), pulmonary (25%), diabetes (12%), and malignancy (7%) being the most frequent.
Cultures and Serology Forty‐one viruses were isolated during 39 of the 149 illnesses (26%) (Table 1). RSV was isolated from 18 patients, followed by rhinovirus in 14, Herpes simplex (HSV) in six, parainfluenza‐1 in two, and parainfluenza‐2 in a single patient. Influenza virus was not isolated. Two patients had dual infections (HSV/RSV and HSV/rhinovirus). RSV group could be determined for 15 of the 18 RSV isolates. Nine of these were group B, and six were group A. One‐hundred‐six of the 133 (80%) had acute and convalescent sera available for analysis. Thirty‐three (31%) showed serologic evidence of RSV infection. Forty RSV infections were documented either by culture alone (5), serology alone (22), or both (13). Fifteen of the 18 individuals from whom RSV was isolated had acute and convalescent sera available. Of these, 13 showed ≥4‐fold rises in RSV titers. The other two patients had acute titers of IgG greater than the highest dilution and, therefore, rises could not be detected. In addition, six patients (5.7%) demonstrated ≥4‐fold rises in PIV titers (3 to PIV‐2 and 3 to PIV‐1). Two patients had a ≥4‐fold rise in HAI titers to influenza which was confirmed by HA‐specific EIA. Overall, a viral etiology of the respiratory infection was documented by culture or serology in 62 of 149 illnesses (42%).
Table 1.
SUMMARY OF CULTURES AND SEROLOGY
| Virus | No. Isolates | Sero‐positive | Respiratory Viral Infection Diagnosed* |
|---|---|---|---|
| RSV | 18 | 33 | 40 |
| Rhinovirus | 14 | ND | 14 |
| Herpes Simplex | 6 | ND | 0** |
| Parainfluenza‐1 | 2 | 3 | 3 |
| Parainfluenza‐2 | 1 | 3 | 3 |
| Influenza | 0 | 2 | 2 |
| Total | 41 | 41 | 62 |
Diagnosis either by positive culture, serology, or both.
Herpes simplex not felt to be the cause of respiratory symptoms.
Epidemiology Respiratory illnesses were reported throughout the study period from 12/11/89 to 3/13/90, with the majority reported in January (Figure 1). Illnesses were noted on all nine floors of the skilled nursing facility and on 18 of 19 floors in the health‐related facility. RSV was isolated throughout most of the study period with a peak occurrence in mid‐January. Although RSV infections were geographically scattered throughout the institution, eight and 14 cases occurred on the 7th and 8th floors of the skilled nursing facility, respectively. On the 8th floor, all five of the typeable RSV isolates were group B, while on the 7th floor four of five typeable isolates were group A. It was also noted that in three of the four wings on these two floors, cases of RSV tended to occur in patients residing on one side of the hallway.
Figure 1.
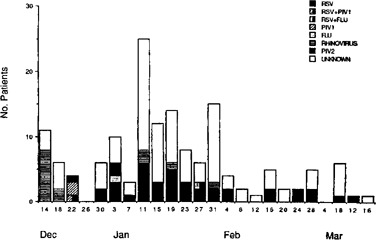
Temporal distribution of respiratory illnesses.
Rhinovirus infections also occurred throughout the study period, although nine of the 14 cases occurred during a 4‐day period in December. Five of the 14 cases also occurred on the 8th floor, with the remainder scattered widely throughout the institution. All six cases of PIV infection occurred during late December or early January. The three PIV‐1 infections occurred within a 5‐day period in patients who lived on the same floor in adjacent rooms.
Clinical Syndromes The clinical features of illness were compared in patients with the two most frequently documented viral infections, RSV and rhinovirus (Table 2). The spectrum of illness associated with RSV infection was broad, ranging from mild nasal congestion only to high fever and respiratory distress. The most prominent complaint was nasal congestion (92%), followed by cough (90%) and sputum production (60%). Appearance on physical examination was also variable with analog scores ranging from 1 to 7 (mean analog score 3.7 ± 1.5). Fifty‐four percent had a score of ≥4, suggesting moderately severe disease. Nine had oral temperatures greater than 101°F. Sixty‐five percent of patients had abnormal lung findings including rales in 40%, wheezing in 35%, and rhonchi in 13%.
Table 2.
CLINICAL CHARACTERISTICS OF RSV AND RHINOVIRUS INFECTIONS
| RSV* (n = 40) | Rhinovirus (n = 14) | ||
|---|---|---|---|
| % | % | ||
| Signs/symptoms | |||
| Constitutional | 21 (53) | 6 (43) | |
| Nasal congestion | 37 (92) | 11 (79) | |
| Headache | 1(3) | 2(14) | |
| Sore throat | 8 (20) | 3(21) | |
| Cough | 36 (90) | 10 (71) | |
| Sputum | 24 (60) | 3 (21) | P ** = 0.05 |
| Shortness of breath | 5 (13) | 2 (14) | |
| Fever (T ≥ 100.0) | 18 (45) | 4 (28) | |
| Analog score ≥4 | 19 (54)*** | 1 (7) | P = 0.01 |
| Conjunctivitis | 4(10) | 0 (0) | |
| Nasal discharge | 15 (38) | 4 (28) | |
| Pharyngitis | 5 (13) | 2 (14) | |
| Wheezing | 14 (35) | 2 (14) | P= 0.10 |
| Rales | 16 (40) | 3 (21) | P = 0.10 |
| Rhonchi | 5 (13) | 2 (14) | |
| Complications | |||
| Pneumonia | 4 (10) | 0 (0) | |
| Antibiotics given | 19 (48) | 2 (14) | P = 0.06 |
| Death | 2 (5) | 0 (0) | |
Includes one patient with apparent asymptomatic unreported illness.
P determined by chi square using Yates correction factor.
Analog scores were recorded in 35 or 40 subjects and 14 of 14 rhinovirus subjects.
The mean room air SaO2 was 95%, and two patients required supplemental oxygen during the acute illness. Twelve patients had chest roentgenograms, four of whom had an infiltrate. Antibiotics were prescribed in 49% of RSV‐infected patients. One patient was hospitalized with pneumonia, and two others died, one during the acute RSV infection, the other 1 month later after a steady decline in health and with an ill‐defined pulmonary process. Most patients recovered without sequelae, although the functional status of two patients deteriorated after their illness.
In contrast, illness associated with rhinovirus infection was generally mild. Rhinorrhea was the most frequent complaint (79%). Cough was also a prominent symptom (71%), although less common than in the RSV‐infected group (90%). Sputum production was significantly less frequent with rhinovirus infection compared to RSV (21% vs 60%, P = 0.05 by Chi square). On physical examination patients generally did not appear acutely ill. Only one person had an analog score >3, and the mean analog score was 1.6 ± 1.5 which was significantly lower than the mean illness score in RSV infection (p < .001 by t test). The most prominent sign was nasal discharge (28%). Only two patients (14%) received antibiotics, and all recovered without sequelae.
PIV infection was documented in six individuals, three with PIV‐1, and three with PIV‐2. Although the numbers are too small to permit comparison of clinical syndromes to other infecting agents, each type of PIV infection appeared to produce a distinct illness. The three individuals with PIV‐1 had a mean analog score of 3.7. All complained of cough and constitutional symptoms. Fever and abnormal chest exams were present in two of three patients, and all three received antibiotics during their illness. PIV‐2 illnesses were somewhat milder, with a mean analog score of 2.0. All three complained of sore throat, and two were hoarse. Pharyngeal erythema was noted in two of three patients, and none had a temperature >99°F or received antibiotics. No cases of pneumonia were documented in either group, and all recovered without sequelae.
DISCUSSION
Viruses are a major cause of acute respiratory infections in the general population. The groups of viruses responsible for most acute respiratory infections are rhinovirus, coronavirus, influenza, RSV, parainfluenza, and adenovirus. 7 An attempt was made to diagnose each of these pathogens, with the exception of coronavirus, utilizing either culture, serology, or both. Several studies of “influenza‐like” illnesses in nursing homes have shown that a variety of infectious agents can be found. 8 – 10 This study provides evidence of the importance of viruses as a cause of respiratory infection in the nursing home since 42% of acute respiratory illnesses during one winter season were viral in origin. The number and severity of respiratory illnesses during the study period was not unusual compared with previous winter months at this nursing home. However, the season was somewhat atypical since influenza was not isolated, despite its prevalence in other nursing homes in the community. It is possible that high rates of influenza vaccination among staff (37%) and residents (78%) may have influenced influenza infection within the institution. 11
Respiratory syncytial virus (RSV), the most common virus isolated in this study, is usually considered a pathogen of infants and young children. While less common in adults, RSV can lead to severe community‐acquired pneumonias in elderly or immunocompromised adults. 12 – 13 A number of outbreaks of RSV have been reported from chronic care facilities. 14 – 18 It is noteworthy that, in our prospective analysis, RSV accounted for 27% of the acute respiratory infections. While it represents only 1 year of study, these data provide further evidence that RSV is an important pathogen in the institutionalized elderly.
The clinical syndrome of nasal congestion, cough with expectoration, and fever is consistent with previous reports of RSV infection in the elderly. Wheezing, which is common in infants with RSV, was also found in 35% of patients in this study, most of whom did not have preexisting pulmonary disease. One of the prominent features of RSV infection in this study was the wide spectrum of illness it produced, ranging from a mild coryzal illness to high fever and respiratory distress. Rates of pneumonia (5%–67%) and death (0%–20%) varied significantly among previously published outbreaks. Factors determining the severity of illness are not completely understood but may be related to the status of the host and to the infecting group or strain of RSV. 4 Recovery of virus did not appear to be associated with more severe illness as the clinical course of culture‐positive cases of RSV did not differ from those with only serologic evidence of infection.
Cases of RSV infection were widely distributed geographically, occurred over a 3‐month period, and were of both A and B groups. The presence of both groups suggests, in contrast with most previous reports, that the present outbreak was not from a single nosocomial source. There was, however, epidemiologic evidence suggesting nosocomial transmission within the institution. Clustering of A group infections on the seventh floor and B group infections on the 8th floor, as well as clustering of cases by sides of the hall on three of the four wings, is suggestive of nosocomial spread. RSV is thought to be transmitted by fomites and requires direct contact with respiratory secretions. 19 Although documentation of illness among staff members was not attempted during this study, nosocomial transmission of RSV by hospital personnel has been described. 20 Therefore, strict handwashing and the use of gowns and gloves, which have been shown to be effective measures for controlling nosocomial RSV infections in hospitalized infants, may be effective in the nursing home as well.
Rhinovirus was the second most frequent virus isolated in this study. In healthy adults rhinoviral infection is a self‐limited illness characterized by sneezing, rhinorrhea, cough, and mild sore throat. 21 There are very limited data on rhinoviral infection in the elderly. Analysis of the 14 culture‐documented infections in this study indicates rhinovirus produces a relatively benign illness in the elderly as well. Infection was characterized by rhinorrhea and cough, but without high fever or signs of lower respiratory tract involvement. Generally, the patients did not appear acutely ill, and all recovered without sequelae. Unlike other respiratory viruses, such as influenza or adenoviruses, rhinovirus infection produces relatively minor damage to the nasal epithelium and probably none to the tracheal mucosa. 22 Since rhinovirus replication is markedly reduced at body temperature, direct invasion of the lower respiratory tract should be unusual at any age. Rhinoviruses are transmitted primarily by contact with infected secretions followed by autoinoculation, and transmission can be decreased by handwashing and environmental disinfection. 23 , 24
PIV is a common cause of croup and bronchitis in young children. 25 Reinfection in young healthy adults typically presents as a URI; however, pneumonia has rarely been described. 26 While PIV infection in the elderly has not been well characterized, there have been several outbreaks of PIV‐1 and −3 infections reported in nursing homes. 27 , 28 The illness has been characterized by fever, rhinorrhea, pharyngitis, and cough, and pneumonia has been common, ranging from 17% to 29%. Although PIV infection was uncommon in the present study, we can state that patients with PIV‐1 infection were all febrile, moderately ill, and received antibiotics. The mode of transmission of PIV appears to be direct person‐to‐person and/or droplet aerosol spread. Therefore, infection control measures similar to those used in RSV outbreaks may be useful in PIV outbreaks.
In summary, viral respiratory tract infections are common in the nursing home during the winter months. Both RSV and rhinovirus were prevalent during this study, with RSV being associated with a significantly more severe illness. Frequently, the diagnosis of “a viral infection” is one of exclusion and of little use to the clinician. However, diagnosis of these agents is important for several reasons. First, infection control policies for RSV, rhinovirus, and PIV are different from those for influenza. Secondly, with specific viral diagnosis, the use of antibiotics and amantadine might be decreased, thereby reducing unnecessary side effects. And, finally, as new vaccines become available, the elderly may represent a potential target population.
ACKNOWLEDGMENTS
We thank Patricia Hennessey, RN, for data collection, Joanne Prives for transcription assistance, and the staff of St. Ann's Home for their help in conducting the study.
From the University of Rochester School of Medicine & Dentistry, Department of Medicine, and The Rochester General Hospital Department of Medicine.
Presented at the American Society of Microbiology 30th Interscience Conference on Antimicrobial Agents and Chemotherapy (Abstract #1288), Atlanta, GA, October 24, 1990.
REFERENCES
- 1. Monto AS, Ullman BM. Acute respiratory illness in an American community: The Tecumseh study. JAMA 1974;227:164–169. [PubMed] [Google Scholar]
- 2. Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis 1980;2:801–810. [DOI] [PubMed] [Google Scholar]
- 3. Swriiwantankul K, Kelvie W, Lasagna L et al. Studies with different types of visual analog scores for measurement of pain. Clin Pharmacol Ther 1983;34:234–239. [DOI] [PubMed] [Google Scholar]
- 4. McConnochie KM, Hall CB, Walsh EE et al. Variation in severity of respiratory syncytial virus with subtype. J Pediatr 1990;117:52–58. [DOI] [PubMed] [Google Scholar]
- 5. Hendry MR, Burns JC, Walsh EE et al. Strain‐specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis 1988;157:640–647. [DOI] [PubMed] [Google Scholar]
- 6. Madore HP, Reichman RC, Dolin R. Serum antibody responses in naturally occurring influenza‐A virus infection determined by enzyme‐linked immunosabsorbent assay, hemagglutination‐inhibition, and complement fixation. J Clin Microbiol 1983;18:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gwaltney JM Jr. The common cold. In: Mandell GI, Douglas RG, Jr, Bennet JE, eds. Principles and Practices of Infectious Diseases, 3rd Ed New York: Churchill Livingston, 1990, pp 489–499. [Google Scholar]
- 8. Gross P, Rodenstein M, LaMontagne JR et al. Epidemiology of acute respiratory illness during an influenza outbreak: A prospective study. Arch Intern Med 1988;148:559–661. [PubMed] [Google Scholar]
- 9. Arroyo JC, Jordan W, Milligan L. Upper respiratory tract infection and serum antibody responses in nursing home patients. Am J Infect Control 1988;16:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholson KG, Baker DJ, Farquhar A et al. Acute upper respiratory tract viral illness and influenza immunization in homes for the elderly. Epidemiol Infect 1990;105:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patriarca PA, Weber SA, Parker RA et al. Risk factors for outbreaks of influenza in nursing homes: A case control study. Am J Epidemiol 1986;124:114–119. [DOI] [PubMed] [Google Scholar]
- 12. Zaroukian MH, Kashyap GH, Wentworth BB. Case report: Respiratory syncytial virus infection—a cause of respiratory distress and pneumonia in adults. Am J Med Sci 1988;295:218–222. [DOI] [PubMed] [Google Scholar]
- 13. Levenson RM, Kantor OS. Fatal pneumonia in an adult due to respiratory syncytial virus. Arch Intern Med 1987;147:791–792. [PubMed] [Google Scholar]
- 14. Agius G, Dindinaud G, Bigger RJ et al. An epidemic of respiratory syncytial virus in the elderly people: Clinical and serological findings. J Med Virol 1990;30:117–127. [DOI] [PubMed] [Google Scholar]
- 15. Garvie DG, Gray J. An outbreak of an influenza‐like illness in a nursing home. J Am Geriatr Soc 1990;36:659–662. [DOI] [PubMed] [Google Scholar]
- 16. Hart RJ. An outbreak of respiratory syncytial virus in an old people's home. J Infect 1984;8:259–261. [DOI] [PubMed] [Google Scholar]
- 17. Sorvillo FJ, Huie SF, Strassburg MA et al. An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. J Infect 1984;9:252–256. [DOI] [PubMed] [Google Scholar]
- 18. Mathur U, Bentley DW, Hall CB. Concurrent respiratory syncytial virus and Influenza A infections in the institutionalized elderly and chronically ill. Ann Intern Med 1980;93:49–52. [DOI] [PubMed] [Google Scholar]
- 19. Hall CB, Geiman JM, Biggar R et al. Respiratory syncytial infections within families. N Engl J Med 1976;1294:414–419. [DOI] [PubMed] [Google Scholar]
- 20. Hall CB, Geiman JM, Douglas RG et al. Control of nosocomial respiratory syncytial viral infections. Pediatrics 1978;62:728–732. [PubMed] [Google Scholar]
- 21. Gwaltney JM. Rhinovirus infections in an industrial population. 1. The occurrence of illness. N Engl J Med 1966;275:1261–1268. [DOI] [PubMed] [Google Scholar]
- 22. Gwaltney J. Rhinovirus. In Mandell GI, Douglas RG, Jr, Bennet JE, eds. Principles and Practices of Infectious Disease, 3rd Ed. New York: Churchill Livingston, 1990, pp 1399–1405. [Google Scholar]
- 23. Hendley JO, Wenzel RP, Gwaltney JM. Transmission of rhinovirus colds by self‐inoculation. N Engl J Med 1973;288:1361–1364. [DOI] [PubMed] [Google Scholar]
- 24. Gwaltney JM, Moskalski PB, Hendley JO. Interruption of experimental rhinovirus transmission. J Infect Dis 1980;142:811–815. [DOI] [PubMed] [Google Scholar]
- 25. Anderson JL, Patriarca PA, Hierholzer JC. Viral respiratory illnesses. Med Clin North Am 1983;67:1009–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wenzel RP, McCormick DP, Beam WE Jr. Parainfluenza pneumonia in adults. JAMA 1972;221:294–295. [PubMed] [Google Scholar]
- 27. Centers for Disease Control. Parainfluenza outbreaks in extended care facilities—United States. MMWR 1978;27:475–476. [Google Scholar]
- 28. Public Health Laboratory Science Communicable Disease Surveillance Centre. Parainfluenza infections in the elderly. Br Med J 1983;287:1619.6315128 [Google Scholar]


