Abstract
Background: Severe acute respiratory syndrome (SARS) is an emerging infectious disease and diarrhea has been reported in up to 76% of cases. The purpose of the present paper was to carry out a retrospective study of the clinical and demographic data of SARS patients with diarrhea in Princess Margaret Hospital.
Methods: From 1 to 31 March 2003, hospital records from 240 patients with confirmed SARS were studied. Patients with watery stool of ≥3 times/day for at least 3 consecutive days were defined as the diarrhea group. Clinical and demographic data were compared between the diarrhea and non‐diarrhea groups. Chest X‐ray (CXR) scores during the peak of diarrhea period were recorded by a respiratory physician. These CXR scores were correlated with the peak frequency of diarrhea by Spearman's correlation coefficient.
Results: Diarrhea occurred in 20.4% of patients after admission. Female patients were predominant with a female to male ratio of 6:1 (P < 0.001) and 69.4% of patients were living in Amoy Gardens Estate (P = 0.01). The proportions of patients requiring ventilatory care and mortality in the diarrhea group were 8.2% and 2%, respectively, which were significantly lower than those in the non‐diarrhea group (27.6% and 16.2%, P < 0.005). The CXR scores during the peak of diarrhea were not correlated with the maximum frequency of diarrhea (r = −0.09, P = 0.5).
Conclusions: A total of 20.4% of SARS patients had the complication of diarrhea after hospital admission. Both female sex and being a resident of Amoy Gardens Estate were associated with diarrhea. The diarrhea group had a better prognosis.
Keywords: complications of SARS, coronavirus, diarrhea, SARS
INTRODUCTION
Severe acute respiratory syndrome (SARS) is an emerging infectious disease and by 31 December 2003, SARS had been described in 29 countries, involving 8096 individuals and causing 774 deaths. 1 Severe pneumonia causing respiratory failure was the major cause of death in these patients. Fever and influenza‐like symptoms were the predominant presenting features but diarrhea, nausea and vomiting were frequently observed. 2 , 3 , 4 , 5 , 6 Diarrhea was an important problem because SARS coronavirus could be identified in stool in up to 100% of cases on day 10 in one study, 5 and up to 70 days after onset of symptoms in another study. 7 Although stool culture for SARS coronavirus failed, 7 the possibility of oral–fecal transmission became imminent. 8 Amoy Gardens was one of the most densely populated private housing estates in Hong Kong and in March 2003, 321 residents from 15 blocks had contracted the disease. The source was traced to a 33‐year‐old patient with watery diarrhea and it was postulated that the virus was spread via a faulty sewage system. 8 Recently, coronavirus protein was identified in the esophagus, stomach and small intestines by immunohistochemical staining 9 and in colon by culture and electron microscopy, 7 suggesting a high intestinal tropism of the SARS coronavirus. Recently coronavirus‐related proteins or toxins were postulated to be the cause of diarrhea. 7 In Hong Kong, the Medical and Geriatric Unit of Princess Margaret Hospital was the quarantine hospital taking care of SARS patients and by 26 May 2003, we had treated 578 patients. The present study was conducted to study the clinical and demographic data of SARS patients with diarrhea in a retrospective manner.
METHODS
From 1 31 March 2003, consecutive case records of patients with confirmed SARS who were admitted to Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong were retrieved. The diagnosis of SARS was based on fever above the temperature of 38°C, cough or shortness of breath, new pulmonary infiltrates on chest radiography (CXR) or high‐resolution computed tomography (CT) thorax in the absence of an alternative diagnosis to explain the clinical presentation. 10 In addition, there must be laboratory evidence of SARS coronavirus (SARS‐CoV) infection by either positive reverse transcription–polymerase chain reaction (RT‐PCR) test 11 in stool or nasopharyngeal aspirates, or fourfold increase in antibody titer to coronavirus upon follow up. All patients were treated with clarithromycin (Abbott Laboratories, IL, USA) 500 mg b.d. for 2 weeks, amoxicillin/clavulanate (GlaxoSmithKline, UK) in intravenous or oral form for 2 weeks, ribavirin (Schering Plough, NJ, USA) 400–800 mg intravenously every 8 h and hydrocortisone (Pfizer, NY, USA) 100–200 mg intravenously every 6 h or prednisolone (Clonmel Healthcare, Ireland) 1 mg/kg orally. Pulse methylprednisolone (Pharmacia) 0.5–1 g per day was given for 1–6 days when patients developed respiratory distress, oxygen desaturation or progression in CXR. Patient data, need of ventilatory care, survival, number of bowel movements per day, total potassium supplement, lowest serum potassium and lowest serum albumin level were retrieved from the records and entered into a database for further analysis. The time of onset of symptoms was recorded and bowel habits before admission were recalled. The intake and output chart of these patients were carefully studied. Those patients with watery or loose stool at least three times a day for at least 3 consecutive days were defined as diarrhea patients and selected for further study. Stool tests for pathogenic bacteria such as Salmonella, Shigella and Clostridium difficile infection were reported. Symptomatic treatment of diarrhea with hyoscine methobromide (Synco, Hong Kong), diphenoxylate/atropine sulfate (Pharmacia) and kaopectate (Universal Pharmaceutical, Hong Kong) were also recorded. Pneumonia was scored by a respiratory physician (WLT) according to the scoring system proposed by So et al. 12 All chest radiographs were semiquantified by separating each lung into six sections (upper, middle, and lower zones and medial and lateral divisions) and scoring them on a four‐point scale: 0, clear; 1, subtle haziness or mild infiltrates; 2, ground‐glass appearance or prominent infiltrates; and 3, confluent or dense opacities. The maximum score would be 36. The CXR scores at the time of maximum frequency of diarrhea were then correlated with maximum frequency of bowel movements to look for an association. Data between patients with diarrhea and patients without diarrhea were compared. Bowel habits were assessed during follow up 3 weeks and 3 months after discharge from hospital.
Statistics
Results are expressed as mean ± 1 SD. Statistical comparison of data was done by χ2 test for categorical data and unpaired Student's t‐test for continuous variables. Log linear analysis was performed when there were several variables that were associated with diarrhea. Correlation of CXR scores during the peak of diarrhea with the peak frequency of diarrhea was tested by Spearman's correlation coefficient. We used SPSS 10.0 (Chicago, IL, USA 1999) for all analyses.
RESULTS
Two hundred and sixty‐seven adult patients with suspected SARS were admitted to Princess Margaret Hospital from 1 to 31 March 2003 and their records were retrieved. Two hundred and forty patients showed clinical features of SARS plus either positive RT‐PCR test from nasopharyngeal aspirate or stool, or fourfold rise in antibody titer upon follow up. Most of them were young patients with a mean age of 40 ± 13.4 years and 60.3% of them were female. More than 95% of them had no other significant medical illness in the past. According to data in the intake and output chart, there were 141 patients (58.8%) who had passed ≥3 loose or watery stool per day during the hospital stay for at least 1 day, and 49 patients (20.4%) with ≥3 watery or loose stool per day for at least 3 consecutive days. The latter group was defined as the diarrhea group in the present study. Comparison of the demographic and clinical data of these patients is shown in Table 1. The age distribution and baseline mean serum hemoglobin and potassium levels were similar between the two groups. The total amount of potassium supplements was similar between the two groups. The baseline serum albumin level was, however, significantly higher in the diarrhea group (38.5 vs 36.8 g/L, P = 0.02). There were significantly more female patients in the diarrhea group and the male : female ratio was 1:6 (P < 0.001). There were also significantly more patients living in the Amoy Gardens Estate in the diarrhea group (69.4%, P = 0.01). Loglinear analysis showed that female gender and being a resident of Amoy Gardens Estate were significantly associated with diarrhea.
Table 1.
Comparison of demographic and clinical data of severe acute respiratory syndrome (SARS) patients with and without diarrhea
| Patients with diarrhea | Patients without diarrhea | |
|---|---|---|
| No. patients | 49 | 191 |
| Sex (male : female) | 1 : 6** | 1 : 1.8 |
| Mean age ± 1 SD | 38 ± 11.7 | 40.5 ± 13.7 |
| Amoy Gardens resident | 34 (69.4%)* | 97 (50.5%) |
| Mean hemoglobin ± 1 SD on admission (g/dL) | 12.9 ± 1 | 13 ± 1.6 |
| Mean albumin ± 1 SD on admission (g/L) | 38.6 ± 3.9* | 36.8 ± 4.6 |
| Mean drop in albumin ± 1 SD (g/L) | 9.9 ± 4.8 | 9.5 ± 5.3 |
| Mean potassium ± 1 SD on admission (mmol/L) | 3.6 ± 0.4 | 3.6 ± 0.4 |
| Lowest potassium ± 1 SD (mmol/L) | 2.5 ± 0.4* | 2.8 ± 0.4 |
| Total potassium supplement ± 1 SD (g) | 33.8 ± 14.9 | 34.8 ± 21.4 |
| Ventilation (%) | 4 (8.2%)** | 53 (27.6%) |
| Death (%) | 1 (2%)** | 31 (16.2%) |
Diarrhea was defined as ≥3 times watery or loose stools/day for at least 3 consecutive days.
P < 0.05;
P < 0.005.
Diarrhea group
Forty‐nine patients (20.4%) were recorded to have more than 3 watery or loose stool per day for at least 3 consecutive days. The proportion of patients having ≥3 bowel movements per day over the first 21 days after onset of symptoms is shown in Fig. 1. The mean frequency of bowel movements plus 1 SD is shown in Fig. 2. The mean onset time of diarrhea was 7.5 ± 2.8 days after onset of fever. Up to 5% of patients had diarrhea in the first week and the peak incidence of 17% occurred on day 12. The pattern for patients living in Amoy Gardens Estate was similar to that of patients living in other areas. The proportion of patients receiving diphenoxylate/atropine sulfate, kaopectate and hyoscine methobromide was 12.2%, 12.2% and 6.1%, respectively, for 1–6 days. The mean lowest potassium level in the diarrhea group (2.5 mmol/L) was also significantly lower than that in the group without diarrhea (2.8 mmol/L; P < 0.001). The mean drop in serum albumin level was 9.9 ± 4.8 g/L in the diarrhea group as compared with 9.5 ± 5.3 g/L in the group of patients without diarrhea (P = 0.6). The proportion of patients in the diarrhea group who required ventilatory care was 8.2% (P < 0.001) and the mortality was only 2% (P < 0.001). Diarrhea leading to renal impairment was not reported. The CXR scores during the period of peak diarrhea was not correlated with the maximum frequency of diarrhea (correlation coefficient = −0.09, P = 0.5) as shown in Fig. 3. None of the patients had evidence of pathogenic bacteria or C. difficile infection in stools.
Figure 1.
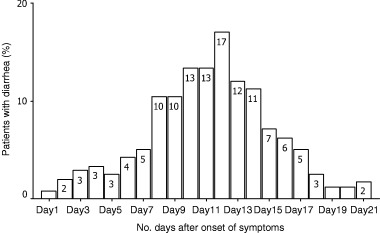
Percentage of patients with diarrhea after onset of symptoms.
Figure 2.
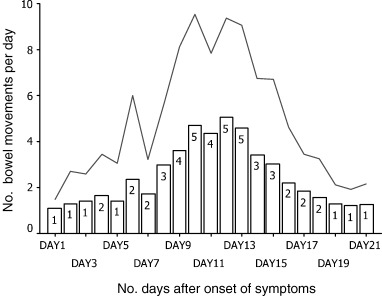
Mean number of bowel movements per day in the diarrhea group (+1 SD). (––) M + 1 SD; (□) mean.
Figure 3.
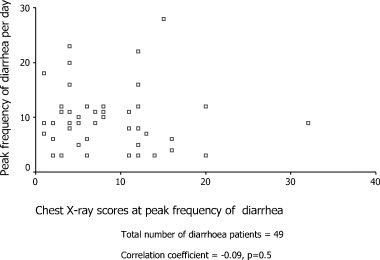
Correlation of peak frequency of diarrhea with chest X‐ray scores. n = 49; correlation coefficient = −0.09; P = 0.5.
Among the 210 patients who were still alive, 144 patients (69%) had attended follow up 3 weeks and 3 months after discharge at Princess Margaret Hospital. There were eight patients who complained of having diarrhea during follow up. The male : female ratio was 1 and the mean age was 33.2 years. Four belonged to the diarrhea group (4/38, 10.5%) and the other four belonged to the non‐diarrhea group (4/106, 3.8%). The odds ratio for having diarrhea during follow up was 3 (95% confidence interval: 0.7–12.6) for the diarrhea group.
DISCUSSION
Although SARS was mainly a respiratory problem, diarrhea was commonly reported in several series 5 , 7 , 13 with a prevalence of 38.4–73% during the hospital stay. The present cohort was based on different criteria, namely watery diarrhea at least 3 times a day for at least 3 days. The prevalence was thus lower than that by Choi et al. 13 The reason for our selection was that short‐lasting diarrhea, although very common, was of uncertain clinical importance. In two papers, diarrhea was reported as the presenting symptom in 5.8% 7 and 6% 2 of patients, respectively. Diarrhea was also noted in 3% of patients on the first day of admission in the present series, based on hospital inpatient records. We also came across several patients who had high fever and frequent watery diarrhea on presentation. They had minimal cough and CXR was completely normal. They were suspected to have SARS because of positive history of contact with other SARS patients. Small consolidation was noted only in high‐resolution CT thorax. A high index of suspicion was needed in these patients.
Peiris et al. described the clinical course of 75 patients in United Christian Hospital in Hong Kong and reported that 73% had watery diarrhea 7.5 ± 2.3 days after the onset of symptoms. 5 The mean maximum frequency of bowel movements was 6.3 ± 3.5 times daily and the mean duration of diarrhea was 3.9 ± 2.3 days. All patients improved by day 13 in that study. The patients with diarrhea in the present cohort also followed a similar pattern as shown in Fig. 2. We noted that female patients and residents of Amoy Gardens Estate were associated with diarrhea, and loglinear analysis showed that there was no significant interaction between these two factors. Several hypotheses might explain these findings. Different genomes of the coronavirus from different sources might lead to different clinical manifestations of the viral infection. Host factors such as sex might also account for the difference. More studies on genotypes of coronavirus and interaction with host factors are needed to answer these questions.
In the Leung et al. study, diarrhea was significantly associated with ventilatory support but not with the use of supplementary oxygen or mortality. 7 In the Peiris et al. study diarrhea was, however, not identified as a risk factor for the development of acute respiratory distress syndrome (ARDS). 5 The association of diarrhea with ventilatory care was not observed in the present study and the diarrhea patients in the present cohort had a significantly better prognosis. Male sex was identified as a poor prognostic factor in two studies by univariate analysis with P = 0.04 5 and 0.06. 13 In the present study, the mortality was 20% for male patients and 8.9% for female patients with P = 0.02. The high female : male ratio of 6:1 in our cohort of diarrhea patients contributed to the better prognosis. We also expected that the CXR scores might be very high during the peak diarrhea period if diarrhea was associated with progression of pneumonia but we failed to find an association as shown in Fig. 3. The Spearman's correlation coefficient was only −0.09 (P = 0.5). Only three out of the 49 patients had CXR scores >18. This implied that pneumonia was not severe when diarrhea worsened. The exact interaction between various host factors and the SARS coronavirus, however, remains to be sorted out by future studies.
It was known that the albumin level and potassium level dropped in patients with acute gastroenteritis related to Salmonella and Shigella, 14 , 15 partly as a result of loss from the gastrointestinal tract. In the present study, patients with diarrhea had a mean drop of serum albumin of 9.9 ± 4.8 g/L but patients without diarrhea also a similar degree of drop, of 9.5 ± 5.3 g/L. The serum potassium level also dropped significantly in both groups of patients and the fall was significantly bigger in the diarrhea group. Hypokalaemia was partly related to the use of high‐dose steroids, 16 and prompt replacement of potassium was mandatory in these patients to prevent life‐threatening cardiac arrhythmia.
It had been reported that 1 year after Salmonella gastroenteritis, approximately 30% of patients went on to develop symptoms typical of irritable bowel syndrome. 17 The patients who developed symptoms of irritable bowel syndrome were predominantly female and had experienced a longer bout of gastroenteritis. 18 In the present cohort, patients with diarrhea during hospital stay had a higher chance of having episodes of diarrhea at 3 months after follow up but a difference in gender was not observed.
CONCLUSIONS
A total of 20.4% of SARS patients had complications of diarrhea after hospital admission. Both female sex and being a resident of Amoy Gardens Estate were significantly associated with diarrhea. Patients with diarrhea had a better prognosis.
REFERENCES
- 1. World Health Organization. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS). Weekly Epidemiol. Rec. 2004; 79: 1–12. http://www.who.int/wer [Google Scholar]
- 2. Booth CM, Matukas LM, Tomlinson GA et al. Clinical features and short term outcomes of 144 patients with SARS in the Greater Toronto area. JAMA 2003; 289: 2801–9. [DOI] [PubMed] [Google Scholar]
- 3. Lee N, Hui D, Wu A et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003; 348: 1986–94. [DOI] [PubMed] [Google Scholar]
- 4. Hsu LY, Lee CC, Green JA et al. Severe acute respiratory syndrome (SARS) in Singapore; clinical features of index patient and initial contacts. Emerg. Infect. Dis. 2003; 9: 713–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peiris JSM, Chu CM, Cheng VCC et al. Prospective study of the clinical progression and viral load of coronavirus pneumonia in a community outbreak. Lancet 2003; 361: 1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong N, Ding Y, Mao Y et al. Chinese Medical Association, China Association of Chinese Medicine. Consensus for the management of severe acute respiratory syndrome. Chin. Med. J. 2003; 116: 1603–35. [PubMed] [Google Scholar]
- 7. Leung WK, To KF, Chan PKS et al. Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology 2003; 125: 1011–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Department of Health, HK Government. Outbreak of severe acute respiratory syndrome (SARS) at Amoy Gardens, Kowloon Bay, Hong Kong. Main findings of the investigation. Health Welfare & Food Bureau SARS Bulletin 18 April 2003;. 1–7. http://www.info.gov.hk/dh/diseases/ap/eng/bulletin0418.htm
- 9. He L, Ding YQ, Che XT et al. Expression of the monoclonal antibody against nucleocapsid antigen of SARS‐asociated coronavirus in autopsy tissues from SARS patients. Di. Yi. Jun Yi. Da. Xue. Xue. Bao 2003; 23: 1128–30. [PubMed] [Google Scholar]
- 10. Peiris JSM, Lai ST, Poon LLM et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003; 362: 1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsang OT‐Y, Chau T‐N, Choi K‐W et al. Coronavirus‐positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg. Infect. Dis. 2003; 9: 1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. So LK, Lau AC, Yam LY et al. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet 2003; 361: 1615–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi KW, Chau TN, Tsang O et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann. Intern. Med. 2003; 139: 715–23. [DOI] [PubMed] [Google Scholar]
- 14. Fontana M, Zuin G, Galli L, Paccagnini S, Villa M, Loguercio L. Fecal alpha‐1‐antitrypsin excretion in acute diarrhea: relationship with causative pathogens. Helv. Paediatr. Acta 1988; 43: 211–18. [PubMed] [Google Scholar]
- 15. Bennish MI, Salem MA, Wahed MA. Enteric protein loss during shigellosis. Am. J. Gastroenterol. 1993; 88: 53–7. [PubMed] [Google Scholar]
- 16. Howlett TA, Drury PL. Endocrine disease In: Kumar P, Clark M, eds. Clinical Medicine, 5th edn UK: WB Saunders, 2002; 1000–68. [Google Scholar]
- 17. McKendrick MW, Read NW. Irritable bowel syndrome. Post Salmonella infection. J. Infect. 1994; 29: 1–3. [DOI] [PubMed] [Google Scholar]
- 18. Gwee KA, Graham JC, McKendrick MW et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 1996; 347: 150–3. [DOI] [PubMed] [Google Scholar]


