Abstract
Objectives
Recombinant feline interferon‐ω therapy is an immunomodulator currently used in the treatment of different retroviral diseases including feline immune deficiency virus and feline leukaemia virus. Although its mechanism of action remains unclear, this drug appears to potentiate the innate response. Acute phase proteins are one of the key components of innate immunity and studies describing their use as a monitoring tool for the immune system in animals undergoing interferon‐ω therapy are lacking. This study aimed to determine whether interferon‐ω therapy influences acute phase protein concentrations namely serum amyloid‐A, α‐1‐glycoprotein and C‐reactive protein.
Methods
A single‐arm study was performed using 16 cats, living in an animal shelter, naturally infected with retroviruses and subjected to the interferon‐ω therapy licensed protocol. Samples were collected before (D0), during (D10 and D30) and after therapy (D65). Serum amyloid‐A and C‐reactive protein were measured by specific enzyme‐linked immunosorbent assay kits and α‐1‐glycoprotein by single radial immunodiffusion.
Results
All the acute phase proteins significantly increased in cats undergoing interferon‐ω therapy (D0/D65: P<0·05)
Clinical Significance
Acute phase proteins appear to be reasonable predictors of innate‐immune stimulation and may be useful in the individual monitoring of naturally retroviral infected cats undergoing interferon‐ω therapy.
INTRODUCTION
The acute phase response (APR) is one of the main reactions of the innate host defence system (Baumann & Gauldie 1994, Paltrinieri 2008). It refers to a non‐specific and complex phenomenon that occurs in the early stages of inflammation, preceding the development of the acquired immune response (Baumann & Gauldie 1994, Ceron et al. 2005). The APR is a consequence of the production and release of several cytokines, of which interleukin (IL)‐1, IL‐6 and tumour necrosis factor (TNF)‐α are the most relevant (Martínez‐Subiela et al. 2001, Paltrinieri 2008). These mediators induce various changes in the body including fever, leucocytosis and a modulation of protein synthesis by hepatocytes (Baumann & Gauldie 1994, Ceciliani et al. 2002).
Positive acute phase proteins (APP), which increase during inflammation, are believed to act as immunomodulators, ‐contributing in different ways to reinforce the body's innate defences during inflammation (Petersen et al. 2004, Eckersall & Bell 2010). The most relevant and well described positive APPs in cats are α‐1‐glycoprotein (AGP) and serum amyloid‐A (SAA) (Petersen et al. 2004, Ceron et al. 2005, Paltrinieri 2008, Eckersall & Bell 2010).
AGP is believed to act as an immunomodulator and anti‐inflammatory protein because it down‐regulates the neutrophilic response secondary to inflammation, stimulates the production of IL‐1R antagonists by macrophages, reduces platelet aggregation and lymphoid proliferation and modulates the production of anti‐inflammatory cytokines by the circulating lymphocytes (Hochepied et al. 2003). Its increase in different cat infectious diseases has been described (Duthie et al. 1997, Paltrinieri et al. 2004, Paltrinieri et al. 2007a,2007b). In retroviral infected cats, while a previous study reported its increase (Duthie et al. 1997), a more recent one found that FIV positive cats have lower concentrations of AGP than healthy ones (Korman et al. 2012).
SAA is a small protein that appears to be the precursor of amyloid protein A, a major protein of α‐amyloid which is potentially involved in a variety of chronic inflammatory diseases (Uhlar & Whitehead 1999). Among its major functions, SAA acts as a scavenger of oxidised metabolites, protecting tissues from excessive damage induced by inflammation (He et al. 2006). As with AGP, its measurement has been reported in different feline diseases (Kajikawa et al. 1999, Sasaki et al. 2001, 2003, Giordano et al. 2004, Tamamoto et al. 2008, 2009).
C‐reactive protein (CRP) was the first APP described and it is considered to be a major protein in different species such as humans and dogs (Ceron et al. 2005, Schultz & Arnold 1990). Among the major functions in the immune system, CRP is involved in the activation of the classical complement pathway, the enhancement of phagocytosis or even the modulation of polymorphonuclear cells (Schultz & Arnold 1990). Because CRP does not appear to be involved in the feline acute phase reaction, it has not been very well studied or documented in the cat (Ceron et al. 2005). In human medicine, several studies describe its increase in human immunodeficiency virus (HIV) positive patients (Jahoor et al. 1999, Treitinger et al. 2001), even after immunomodulation therapy with exogenous IL‐2 (Barbai et al. 2010). Despite the similarity between HIV and FIV (Hosie et al. 2009, Hartmann 2011), the CRP behaviour in FIV positive cats undergoing immunomodulating therapy remains unknown.
Recombinant feline interferon‐ω (rFeIFN‐ω) therapy (Virbagen, Virbac) is an immunomodulating drug that plays an important role in the therapeutic approach for various feline diseases including cat retrovirus infections (Collado et al. 2006). There are only a few studies describing the clinical improvement of retroviral infected cats with rFeIFN‐ω therapy (de Mari et al. 2004, Domenech et al. 2011, Gil et al. 2013) and little is known about the immunological bases that support these findings. Because of reported clinical improvement, increased survival time and reduction of concurrent viral excretion, rFeIFN‐ω appears to be involved in the innate response (de Mari et al. 2004, Domenech et al. 2011, Gil et al. 2013).
Studies that describe the use of APPs as a clinical monitoring tool for the immune system in animals undergoing ‐IFN‐therapy are scarce. Therefore, the main objective of this study was to determine whether rFeIFN‐ω therapy influences APPs (namely SAA, AGP and CRP) in naturally retroviral infected cats and whether these parameters may be good predictors of innate‐immune stimulation.
MATERIALS AND METHODS
Animals
Sixteen naturally retroviral infected cats living in an animal shelter (União Zoófila, Lisbon) were selected for the study. All the cats were accustomed to the shelter environment having lived there for at least 8 weeks before the start of the protocol. In accordance with previous studies (de Mari et al. 2004; Gil et al. 2013), the inclusion criteria included the following: (1) cats of any age, breed or sex (heterogeneous population), (2) cats that showed at least one clinical sign potentially related to retroviral infections, (3) cats that had previously shown a positive rapid immuno‐migration FIV and/or FeLV test result. Exclusion criteria were (1) cats with any type of malignancy/neoplasia (such as lymphoma or lymphoid leukaemia), (2) cats having received immunomodulating drugs (such as corticosteroids) during the 4 weeks before the study, (3) cats having received antibiotics or non‐steroidal anti‐inflammatory drugs during the 2 weeks before the study and (4) cats that did not complete the therapeutic protocol. Initially, all cats were retested to confirm their FIV/FeLV infections by using commercially available enzyme‐linked immunoabsorbent assays (ELISA) (ViraCHEK/FIV and ViraCHEK/FeLV, Synbiotics).
Animals were housed in two different catteries, according to their FIV or FeLV status. Animals concurrently infected with FIV and FeLV were housed in the FeLV cattery. All the cats in each cattery were treated and during the study, no incoming animals were allowed. For the purposes of analysis, cats were divided into three different groups according to their retroviral status: FIV positive cats (n=7), FeLV positive cats (n=6) and co‐infected animals (n=3).
The cats were living in good conditions, in agreement with current ethical and European welfare standards. All the procedures involving the manipulation of these animals were consented and approved not only by the Local Committee for Ethics and Animal Welfare (CEBEA‐Faculty of Veterinary Medicine/Technical University of Lisbon) but also by the clinical director of the animal shelter.
Treatment protocol
On the basis of the assumptions derived from two previously published double arm trials with rFeIFN‐ω (de Mari et al. 2004, Domenech et al. 2011), a single‐arm study was performed. In this study model, a time point before therapy is considered to be the animal's own control. In this study, assessments before therapy were designated as D0 and considered representative of the stage of each animal before treatment.
All the animals were treated with rFeIFN‐ω, according to the licensed protocol (three cycles of injections at Day (D) 0, D14 and D60. Each treatment cycle consists of five subcutaneous (sc) injections: 1 µ/kg once a day for 5 days). Vials of rFeIFN‐ω (‐Virbagen Omega; Virbac) were reconstituted with the accompanying saline diluent according to the manufacturer's recommendations immediately before each treatment.
Treatment was administered by two veterinary clinicians from the research team of the project where this study was conducted.
Supportive treatment
Despite the exclusion criteria applied, some animals needed supportive treatment during therapy. Consequently, potentiated amoxycillin, hepatic protectants (ursodeoxycholic acid, sylimarin or S‐adenylmethionine) and/or fluid therapy were allowed. Although any antibiotic may have direct immunomodulator effects, potentiated amoxycillin was allowed taking into account its empirical use and frequent administration in retrovirus infected cats with suspected bacterial infections. Antibiotics (other than potentiated amoxycillin), corticosteroids and non‐steroidal anti‐inflammatory drugs were not permitted to avoid any possible immunomodulation effects.
Blood collection and analysis
Blood samples were collected by venipuncture of the jugular vein at four specific time points namely: before (D0), during (D10 and D30) and after therapy (D65).
To allow a better evaluation and simpler blood sample collections, cats were subjected to mild sedation with 0·2 to 0·5 mg/kg butorphanol solution sc (Dolorex, Intervet Portugal).
Serum samples were collected after clotting of the sample had occurred by centrifugation (5000g, 10 minutes), and were subsequently frozen at −20°C until analysed.
SAA and CRP were measured by specific ELISA kits (Phase SAA Multispecies/Tridelta and Cat CRP ELISA/Kamiya Biomedical Company, respectively). AGP was determined by single radial immunodiffusion (Feline AGP, SRID, Tridelta).
All the measurements were performed according the manufacturer's instructions.
Statistical analysis
Statistical evaluation was performed using R Statistical Software. Because of the small sample size, nonparametric statistical tests were used. Kruskall–Wallis Tests were applied to assess differences among groups at each time point. When differences were observed, a pairwise comparison was applied. To assess group variations during time, a Friedman Rank Sum Test was used. The nonparametric tests applied took into account not only the magnitude but also the predominant “sign” (positive or negative) of the effect. Significance was set at P<0·05.
RESULTS
All 16 cats completed the licensed therapeutic protocol. No statistical differences between groups were observed apart from CRP on D65 and AGP on D30.
For SAA, groups were similar at all time points and all the cats behaved similarly. Therefore, for analysis over time, the groups were all considered together. Global results are presented in Fig 1. A significant increase of SAA concentration was observed (P=0·0005; increased SAA in 15 animals and decreased SAA in 1). On D65, mean SAA concentration was 1·6 times higher than D0. All values remained below the upper limit of the reference interval (RI) of 10 µg/mL.
Figure 1.
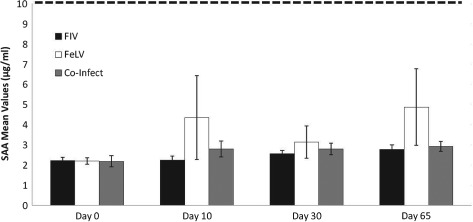
Mean ±standard error (se) of serum concentrations of serum amyloid‐A (SAA) in naturally retroviral infected cats, before (D0) during (D10 and D30) and after (D65) rFeIFNω therapy. The reference interval is less than 10 µg/mL. The observed increase was statistically significant (Friedman test D0 versus D65 P=0·0005)
For AGP, the three groups were also similar at all time points, apart from D30 (P=0·016), where co‐infected and FeLV cats showed higher mean values than FIV cats (FIV versus ‐co‐infected: P=0·029; FIV versus FeLV: P=0·067; FeLV versus ‐co‐infected: P=0·12) (Fig 2). There was a significant overall increase (1·7 times) of AGP concentrations (P=0·012; AGP increased in 13 cats and decreased in 3) from D0 to D65. Basal values were within the RI (260 to 580 µg/mL). During therapy, mean values of FeLV and co‐infected cats exceeded the upper limit of the RI while for FIV, all the results remained within the RI.
Figure 2.
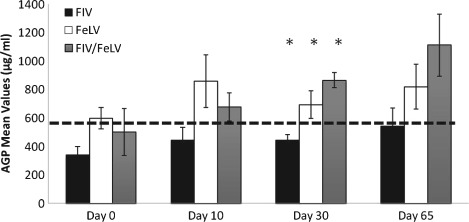
Mean ±standard error (se) serum concentrations of α‐glycoprotein‐1 (AGP) in naturally retroviral infected cats, before (D0) during (D10 and D30) and after (D65) rFeIFNω therapy. The horizontal line represents the upper limit of the reference interval (260 to 580 µg/mL). The observed increase was statistically significant (Friedman test D0 versus D65 P=0·012). Groups are statistically similar except at D30 (*). Kruskall–Wallis P=0·016; Pairwise comparison: FIV versus co‐infected: P=0·029; FIV versus FeLV: P=0·067; FeLV versus co‐infected: P=0·12
For CRP, the three groups were statistically indistinguishable during the study apart from D65 (P=0·019), where there was a statistically significant difference. At this time point, co‐infected and FeLV cats had higher CRP concentrations than FIV cats (FIV versus co‐infected: P=0·009; FIV versus FeLV: P=0·052; FeLV versus co‐infected: P=0·36). Results of CRP values are presented in Fig 3. From D0 to D65 there was an increase in CRP concentrations in all the cats (P=0·0001; increased CRP in 16 cats). This increase was approximately 1·8 times the baseline value. Values on D30 and D65 were above the upper limit of the RI (38 to 186 µg/mL) for FIV, FeLV and co‐infected cats,
Figure 3.
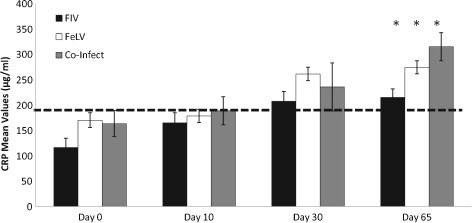
Mean ±standard error (se) of serum concentrations of c‐reactive protein (CRP) in naturally retroviral infected cats, before (D0) during (D10 and D30) and after (D65) rFeIFNω therapy. The horizontal line represents the upper limit of the reference interval (38 to 186 µg/mL). The observed increase was statistically significant (Friedman test D0 versus D65 P=0·0001). Groups are similar except at D65 (*). Kruskall–Wallis P=0·019; Pairwise comparison: FIV versus co‐infected: P=0·009; FIV versus FeLV: P=0·052; FeLV versus co‐infected: P=0·36
Only one cat of the FeLV group (cat 9) received supportive therapy at D65, namely intravenous fluids, because of suspected otitis/vestibular syndrome and mild‐dehydration. This fact was considered as a co‐morbidity factor and was included in the clinical‐evaluation and considered in the clinical score. This animal also received potentiated amoxycilin but after the end of the study. The APP profile of this cat was similar to the wider group.
Data related to clinical signs and concurrent viral loads (namely calicivirus, herpesvirus, coronavirus and parvovirus) in these cats over the same time period have been previously reported (Gil et al. 2013). Herpesvirus and coronavirus viral load were assessed by real‐time polymerase chain reaction (PCR), while calicivirus and parvovirus status were determined by conventional‐PCR (Gil et al. 2013). These results are summarised in Table 1, which also includes the detailed APP results. Clinical signs improved and concurrent viral excretion decreased in the majority of the cats. Only two cats increased coronovirus excretion but they remained asymptomatic. All the cats that were positive for calicivirus and a single cat that was excreting parvovirus became negative at the end of the study meaning an overall significant decrease of concurrent viral infections. Simultaneously, the majority of cats had increased APP concentrations.
Table 1.
Individual variation of clinical scores, concurrent viral excretion and acute phase proteins in FIV, FeLV and FIV/FeLV cats treated with rFeIFN‐ω
| Cats | FIV/FeLV status | Clinical scores (D0 versus D65) | Concurrent viral excretion (individual tendency D0 versus D65) | Acute phase proteins (individual tendency D0 versus D65) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Calicivirus | Herpesvirus | Coronavirus | Parvovirus | SAA | AGP | CRP | |||
| 1 | FIV | ↓ | BN | ↓ | ↓ | − | ↑ | ↑ | ↑ |
| 2 | FIV | → | − | ↓ | − | − | ↑ | ↑ | ↑ |
| 3 | FIV | ↓ | − | ↓ | ↓ | − | ↑ | ↑ | ↑ |
| 4 | FIV | ↓ | BN | * | ↑ | − | ↑ | ↑ | ↑ |
| 5 | FIV | ↓ | − | ↓ | − | − | ↑ | ↑ | ↑ |
| 6 | FIV | ↓ | BN | − | ↓ | − | ↓ | ↑ | ↑ |
| 7 | FIV | → | BN | − | ↓ | − | ↑ | ↓ | ↑ |
| 8 | FeLV | → | BN | ↓ | ↓ | − | ↑ | ↑ | ↑ |
| 9 | FeLV | → | BN | ↓ | * | − | ↑ | ↑ | ↑ |
| 10 | FeLV | ↓ | BN | * | ↑ | − | ↑ | ↑ | ↑ |
| 11 | FeLV | ↓ | BN | * | * | − | ↑ | ↑ | ↑ |
| 12 | FeLV | → | BN | ↓ | ↓ | − | ↑ | ↓ | ↑ |
| 13 | FeLV | ↓ | BN | ↓ | ↓ | − | ↑ | ↓ | ↑ |
| 14 | FIV/FeLV | ↓ | BN | − | ↓ | − | ↑ | ↑ | ↑ |
| 15 | FIV/FeLV | → | BN | ↓ | * | − | ↑ | ↑ | ↑ |
| 16 | FIV/FeLV | ↓ | BN | ↓ | ↓ | BN | ↑ | ↑ | ↑ |
Comparing D0 (before) to D65 (end of the therapy): (↓) refers to a decrease of the parameter; () refers to an increase of the parameter; (→) refers to a stable parameter; (−) refers to negative samples; (*) refers to intermittent excretion during therapy, despite a negative result at D0 and D65. (BN) refers to animals that were positive at D0 and became negative during therapy. Regarding Clinical Scores, a reduction of the parameter refers to a clinical improvement. FIV feline immunodeficiency virus, FeLV feline leukemia virus, rFeIFN‐ω Recombinant feline interferon‐ω
DISCUSSION
This study describes the changes observed in serum concentrations of three different APPs in 16 cats naturally infected with retroviruses and undergoing rFeIFN‐ω therapy. A single‐arm study was considered and performed as this is likely to be the most reliable approach when studying naturally infected ‐animals, for which time of infection and sub‐types of the virus are uncertain. In this study, rFeIFN‐ω was used according to a licensed protocol, approved for veterinary use in feline retrovirus infections. Although only a few studies have been published, clinical improvement of cats undergoing rFeIFNω therapy has been well documented in double arm trials (de Mari et al. 2004, Domenech et al. 2011). This study provides further information regarding parameters complementary to the main clinical signs and concurrent viral excretion data previously published (Gil et al. 2013), clarifying the pathophysiological phenomena behind immune stimulation.
As several authors have suggested (Ceron et al. 2005) the concentration of APPs may be within the RI even in animals with disease. As such measurement of these parameters for monitoring inflammation stimulus is not without difficulty. Using each individual animal as its own reference was considered to be a practical and reasonable approach to bypass this problem (Ceron et al. 2005). Therefore, as in previous human studies that evaluated APPs (Wasunna et al. 1995, Barbai et al. 2010), animals acted as their own controls and the time point before therapy (D0) was considered the baseline value for each cat. Subsequently, it was possible to study the individual trends during therapy.
Although there is no consensus on upper and lower limit values for the measured APPs, the suggested ranges for each kit were used. Particularly for AGP and CRP, cats had higher mean values than the upper limit at some time points. However, previous studies describe higher values in healthy cats (Kajikawa et al. 1999, Selting et al. 2000), which if adapted would expand the recommended RI. Only the comparison between individual values before (D0), during and after therapy (D65) with particular relevance to differences between D0 and D65 were considered relevant for the study, whether or not they were above the RI.
For concentrations of SAA and AGP, the three groups studied (FIV, FeLV and co‐infected animals) behaved similarly, demonstrating increased values during rFeIFN‐ω therapy. Groups were not statistically different with the exception of AGP on D30. This particular variation is secondary to the fact that the FIV group demonstrated lower AGP values compared to FeLV and co‐infected cats. Despite the low number of animals a possible explanation may be sub‐clinical diseases leading to a particular alteration at this time point. However, considering that the statistical difference was only evident at this particular time point, this finding was not considered biologically significant. According to previous studies which reported that AGP and SAA are good predictors of immunomodulation (Kajikawa et al. 1999, Hochepied et al. 2003), these results support the hypothesis that rFeIFN‐ω therapy may modulate pro‐inflammatory innate mechanisms. These APPs may be a useful monitoring tool for demonstrating modulation of the innate‐immune response.
For CRP, with the exception of D65 the three groups of cats were also similar throughout the study. The particular variation at D65 was due to the fact that FeLV and co‐infected cats had higher values than FIV cats. As for AGP at D30, this may have been due to various causes such as sub‐clinical uncontrolled infections in the shelter or even a natural progression of retroviral disease. From the beginning (D0) until the end of the treatment (D65) a significant increase of CRP concentrations was noted. Although CRP has not been considered as a useful biomarker of inflammation in cats, this study shows that this APP behaves similarly to SAA and AGP. Therefore, in contrast with previous studies, CRP may also have value as a biomarker of feline inflammation, being increased in a similar magnitude to the other APPs measured.
A recent study described the evolution of different APPs, namely SAA and AGP, in FIV and non‐FIV cats following Mycoplasma haemofelis and Candidatus Mycoplasma ‐Haemominutum infections (Korman et al. 2012). This study revealed that ‐pre‐existing FIV infection did not significantly affect the APR to mycoplasma. Despite remaining within the RI, FIV positive cats demonstrated lower concentrations of AGP than non‐FIV cats. This contrasted with previous studies suggesting that AGP was increased in FIV cats (Duthie et al. 1997). Taking all these into account, it seems reasonable to consider that FIV cats are able to develop an efficient acute phase, which may lead to a rise of APPs. Regarding the more conventional assessments of the immune system in retroviral infected cats, a previous study concluded that the hyperglobulinaemia commonly observed in FIV cats may be due to hyperactivation of B‐cells which is more evident when the disease progresses (Gleich & Hartmann 2009). In contrast, and due to a progressive defect of helper T‐cells, FeLV cats do not usually present with hyperglobulinaemia being more prone to have severe cytopenias (Gleich & Hartmann 2009). According to this study, the increase of APPs was similar in FIV and FeLV, suggesting that the innate‐immune stimulation must have the same basis in these two groups. No studies were performed to correlate γ‐globulins with APPs in retroviral infected cats, and whether rFeIFN‐ω interferes with serum protein electrophoresis profiles remains unclear.
It has been previously demonstrated that rFeIFNω results in an overall clinical improvement not only of FIV but also FeLV and co‐infected cats (Gil et al. 2013). Clinical signs, evaluated by a score‐scale, decreased in the three groups (FIV, FeLV and co‐infected) meaning an overall improvement of symptomatic cats. In total, 10 of 16 improved their clinical signs while 6 of 16 remained stable. Furthermore, a significant decrease in excretion of other viruses was also observed concomitantly (Gil et al. 2013). Correlating the APP profile with these previous results, it was observed that APP concentrations increased in cats with concurrently improved clinical signs and reduced viral excretion. This finding was consistent even in cats with low clinical scores and in those where scores remained stable with therapy. Recognising that APPs may be increased in different situations such as chronic infections and severe inflammation (Ceron et al. 2005, Paltrinieri 2008), this concurrent clinical improvement and the decrease in the loads of other viruses reinforce and sustain the hypothesis that rFeIFN‐ω therapy potentiates the immune response and may involve a beneficial APP increase in treated animals.
In conclusion, all the measured APPs significantly increased, revealing a potential innate‐immune response in naturally infected cats during rFeIFN‐ω therapy. In humans, it has been described that the administration of IL‐2 in HIV patients induced an increase of CRP, which was positively correlated to an increase of CD4+ cell count (Barbai et al. 2010). These findings reported a possible involvement of CRP in the IL2‐induced immune stimulation (Barbai et al. 2010). Conversely, it was previously described that CD4/CD8 ratios do not change in cats under rFeIFN‐ω (Domenech et al. 2011). Therefore, the true mechanism by which rFeIFN‐ω induces an increase in APP remains unclear. To further characterise the immune response during rFeIFN‐ω therapy, further studies are required to correlate these findings with other parameters such as the cytokine profile. For now, the results of this study suggest that APPs may be promising predictors of innate‐immune stimulation in naturally retroviral infected cats undergoing rFeIFN‐ω therapy. In the future, they could be combined together in an APP‐panel that may help with the individual monitoring and assessment of rFeIFN‐ω therapy.
Acknowledgements
This work was supported by the project CIISA 50; Rodolfo Leal is a PhD fellow (FCT SFRH/BD/62917/2009, Portugal); Solange Gil is a research assistant under Programa Ciência 2007 (FCT‐Portugal). Authors would like to thank Joana Cravo, Inês Siborro, Clara Cartaxeiro for their contribution with laboratory work.
Parts of this work were presented in abstract on the 21st European College of Veterinary Internal Medicine (ECVIM) ‐Congress, September 2011, on the 11th International Feline Retrovirus Research Symposium, August 2012 and on the 22nd ECVIM Congress, September 2012.
Conflict of interest
D. McGahie is an employee of Virbac, Carros, France. His contribution for this work was mainly as a Consultant.
References
- Barbai, V. H. , Ujhelyi, E. , Szlavik, J. , et al (2010) Changes in the levels of some acute‐phase proteins in human immunodeficiency virus‐1 infected patients, following interleukin‐2 treatment. Clinical and Experimental Immunology 161, 134‐141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, H. & Gauldie, J. (1994) The acute phase response. Immunology Today 15, 74‐80 [DOI] [PubMed] [Google Scholar]
- Ceciliani, F. , Giordano, A. & Spagnolo, V. (2002) The systemic reaction during inflammation: the acute‐phase proteins. Protein and Peptide Letters 9, 211‐223 [DOI] [PubMed] [Google Scholar]
- Ceron, J. J. , Eckersall, P. D. & Martynez‐Subiela, S. (2005) Acute phase proteins in dogs and cats: current knowledge and future perspectives. Veterinary Clinical Pathology 34, 85‐99 [DOI] [PubMed] [Google Scholar]
- Collado, V. M. , Doménech, A. , Gómez‐Lucía, E. , et al (2006) Usos de interferón en la clínica de pequeños animales. Pequeños Animales 63, 68‐75 [Google Scholar]
- de Mari, K. , Maynard, L. , Sanquer, A. , et al (2004) Therapeutic effects of recombinant feline interferon‐omega on feline leukemia virus (FeLV)‐infected and FeLV/feline immunodeficiency virus (FIV)‐coinfected symptomatic cats. Journal of Veterinary Internal Medicine 18, 477‐482 [DOI] [PubMed] [Google Scholar]
- Domenech, A. , Miro, G. , Collado, V. M. , et al (2011) Use of recombinant interferon omega in feline retrovirosis: from theory to practice. Veterinary Immunology and Immunopathology 143, 301‐306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie, S. , Eckersall, P. D. , Addie, D. D. , et al (1997) Value of alpha 1‐acid glycoprotein in the diagnosis of feline infectious peritonitis. Veterinary Record 141, 299‐303 [DOI] [PubMed] [Google Scholar]
- Eckersall, P. D. & Bell, R. (2010) Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Veterinary Journal 185, 23‐27 [DOI] [PubMed] [Google Scholar]
- Gleich, S. & Hartmann, K. (2009) Hematology and serum biochemistry of feline immunodeficiency virus‐infected and feline leukemia virus‐infected cats. Journal of Veterinary Internal Medicine 23, 552‐558 [DOI] [PubMed] [Google Scholar]
- Gil, S. , Leal, R. O. , Duarte, A. , et al (2013) Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Research in Veterinary Science 94, 753‐763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano, A. , Spagnolo, V. , Colombo, A. , et al (2004) Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Veterinary Journal 167, 38‐44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, K. (2011) Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Veterinary Immunology and Immunopathology 143, 190‐201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, R. , Shepard, L. W. , Chen, J. , et al (2006) Serum amyloid A is an endogenous ligand that differentially induces IL‐12 and IL‐23. Journal of Immunology 177, 4072‐4079 [DOI] [PubMed] [Google Scholar]
- Hochepied, T. , Berger, F. G. , Baumann, H. , et al (2003) Alpha(1)‐acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine and Growth Factor Reviews 14, 25‐34 [DOI] [PubMed] [Google Scholar]
- Hosie, M. J. , Addie, D. , Belak, S. , et al (2009) Feline immunodeficiency. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 11, 575‐584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor, F. , Gazzard, B. , Phillips, G. , et al. (1999) The acute‐phase protein response to human immunodeficiency virus infection in human subjects. American Journal of Physiology 276, E1092‐E1098 [DOI] [PubMed] [Google Scholar]
- Kajikawa, T. , Furuta, A. , Onishi, T. , et al (1999) Changes in concentrations of serum amyloid A protein, alpha 1‐acid glycoprotein, haptoglobin, and C‐reactive protein in feline sera due to induced inflammation and surgery. Veterinary Immunology and Immunopathology 68, 91‐98 [DOI] [PubMed] [Google Scholar]
- Korman, R. M. , Ceron, J. J. , Knowles, T. G. , et al (2012) Acute phase response to Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ infection in FIV‐infected and non‐FIV‐infected cats. Veterinary Journal 193, 433‐438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Subiela S, T. V. F., Parra Muñoz MD, Cerón JJM; (2001) Proteínas de fase aguda: conceptos básicos y principales aplicaciones clínicas en medicina veterinaria/Acute phase proteins: general concepts and main clinical applications in veterinary medicine. Anales de Veterinaria de Murcia 17, 97‐113 [Google Scholar]
- Paltrinieri, S. (2008) The feline acute phase reaction. Veterinary Journal 177, 26‐35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri, S. , Giordano, A. , Ceciliani, F. , et al (2004) Tissue distribution of a feline AGP related protein (fAGPrP) in cats with feline infectious peritonitis (FIP). Journal of Feline Medicine and Surgery 6, 99‐105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri, S. , Giordano, A. , Tranquillo, V. , et al (2007a) Critical assessment of the diagnostic value of feline alpha1‐acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. Journal of Veterinary Diagnostic Investigation 19, 266‐272 [DOI] [PubMed] [Google Scholar]
- Paltrinieri, S. , Metzger, C. , Battilani, M. , et al (2007b) Serum alpha1‐acid glycoprotein (AGP) concentration in non‐symptomatic cats with feline coronavirus (FCoV) infection. Journal of Feline Medicine and Surgery 9, 271‐277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, H. H. , Nielsen, J. P. & Heegaard, P. M. (2004) Application of acute phase protein measurements in veterinary clinical chemistry. Veterinary Research 35, 163‐187 [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Ma, Z. , Okazaki, K. , et al (2001) Characterization of monoclonal antibodies specific for feline serum amyloid (SAA) protein. Hybridoma 20, 103‐108 [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Ma, Z. , Khatlani, T. S. , et al (2003) Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. Journal of Veterinary Medical Science 65, 545‐548 [DOI] [PubMed] [Google Scholar]
- Schultz, D. R. & Arnold, P. I. (1990) Properties of four acute phase proteins: C‐reactive protein, serum amyloid a protein, α1‐acid glycoprotein, and fibrinogen. Seminars in Arthritis and Rheumatism 20, 129‐147 [DOI] [PubMed] [Google Scholar]
- Selting, K. A. , Ogilvie, G. K. , Lana, S. E. , et al (2000) Serum alhpa 1‐acid glycoprotein concentrations in healthy and tumor‐bearing cats. Journal of Veterinary Internal Medicine 14, 503‐506 [DOI] [PubMed] [Google Scholar]
- Tamamoto, T. , Ohno, K. , Ohmi, A. , et al (2008) Verification of measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. Journal of Veterinary Medical Science 70, 1247‐1252 [DOI] [PubMed] [Google Scholar]
- Tamamoto, T. , Ohno, K. , Ohmi, A. , et al (2009) Time‐course monitoring of serum amyloid A in a cat with pancreatitis. Veterinary Clinical Pathology 38, 83‐86 [DOI] [PubMed] [Google Scholar]
- Treitinger, A. , Spada, C. , da Silva, L. M. , et al (2001) Lipid and acute‐phase protein alterations in HIV‐1 infected patients in the early stages of infection: correlation with CD4+ lymphocytes. Brazilian Journal of Infectious Disease 5, 192‐199 [DOI] [PubMed] [Google Scholar]
- Uhlar, C. M. & Whitehead, A. S. (1999) Serum amyloid A, the major vertebrate acute‐phase reactant. European Journal of Biochemistry 265, 501‐523 [DOI] [PubMed] [Google Scholar]
- Wasunna, K. M. , Raynes, J. G. , Were, J. B. , et al (1995) Acute phase protein concentrations predict parasite clearance rate during therapy for visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine & Hygiene 89, 678‐681 [DOI] [PubMed] [Google Scholar]


