Abstract
The Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) is a novel Coronavirus which was responsible of the first case of human acute respiratory syndrome in the Kingdom of Saudi Arabia (KSA), 2012. Dromedary camels are considered as potential reservoirs for the virus and seem to be the only animal host which may transmit the infection to human. Further studies are required to better understand the animal sources of zoonotic transmission route and the risks of this infection. A primary sero‐prevalence study of MERS‐CoV preexisting neutralizing antibodies in Dromedary camel serum was conducted in Tabuk, western north region of KSA, in order to assess the seopositivity of these animals and to explain their possible role in the transmission of the infection to Human. One hundred seventy one (171) serum samples were collected from healthy dromedary camels with different ages and genders in Tabuk city and tested for specific serum IgG by ELISA using the receptor‐binding S1 subunits of spike proteins of MERS‐CoV. 144 (84,21%) of the total camel sera shown the presence of protein‐specific antibodies against MERS‐CoV. These results may provide evidence that MERS‐CoV has previously infected dromedary camels in Tabuk and may support the possible role of camels in the human infection.
Keywords: blood, Coronavirus, enzyme assays, immunoglobulin, reservoir
1. INTRODUCTION
The Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) is positive‐sense, single‐stranded RNA novel species of the genus Beta‐coronavirus and the family of Coronaviridae.
Since Middle East Respiratory Syndrome (MERS) was described in September 2012, 1794 laboratory‐confirmed cases of infection including at least 731 (40,8%) related deaths have been reported by WHO in the kingdom of Saudi Arabia (KSA).1
Most MERS cases have been reported in the Arab peninsula, in Saudi Arabia, Jordan, Qatar, and the United Arab Emirates, and other imported index cases, have been reported in France, Germany, Italy, Tunisia, and the United Kingdom.2, 3
Although instances of human‐to‐human transmission of MERS‐CoV infection have been documented between case‐patients and others in close contact, including patients and family members, the sources of infection for most patients remain usually unknown.
The epidemiology of the disease, so far is suggestive of multiple zoonotic transmissions from an animal reservoir leading to human infection, sometimes with secondary transmission events in human.
Phylogenetically closely related, viruses have been detected in bats in some countries in Africa and Europe.4, 5 A very short fragment of the RNA‐dependent RNA polymerase gene that was genetically identical to MERS‐CoV has been detected in a bat captured surrounding the residence of a human case with MERS.6
Evidence also provides that MERS‐CoV has been circulating in camels at least since 1992,7 while human infected cases were newly reported in KSA.8 This suggests that camels may play a role of primary reservoir for transmitting the viral infection to human.
The hypothesis of MERS‐CoV transmission from dromedary camels to humans was reported in a study undertaken on human infection with MERS‐CoV after exposure to infected camels in Saudi Arabia.9 Similarities in genome sequence between MERS‐CoVs isolated from dromedary camels and humans were described in different studies and suggest the potential role of camels in human infection.9, 10, 11, 12
Since MERS‐CoV specific antibodies following Coronavirus infection remain detectable for many years,13 seroepidemiology of potential animal species for MERS‐CoV‐specific antibody is a necessary approach to identify candidate species for further investigation. Serological approaches, including ELISA and immunoflourescence assays are well used for specific antibodies detection in human and camels, while virus neutralization which was considered by some authors as a gold‐standard test, is used for infectious virus detection.14, 15
Even if MERS‐CoV circulation was well studied in many regions in KSA since its emergence in 2012, no data are available about camel infection in Tabuk, north‐west region of the kingdom. The main objective of this study was to assess the sero‐prevalence of MERS‐CoV preexisting neutralizing antibodies in the serum of healthy dromedary camels in Tabuk city in order to evaluate the immune status of these animals and their protection against new MERS‐CoV infections. The eventual presence of MERS‐CoV antibodies in Dromedary camels can explain the possible role of these animals in the transmission of infection to Human which is not clearly described till now.
2. MATERIALS AND METHODS
2.1. Serum sample collection
A serological study was carried from January to April 2016. One hundred seventy one (171) serum samples were collected from three herds of Dromedary camels (Camelus dromedarius) located around Tabuk city. The three sites of sample collection had been chosen for their localization near Tabuk city and camels are in continues contact with humans (owners, farmers, and other people). The areas of sample collection are not considered as geographically separated and don't present any difference of environmental conditions which can have an influence on camels infection by the virus. According to the farmers and the animal owners, all selected dromedary camels were born and bred in Tabuk and aged from 1 to 5 years. Serum samples were collected by a veterinarian from only healthy camels in order to avoid unnecessary suffering of the animals or possible accidents of the handling personnel. Camels showing clinical signs of any disease were excluded from this study.
Among 171 samples, 36 were collected in January 2016, 74 in February, 30 in March and 31 in April. In total, 93 camels were males, 78 females, 71 juveniles (<2 years) and 100 adults (3–5 years).
2.2. Serological analysis
Blood sera were separated, diluted at 1:100 and analyzed for MERS‐CoV specific antibodies using the anti‐MERS‐CoV ELISA Camel (IgG) kit manufactured by EUROIMMUN AG (Lübeck, Germany). This test is based on the recombinant MERS‐CoV spike protein subunit‐1 and has successfully been used by other authors evaluating MERS‐CoV in camels.16
Optical Density (OD) was measured at 450 nm using a MINDRAY MR‐96 ELISA reader.
2.3. Statistical analysis
Statistical analysis was performed on SPSS v. 22.0 software (SPSS Inc., Chicago, IL). Data were expressed as percentage for continuous variables, which were normally distributed, or as percentages of total for categorical variables. Pearson χ 2 test was used to assess inter–group significance.
2.4. Ethical approval
This research was ethically approved by the research ethic committee at the University of Tabuk.
3. RESULTS
One hundred seventy one serum samples were collected from three areas in Tabuk city and analyzed by ELISA technique for MERS‐CoV specific antibodies detection. The three farms are located around the city of Tabuk and are not considered as geographically separated. A total of 93 of 171 dromedary camels were males and 78 were females, 71 were juveniles, and 100 were adults.
According to the manufacture instruction of the ELISA kit, results are interpreted as: Positive (Ratio ≥ 1.1), Negative (Ratio < 0.8), and borderline (0.8 < Ratio <1.1) with Ratio = Control or Sample OD/Calibrator OD (cut‐Off).
In total, 144 samples (84.21%) were MERS‐CoV antibody positive, 22 (12.86%) were negative and 5 (2.92%) were borderline. In males, 77 (82.8%) were positive and 14 (15%) were negative. In females, 67 (85.9%) were positive and 08 (10.30%) were negative. The statistical analysis, using Pearson χ 2 test, didn't show any significant difference of antibody prevalence between males and females (P = 0.542). According to the age, 66 (92.95%) of juveniles were positive and 04 (5.63%) were negative. From adults, 78 (78%) were positive and 18 (18%) were negative (Table 1 and Figure 1). The prevalence of MERS‐CoV antibodies in juveniles was significantly higher than prevalence in adults (P = 0.030).
Table 1.
Seroprevalence of MERS‐CoV antibody in Dromedary camels according to the sex and the age
| Results | |||
|---|---|---|---|
| Gender | Pos | Neg | Brd |
| Male (93) | 77 (82.8%) | 14 (15.1%) | 02 (02.2%) |
| Female (78) | 67 (85.90%) | 08 (10.25%) | 03 (03.85%) |
| P * | 0.542 | 0.542 | 0.542 |
| Age | |||
| Juvenile (71) | 66 (92.95%) | 04 (05.63%) | 01 (01.4%) |
| Adult (100) | 78 (78%) | 18 (18%) | 04 (04%) |
| P * | 0.030 | 0.030 | 0.030 |
| Total (171) | 144 (84.21%) | 22 (12.86%) | 05 (02.12%) |
Pos, positive; Neg, negative; Brd, borderline; Ab, antibody.
Chi Square Test for continuous variables (variables with normal distribution).
Figure 1.
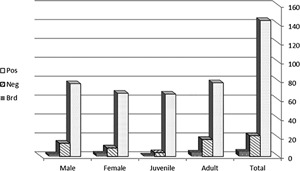
Distribution of results of MERS‐CoV antibody detection in Dromedary camels according to the sex and the age. Statistical analysis does not show any significant difference in MERS‐CoV antibody prevalence between male and female genders (P = 0.542) but prevalence of antibodies in juveniles was significantly higher than prevalence in adults (P = 0.030). Pos, positive; Neg, negative; Brd, borderline; M, male; F, female; JUV, juvenile; AD, adult
During sample collection, the veterinarian and the farmers confirmed the total absence of clinical signs and symptoms related to MERS such as respiratory distress or nasal or ocular discharges in selected animals.
No variations in result distribution of MERS‐CoV antibody seroprevalence were observed among the three sites of sample collection as there was no geographic separation or origin difference among animals (Data not shown).
4. DISCUSSION
A primary study was conducted in Tabuk, northwest region of KSA, to evaluate the prevalence of specific MERS‐CoV antibodies in the serum of healthy dromedary camels in and to interpret the results according to the epidemiological information. This study is the first to present data about the camel MERS‐CoV in the region of Tabuk, in the north‐west of the country. Published studies about KSA were mainly undertaken in the central and southern regions of the Kingdom (Riyadh, Gizan, Hafuf…). Blood sera collected from 171 camels were analyzed by CoV ELISA camel (IgG) test for antibody detection against the S1 antigen of MERS‐CoV according to the manufacture instructions. This test has been successfully used and evaluated by many authors for MERS‐CoV detection in camels.11, 16, 17
Results have shown that a high number (85%) of dromedary camels from the different farms of Tabuk city have MERS‐CoV neutralizing antibodies. Specific antibodies were detected in serum samples collected in 2016 from healthy camels, all originated from Tabuk and with different ages and genders. This first finding suggests a longstanding presence of MERS‐CoV in camels in this region of KSA which has known an epidemic human MERS‐CoV infection since 2012.8 Our results do not provide proof for the presence of MERS‐CoV in dromedary camels but they do support the finding of Meyer et al18 suggesting that MERS‐CoV or a closely related coronavirus antibody detection, is not a new infection in dromedary camels as they don't present signs and symptoms for the CoV disease. A current infection and active viral excretion need to be confirmed by respiratory sample analysis, viral isolation on cell culture and/or by RNA material detection by molecular approaches.
Our results are in agreement with those published in a study conducted in the central and southern regions of KSA 2013 showing a high rate of camels with MERS‐CoV specific antibodies.7 Indeed, 203 serum samples from dromedary camels collected from Hafuf, Gizan, and Riyadh and screened by ELISA test showed that 74% of the animals were found to have antibodies to MERS‐CoV.7 In the same study, 264 archived serum samples collected from dromedary camels from 1992 to 2010 in Riyadh and Kharj were also analyzed by ELISA and showed a high seroprevalence (92%) of MERS‐CoV neutralizing antibodies.7 Our data agree also with previous studies reporting wide antibody prevalence in camels in many countries, including Egypt and Oman.12, 19 In another study conducted in Oman, all serum samples from 50 dromedary camels were positive for MERS‐CoV specific antibodies.12 Similar results were also reached from a larger study conducted in United Arab Emirates (UAE), where 500 dromedary camels’ sera screened in 2013 revealed 96% seropositivity.18 In Africa, an outbreak investigating serum samples for MERS‐CoV assessment in camels has also shown similar results in Nigeria (94%), Tunisia (48.5%), and Ethiopia (96.3%).20 Likewise, in a study conducted on 189 archived camel sera samples collected in 1997 from Egypt and between 1983 and 1984 from Sudan and Somalia, 81% were found to have neutralizing antibodies to MERS‐CoV.16 Similar results were also obtained in a study from Kenya.17 Taken together, our findings and all those from the Arabian Peninsula and Africa, suggest a wide exposure of Dromedary camels to MERS‐CoV which may be infected by the virus at some point in their life.
While camels in the Middle East and Africa were highly exposed to MERS‐CoV infection, camels from Europe and Australia which are geographically isolated were not exposed to the virus.12 However, serum samples collected from 105 dromedary camels living in the archipelago of Canary Islands, between 2012 and 2013 showed that 14% have antibodies against MERS‐CoV.12 In another study carried out in the same islands, 170 dromedary camel sera were analyzed and only 4.1% were seropositive for MERS‐CoV. Data showed that all the seropositive dromedary camels for MERS‐CoV were all imported from Africa. In addition, 307 dromedary camels’ sera collected from different regions between 2013 and 2014 in the Islands were all tested negative for specific MERS‐CoV antibodies.21 All these findings indicate that active MERS‐CoV infection did not occur in the Canary Islands.22
The high prevalence of camel MERS antibodies in KSA and the Arab peninsula, countries with human epidemic MERS, and the absence or low prevalence of camel MERS‐CoV antibodies in Europe and Australia, countries without or with few human MERS cases, could explain the potential role of camels as reservoirs of MERS‐CoV infection. In our study, the high prevalence of MERS‐CoV antibodies in camels could be explained by the acquisition of maternal immunity or by the occurrence of viral infection at the juvenile age. As the sample collection was done in 2016, the animal infection would have occurred in 2011–2013, before or during MERS emergence in the country. This finding can also constitute another argument supporting the hypothesis of the role of dromedary camels as potential reservoir for MERS transmission to human. Also, complete genomic sequences of dromedary camels MERS‐CoV were found to be similar to sequences of Human MERS‐CoV.10, 11 Serological studies detecting MERS‐CoV antibodies in archived serum samples suggested the potential role of dromedary camels in Human MERS‐CoV infection since similar genomic sequences of MERS‐CoV were found in contemporary dromedary camels and Humans.7 The only archived specimens were sera and MERS‐CoV sequencing has been unsuccessful.7
Our results, although restricted to serology, provide arguments supporting the camel role in Human MERS infection but do not confirm this relationship according to the current data. Further epidemiological investigations on human exposure to dromedary camels are required to confirm this relationship. In case of implication of dromedary camels in Human MERS, some questions will arise about the absence of human infection before 2012 since high MERS‐CoV seroprevalence was noted in archived camel sera. Did MERS‐CoV emerge only as a human pathogen in 2012, or was‐it because of a lack of diagnostic approaches to identify the human MERS? Or also mutation has occurred to facilitate cross‐species transmission? More analysis of archived specimens and genome sequencing can provide clear answer to these questions.
By looking at the age, our results have shown a high seoprevalence of MERS‐CoV antibodies in all the age stages of dromedary camels but with difference between young (<2 years) and adult (>3 years) animals. Seroprevalence was significantly higher in juveniles (92%) than in adults (78%). The high frequency of seropositive camels was noted in all farms, from 85% to 100% for juveniles and from 65% to 85% for adults.
Available data describing the association of camel age to MERS‐CoV antibodies expression are till now limited and explanations about the relation between the age and the antibody prevalence remain usually hypothetical. In our study, adult camels might be infected by the virus as juveniles as was reported by Benjamin in 2016, suggesting that MERS‐CoV infection appears to predominantly affect young dromedary camels.23 The high seroprevalence in juveniles and adults confirms also results of Hemida et al24 but with lower rates in juveniles. The same author explained the increase of the seropositivity in adult camels by the age range of the animals which were exposed for long time to the virus infection.24
For young camels, specific MERS‐CoV antibodies could be acquired from their mothers as it was reported by Kamber25 who suggests that maternal IgG antibodies in camels are acquired through the colostrum intake during the first 24 h post‐parturition and not via the trans‐placental route. After 24 h, antibody levels in the dam's milk decrease rapidly, and IgG levels in serum cease to rise.25 We also suggest that maternal antibodies might not have been sufficient to mediate protective immunity for them. Animals would then be exposed to new MERS‐CoV infection and specific antibodies would consequently increase in 1–2 years after, which can explain the higher seroprevalence in juveniles then in adults. The predominance of viral antibodies in young camels could also be explained by animal exposure to the virus few times post‐parturition and not by mother immunity, which will stimulate antibody secretion in the blood. In the two last possibilities, young camels could contract viral infection after 2012, period of epidemic MERS circulation in KSA, and production of specific antibodies would consequently increase in some months after birth. Respiratory specimens, as nasal swabs, and rectal swabs will be required for analysis to know whether anti‐MERS antibodies are issued from recent viral infection.
In relation to sex, our results have shown a high seropositivity in both males (82,8%) and females (85.9%) without a significant difference between the two genders. This information was not reported in previous studies and indicates that the sex cannot be a limiting factor in camel's infection by the virus.
In conclusion, our findings in Dromedary camel's sera, even if limited to serology, constitute a strong argument suggesting that MERS‐CoV has previously infected dromedary camels in Tabuk region. We speculate that Dromedary camels may support the role of these animals as potential reservoirs for human infection but we cannot confirm this relationship from the current data alone. Further studies including more animals and respiratory samples are needed for a larger evaluation of camel population in this region. In addition, a whole genome sequencing of camel MERS‐CoV is required to confirm its incrimination in human infection.
ACKNOWLEDGMENT
The work was financially supported by the Deanship of Scientific Research (DSR) at the University of Tabuk.
Harrath R, Abu Duhier FM. Sero‐prevalence of Middle East respiratory syndrome coronavirus (MERS‐CoV) specific antibodies in dromedary camels in Tabuk, Saudi Arabia. J Med Virol. 2018;90:1285–1289. 10.1002/jmv.25186
REFERENCES
- 1.Saudi Ministry of Health (MOH). Middle East respiratory syndrome coronavirus (MERS‐CoV) update. November 6, 2017. https://www.moh.gov.sa
- 2. Bermingham A, Chand MA, Brown CS, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012; 17:pii=20290. [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS‐CoV) summary and literature update. July 9, 2013. Geneva: WHO. [Accessed 20 Aug 2013].
- 4. Annan A, Baldwin HJ, Corman VM, et al. Human betacoronavirus 2c EMC/2012‐ related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013; 19:456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ithete NL, Stoffberg S, Corman VM, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013; 19:1697–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Memish ZA, Mishra N, Olival KJ, et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013; 19:1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alagaili AN, Briese T, Mishra N, et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014; 5:e01002–e01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cotten M, Watson SJ, Kellam P, et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet. 2013; 382:1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS‐CoV) in dromedary camels, Oman, 2013. Euro Surveill. 2014; 19:pii: 20781. [DOI] [PubMed] [Google Scholar]
- 10. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014; 14:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014; 20:1012–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reusken CB, Haagmans BL, Muller MA, et al. Middle East respiratory syndrome coronavirus neutralizing serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013; 13:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007; 357:1162–1163. [DOI] [PubMed] [Google Scholar]
- 14. Corman VM, Muller MA, Costabel U, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV‐EMC) infections. Euro Surveill. 2012; 17:20334. [DOI] [PubMed] [Google Scholar]
- 15. Reusken C, Mou H, Godeke GJ, et al. Specific serology for emerging human coronaviruses by protein microarray. Euro Surveill. 2013; 18:20441. [DOI] [PubMed] [Google Scholar]
- 16. Müller MA, Corman VM, Jores J, et al. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis 2014; 20:2093–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corman VM, Jores J, Meyer B, et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg Infect Dis. 2014; 20:1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer B, Müller MA, Corman VM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014; 20:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perera RA, Wang P, Gomaa M, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013; 18:20574. [DOI] [PubMed] [Google Scholar]
- 20. Reusken CB, Messadi L, Feyisa A, et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014; 20:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crameri G, Durr P, Barr J, et al. Absence of MERS‐CoV antibodies in feral camels in Australia: implications for the pathogen's origin and spread. One Health. 2015; 1:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gutiérrez C, Tejedor‐Junco MT, González M, Lattwein E, Renneker S. Presence of antibodies but no evidence for circulation of MERS‐CoV in dromedaries on the Canary Islands. Euro Surveill. 2015; 20. [DOI] [PubMed] [Google Scholar]
- 23. Benjamin M, Judit J, Rajib B, et al. Time course of MERS‐CoV infection and immunity in dromedary camels. Emerg Infect Dis. 2016; 22:2171–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hemida MG, Perera RA, Wang P, et al. Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013; 18:20659. [DOI] [PubMed] [Google Scholar]
- 25. Kamber R, Farah Z, Rusch P, Hassig M. Studies on the supply of immunoglobulin G to newborn camel calves (Camelus dromedarius). J Dairy Res. 2001; 68:1–7. [DOI] [PubMed] [Google Scholar]


