Abstract
Ebola virus disease (EVD) is a zoonotic disease that causes severe haemorrhagic fever, with high fatality rates of up to 90% in humans. Today, there is no effective treatment available. Person‐to‐person transmission occurs through exposure to blood or body fluids, which can threaten other household members and first‐line healthcare workers. The first cases of EVD in Guinea were identified on 22 March 2014. It was initially believed that this like previous outbreaks would be self‐limiting. However, lack of public health infrastructure, delays in virus detection and late implementation of control interventions contributed to widespread transmission of EVD in a region inexperienced in dealing with the disease. Socio‐cultural and economic factors probably also played a key role in the spread of the disease, resulting in the current large‐scale outbreak. Some promising candidate treatments for this disease are now being developed.
Keywords: ebola virus, outbreak, societal challenges, treatment, vaccine
Emerging infectious diseases: a challenge to both human and veterinary health
Outbreaks of emerging infectious diseases continue to challenge both human and veterinary health worldwide. Events such as the outbreaks of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus, H5N1 avian influenza, Rift valley fever, foot and mouth disease and Crimean–Congo haemorrhagic fever virus in Europe and, recently, Ebola virus disease (EVD) in West Africa are just a few examples 1, 2, 3, 4, 5, 6. There are multiple causes of these epidemics, including factors such as increased population mobility, trade globalization and climate change potentially affecting the geographical distribution of viral vectors 7, 8. The potential for bioterrorism through the deliberate release of an infectious agent in an area not previously affected has been raised and adds a further dimension to the emergence of infectious disease and its control. Zoonotic diseases caused by microorganisms whose principal reservoirs are wild and domestic animals are a leading cause of death and disease. In the past 60 years, more than 50% of emerging infectious diseases in humans have originated from zoonoses 9. The zoonotic infection arena contains several billion humans, a growing number of domestic animals and many wild animals and vectors, which contribute to a large number of interactions between different players and zoonotic infections. Complex interactions between epidemiological, ecological and social processes will promote the emergence and transmission of zoonotic diseases. The latest outbreak of EVD highlights the complexity of the interface between the elements mentioned above for zoonotic diseases. In this review, several crucial questions raised during the current outbreak will be discussed, such as: why EVD outbreaks occur in West Africa, far away from the endemic area; why this outbreak has occurred now; and which factors have contributed to the current widespread transmission of EVD, in contrast to all previous known outbreaks. In addition, the most promising candidate treatments will be summarized.
Filoviridae
The Filoviridae family belongs to the order Mononegavirales, but its members are significantly separated from other Mononegavirales on the basis of physiochemical, morphological, biological features and genomic structure 10. Members of the Filoviridae are nonsegmented, RNA viruses with negative polarity 10. These viruses are filamentous (as indicated by their name, derived from the Latin filum meaning ‘thread’). They form a thread‐like shape, with a uniform diameter of about 80 nm 11 and a typical length of about 1200 nm 12, 13.
At present, Ebola Virus and Marburg virus are the only two recognized genera of the family Filoviridae. Lloviu virus (LLOV) can be classified as a distinct genus, Lloviu, with one species, Lloviu cuevavirus. LLOV was recently detected in Schreiber's long‐fingered bats (Miniopterus schreibersii Kuhl 1817), and phylogenetic analyses have demonstrated that LLOV is distant from both Marburg virus and EBOV 14, 15. However, this virus has not yet been isolated. The genus Ebola virus (EBOV) contains the following species: Ebola virus, previously Ebola Zaire, Sudan Ebola virus, Reston Ebola virus, Taï Forest Ebola virus (formerly Côte d'Ivoire Ebola virus) and Bundibugyo Ebola virus 14, 15. Within the genus Marburg virus, there is a single species, Marburg virus (formerly Lake Victoria Marburg virus), which consists of two very divergent ‘viruses’, Marburg virus and Ravn virus, which are approximately 20% divergent at the genetic level 14, 15, 16. Of interest, this is in contrast to the known diversity for EBOV species, with a nucleotide difference between sequences of only 2.7%, 5.2% and 4.5% for EBOV, Sudan Ebola virus and Reston Ebola virus, respectively 16, 17. Classification of the Filoviridae family will most probably continue to develop as further information becomes available through genome sequencing. Phylogenetic studies have been applied to estimate the age of filoviruses. The estimates of common ancestor age range from thousands to millions of years 18, 19, which indicates that these studies should be repeated using novel techniques and increased sample size to obtain more consistent estimates of the age of filoviruses. Recent studies of almost 100 whole‐genome sequences using Bayesian coalescent phylogenetic analyses indicate nucleotide substitutions/site/year for different viruses ranging from 0.46 × 10−4 for Sudan Ebola virus to 8.21 ×10−4 for Reston Ebola virus. Studies by Carroll et al. 16 estimated recent common ancestry (approximately 50 years ago) for both Reston Ebola virus and EBOV.
The filovirus genome is approximately 19 kb in length. Filoviruses express seven different proteins 20: nucleoprotein (NP), glycoprotein (GP), RNA‐dependent RNA polymerase (RdRP) and the structural proteins virus protein (VP)24, VP30, VP35 and VP40. In addition, EBOV is able to express a truncated soluble GP through RNA editing and small soluble GP, which are secreted from the host cell 20, 21. The surfaces of the viral membranes are spiked with GP trimers. These trimers are formed from GP1 and GP2 (product of cleavage of precursor GP). The GP trimers mediate receptor binding and are the target for neutralizing antibodies. GP spikes, which embed on the virion surface, mediate virus entry 22, 23. GP1 contains an excessively O‐linked glycosylated mucin‐like domain and a heavily N‐linked glycosylated glycan cap domain, and these exterior domains are responsible for binding to a variety of host cell surface factors, as well as covering the receptor‐binding domain under them 24. NP and VP30 are required for RNA encapsidation 25, 26. However, it has also been suggested that VP30 may act as a viral transcription activator 27, 28. VP35 links NPs with the viral RdRP to construct the viral RNA synthesis complex for transcription and genome replication 29. The VP35 protein is also known to interfere with interferon induction in both Marburg virus and EBOV 30, 31. The matrix proteins VP40 and VP24 play essential roles in later steps of the replication cycle, such as assembly and budding 32, 33, 34. VP 24 may also act as an interferon antagonist 35, 36. It has been demonstrated that different forms of Ebola GPs can be released from infected cells and that these secreted GPs may activate noninfected dendritic cells and macrophages, causing massive release of pro‐ and anti‐inflammatory cytokines and thereby affecting vascular permeability 37. These GPs thus contribute to high virus pathogenicity. As mentioned above, EBOV is also able to express a truncated soluble GP, which contributes to a mechanism of host immune system evasion through absorption of antibodies and interference with antibody‐mediated clearance 21, 38, 39, 40.
Ebola virus disease was first described in 1976 in Zaire [now the Democratic Republic of Congo (DRC)]. Since then, there have been multiple EBOV transmission events 41, 42 and several EVD outbreaks 43, 44. In August 2014, the largest, most sustained and most widespread EVD outbreak in history became apparent and was declared a Public Health Emergency of International Concern by the World Health Organization (WHO). The WHO was notified of the outbreak in March 2014 45, after a febrile illness cluster with high case fatality rate in the area of Gueckedou, Guinea. This attracted international attention and was subsequently identified as the viral zoonosis EBOV. Sequencing data showed that the 2014 outbreak in West Africa was due to infection with a strain of EBOV that differed from the viral strains identified in earlier outbreaks 45.
The suspected index case in the current outbreak is believed to be a 2‐year‐old boy in Guinea, who died on 6 December 2013; it was initially suggested that he contracted the disease after exposure to an infected fruit bat 46; however, new data indicate that EBOV transmission to this boy was instead through insectivorous bats 47. His case became the starting point source for person‐to‐person spread of EVD into the population in several West African countries. Guinea, Liberia and Sierra Leone have been most affected, and these countries continue to report new cases. Since the beginning of this current outbreak, EBOV has infected 24 666 individuals and has caused 10 179 deaths, according to a WHO report issued on 18 March 2015 48 (Fig. 1). However, the exact number of cases is difficult to determine, and the magnitude of the outbreak is believed to have been significantly under estimated. One case in Senegal was a traveller from Guinea, and a small number of cases in Nigeria and Mali originated from Liberia and Guinea, respectively. All these countries have since been declared free from EVD, largely as a result of rigorous control measures. There have also been a very few cases of EVD amongst travellers returning to Western Europe and the USA.
Figure 1.
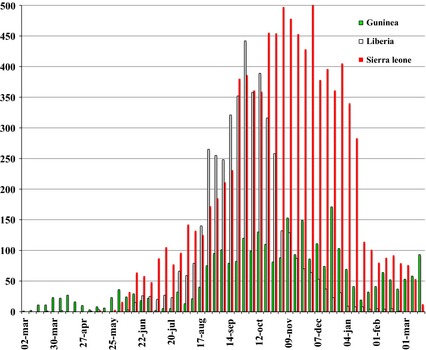
The number of confirmed, probable and suspected cases of Ebola virus disease over time.
It is important to note that the symptoms, transmissibility, incubation period and death rate in the current outbreak are similar to those reported for previous outbreaks. Genetic analysis of samples from the current outbreak suggests a single transmission event from the natural reservoir, followed by human‐to‐human transmission during the outbreak 49.
Clinical symptoms, transmission and infection control measures
Patients with EVD usually demonstrate clinical symptoms after an incubation period of 4–10 days, with a range of 2–21 days 20. After a sudden onset of fever, vomiting, chills, myalgia and diarrhoea, the disease can evolve into a severe state with a rapid clinical decline. This disease phase is characterized by multisystem involvement and includes systemic, gastrointestinal, respiratory, vascular and neurological symptoms (Table 1). Haemorrhagic manifestations include petechiae, ecchymosis and mucosal haemorrhages. In later stages, patients demonstrate shock, convulsions and severe metabolic disturbances 20, 50. Patients with fatal disease develop clinical signs early during infection and die typically between day 6 and 16 with hypovolaemic shock and multi‐organ failure. Haemorrhages can be severe but are only present in fewer than half of all patients and in the current outbreaks have been observed in less than 30% of patients. In nonfatal cases, fever is present for several days and patients typically improve around day 6–11, about the time that the humoral antibody response is noted 51. The most common symptoms in the current outbreak are fever (87%), fatigue (76%), vomiting (68%), diarrhoea (66%), loss of appetite (65%), headache (53%), abdominal pain (44%) and myalgias (39%) 52. Figure 2 shows the data from a previous study to prospectively determine whether body fluids contain EBOV RNA at different periods during the acute and convalescent phases 53.
Table 1.
Describing clinical symptoms related to the Ebola virus disease
| Systemic symptoms | Prostration |
|---|---|
| Gastrointestinal symptoms | Anorexia, nausea, vomiting, abdominal pain, diarrhoea |
| Respiratory symptoms | Chest pain, shortness of breath, cough, nasal discharge |
| Vascular symptoms | Conjunctival injection, postural hypotension, oedema |
| Neurological symptoms | Headache, confusion, coma |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
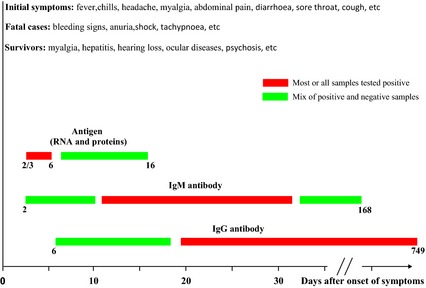
Antigen and antibody responses amongst patients with Ebola virus disease. Under bars denote days after symptom onset.
Currently, it is unclear whether small droplets containing EBOV form within the human respiratory tract in patients with EVD, even though EBOV particles have been found in human alveoli 54. However, epidemiological data have clearly demonstrated that EBOV does not undergo traditional airborne transmission. The majority of patients in previous and current epidemics have been infected by direct contact 55, 56, and all EVD outbreaks in Africa have been contained without precautions against airborne transmission in the affected populations 57.
However, early experiments in nonhuman primates (NHPs) examining routes of infection of EBOV demonstrated that the virus could be aerosolized to small droplet or droplet nuclei size (mechanically) and cause lethal disease in rhesus macaques after inhalation of at least 400 pfu 58. More recent experiments have shown that inhalation of less than 10 infectious particles of EBOV is sufficient to cause lethal disease in NHPs 59. However, it is important to note that these studies do not address the question of whether EBOV is aerosolized naturally. Direct contact with body fluids from patients with EVD is the most likely way of transmitting EBOV. The evidence from previous and current outbreaks, epidemiological data and animal experiments all clearly demonstrate that contact with EBOV‐infected fluids can lead to infection. Despite strong evidence to suggest that contact with body fluids is the route of EBOV transmission, it remains unclear when (i.e. how long postdisease onset) and which (e.g. sweat and tears) fluids contain infectious virus. In previous studies, EBOV has been isolated from blood, saliva, breast milk and semen 60, whereas only viral RNA has been detected in sweat, tears, faeces and skin, rectal and vaginal swabs. It remains unclear how much infectious virus is secreted in different body fluids at different periods postdisease onset. Assessing the concentration of infectious virus during different time‐points from disease onset in infected patients may help to determine when and how much virus is shed in different body fluids.
Very recent data from a 36‐year‐old male patient (treated in Hamburg, Germany) demonstrated high levels of viral RNA in plasma until 2 weeks after disease onset; RNA was also been detected in sweat until 40 days postdisease onset, whilst the level of viral RNA decreased below the detection level in urine at 31 days postonset 61.
As described above, EBOV is spread through direct contact with body fluids from an infected person and by contact with contaminated surfaces or equipment; therefore, patients with suspected or confirmed EVD should be isolated either in a isolation/specific room or a restricted area. Healthcare workers should also use dedicated equipment, which should be exclusively assigned to patient with EVD care areas. All healthcare workers and visitors should carry personal protective equipment (according to the expected level of risk, based on WHO recommendations and national/institutional guidelines) and hand hygiene should be performed (alcohol‐based hand rub solution or soap and running water). Further details are available in the WHO and/or national regulations and recommendations 62.
Factors contributing to the magnitude of the current EVD outbreak
As mentioned above, the causative EBOV strain in this current outbreak is closely related to that in previous EBOV (Ebola Zaire) outbreaks in Central Africa 49. However, by August 2014, the current outbreak had become the largest, most sustained and most widespread EVD outbreak in history. It has been suggested that EBOV could have been circulating in West Africa for about a decade 49, which raises the question of why there is an EVD outbreak in West Africa now. Another pressing issue concerns the factors that contributed to the current widespread transmission of EVD, in contrast to all previous known outbreaks which were limited in scope. The huge EVD epidemic in West Africa presents unique challenges because of spread into crowded urban environments and occurrence in remote communities. One year after the first case of EVD in Guinea, it can be concluded that health teams at urban, county and district levels, particularly in rural counties with remote regions, need adequate training in: (i) case reporting, investigation and management, (ii) contact tracing, (iii) safe burial, and (iv) safe sample collection, processing and transport for diagnostic testing. Control of the epidemic is much more challenging in rural counties, as there are few roads, most of which are in poor condition, an overall lack of vehicles, no internet connectivity and limited telephone network coverage. Therefore, the development of novel communication and transportation network strategies for these remote communities is critical for management of EVD in such areas.
In general, the following three elements can be cited as crucial factors contributing to the current substantial and widespread outbreak in West Africa (Fig. 3): (i) delays in outbreak detection, (ii) lack of public health infrastructure, and (iii) socio‐cultural factors.
Figure 3.
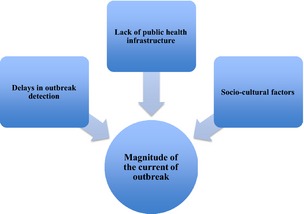
Schematic representation of the most important factors contributing to the current and widespread outbreak of Ebola virus disease in West Africa.
Delays in outbreak detection
The suspected index case in the current outbreak, the 2‐year‐old boy in Guinea, contracted the disease and died at the end of 2013 46. However, the outbreak was not identified until March 2014 45, which enabled several transmission chains to advance in the region and to cross its borders. A number of factors impede the early identification of EVD outbreaks in Africa in general and in West Africa in particular. First, only a very few EVD outbreaks have occurred in Africa (East and Central Africa) since the first outbreak was identified in 1976, so awareness of the disease tends to be low. In addition, some areas at risk of EVD have not yet experienced an outbreak, which has strongly limited community‐level knowledge of the disease 63. Secondly, the early symptoms of EVD are nonspecific 64, which increases the likelihood of misdiagnosis. Thirdly, there is a lack of diagnostic testing in low‐income countries. Fourthly, there is a lack of epidemiological monitoring for the timely identification of case clusters.
Lack of public health infrastructure
An insufficiency of resources at an early stage following outbreaks in the region is most probably the key factor responsible for the excessive scale of the ongoing EVD epidemic in West Africa 65. In particular, the lack of sufficient quantities of essential supplies to implement infection control measures in healthcare settings and the low number of healthcare workers and staff available to manage a growing case burden contributed significantly to the widespread transmission of EVD in the current outbreak.
Socio‐cultural factors
Socio‐cultural and economic factors in the continent of Africa are a very important element contributing to the spread of infectious disease and also complicate the implementation of control interventions. Therefore, these factors have probably had a key role in the current EVD outbreak. In particular, cultural practices involving touching and washing the body of the deceased contribute to the dissemination of the EBOV. The associated potential for transmission to neighbouring and distant areas via exposed funeral attendants can facilitate the development of major epidemics 66. Moreover, the lack of prior experience and knowledge of the disease can lead communities to deny its existence and to associate illness with witchcraft or an alleged government conspiracy to gain control of the population or attract resources from the international community 67. For instance, during the ongoing epidemic in West Africa, a group of individuals looted equipment and potentially contaminated materials from an isolation facility in a quarantined neighbourhood. Finally, the stigma carried by EVD survivors and the family members of EVD victims could exacerbate disease spread. In particular, uninformed families tend to hide relatives and friends infected with EVD, to avoid being shunned by their own communities, which enhances transmission rates. The problem is compounded by the high case fatality ratio of EVD, which leads misinformed communities to associate case isolation with a death sentence.
Therapeutic agents and vaccines
To date, there are no approved antiviral medicines or vaccines for EBOV. However, there are several candidate treatments with promising results in vitro and in vivo. Current research programmes for developing new therapeutic tools against EVD include the use of antibodies, plasma transfusions from convalescent patients, novel small‐molecule antiviral agents and vaccines. The most promising medicines and vaccines available at present are summarized below (see Table 2).
Table 2.
Antiviral and vaccine candidates to treat Ebola virus disease
| Name (manufacturer) | Mechanism of action | Phase of development |
|---|---|---|
| ZMapp (Mapp Biopharmaceutical Inc) | Combination of three monoclonal antibodies | Phase 1 |
| Brincidofovir (Chimerix) |
Ebola: unknown CMV: incorporated into DNA chain, inhibiting DNA synthesis |
Phase 1 (Ebola) Phase 3 (CMV and ADV) |
| Favipiravir (Fuji Film/Toyama Chemical) |
Nucleotide analogue that inhibits RNA Polymerases and causes lethal mutagenesis after incorporation into viral RNA3333 |
Phase 3 (influenza) |
| TKM‐Ebola (Tekmira) | siRNA; interferes with proteins L, VP24 and VP35 | Phase 1 |
| AVI‐7537 (Sarepta) | PMO, which inhibits protein VP24 | Phase 1 |
| BCX‐4430 (Biocryst) | Nucleoside analogue | Preclinical |
| cAd3 (GSK/USNAIAD) | Stimulates immune response to Ebola glycoprotein using chimpanzee adenovirus | Phase 1 |
| rVSV‐Ebola (Newlink Genetics/PHAC) | Stimulates immune response to Ebola glycoprotein using rVSV | Phase 1 |
CMV, cytomegalovirus; ADV, adenovirus; VP, virus protein; siRNA, small interfering RNA; PMO, phosphorodiamidate morpholino oligomers; US NAIAD, National Institute of Allergy and Infectious Diseases; GSK, GlaxoSmithKline; PHAC, Public Health Agency of Canada.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Neutralizing antibody therapy
A neutralizing antibody targets the virus and inhibits virus replication at a very early stage of the replication cycle. Antibody‐based EVD therapies are being developed using either convalescent serum from recovered patients or engineered monoclonal antibody (see below).
Transfusion therapy
The use of hyperimmune globulin has been well documented in other diseases (e.g. hepatitis B, rabies and varicella‐zoster virus). In 1995, during an EVD outbreak in Kikwit, DRC, eight patients with EVD symptoms were treated with convalescent serum containing IgG EBOV antibodies from recovered patients with EVD. This treatment led to the survival of seven of these patients, a significantly improved survival rate compared with the average fatality rate of 80% in this particular outbreak 68. However, it should be noted that these patients also received supportive treatment, and therefore, it is impossible to determine whether the serum transfusion was the crucial factor for patient survival 68. The WHO has stated that blood or plasma transfusions from convalescent patients may be used for treatment of patients infected with EBOV in the current outbreak 69. However, the use of transfusion therapy is complicated due to: (i) limited/nonexistent laboratory infrastructure, (ii) lack of resources for safe collection and screening of blood from convalescent patients 70 and (iii) the requirement for a blood type match between donor and recipient. For these reasons, the use of plasma transfusion therapy is probably less promising than treatment with neutralizing monoclonal antibodies.
Monoclonal antibody treatment
The prototype product ZMapp is composed of three humanized monoclonal antibodies which target EBOV GPs. These antibodies have been chimerized by genetic engineering and manufactured in tobacco plants. The components of ZMapp are monoclonal antibody c13C6 from an existing antibody cocktail called MB‐003 and two monoclonal antibodies (c2G4 and c4G7) from a different antibody cocktail, ZMab 71, 72. Although it is still in the early phase of development, ZMapp has been used to treat seven patients with EVD, with five of those patients surviving 73. However, there is a lack of adequate data about its safety and efficacy in animal models and in humans. In an early study involving EBOV in rhesus macaques, use of ZMapp produced promising results 72. Nevertheless, there are still several barriers to the use of ZMapp for clinical treatment, for example the manufacturer of ZMapp is not yet prepared for mass production and the product is still in the early phase of development. However, efforts are being made to scale up production 73.
Small‐molecule (nucleoside analogue) antiviral agents
Brincidofovir (CMX‐001)
This antiviral agent is an orally available lipid conjugate of cidofovir. Brincidofovir is being tested in early and late phase clinical trials for its effect on a number of viral diseases caused by different DNA viruses, including adenovirus, herpes viruses, orthopoxviruses, papillomavirus and polyomaviruses 74, 75, 76. This drug has shown potent anti‐EBOV activity at cell culture level and has also been used to treat patients with EVD. Brincidofovir has a long half‐life, which means fewer doses and thereby fewer renal side effects compared with cidofovir 77. However, there are currently no published data regarding the safety or efficacy of this molecule in treating EVD in either animal models or humans.
Favipiravir (T‐705)
The pyrazinecarboxamide derivative favipiravir is a nucleotide analogue that inhibits the viral RdRP, either by interacting with and occupying its catalytic domain or by incorporation into the newly synthesized viral RNA to cause lethal mutagenesis 78. Favipiravir has primarily been studied for the treatment of influenza, but it has also demonstrated activity against arenaviruses and bunyaviruses 79, 80, 81. Recent work demonstrated that this molecule inhibits the replication of EBOV in both cell culture and small animal models 82, 83. Favipiravir could be administered orally 48, 81, which could prevent potential risks arising during drug injection. More importantly, phase III clinical trials of favipiravir for influenza treatment have been completed 84, making it possible for it to be quickly available for EVD therapy as long as anti‐EBOV activity can be proved in NHP model.
Bcx‐4430
This novel adenosine analogue has demonstrated efficacy in treating both EBOV and Marburg virus 85. BCX‐3340 indirectly inhibits RNA polymerase activity, resulting in termination of transcription and viral RNA replication. Preliminary data from animal experiments demonstrate very promising results.
Oligonucleotide‐based antiviral agents
TKM‐Ebola
This antiviral agent (intravenous formulation) is a small interfering RNA (siRNA) in early clinical trials 86. This siRNA specifically recognize the RNA sequences of RdRP (EK‐1), VP24 and VP35 and are packaged with polyethylenimine or lipid particles for in vivo delivery 86, 87.
Avi‐6002
Another antisense oligonucleotide‐based technology, termed phosphorodiamidate morpholino oligomers (PMOs), is also being applied for EVD therapy. These molecules inhibit viral replication by recognizing the specific single‐stranded RNA or DNA of viruses and binding with them to form stable complexes 88. The EBOV‐specific PMO drug AVI‐6002 is a mixture of positively charged PMOs targeting mRNA sequences of VP24 and VP35. Both TKM‐Ebola and AVI‐6002 have demonstrated promising in vitro and in vivo effects. However, there are two major issues to consider: (i) the mutation rate at the nucleic acid level is usually high for RNA viruses and this can lead to problems regarding genetic variation in the virus for antisense oligonucleotide‐based drugs and (ii) both these molecules should be delivered into the cytoplasm more efficiently to reduce drug dosage and frequency.
Vaccines
At present, two vaccine candidates are receiving most of the attention in the research world. The first candidate is based on recombinant vesicular stomatitis virus (rVSV), which has been genetically engineered to invoke an immune response against EBOV GP 89, 90. Newlink Genetics and the Public Health Agency of Canada are jointly developing this candidate vaccine 90, which has been named rVSV (rVSV‐EBO). The second candidate, which is based on chimpanzee adenovirus serotype 3, is called chimpanzee adenovirus serotype 3 (cAd3‐EBO) and is being jointly developed by GlaxoSmithKline and the United States National Institute of Allergy and Infectious Diseases 91. Both vaccine candidates have demonstrated very good efficacy in preventing EBOV infection in NHPs 92, 93.
rVSV‐EBO
Vesicular stomatitis virus is a nonsegmented, negative‐stranded RNA virus that is a member of the Rhabdoviridae family. Attenuated rVSV has been studied as a potential vector for several viruses, for example in the development of vaccines for the treatment of HIV, influenza and Marburg virus. rVSV‐EBO has demonstrated efficacy for pre‐exposure prophylaxis in small animal models and in NHP models.
cAd3‐EBO
Recombinant adenovirus technology is the basis for this vaccine candidate. Initial studies using recombinant human adenovirus serotype 5 have generated promising results in animal models. However, widespread use is complicated by the fact that many adults are seropositive for Ad5, depending on country of origin. cAd3 is a rare adenovirus serotype, and therefore, humans do not have pre‐existing immunity to it, which makes this serotype very interesting for developing new vaccine candidates. In a recent trial in chimpanzees, pre‐exposure prophylaxis with cAd3 resulted in 100% protection. However, cAd3 has not yet been studied as a postexposure prophylaxis for EVD infection.
Conclusion
As illustrated above, there are several candidate treatments with promising results in vitro and in vivo as regards controlling EVD. It is hoped that some of these will soon be commercially available to help treat patients who contract this devastating disease.
Conflict of interest statement
The author has no conflicts of interest to declare.
Mirazimi A (Karolinska Institutet, Stockholm; and National Veterinary Institute, Uppsala, Sweden). Ebola virus disease: societal challenges and new treatments. J Intern Med 2015; 278: 227–237.
References
- 1. Apisarnthanarak A, Mundy LM. Influenza outbreak among health care workers in an avian influenza (H5N1)‐endemic setting. Clin Infect Dis 2006; 43: 1493–4. [DOI] [PubMed] [Google Scholar]
- 2. Watts J. Report details lessons from SARS outbreak. Lancet 2003; 362: 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haydon DT, Kao RR, Kitching RP. The UK foot‐and‐mouth disease outbreak – the aftermath. Nat Rev Microbiol 2004; 2: 675–81. [DOI] [PubMed] [Google Scholar]
- 4. Flick R, Bouloy M. Rift Valley fever virus. Curr Mol Med 2005; 5: 827–34. [DOI] [PubMed] [Google Scholar]
- 5. Anyamba A, Chretien JP, Small J et al Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA 2009; 106: 955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ergonul O. Crimean‐Congo haemorrhagic fever. Lancet Infect Dis 2006; 6: 203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. Am J Prev Med 2008; 35: 436–50. [DOI] [PubMed] [Google Scholar]
- 8. Sutherst RW. Global change and human vulnerability to vector‐borne diseases. Clin Microbiol Rev 2004; 17: 136–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones KE, Patel NG, Levy MA et al Global trends in emerging infectious diseases. Nature 2008; 451: 990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters CJ, Khan AS. Filovirus diseases. Curr Top Microbiol Immunol 1999; 235: 85–95. [DOI] [PubMed] [Google Scholar]
- 11. Bharat TA, Noda T, Riches JD et al Structural dissection of Ebola virus and its assembly determinants using cryo‐electron tomography. Proc Natl Acad Sci USA 2012; 109: 4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geisbert TW, Jahrling PB. Differentiation of filoviruses by electron microscopy. Virus Res 1995; 39: 129–50. [DOI] [PubMed] [Google Scholar]
- 13. Jaax N, Jahrling P, Geisbert T et al Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet 1995; 346: 1669–71. [DOI] [PubMed] [Google Scholar]
- 14. Barrette RW, Xu L, Rowland JM, McIntosh MT. Current perspectives on the phylogeny of Filoviridae. Infect Genet Evol 2011; 11: 1514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn JH, Becker S, Ebihara H et al Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol 2010; 155: 2083–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carroll SA, Towner JS, Sealy TK et al Molecular evolution of viruses of the family Filoviridae based on 97 whole‐genome sequences. J Virol 2013; 87: 2608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauber C, Gorbalenya AE. Genetics‐based classification of filoviruses calls for expanded sampling of genomic sequences. Viruses 2012; 4: 1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki Y, Gojobori T. The origin and evolution of Ebola and Marburg viruses. Mol Biol Evol 1997; 14: 800–6. [DOI] [PubMed] [Google Scholar]
- 19. Taylor DJ, Leach RW, Bruenn J. Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol 2010; 10: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377: 849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volchkova VA, Feldmann H, Klenk HD, Volchkov VE. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel‐orientated homodimer. Virology 1998; 250: 408–14. [DOI] [PubMed] [Google Scholar]
- 22. Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J Virol 2006; 80: 4174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yonezawa A, Cavrois M, Greene WC. Studies of ebola virus glycoprotein‐mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol 2005; 79: 918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tran EE, Simmons JA, Bartesaghi A et al Spatial localization of the Ebola virus glycoprotein mucin‐like domain determined by cryo‐electron tomography. J Virol 2014; 88: 10958–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Y, Xu L, Sun Y, Nabel GJ. The assembly of Ebola virus nucleocapsid requires virion‐associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol Cell 2002; 10: 307–16. [DOI] [PubMed] [Google Scholar]
- 26. John SP, Wang T, Steffen S, Longhi S, Schmaljohn CS, Jonsson CB. Ebola virus VP30 is an RNA binding protein. J Virol 2007; 81: 8967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modrof J, Becker S, Muhlberger E. Ebola virus transcription activator VP30 is a zinc‐binding protein. J Virol 2003; 77: 3334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weik M, Modrof J, Klenk HD, Becker S, Muhlberger E. Ebola virus VP30‐mediated transcription is regulated by RNA secondary structure formation. J Virol 2002; 76: 8532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trunschke M, Conrad D, Enterlein S, Olejnik J, Brauburger K, Muhlberger E. The L‐VP35 and L‐L interaction domains reside in the amino terminus of the Ebola virus L protein and are potential targets for antivirals. Virology 2013; 441: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basler CF, Mikulasova A, Martinez‐Sobrido L et al The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol 2003; 77: 7945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basler CF, Wang X, Muhlberger E et al The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci USA 2000; 97: 12289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bamberg S, Kolesnikova L, Moller P, Klenk HD, Becker S. VP24 of Marburg virus influences formation of infectious particles. J Virol 2005; 79: 13421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoenen T, Jung S, Herwig A, Groseth A, Becker S. Both matrix proteins of Ebola virus contribute to the regulation of viral genome replication and transcription. Virology 2010; 403: 56–66. [DOI] [PubMed] [Google Scholar]
- 34. Jasenosky LD, Kawaoka Y. Filovirus budding. Virus Res 2004; 106: 181–8. [DOI] [PubMed] [Google Scholar]
- 35. Zhang AP, Abelson DM, Bornholdt ZA, Liu T, Woods VL Jr, Saphire EO. The ebolavirus VP24 interferon antagonist: know your enemy. Virulence 2012; 3: 440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang AP, Bornholdt ZA, Liu T et al The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8: e1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Escudero‐Perez B, Volchkova VA, Dolnik O, Lawrence P, Volchkov VE. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog 2014; 10: e1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kindzelskii AL, Yang Z, Nabel GJ, Todd RF III, Petty HR. Ebola virus secretory glycoprotein (sGP) diminishes Fc gamma RIIIB‐to‐CR3 proximity on neutrophils. J Immunol 2000; 164: 953–8. [DOI] [PubMed] [Google Scholar]
- 39. Sui J, Marasco WA. Evidence against Ebola virus sGP binding to human neutrophils by a specific receptor. Virology 2002; 303: 9–14. [DOI] [PubMed] [Google Scholar]
- 40. Volchkova VA, Klenk HD, Volchkov VE. Delta‐peptide is the carboxy‐terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 1999; 265: 164–71. [DOI] [PubMed] [Google Scholar]
- 41. Leroy EM, Rouquet P, Formenty P et al Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 2004; 303: 387–90. [DOI] [PubMed] [Google Scholar]
- 42. Chowell G, Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med 2014; 12: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klompas M, Diekema DJ, Fishman NO, Yokoe DS. Ebola fever: reconciling planning with risk in U.S. hospitals. Ann Intern Med 2014; 161: 751–2. [DOI] [PubMed] [Google Scholar]
- 44. Towner JS, Sealy TK, Khristova ML et al Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 2008; 4: e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Team WHOER . Ebola virus disease in West Africa – the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371: 1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baize S, Pannetier D, Oestereich L et al Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371: 1418–25. [DOI] [PubMed] [Google Scholar]
- 47. Mari Saez A, Weiss S, Nowak K et al Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med 2014; 7: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. WHO . http://whoint/csr/disease/ebola/situation-reports/en/. WHO; 2015. [Google Scholar]
- 49. Gire SK, Goba A, Andersen KG et al Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345: 1369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeffs B. A clinical guide to viral haemorrhagic fevers: Ebola, Marburg and Lassa. Trop Doct 2006; 36: 1–4. [DOI] [PubMed] [Google Scholar]
- 51. West TE, Von Saint Andre‐von Arnim A. Clinical presentation and management of severe Ebola virus disease. Ann Am Thorac Soc 2014; 11: 1341–50. [DOI] [PubMed] [Google Scholar]
- 52. Team WHOER , Agua‐Agum J, Ariyarajah A, Aylward B, et al West African Ebola epidemic after one year – slowing but not yet under control. N Engl J Med 2015; 372: 584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rowe AK, Bertolli J, Khan AS et al Clinical, virologic, and immunologic follow‐up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis 1999; 179(Suppl. 1): S28–35. [DOI] [PubMed] [Google Scholar]
- 54. Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 2015; 235: 153–74. [DOI] [PubMed] [Google Scholar]
- 55. Roels TH, Bloom AS, Buffington J et al Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis 1999; 179(Suppl. 1): S92–7. [DOI] [PubMed] [Google Scholar]
- 56. Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis 1999; 179(Suppl. 1): S87–91. [DOI] [PubMed] [Google Scholar]
- 57. Borio L, Inglesby T, Peters CJ et al Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 2002; 287: 2391–405. [DOI] [PubMed] [Google Scholar]
- 58. Johnson E, Jaax N, White J, Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol 1995; 76: 227–36. [PMC free article] [PubMed] [Google Scholar]
- 59. Reed DS, Lackemeyer MG, Garza NL, Sullivan LJ, Nichols DK. Aerosol exposure to Zaire ebolavirus in three nonhuman primate species: differences in disease course and clinical pathology. Microbes Infect 2011; 13: 930–6. [DOI] [PubMed] [Google Scholar]
- 60. Bausch DG, Towner JS, Dowell SF et al Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(Suppl. 2): S142–7. [DOI] [PubMed] [Google Scholar]
- 61. Kreuels B, Wichmann D, Emmerich P et al A case of severe Ebola virus infection complicated by gram‐negative septicemia. N Engl J Med 2014; 371: 2394–401. [DOI] [PubMed] [Google Scholar]
- 62. WHO hwwicrpefice . Infection prevention and control guidance for care of patients in health‐care settings, with focus on Ebola. 2014.
- 63. Fauci AS. Ebola – underscoring the global disparities in health care resources. N Engl J Med 2014; 371: 1084–6. [DOI] [PubMed] [Google Scholar]
- 64. Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ 1983; 61: 997–1003. [PMC free article] [PubMed] [Google Scholar]
- 65. Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8: e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pandey A, Atkins KE, Medlock J et al Strategies for containing Ebola in West Africa. Science 2014; 346: 991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Borchert M, Mutyaba I, Van Kerkhove MD et al Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis 2011; 11: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mupapa K, Massamba M, Kibadi K et al Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis 1999; 179(Suppl. 1): S18–23. [DOI] [PubMed] [Google Scholar]
- 69. Gulland A. First Ebola treatment is approved by WHO. BMJ 2014; 349: g5539. [DOI] [PubMed] [Google Scholar]
- 70. Burnouf T, Emmanuel J, Mbanya D et al Ebola: a call for blood transfusion strategy in sub‐Saharan Africa. Lancet 2014; 384: 1347–8. [DOI] [PubMed] [Google Scholar]
- 71. Qiu X, Audet J, Wong G et al Successful treatment of ebola virus‐infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 2012; 4: 138ra81. [DOI] [PubMed] [Google Scholar]
- 72. Qiu X, Wong G, Audet J et al Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCarthy M. US signs contract with ZMapp maker to accelerate development of the Ebola drug. BMJ 2014; 349: g5488. [DOI] [PubMed] [Google Scholar]
- 74. Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther 2014; 12: 1171–8. [DOI] [PubMed] [Google Scholar]
- 75. Olson VA, Smith SK, Foster S et al In vitro efficacy of brincidofovir against variola virus. Antimicrob Agents Chemother 2014; 58: 5570–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parker S, Crump R, Foster S et al Co‐administration of the broad‐spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus. Antiviral Res 2014; 111: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lanier R, Trost L, Tippin T et al Development of CMX001 for the Treatment of Poxvirus Infections. Viruses 2010; 2: 2740–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sangawa H, Komeno T, Nishikawa H et al Mechanism of action of T‐705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother 2013; 57: 5202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mendenhall M, Russell A, Smee DF et al Effective oral favipiravir (T‐705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic Fever. PLoS Negl Trop Dis 2011; 5: e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gowen BB, Wong MH, Jung KH et al In vitro and in vivo activities of T‐705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 2007; 51: 3168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Scharton D, Bailey KW, Vest Z et al Favipiravir (T‐705) protects against peracute Rift Valley fever virus infection and reduces delayed‐onset neurologic disease observed with ribavirin treatment. Antiviral Res 2014; 104: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oestereich L, Ludtke A, Wurr S, Rieger T, Munoz‐Fontela C, Gunther S. Successful treatment of advanced Ebola virus infection with T‐705 (favipiravir) in a small animal model. Antiviral Res 2014; 105: 17–21. [DOI] [PubMed] [Google Scholar]
- 83. Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Post‐exposure efficacy of oral T‐705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res 2014; 104: 153–5. [DOI] [PubMed] [Google Scholar]
- 84. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T‐705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100: 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Warren TK, Wells J, Panchal RG et al Protection against filovirus diseases by a novel broad‐spectrum nucleoside analogue BCX4430. Nature 2014; 508: 402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McCarthy M. FDA allows second experimental drug to be tested in Ebola patients. BMJ 2014; 349: g5103. [DOI] [PubMed] [Google Scholar]
- 87. Geisbert TW, Lee AC, Robbins M et al Postexposure protection of non‐human primates against a lethal Ebola virus challenge with RNA interference: a proof‐of‐concept study. Lancet 2010; 375: 1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Warren TK, Shurtleff AC, Bavari S. Advanced morpholino oligomers: a novel approach to antiviral therapy. Antiviral Res 2012; 94: 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marzi A, Engelmann F, Feldmann F et al Antibodies are necessary for rVSV/ZEBOV‐GP‐mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA 2013; 110: 1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Attaran A, Nickerson JW. Is Canada patent deal obstructing Ebola vaccine development? Lancet 2014; 384: e61. [DOI] [PubMed] [Google Scholar]
- 91. Ledgerwood JE, DeZure AD, Stanley DA et al Chimpanzee adenovirus vector Ebola vaccine – preliminary report. N Engl J Med 2014. DOI: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 92. Stanley DA, Honko AN, Asiedu C et al Chimpanzee adenovirus vaccine generates acute and durable protective immunity against Ebola virus challenge. Nat Med 2014; 20: 1126–9. [DOI] [PubMed] [Google Scholar]
- 93. Geisbert TW, Geisbert JB, Leung A et al Single‐injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol 2009; 83: 7296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


