Abstract
Background
In the recent outbreak of novel coronavirus infection in Wuhan, China, significantly abnormal coagulation parameters in severe novel coronavirus pneumonia (NCP) cases were a concern.
Objectives
To describe the coagulation feature of patients with NCP.
Methods
Conventional coagulation results and outcomes of 183 consecutive patients with confirmed NCP in Tongji hospital were retrospectively analyzed.
Results
The overall mortality was 11.5%, the non‐survivors revealed significantly higher D‐dimer and fibrin degradation product (FDP) levels, longer prothrombin time and activated partial thromboplastin time compared to survivors on admission (P < .05); 71.4% of non‐survivors and 0.6% survivors met the criteria of disseminated intravascular coagulation during their hospital stay.
Conclusions
The present study shows that abnormal coagulation results, especially markedly elevated D‐dimer and FDP are common in deaths with NCP.
Keywords: coagulation parameter, D‐dimer, disseminated intravascular coagulation, fibrin degradation product, novel coronavirus pneumonia
Essentials
-
•
The role of coagulopathy in severe novel coronavirus pneumonia (NCP) remains to be clarified.
-
•
Conventional coagulation parameters of consecutive patients with NCP were retrospectively analyzed.
-
•
Abnormal coagulation results, are associated with poor prognosis.
-
•
Existence of disseminated intravascular coagulation is common in deaths with NCP.
Alt-text: Unlabelled Box
1. INTRODUCTION
Since December 2019, novel coronavirus pneumonia (NCP) cases have emerged in Wuhan, China, and the 2019 novel coronavirus (2019‐nCoV) was confirmed as the cause of the NCP.1 The number of infected patients in China has increased rapidly and exceeded 60 000 in mid‐February 2020. In previous reports,2., 3., 4. the clinical characteristics of NCP patients have been investigated, the reported mortalities were 4.3%, 11.0%, and 14.6%, respectively; organ dysfunction and coagulopathy were associated with high mortality. However, the complete coagulation parameters of NCP cases were not fully reported, which may have prognostic values and be important therapeutic targets. In this study, the coagulation parameters of consecutive NCP cases in our hospital were shown and the differences between survivors and non‐survivors were investigated.
2. METHODS
Consecutive patients with confirmed NCP admitted to Tongji Hospital of Huazhong University of Science and Technology in Wuhan from January 1 to February 3, 2020, were enrolled. This study was approved by the Ethics Committee of Tongji Hospital (Wuhan, China). The diagnosis of NCP was according to World Health Organization interim guidance5 and confirmed by RNA detection of the 2019‐nCoV in the clinical laboratory of Tongji Hospital. The clinical outcomes were monitored up to February 13, 2020.
The samples for coagulation tests were collected on admission and during the hospital stay: prothrombin time (PT), activated partial thromboplastin time (APTT), antithrombin activity (AT), fibrinogen, fibrin degradation product (FDP), and D‐dimer were detected using a STA‐R MAX coagulation analyzer and original reagents (Diagnostica Stago, Saint‐Denis, France).
Between survivors and non‐survivors, normally and abnormally distributed quantitative variables were compared using the Student's t test and the Mann‐Whitney U test, respectively. Categorical variables were compared using the chi‐squared test. The results were given as the mean ± standard deviation, median (interquartile range), or number (percentage), wherever appropriate. A P‐value of < .05 was considered statistically significant. Data were analyzed using SPSS 21.0 for Windows (SPSS Inc.).
3. RESULTS AND DISCUSSION
There were 183 patients (85 females and 98 males) with NCP enrolled into the study, and these patients had complete clinical information and the laboratory data required for this study. The mean age at disease onset was 54.1 years (range, 14‐94 years). Seventy‐five (41.0%) patients had chronic diseases, including cardiovascular and cerebrovascular diseases, respiratory system disease, malignant tumor, chronic liver and kidney disease, and others. All patients received antiviral and supportive therapies after diagnosis. By the end of February 13, 78 (42.6%) patients had been discharged and 21 (11.5%) patients had died, the rest 84 (45.9%) of the patients remain hospitalized in stable condition.
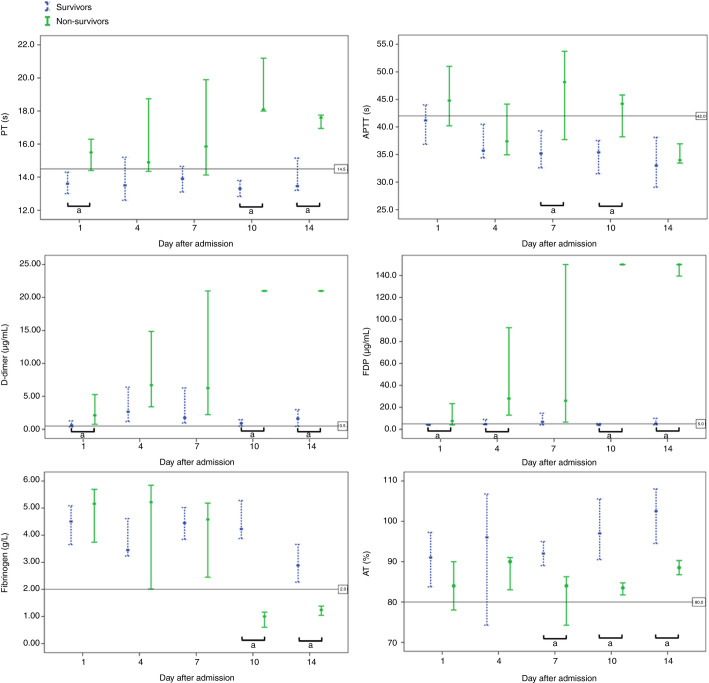
The coagulation parameters on admission between survivors and non‐survivors were compared (Table 1 ). Based on our detection system, the reportable range of D‐dimer and FDP were 0.22‐21.00 µg/mL and 4.0‐150.0 µg/mL, respectively. The dynamic changes in coagulation parameters were tracked from day 1 to day 14 after admission at three‐day intervals (Figure 1 ).
Table 1.
Coagulation parameters of NCP patients on admission
| Parameters | Normal range | Total (n = 183) | Survivors (n = 162) | Non‐survivors (n = 21) | P values |
|---|---|---|---|---|---|
| Age (years) | 54.1 ± 16.2 | 52.4 ± 15.6 | 64.0 ± 20.7 | <.001 | |
| Sex (male/female) | 98/85 | 82/80 | 16/5 | .035 | |
| With underlying diseases | 75 (41.0%) | 63 (38.9%) | 12 (57.1%) | .156 | |
| On admission | |||||
| PT (sec) | 11.5‐14.5 | 13.7 (13.1‐14.6) | 13.6 (13.0‐14.3) | 15.5 (14.4‐16.3) | <.001 |
| APTT (sec) | 29.0‐42.0 | 41.6 (36.9‐44.5) | 41.2 (36.9‐44.0) | 44.8 (40.2‐51.0) | .096 |
| Fibrinogen (g/L) | 2.0‐4.0 | 4.55 (3.66‐5.17) | 4.51 (3.65‐5.09) | 5.16 (3.74‐5.69) | .149 |
| D‐dimer (µg/mL) | <0.50 | 0.66 (0.38‐1.50) | 0.61 (0.35‐1.29) | 2.12 (0.77‐5.27) | <.001 |
| FDP (µg/mL) | <5.0 | 4.0 (4.0‐4.9) | 4.0 (4.0‐4.3) | 7.6 (4.0‐23.4) | <.001 |
| AT (%) | 80‐120 | 91 (83‐97) | 91 (84‐97) | 84 (78‐90) | .096 |
Abbreviations: APTT, activated partial thromboplastin time; AT, antithrombin activity; FDP, fibrin degradation product; NCP, novel coronavirus pneumonia; PT, prothrombin time (PT).
Figure 1.
Dynamic profile of coagulation parameters in patients with novel coronavirus pneumonia (NCP). Timeline charts illustrate the changes of coagulation parameters in 183 patients with NCP (21 non‐survivors and 162 survivors) after admission. The error bars show medians and 25% and 75% percentiles. The horizontal lines show the upper normal limits of prothrombin time, activated partial thromboplastin time, D‐dimer and fibrin degradation product, and the lower normal limits of fibrinogen and antithrombin activity, respectively. aP < 0.05 for survivors versus non‐survivors
According to the International Society on Thrombosis and Haemostasis (ISTH) diagnostic criteria for disseminated intravascular coagulation (DIC),6 15 (71.4%) of the non‐survivors matched the grade of overt‐DIC (≥5 points) in later stages of NCP (Table 2 ), the median time from admission to DIC was 4 days (range, 1‐12 days). On the contrary, only one (0.6%) survivor matched the DIC criteria during hospital stay.
Table 2.
The grade of DIC in non‐survivors with NCP (n = 21)
| Number of patients (%) | |
|---|---|
| Platelet counts (×109/L) | |
| 50‐100 (1 point) | 7 (33.3) |
| <50 (2 points) | 5 (23.8) |
| D‐dimer (µg/mL) | |
| 1.0‐3.0 (2 points) | 3 (14.3) |
| >3.0 (3 points) | 18 (85.7) |
| Fibrinogen (g/L) | |
| <1.0 (1 point) | 6 (28.6) |
| Prolongation of PT (sec) | |
| 3‐6 (1 point) | 5 (23.8) |
| >6 (2 points) | 10 (47.6) |
| Meeting the ISTH criteria of DIC (Total points ≥5) | 15 (71.4) |
Note:D‐dimer cutoff levels were defined according to a previous report derived from more than 1000 samples in intensive care.7
Abbreviations: DIC, disseminated intravascular coagulation; ISTH, International Society on Thrombosis and Haemostasis; NCP, novel coronavirus pneumonia
In our enrolled patients with NCP, the non‐survivors revealed significantly higher D‐dimer and FDP levels, and longer PT compared to survivors on admission. By the late hospitalization, the fibrinogen and AT levels were also significantly lower in non‐survivors; this suggested that conventional coagulation parameters during the course of NCP were significantly associated with prognosis.
DIC appeared in most of the deaths. Patients presenting with a virus infection may develop into sepsis associated with organ dysfunction. Sepsis is well established as one of the most common causes of DIC; development of DIC results when monocytes and endothelial cells are activated to the point of cytokine release following injury, with expression of tissue factor and secretion of von Willebrand factor. Circulation of free thrombin, uncontrolled by natural anticoagulants, can activate platelets and stimulate fibrinolysis.8 At the late stages of NCP, levels of fibrin‐related markers (D‐dimer and FDP) moderately or markedly elevated in all deaths, which suggested a common coagulation activation and secondary hyperfibrinolysis condition in these patients.
In a previous study,9 Gralinski et al investigated viral pathogenesis and identified a novel host pathway involved in severe acute respiratory syndrome (SARS)‐coronavirus disease progression. Their data suggest that dysregulation of the urokinase pathway during SARS‐coronavirus infection contributes to more severe lung pathology and that plasminogen activator inhibitor‐1 plays a protective role following infection. In addition, Fatma Berri et al10 reported that plasminogen contributes to inflammation caused by influenza through fibrinolysis, and 6‐aminocaproic acid can protect against influenza. Presumably, fibrinolysis may also be induced following severe 2019‐nCoV infection.
The limitations of this report included that, as a relatively small, single‐center study, the mortality and characteristics of enrolled patients may not be representative; our findings should be confirmed in an adequately powered clinical study. In addition, some patients are still hospitalized at the time of manuscript submission. Nonetheless, the present study has shown that existence of DIC is common in deaths with NCP; abnormal coagulation results, especially markedly elevated D‐dimer and FDP, may have the potential to guide therapy and evaluate prognosis.
AUTHOR CONTRIBUTIONS
N. Tang and X. Wang collected the clinical data and processed statistical data. N. Tang and D. Li drafted and revised the manuscript. Z. Sun designed and guided the study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
We thank all patients involved in the study.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 18 February 2020
REFERENCES
- 1.Zhu N., Zhang D., Wang W., et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Published January 28, 2020. Accessed January 31, 2020. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected
- 6.Taylor F.B., Jr, Toh C.H., Hoots W.K., et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 7.Dempfle C.E., Wurst M., Smolinski M., et al. Use of soluble fibrin antigen instead of D‐dimer as fibrin‐related marker may enhance the prognostic power of the ISTH overt DIC score. Thromb Haemost. 2004;91(4):812–818. doi: 10.1160/TH03-09-0577. [DOI] [PubMed] [Google Scholar]
- 8.Kitchens C.S. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC) Educ Program Am Soc Hematol. 2009;2009(1):240–246. doi: 10.1182/asheducation-2009.1.240. [DOI] [PubMed] [Google Scholar]
- 9.Gralinski L.E., Bankhead A., 3rd, Jeng S., et al. Mechanisms of severe acute respiratory syndrome coronavirus‐induced acute lung injury. mBio. 2013;4(4):e00271–e313. doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berri F., Rimmelzwaan G.F., Hanss M., et al. Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]