Abstract
Angiotensin converting enzyme (ACE) 2 is a carboxypeptidase that shares 42% amino acid homology with ACE. Little is known about the regulation or pattern of expression of ACE2 in the mouse lung, including its definitive cellular distribution or developmental changes. Based on Northern blot and RT‐PCR data, we report two distinct transcripts of ACE2 in the mouse lung and kidney and describe a 5′ exon 1a previously unidentified in the mouse. Western blots show multiple isoforms of ACE2, with predominance of a 75–80 kDa protein in the mouse lung versus a 120 kDa form in the mouse kidney. Immunohistochemistry localizes ACE2 protein to Clara cells, type II cells, and endothelium and smooth muscle of small and medium vessels in the mouse lung. ACE2 mRNA levels peak at embryonic day 18.5 in the mouse lung, and immunostaining demonstrates protein primarily in the bronchiolar epithelium at that developmental time point. In murine cell lines ACE2 is strongly expressed in the Clara cell line mtCC, as opposed to the low mRNA expression detected in E10 (type I‐like alveolar epithelial cell line), MLE‐15 (type II alveolar epithelial cell line), MFLM‐4 (fetal pulmonary vasculature cell line), and BUMPT‐7 (renal proximal tubule cell line). In summary, murine pulmonary ACE2 appears to be primarily epithelial, is developmentally regulated, and has two transcripts that include a previously undescribed exon. J. Cell. Biochem. 101:1278–1291, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: angiotensin converting enzyme 2 (ACE2), alveolar epithelium, Clara cell, mouse, lung, exon
Angiotensin converting enzyme (ACE) 2 was first identified in 2000 [Donoghue et al., 2000; Tipnis et al., 2000] as a zinc metalloprotease with 42% amino acid homology to ACE. The ACE2 gene is highly conserved in non‐vertebrates and mammals [Chou et al., 2006] and has 18 well‐characterized exons. In humans a 19th exon of ACE2 (exon 1a, National Institutes of Health GenBank accession No. AB193259) has recently been identified as well [Itoyama et al., 2005]. The murine and human ACE2 genes are both located on chromosome X and share 83% nucleotide identity [Komatsu et al., 2002]. There is potential for multiple splice variants of ACE2, as has been described in the homologous gene ACE that has distinct somatic and testicular isoforms [Soubrier et al., 1988]. Komatsu et al. 2002 have previously reported two transcripts of 2.8 and 2.0 kilobases (kb) of ACE2 (AB053181 and AB053182, respectively) produced by alternate splicing in the mouse lung and kidney. The GenBank includes two additional mRNA transcripts of murine ACE2 of 2,739 bp (BC026801) and 3,268 bp (NM_027286); the larger transcript differs primarily in the addition of untranslated 3′ sequence to the end of exon 18.
ACE2 is an 805 amino acid glycoprotein that exists as a type I integral membrane protein but can also be cleaved and secreted as the N‐terminal ectodomain [Donoghue et al., 2000]. In the published literature multiple molecular weights have been reported for ACE2 in the mouse, ranging from 75 kiloDaltons (kDa) [Gembardt et al., 2005], to 89 kDa [Ye et al., 2004; Wysocki et al., 2006], to 125 kDa [Imai et al., 2005]. Analyses of proteins produced by cell lines transduced with human ACE2 mRNAs indicate that the differences in molecular weight are attributable to variable glycosylation and cleavage events, with the 120 kDa form representing the membrane‐bound, fully glycosylated protein, the 105 and 95 kDa forms representing partial glycosylation of the shed N‐terminal ectodomain, and the 80 and 85 kDa forms representing the deglycosylated shed and membrane‐bound proteins, respectively [Lambert et al., 2005]. It is possible that other protein species exist as well that have yet to be identified.
Studies using quantitative RT‐PCR [Harmer et al., 2002], RNase protection assays [Gembardt et al., 2005], and Northern blots [Donoghue et al., 2000; Tipnis et al., 2000; Komatsu et al., 2002] have demonstrated moderate ACE2 expression in lungs of both humans [Harmer et al., 2002] and mice [Komatsu et al., 2002; Gembardt et al., 2005], with high levels of ACE2 in kidney, heart, testis, and small intestine in both species [Donoghue et al., 2000; Tipnis et al., 2000; Harmer et al., 2002; Gembardt et al., 2005]. In many of these tissues the primary cell types expressing ACE2 appear to be vascular endothelial cells [Donoghue et al., 2000], and therefore ACE2 has been assumed to be primarily an endothelial protein, much like ACE. In the human lung immunostaining has localized ACE2 to endothelial and smooth muscle cells of large and small blood vessels, as well as to types I and II alveolar epithelial cells and bronchial epithelial cells [Hamming et al., 2004; Ren et al., 2006]. In human bronchial epithelium in vivo ACE2 appears to reside in the apical plasma membrane [Ren et al., 2006], and it has been reported that ACE2 is apically distributed in ciliated cells of cultured human airway epithelium [Jia et al., 2005]. In the mouse lung Gembardt et al. 2005 have suggested that ACE2 protein is localized to alveolar macrophages and type II cells based on immunohistochemistry. In situ hybridization of the mouse lung shows that ACE2 mRNA is localized to vascular endothelial and airway epithelial cells [Imai et al., 2005]. It is not definitively known based on these studies which particular cell types express ACE2. ACE2 has clearly been demonstrated to act as the primary receptor for the Severe Acute Respiratory Syndrome (SARS) coronavirus [Li et al., 2003], the virus responsible for 8,096 cases in 29 countries during the 2002–2003 SARS outbreak [World Health Organization, 2004]. Therefore, one can infer the sites of ACE2 expression in the lung from studies localizing SARS coronavirus particles in infected animals. In mouse models of SARS coronavirus infection, in situ hybridization reveals viral RNA in the terminal bronchiolar epithelium [Glass et al., 2004]. In macaque models of SARS coronavirus infection, immunostaining shows extensive SARS coronavirus antigen in types I and II alveolar epithelial cells [Haagmans et al., 2004]. To date there have not been immunohistochemical or in situ hybridization studies that unequivocally identify which cell types express ACE2 in the mouse lung.
Acting as a carboxypeptidase with angiotensin II as its primary substrate [Vickers et al., 2002], ACE2 counterbalances the enzymatic effects of ACE by converting angiotensin II to angiotensin 1–7 [Donoghue et al., 2000]. Through degradation of pulmonary angiotensin II, ACE2 appears to protect against acute lung injury in murine models of acid aspiration [Imai et al., 2005], sepsis [Imai et al., 2005], and SARS coronavirus infection [Kuba et al., 2005]. Because acute lung injury is a major cause of morbidity and mortality among critically ill patients, with death rates of 25–40% [Bernard, 2005], and because SARS coronavirus infection itself has mortality of 10% and respiratory failure due to acute lung injury in up to 25% [World Health Organization, 2004], it is important to understand the pattern of expression and regulation of pulmonary ACE2, which may ultimately contribute to the development of potential therapies for acute lung injury or SARS coronavirus infection.
Little is definitively known about the function, regulation, or precise cellular distribution of ACE2 in the healthy lung. We sought to determine the pattern of message and protein expression, cellular localization, and developmental regulation of ACE2 in the normal mouse lung, because this species has been [Imai et al., 2005; Kuba et al., 2005] and is likely to be the species used as a model to understand pulmonary ACE2 physiology and pathology.
MATERIALS AND METHODS
Culture and Characterization of Murine Cell Lines
The following cell lines were studied: E10, a spontaneously immortalized adult peripheral lung epithelial cell line that expresses ∼15 differentiation genes that are specific markers of type I cells, provided by Dr. A. Malkinson (University of Colorado, Denver) and Dr. R. Ruch (Medical College of Ohio); MLE‐15, an SV40T antigen immortalized adult type II cell line provided by Dr. J. A. Whitsett (University of Cincinnati); mtCC‐DJS2, an SV40T antigen immortalized Clara cell line [Magdaleno et al., 1997] provided by Dr. Francesco DeMayo (Baylor College of Medicine); MFLM‐4, an SV40T antigen immortalized fetal lung endothelial cell line provided by Dr. Ann Akeson (Children's Hospital, Cincinnati); and BUMPT‐7, a renal proximal tubule cell line conditionally immortalized with a temperature‐sensitive SV40T antigen [Sinha et al., 2003] provided by Dr. John Schwartz (Boston University School of Medicine, Boston). E10 cells were maintained in CMRL 1066 medium, 10% fetal bovine serum, 0.5 mM glutamine, 100 units/ml penicillin G, and 100 µg/ml streptomycin sulfate. All other cell lines were cultured in Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin G, and 100 µg/ml streptomycin sulfate. All media and additives were from Invitrogen (Carlsbad, CA).
Collection of Murine Tissues
CD1 wild‐type adult, newborn, and fetal mice (Charles River Laboratories, Wilmington, MA) were euthanized by intraperitoneal injection of sodium methohexital (Eli Lilly, Indianapolis, IN), followed by transection of the aorta. Lung, kidneys, heart, and testes were collected and snap‐frozen on dry ice for subsequent RNA or protein isolation.
RNA Isolation
Total RNA from both tissues and cell lines was extracted by mechanical homogenization and scraping of confluent plates, respectively, and isolated in TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA for use in RT‐PCR and QRT‐PCR was DNase treated using DNA‐free™ (Ambion, Inc., Austin, TX) according to the manufacturer's instructions. RNA concentration was measured using a NanoDrop ND‐1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Northern Blots
Northern blots were prepared by the glyoxal/DMSO denaturation method. Total RNA (20 µg) was loaded onto a 1.5% agarose gel, electrophoresed, and transferred onto a nylon membrane (Hybond‐N+, Amersham Biosciences Co., Piscataway, NJ). RNA was crosslinked using ultraviolet light. The 472 bp internal ACE2 RT‐PCR product from primers described below (5′‐CCTTCTCAGCCTTGTTGCTGTTAC‐3′; 5′‐TGCCCAGAGCCTAGAGTTGTAGTC‐3′) was purified with Qiaquick PCR purification and gel extraction kits (Qiagen, Inc., Valencia, CA) and used as a template for a [32P]‐labeled probe prepared by the random hexamer primer method. Prehybridization and hybridization were performed as described previously [Ramirez et al., 1997]. Membranes were stripped and reprobed for the 219 bp product from primers specific for ACE2 exon 1a as described below (5′‐TACACTCTGGGAATGAGGAC‐3′; 5′‐TCTCATCAGCCTTTGAACTT‐3′). Films were exposed for 7–10 days at −70°C prior to developing.
Reverse Transcription Polymerase Chain Reaction (RT‐PCR)
The reverse transcription step was performed using poly‐dT (Roche Diagnostics, Indianapolis, IN) and avian myeoloblastosis virus reverse transcriptase (Promega Corp., Madison, WI) in a final volume of 25 µl for RT‐PCR or using the random hexamer reverse transcription technique with Taqman reagents (Applied Biosystems, Foster City, CA) in a final volume of 20 µl for QRT‐PCR. RT‐PCR was performed using a master mix containing Taq DNA Polymerase (Qiagen, Inc.) and primers (5′‐CCTTCTCAGCCTTGTTGCTGTTAC‐3′; 5′‐TGCCCAGAGCCTAGAGTTGTAGTC‐3′) designed to produce a 472 bp amplicon near the 5′ terminus of the published murine ACE2 mRNA sequence. β‐actin mRNA was amplified as a control for RNA integrity and loading. For certain purposes T1α mRNA, which is type I cell specific, and caveolin 1α mRNA, which is expressed by both type I and endothelial cells in the peripheral lung, were amplified as described previously [Rishi et al., 1995; Ramirez et al., 2002]. Nested RT‐PCR for ACE2 mRNA was performed using the above primers for the initial amplification (30 cycles) and internal primers (5′‐AGTCCCTCACCGAGGAAAAT‐3′; 5′‐AATGGTGCTCATGGTGTTCA‐3′) for a 326 bp final product (25 cycles). PCR products were gel‐purified using the Qiaquick gel extraction kit (Qiagen) and sequenced by the Boston University Core Sequencing Facility. Sequence data confirmed the products to be ACE2.
Identification of Additional 5′ Sequence
Northern blots of total lung RNA revealed a disparity in message sizes compared to those reported earlier for mouse lung [Komatsu et al., 2002]; specifically, the larger transcript we observed was ∼3.4 kb versus 2.8 kb reported earlier, suggesting that the reported 5′ sequence may be incomplete. Based on the recent identification in human ACE2 of exon 1a, 5′ to the 18 known exons [Itoyama et al., 2005], we performed a BLAST search (NCBI website; http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) that identified a region of 74% identity between human exon 1a (AB193260) and sequence in the mouse genome 5′ to the known ACE2 transcript (BC026801). A homologous forward primer (5′‐TACACTCTGGGAATGAGGAC‐3′) and a reverse primer (5′‐TCTCATCAGCCTTTGAACTT‐3′) producing a 219 bp amplicon at the 5′ end of the previously described exon 1 were designed for RT‐PCR using Taq DNA Polymerase (Qiagen).
5′‐Rapid Amplification of cDNA Ends (5′‐RACE) and Cloning of Full‐Length ACE2 Transcript
Total RNA (1 µg) from adult mouse lung was reverse transcribed using a modified oligo (dT) primer and the BD PowerScript Reverse Transcriptase (Clontech Laboratories, Inc., Mountain View, CA) as described by the manufacturer, to add several dC residues to the first‐strand cDNA 3′ end. PCR for 5′‐RACE was then performed with Advantage 2 Polymerase Mix (Clontech), a universal forward primer, and an ACE2‐specific reverse primer (5′‐GCCTTTGAACTTGGGTTGGGCACTG‐3′) using a touchdown PCR protocol [(94°C 30 s, 72°C 3 min) × 5 cycles, (94°C 30 s, 70°C 30 s, 72°C 3 min) × 5 cycles, (94°C 30 s, 68°C 30 s, 72°C 3 min) × 35 cycles]. The products were electrophoresed on a 1.2% agarose gel and gel‐purified using a gel extraction kit (Qiagen). The purified product was ligated into the PCR2.1 cloning vector (Invitrogen) using T4 DNA ligase at 14°C overnight. The ligation reaction products were inserted as a plasmid into OneShot competent E. coli cells (Invitrogen) by heat‐shocking the cells at 42°C in SOC medium. Transformed bacteria were grown overnight at 37°C on LB‐agar plates containing ampicillin. Clones were selected and grown overnight in LB‐ampicillin media, after which the plasmid was purified through a mini‐prep procedure (Qiagen) following manufacturer's protocol. Clones were sent for sequencing. After obtaining sequence of exon 1a, a second 5′‐RACE was performed using an ACE2 specific reverse primer (5′‐GAAGTTCAGCAGCTGGCTCCGTGTC‐3′) within the new exon to ensure there was no further 5′ sequence of ACE2.
Using RT‐PCR, a forward primer (5′‐GCTGAACTTCACCAGGATAACCAT‐3′) near the 5′ end of exon 1a and a reverse primer (5′‐CCATTGCTCAGACCCTGTGA‐3′) near the 3′ end of the published ACE2 transcript (NM_027286), or forward (5′‐ATTGGTCCAGCAGCTTGTTT‐3′) and reverse (5′‐GGCTTTGTGTTCACCATTGC‐3′) primers at the 5′ and 3′ ends of the published mRNA transcripts (BC026801 and NM_027286), respectively, were used to amplify full‐length ACE2 transcripts using the Advantage 2 PCR kit containing a Taq DNA Polymerase mix (Clontech). The PCR products were electrophoresed on a 0.7% agarose gel, gel‐purified using the Qiaquick gel extraction kit (Qiagen), cloned, and sequenced by the Boston University Core Sequencing Facility. We performed a BLAT search of the mouse genome (University of California Santa Cruz Bioinformatics web page; http://genome.ucsc.edu/cgi-bin/hgblat) to identify the location of exon 1a within the X chromosome. The DNA Strider (Version 1.3F9) program was used to analyze sequence for coding regions.
Quantitative Reverse Transcription Polymerase Chain Reaction (QRT‐PCR)
QRT‐PCR was performed using oligonucleotide primers (5′‐CCCCTTATGACTCGACTGGACA‐3′; 5′‐CTGTTTTGGAATCCTGCATTGC‐3′) specific for a sequence near the 3′ end of the ACE2 mRNA (BC026801) with SYBR Green master mix (Applied Biosystems) in an ABI Prism 7000 sequence detector. Data were normalized to the 18S amplicon, for which primers and probes were purchased from Applied Biosystems and used in conjunction with TaqMan master mix (Applied Biosystems). Samples were run in triplicate with conditions optimized to generate a single product; controls lacking template were run simultaneously. A calibration curve spanning 5 logs was generated using adult mouse kidney as a reference standard.
Protein Isolation
Murine tissues were mechanically homogenized in RIPA buffer containing three protease inhibitors (2 µg/ml aprotinin, 2 µg/ml leupeptin, and 100 µg/ml phenylmethylsulfonyl fluoride). Confluent cell plates were washed 3× with ice‐cold phosphate buffered saline, and then cells were scraped into 1 ml RIPA buffer. The RIPA buffer‐cell lysate suspension was incubated at 4°C on a rotator for 1 h and then centrifuged at 10,000 RPM × 20 min. The supernatant was collected, and protein concentration was determined spectrophotometrically using the Bradford Protein assay (Bio‐Rad Laboratories, Inc., Hercules, CA).
Western Blots
Protein (20–100 µg) was electrophoresed on 7.5% SDS–polyacrylamide gels. Proteins were transferred onto polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA), blocked in 5% milk in 1 × Tris‐buffered saline with 0.05% Tween‐20 (TBST), washed in 1 × Tris‐buffered saline (TBS) or 1 × TBST, and exposed to primary antibody overnight at 4°C on a shaker. Primary antibodies were rabbit polyclonal anti‐ACE2 (ab15348, Abcam, Inc., Cambridge, MA) 1:1,000 in 1% milk in 1 × TBS; goat polyclonal anti‐ACE2 (AF933, R&D Systems, Inc., Minneapolis, MN) 1:750 in 1 × TBS; or goat polyclonal anti‐ACE2 (sc21834, Santa Cruz Biotechnology, Inc, Santa Cruz, CA) 1:200 in 1% milk in 1 × TBS. Peptide inhibition studies were performed with ACE2 specific peptide (sc21834‐P, Santa Cruz) to test the specificity of anti‐ACE2 (sc21834, Santa Cruz) binding on Western blots. Primary antibody was incubated overnight with ACE2 specific peptide or with the non‐specific tuberin peptide (sc893‐P, Santa Cruz) at a concentration of 1:5 by weight prior to adding the primary antibody‐peptide solution to the membrane. Secondary antibodies (horseradish peroxidase‐labeled anti‐rabbit IgG or anti‐goat IgG) were used at 1:10,000 in 1% milk in 1 × TBST, 1 h, room temperature. Signal was detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc., Rockford, IL).
Immunohistochemistry
Lungs were fixed by intratracheal instillation (adult) or immersion (fetal) in freshly prepared 4% paraformaldehyde/0.1% glutaraldehyde (Ladd Research Industries, Williston, VT) in 0.1 M phosphate buffer, pH 7.4. Tissues were stored overnight in fixative at 4°C and processed into paraffin using standard methods. The heart was excised, sliced into fixative, and handled similarly. Kidney tissues were fixed overnight as slices in fixative lacking glutaraldehyde, cryoprotected with 30% sucrose in PBS and frozen for cryosectioning in O.C.T. compound cooled with Freon 22.
In most cases antigen retrieval was performed on rehydrated paraffin sections using 0.05% citraconic anhydride, pH 7.4, for 45 min at 98°C, followed by cooling at room temperature for 30 min [Namimatsu et al., 2005]. Endogenous peroxidases were quenched with 3% H2O2 in MeOH for 15 min at room temperature, and sections were blocked with CAS‐Block (Zymed Laboratories, San Francisco, CA) for 1 h at room temperature. Sections were incubated overnight at 4°C in anti‐ACE2 antibodies (sc21834, Santa Cruz, at 1:100–1:500 dilutions; ab15348, Abcam, at 1:1,500–1:5,000 dilutions). Antibody binding was detected using the Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA) as directed with diaminobenzidine as the chromogenic substrate. Control slides lacking primary antibody were included in all procedures. To test specificity of sc21834 (Santa Cruz) staining, peptide inhibition was performed by preincubating a 1:250 dilution of antibody with a tenfold excess of the antigenic peptide (sc21834‐P, Santa Cruz).
Cryosections of kidney were handled similarly except for omission of the antigen retrieval step in some cases. Sections were counterstained with dilute hematoxylin or left unstained and photographed in a Leitz Aristoplan microscope (Leica Microsystems, Bensheim, Germany) using Improvision software.
Statistics
The Student's t‐test was used to compare groups and calculate P‐values. A P‐value ≤0.05 was used as the level of statistical significance. Standard deviation bars are shown on all graphs. N = 3 or more for all experiments.
RESULTS
Two Transcripts of ACE2 in Mouse Lung and Kidney
Northern blot analysis confirmed the presence of ACE2 expression in adult mouse lung and kidney, showing two bands of ∼2.8 and ∼3.4 kb (Fig. 1A, left panel). After 7 days exposure there was a faintly visible band at ∼3.4 kb in the mtCC Clara cell line (Fig. 1A, left panel), which was evident after a longer exposure (Fig. 1A, right panel). ACE2 mRNA was undetectable in all of the other murine cell lines tested by Northern blot (data not shown), likely due to low ACE2 expression in these cell lines. While the finding of two transcripts in mouse lung and kidney has been previously described [Komatsu et al., 2002], the prior report describes the sizes of these transcripts as 2.8 and 2.0 kb (AB053181, AB053182).
Figure 1.
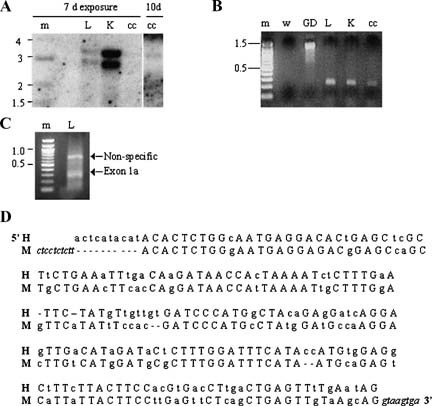
Identification of murine exon 1a. A: Northern blot for ACE2 expression in murine tissues and cell lines. The left panel represents Northern blot developed after 7 days exposure, the right panel shows blot developed after 10 days exposure. Lane m: 1 kb DNA marker (indicated in kb pairs); L: adult lung; K: adult kidney; cc: mtCC cells. B: RT‐PCR for putative 5′ exon 1a of murine ACE2. Lane m: 100 bp DNA marker (indicated in kb pairs); w: water; GD: genomic DNA; L: adult lung; K: adult kidney; cc: mtCC cells. C: 5′‐RACE for putative exon 1a in adult lung. By sequencing ∼800 bp band represents non‐specific amplification and ∼300 bp band includes the 188 bp exon 1a of murine ACE2. Lane m: 100 bp DNA marker; L: adult lung. D: Sequence of human (H) and mouse (M) exon 1a. Capital letters: identitical nucleotides in both species; lower case letters: divergent nucleotides in mouse and human; hyphen (‐): gap in sequence. Italics indicate bordering intron sequence in mouse genome.
Identification of Exon 1a and Additional Transcripts of Murine ACE2
Because of the discrepancy between the published murine ACE2 mRNA transcript sizes (2.7 and 3.2 kb, BC026801 and NM_027286, respectively) and the larger transcript suggested by our Northern blot (∼3.4 kb), we hypothesized that there may be additional undescribed 5′ sequence of ACE2, particularly in light of the recent identification of a 5′ exon 1a in human ACE2 (AB193259) [Itoyama et al., 2005]. By RT‐PCR using a forward primer in the 5′ region of homology to human exon 1a and a reverse primer in the 5′ end of exon 1, we amplified a 219 bp product in adult mouse lung (Fig. 1B). Using genomic DNA as a template with the same primers produced a ∼1,200 bp product (Fig. 1B), suggesting that the intron between the putative exon 1a and exon 1 is approximately 1–1.5 kb. With 5′‐RACE (Fig. 1C) we confirmed the presence of a 188‐bp exon 1a of murine ACE2 that shares 74% nucleotide identity with human ACE2 (sequence and homology to human ACE2 exon 1a shown in Fig. 1D). By sequence analysis this exon is located on chromosome X from position 159,483,486 to 159,483,673 and does not appear to be a coding sequence. The various ACE2 transcripts we have identified both with and without exon 1a are shown in a schematic (Fig. 2A) and confirmed by RT‐PCR in adult lung (Fig. 2B). Because of the RT‐PCR primers used (illustrated in Fig. 2A), transcripts 1 and 3 may not be full‐length transcripts but rather incomplete fragments of the longer transcripts 2 and 4, respectively, lacking the 3′ untranslated region (Fig. 2A,B). However, the presence of the polyA tail in the 3′ end of the 2.7 kb transcript 3 (BC026801) does suggest a complete mRNA transcript. Nonetheless, only transcripts 2 and 4 (Fig. 2A,B) have been submitted as novel mRNAs of ACE2 to the NIH GenBank for registration. By reprobing our initial Northern blot with a probe specific for exon 1a, we confirmed that exon 1a is present in both the 2.8 and 3.4 kb transcripts identified in the mouse lung and kidney (data not shown). By RT‐PCR the 3.4 kb transcript containing exon 1a (transcript 2 from Fig. 2A) is present in all tissues tested including adult kidney, heart (in low levels), testis, and lung, embryonic day (E) 18.5 lung, and the Clara cell line mtCC (Fig. 2C).
Figure 2.

Transcripts of murine ACE2. A: Schematic showing splice products of published ACE2 mRNA and novel exon 1a into possible transcripts. Arrows indicate forward and reverse primers used for RT‐PCR. B: RT‐PCR for ACE2 transcripts in adult lung. m: 500 bp marker (indicated in kb pairs); 1–4: transcripts 1–4 as shown in (A), with transcript 1 amplified using forward primer 1a‐F and reverse primer BC‐R, transcript 2 using forward primer 1a‐F and reverse primer NM‐R, transcript 3 using forward primer BC‐F and reverse primer BC‐R, transcript 4 using forward primer BC‐F and reverse primer NM‐R. C: RT‐PCR showing presence of ACE2 transcript 2 (amplified using forward primer 1a‐F and reverse primer NM‐R), as shown in (A) in various murine tissues and cell lines. w: water; K: adult kidney; H: adult heart; T: adult testis; L: adult lung; 18L: embryonic day 18.5 lung; cc: mtCC cells.
Multiple Protein Species of ACE2 in Murine Tissues and Cell Lines
Western blot analysis confirms the presence of ACE2 protein in adult mouse lung, kidney, heart, and testis, as well as in all cell lines tested (Fig. 3A). The published literature describes five different molecular weights for human ACE2, based on glycosylation and cleavage [Lambert et al., 2005]. In the mouse lung, 125 kDa [Imai et al., 2005] and 75 kDa [Gembardt et al., 2005] species of ACE2 have been reported, and an 89 kDa [Ye et al., 2004; Wysocki et al., 2006] form of ACE2 has been reported in the mouse kidney. By Western blot analyses using an antibody to the membrane‐bound C‐terminus of ACE2 (Fig. 3A) or an antibody against the N‐terminal ectodomain of ACE2 (Fig. 3B), we have identified all of the previously described protein species of ACE2. The major protein species in the lung appears to be 75–80 kDa, although the 120 kDa form is also present at lower levels. In contrast, the major species in other tissues tested is the 105–120 kDa isoform, although the kidney also expresses the 80 kDa form. The mtCC Clara cell line appears to express three protein species, 80, 85, and 120 kDa. The other pulmonary and proximal tubule cell lines appear to contain primarily the 80 kDa (MLE‐15, BUMPT‐7) or 85 kDa (E10, MFLM‐4) forms of ACE2. Western analysis with either antibody reveals a strongly immunoreactive species at 180 kDa (Fig. 3A,B). In order to provide evidence that the immunoreactivity of these bands is specific for ACE2, we used a third antibody against an internal ACE2 peptide for which the antigenic peptide is available (Fig. 3C–E). Preincubation of the antibody with an excess of peptide blocked binding to the 75–80 kDa protein species observed in the E10 cells, adult lung, and adult kidney, as well as to the 180 kDa species in the E10 cell line. This lends considerable support to the interpretation that all of the observed bands represent ACE2.
Figure 3.

Multiple protein species specific for ACE2. A: Western blot for ACE2 protein in murine tissues and cell lines using anti‐ACE2 Abcam ab15348 against cytoplasmic C‐terminus. Lane m: p7708s protein marker (in kDa); L: adult lung; K: adult kidney; H: adult heart; T: adult testis; cc: mtCC; e10: E10; mle: MLE‐15; mflm: MFLM‐4; pt: BUMPT‐7. B: Western blot for ACE2 protein in murine tissues and cell lines using anti‐ACE2 R&D Systems AF933 against N‐terminal ectodomain. Lane m: p7708s protein marker (in kDa); cc: mtCC; L: adult lung; K: adult kidney. C: Western blot for ACE2 protein using anti‐ACE2 Santa Cruz sc21834 alone. Lane m: p7708s protein marker (in kDa); e10: E10; L: adult lung; K: adult kidney. D: Competitive inhibition Western blot for ACE2 protein using anti‐ACE2 Santa Cruz sc21834 in the presence of specific ACE2 control peptide sc21834‐P. Lane m: p7708s protein marker (in kDa); e10: E10; L: adult lung; K: adult kidney. E: Competitive inhibition Western blot for ACE2 protein using anti‐ACE2 Santa Cruz sc21834 in the presence of non‐specific tuberin control peptide sc893‐P. Lane m: p7708s protein marker (in kDa); e10: E10; L: adult lung; K: adult kidney.
Moderate ACE2 Expression in Murine Tissues and Clara Cell Line
By RT‐PCR ACE2 expression is present in adult mouse lung, heart, and kidney, as well as in mtCC cells (Fig. 4A). By contrast, ACE2 mRNA expression was not detected by RT‐PCR in E10, MLE15, MFLM‐4, or BUMPT‐7 cell lines (Fig. 4A). However, transcripts can be detected using nested RT‐PCR (Fig. 4B) and QRT‐PCR (Fig. 4C), which showed high ACE2 expression in the mtCC cell line and very low expression in the other cell lines tested.
Figure 4.

ACE2 expression in murine tissues and cell lines. A: RT‐PCR for ACE2 and β‐actin expression in murine tissues and cell lines (40 cycles). Lane w: water; L: adult lung; H: adult heart; K: adult kidney; pt: BUMPT‐7; e: E10; le: MLE‐15; cc: mtCC; mf: MFLM‐4. B: Nested RT‐PCR for ACE2 expression in murine tissues and cell lines. Lane w: water; L: adult lung; K: adult kidney; H: adult heart; T: adult testis; cc: mtCC; e: E10; le: MLE‐15; mf: MFLM‐4; pt: BUMPT‐7. C: QRT‐PCR for ACE2 expression in murine cell lines, normalized to 18S. cc: mtCC; mle: MLE‐15; mflm: MFLM‐4; pt: BUMPT‐7. *P<0.005 compared to other cell lines.
Localization of ACE2 Expression to Clara Cells, Type II Cells, and Pulmonary Vessels
By immunohistochemistry (Fig. 5) ACE2 protein localizes to bronchiolar epithelium, small‐to‐medium pulmonary vessels, and the distal lung in the adult mouse. Specifically, by our methods ACE2 protein is present in Clara cells but not detectable in ciliated bronchiolar epithelium (Fig. 5A–D), present in smooth muscle cells and endothelium of some but not all medium to large arterioles and venules but not detectable in alveolar capillaries (Fig. 5B,C,E,I), and present in type II alveolar epithelial cells (Fig. 5F–H) but not detectable in type I cells. Antibody staining shows that ACE2 protein is preferentially localized in the apical plasma membrane of Clara cells (Fig. 5C,D) but is more diffusely distributed with an intracellular pattern in type II cells (Fig. 5F–H). This raises the possibility that the two cells may express different transcripts or different protein isoforms. Alveolar macrophages stain positive for ACE2 in some sections, as has been reported previously [Imai et al., 2005; Ren et al., 2006], but our competitive inhibition studies with ACE2 control peptide reveal this staining to be non‐specific (data not shown). On E18.5 ACE2 is present in the non‐ciliated bronchiolar epithelium but was not detected in the distal lung or vasculature (Fig. 5J–K) by our methods. Given that there has been definitive localization of ACE2 in the kidney and heart reported previously [Donoghue et al., 2000; Lely et al., 2004; Ye et al., 2004; Gembardt et al., 2005; Warner et al., 2005; Wysocki et al., 2006], we also localized ACE2 in the adult mouse kidney (Fig. 5L–P) and heart (Fig. 5Q) as a validation of our immunohistochemical methods and antibodies. ACE2 localization in the mouse kidney is similar to that previously reported for the human [Lely et al., 2004; Warner et al., 2005] and mouse [Ye et al., 2004; Gembardt et al., 2005] with specific staining in the proximal tubular epithelium brush border (Fig. 5N–O) and in vascular smooth muscle and endothelial cells of small to medium vessels (Fig. 5P). There was no staining in the renal medulla and very weak staining in glomeruli (Fig. 5L–M). In the mouse heart the myocardial capillaries show strong staining for ACE2 (Fig. 5Q), as has been previously reported in the human [Donoghue et al., 2000].
Figure 5.

Immunohistochemistry for ACE2 in murine tissues. Red arrow: positive staining for ACE2; yellow arrow: no staining for ACE2. Br: bronchiole; BV: blood vessel; A: alveolar space; M: medulla; C: cortex; PT: proximal tubule. A: Adult lung. Red arrow: bronchiolar epithelium. B: Adult lung. Red arrow: Clara cell; yellow arrow: arteriolar endothelium. C: Adult lung. Red arrow: Clara cell; yellow arrow: ciliated epithelium. D: Adult lung. Red arrow: Clara cell; yellow arrow: ciliated epithelium. E: Adult lung. Red arrow: vascular endothelium. F–H: Adult lung type II alveolar epithelial cells. I: Adult lung. Red arrow: endothelium of venule. J: E18.5 lung. Red arrow: bronchiolar epithelium. K: E18.5 lung. Red arrow: Clara cell; yellow arrow: ciliated epithelium. L,M: Adult kidney. Cortex stains positive for ACE2; medulla is negative for ACE2. N,O: Adult kidney. Red arrow: proximal tubule brush border. P: Adult kidney. Red arrow: endothelium and smooth muscle of small artery. Q: Adult myocardium. Red arrows: capillary endothelium. Panels A–C,F–H,L,N,P: antibody sc21834; other panels ab15348. Glutaraldehyde (0.1%) was added to fixative for panels A–C,F–K,Q. Panels L,M,O,P are frozen rather than paraffin‐embedded tissues. Antigen retrieval was performed on tissues in panels A–C,F–K,N,Q.
Developmental Pattern of ACE2 Expression in the Mouse Lung
By RT‐PCR ACE2 mRNA was detectable in the fetal mouse lung at E18.5, but not at E11.5 or E15.5 (Fig. 6A). By contrast, in the embryonic kidney, ACE2 mRNA was detectable by RT‐PCR as early as E15.5 (Fig. 6A). By the more sensitive method of QRT‐PCR we show a low level of ACE2 expression in embryonic lung at E15.5 and E17.5 that increases 2.8‐fold (P = 0.001) between E17.5 and E18.5 when normalized to the 18S amplicon (Fig. 6B).
Figure 6.

Developmental pattern of ACE2 expression. A: RT‐PCR for ACE2, β‐actin, T1α, and caveolin 1α in mouse lung and kidney at various developmental time points (30× cycles). Lane w: water; 11: E11.5; 15: E15.5; 18: E18.5; nb: newborn; ad: adult. B: QRT‐PCR for ACE2 expression in mouse lung and kidney at various developmental time points, normalized to the 18S amplicon. nb: newborn. *P = 0.001 when compared to E17.5.
DISCUSSION
In this article we describe exon 1a of ACE2 and show that this exon is expressed in a 3.4 kb transcript in many murine tissues, including the lung. We also demonstrate that there are multiple protein isoforms of ACE2, and that the major isoform in the lung differs from those in the other tissues we analyzed. We show through immunostaining that ACE2 in the lung is primarily epithelial, as opposed to endothelial in other tissues, and is specifically localized to Clara cells and type II alveolar epithelial cells. The finding of high levels of both ACE2 mRNA and protein in the Clara cell line mtCC supports our conclusion that Clara cells are a key source of ACE2 in the intact mouse lung. While message levels of ACE2 were low by QRT‐PCR in the other cell lines studied, including the types I and II cell lines, the pulmonary endothelial cell line, and the proximal tubule cell line, Western blot analyses demonstrate the presence of ACE2 protein in these cell lines. Finally, we demonstrate that ACE2 is developmentally regulated in the embryonic mouse lung with an expression pattern that matches those of other Clara cell and peripheral lung epithelial genes with a large increase in message levels between E17.5 and E18.5.
Although the finding of two distinct transcripts on Northern blot of mouse lung and kidney has been previously reported [Komatsu et al., 2002], the prior study describes transcripts of 2.0 and 2.8 kb, while we have identified transcripts of 2.8 and 3.4 kb based on markers. The size discrepancy between the larger of these transcripts and the published ACE2 mRNAs in the GenBank (AB053182, 1,993 bp; AB053181, 2,760 bp; 2,739 bp, BC026801; and 3,268 bp, NM_027286) led us to seek additional 5′ sequence here identified as exon 1a of murine ACE2. We initially speculated that the 3.4 kb transcript containing exon 1a might code for the previously undescribed 180 kDa protein isoform that we identified in lung, kidney, and E10 cells using multiple different antibodies to ACE2. However, sequence analysis shows exon 1a to be non‐coding, and its 188 bp sequence is likely too short to explain the larger molecular weight if it were translated. The largest molecular weight reported for ACE2 based on the amino acid sequence when fully glycosylated is 125 kDa [Imai et al., 2005]. The 180 kDa protein species is similar in molecular weight to ACE, which has a molecular weight in the mouse of 170 kDa [Ye et al., 2004; Wysocki et al., 2006], raising the possibility that ACE2 antibodies might not discriminate between these two proteins. However, we do not believe that this is the case because the manufacturer indicates that the Abcam anti‐ACE2 we used does not cross‐react with ACE. Furthermore we performed peptide inhibition studies using a different antibody (Santa Cruz) that show the 180 kDa isoform to be specific for ACE2. We do not yet know what accounts for this larger molecular weight. One possibility is that the ACE2 protein is tightly bound to another protein(s) from which it is not dissociated in the Western conditions we used, a possibility supported by previous information on ACE2 [Lin et al., 2004]. Lin et al. 2004 have shown that human ACE2 from failing myocardium co‐precipitates with integrin β1 and that this complex migrates at a molecular weight of 130 kDa, suggesting that one of the lower molecular weight ACE2 isoforms is part of the complex. Further studies of murine lung ACE2 will be required to resolve this issue. The other ACE2 isoforms we found in mouse lung (75–80, 85, 105, 120 kDa) are likely due to glycosylation and cleavage events, as has been previously described for human ACE2 [Lambert et al., 2005].
We explored the expression of ACE2 in several murine lung and kidney epithelial cell lines to identify a model system that could be useful for in vitro studies of ACE2 such as transcriptional regulation. We found low mRNA levels in the E10, MLE‐15, MFLM‐4, and BUMPT‐7 cell lines and higher levels in the mtCC cell line. When ACE2 proteins in these cells were examined, we observed an apparent discrepancy: mRNA levels were very low but proteins were easily detected. This relationship has been observed previously for murine ACE2 by Gembardt et al. 2005, who found low levels of ACE2 mRNA in the mouse heart by RNase protection assay but very strong protein expression in this organ by Western blot. This suggests that the ACE2 message may be very stable but not abundant in some murine cell types, with low levels of mRNA being translated into moderate amounts of protein. Alternatively, the ACE2 protein may be relatively resistant to degradation and thereby accumulate in higher amounts than the lower message level would ordinarily predict.
We found the principal protein species in the lung (75–80 kDa) to differ from that seen in the other organs (105–120 kDa), though the lung expresses the 120 kDa isoform at lower levels as well. One possible explanation is that the smaller isoform represents the epithelial variant of ACE2 that predominates in the lung, while the 120 kDa form represents the endothelial variant of ACE2 that predominates in most other tissues. This would be consistent with the finding that the lung, which has endothelial ACE2 expression in some but not all vessels, also contains lesser amounts of the 120 kDa isoform, while the kidney, which expresses ACE2 in the proximal tubule epithelium and in the endothelium of the blood vessels, contains both the 120 kDa and the 80 kDa isoforms. The mtCC Clara cell line expresses three protein isoforms of ACE2, which does not necessarily fit with this model. Insight into possible differential expression of mRNA and protein isoforms in epithelial and endothelial cells will require a number of approaches including in situ hybridization and analysis of primary cells isolated from the lung.
The finding that ACE2 is predominantly epithelial in the lung is supported by other observations. ACE2 has been identified as a target gene of the transcription factor hepatocyte nuclear factor 1 beta (HNF1β) [Senkel et al., 2005]. This transcription factor is exclusively expressed in epithelial cells [Coffinier et al., 1999]. In the lung Coffinier et al. 1999 have shown HNF1β to localize by immunostaining most strongly to the bronchial and bronchiolar epithelium with weaker staining in the alveolar epithelium in both E16 and adult mouse lung. In situ hybridization of lungs of mice infected with SARS coronavirus, which binds to ACE2, show viral RNA in the distal bronchiolar epithelium [Glass et al., 2004]. Moreover, the few immunohistochemical [Gembardt et al., 2005] and in situ hybridization [Imai et al., 2005] studies of the mouse lung suggest that ACE2 is present in alveolar [Gembardt et al., 2005] and airway [Imai et al., 2005] epithelium, although the specific cell types expressing ACE2 are not clear based on these studies. With the methods we used ACE2 protein is detected in an apical distribution in Clara cells but not ciliated cells of the bronchiolar epithelium, in type II but not type I alveolar epithelial cells, and in the endothelium and smooth muscle of some but not all small to medium blood vessels in the adult mouse lung. In the fetal lung ACE2 mRNA expression peaks at E18.5 and ACE2 is detectable in the distal, non‐ciliated bronchiolar epithelial cells. This developmental increase in ACE2 expression correlates well with the known time course of fetal Clara cell differentiation, as determined by marked increase in CC10 expression, which occurs between E15.5 and E18.5 [Keijzer et al., 2001].
The function of ACE2 in the normal, uninjured lung remains unknown and will be the focus of our future studies. The ACE2 knock‐out mouse has not been reported to have any lung pathology in either fetuses or adults, although the detailed reports on the phenotype of the unstressed ACE2 knock‐out mouse focused specifically on the heart [Crackower et al., 2002] and kidney [Oudit et al., 2006]. This suggests that there may be redundant proteins with overlapping functions to ACE2 in normal lung biology. Although ACE2 is known to cleave substrates other than angiotensin II [Vickers et al., 2002], its function in degrading angiotensin II is presumed to be the most significant [Vickers et al., 2002] and is the best studied. In this role ACE2 acts as a counterbalance to ACE in the regulation of the renin‐angiotensin system. The presence of ACE2 in the pulmonary epithelium is likely to relate to this role as a critical component of the renin‐angiotensin system.
Angiotensin II has been reported to induce apoptosis in both pulmonary and non‐pulmonary cell lines [Wang et al., 1999b; Yamada et al., 1996] and primary cultures of rat type II cells [Wang et al., 1999b; Krick et al., 2005]. The relevance of these observations to normal lung biology is unclear because many of these studies have used angiotensin II concentrations that far exceed those found in the normal mouse lung [Wei et al., 2002]. While apoptotic cells have been demonstrated by TUNEL staining in the healthy rat lung during post‐natal development [Schittny et al., 1998], the overall importance of apoptosis in the normal lung remains uncertain. By contrast, strong evidence supports the role of apoptosis in the injured lung, in which the hyperplastic type II cells commonly observed during alveolar epithelial repair are removed by programmed cell death [Bardales et al., 1996]. Whether angiotensin II is a critical mediator of apoptosis in these settings is not known, but studies have shown that autocrine generation of angiotensin II is required for both Fas‐induced [Wang et al., 1999a] and TNF‐α‐induced apoptosis [Wang et al., 2000] in primary cultures of type II cells. Using the ACE2 knock‐out mouse as a model, Imai et al. 2005 and Kuba et al. 2005 have clearly demonstrated that ACE2 is able to protect against acute lung injury by catalyzing angiotensin II. This lends support to the possibility that angiotensin II is an important mediator of alveolar epithelial cell injury and death, and that by regulating angiotensin II levels, ACE2 itself plays a critical role in the injured lung. Further studies need to be done to elucidate more definitively the normal function of ACE2 in the mouse lung, and in the pulmonary epithelium in particular.
Acknowledgements
We thank Dr. A. Malkinson (University of Colorado, Denver) and Dr. Randy Ruch (Medical College of Ohio) for the E10 cells, Dr. J.A. Whitsett (University of Cincinnati, Cincinnati, OH) for the MLE‐15 cells, Dr. Francesco DeMayo (Baylor College of Medicine, Houston, TX) for the mtCC‐DJS2 cells, Dr. Ann Akeson (Children's Hospital, Cincinnati, OH) for the MFLM‐4 cells, and Dr. John Schwartz (Boston University School of Medicine, Boston, MA) for the BUMPT‐7 cells. This work was supported by the National Institutes of Health grant NHLBI 47049 (to M.C.W., M.I.R.) and NHLBI T32 07035 (to R.S.W).
REFERENCES
- Bardales RH, Xie SS, Schaefer RF, Hsu SM. 1996. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol 149: 845–852. [PMC free article] [PubMed] [Google Scholar]
- Bernard GR. 2005. Acute respiratory distress syndrome: A historical perspective. Am J Respir Crit Care Med 172: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CF, Loh CB, Foo YK, Shen S, Fielding BC, Tan TH, Khan S, Wang Y, Lim SG, Hong W, Tan YJ, Fu J. 2006. ACE2 orthologues in non‐mammalian vertebrates (Danio, Gallus, Fugu, Tetraodon and Xenopus). Gene 377: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Barra J, Babinet C, Yaniv M. 1999. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 89: 211–213. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira‐dos‐Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. 2002. Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828. [DOI] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. 2000. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E19. [DOI] [PubMed] [Google Scholar]
- Gembardt F, Sterner‐Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. 2005. Organ‐specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Subbarao K, Murphy B, Murphy PM. 2004. Mechanisms of host defense following severe acute respiratory syndrome‐coronavirus (SARS‐CoV) pulmonary infection of mice. J Immunol 173: 4030–4039. [DOI] [PubMed] [Google Scholar]
- Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan KH, Tashiro M, Osterhaus AD. 2004. Pegylated interferon‐alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 10: 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D, Gilbert M, Borman R, Clark KL. 2002. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107–110. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong‐Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. 2005. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoyama S, Keicho N, Hijikata M, Quy T, Phi NC, Long HT, Ha le D, Ban VV, Matsushita I, Yanai H, Kirikae F, Kirikae T, Kuratsuji T, Sasazuki T. 2005. Identification of an alternative 5′‐untranslated exon and new polymorphisms of angiotensin‐converting enzyme 2 gene: Lack of association with SARS in the Vietnamese population. Am J Med Genet A 136: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford‐Lenane C, Perlman S, McCray PB Jr. 2005. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. 2001. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 128: 503–511. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Suzuki Y, Imai J, Sugano S, Hida M, Tanigami A, Muroi S, Yamada Y, Hanaoka K. 2002. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin‐converting enzyme‐related carboxypeptidase (mACE2). DNA Seq 13: 217–220. [DOI] [PubMed] [Google Scholar]
- Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. 2005. Role of hypoxia‐inducible factor‐1alpha in hypoxia‐induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol 32: 395–403. [DOI] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 11: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. 2005. Tumor necrosis factor‐alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe‐acute respiratory syndrome‐coronavirus (SARS‐CoV) receptor, angiotensin‐converting enzyme‐2 (ACE2). J Biol Chem 280: 30113–30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lely AT, Hamming I, van Goor H, Navis GJ. 2004. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593. [DOI] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. 2003. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Keller RS, Weaver B, Zisman LS. 2004. Interaction of ACE2 and integrin beta1 in failing human heart. Biochim Biophys Acta 1689: 175–178. [DOI] [PubMed] [Google Scholar]
- Magdaleno SM, Wang G, Jackson KJ, Ray MK, Welty S, Costa RH, DeMayo FJ. 1997. Interferon‐gamma regulation of Clara cell gene expression: In vivo and in vitro. Am J Physiol 272: L1142–L1151. [DOI] [PubMed] [Google Scholar]
- Namimatsu S, Ghazizadeh M, Sugisaki Y. 2005. Reversing the effects of formalin fixation with citraconic anhydride and heat: A universal antigen retrieval method. J Histochem Cytochem 53: 3–11. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. 2006. Loss of angiotensin‐converting enzyme‐2 leads to the late development of angiotensin II‐dependent glomerulosclerosis. Am J Pathol 168: 1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MI, Rishi AK, Cao YX, Williams MC. 1997. TGT3, thyroid transcription factor I, and Sp1 elements regulate transcriptional activity of the 1.3‐kilobase pair promoter of T1alpha, a lung alveolar type I cell gene. J Biol Chem 272: 26285–26294. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Pollack L, Millien G, Cao YX, Hinds A, Williams MC. 2002. The alpha‐isoform of caveolin‐1 is a marker of vasculogenesis in early lung development. J Histochem Cytochem 50: 33–42. [DOI] [PubMed] [Google Scholar]
- Ren X, Glende J, Al‐Falah M, de Vries V, Schwegmann‐Wessels C, Qu X, Tan L, Tschernig T, Deng H, Naim HY, Herrler G. 2006. Analysis of ACE2 in polarized epithelial cells: Surface expression and function as receptor for severe acute respiratory syndrome‐associated coronavirus. J Gen Virol 87: 1691–1695. [DOI] [PubMed] [Google Scholar]
- Rishi AK, Joyce‐Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, Williams MC. 1995. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol 167: 294–306. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Djonov V, Fine A, Burri PH. 1998. Programmed cell death contributes to postnatal lung development. Am J Respir Cell Mol Biol 18: 786–793. [DOI] [PubMed] [Google Scholar]
- Senkel S, Lucas B, Klein‐Hitpass L, Ryffel GU. 2005. Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta 1731: 179–190. [DOI] [PubMed] [Google Scholar]
- Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. 2003. Chemical anoxia of tubular cells induces activation of c‐Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497. [DOI] [PubMed] [Google Scholar]
- Soubrier F, Alhenc‐Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. 1988. Two putative active centers in human angiotensin I‐converting enzyme revealed by molecular cloning. Proc Natl Acad Sci USA 85: 9386–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. 2000. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem 275: 33238–33243. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. 2002. Hydrolysis of biological peptides by human angiotensin‐converting enzyme‐related carboxypeptidase. J Biol Chem 277: 14838–14843. [DOI] [PubMed] [Google Scholar]
- Wang R, Zagariya A, Ang E, Ibarra‐Sunga O, Uhal BD. 1999a. Fas‐induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am J Physiol 277: L1245–L1250. [DOI] [PubMed] [Google Scholar]
- Wang R, Zagariya A, Ibarra‐Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD. 1999b. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 276: L885–L889. [DOI] [PubMed] [Google Scholar]
- Wang R, Alam G, Zagariya A, Gidea C, Pinillos H, Lalude O, Choudhary G, Oezatalay D, Uhal BD. 2000. Apoptosis of lung epithelial cells in response to TNF‐alpha requires angiotensin II generation de novo. J Cell Physiol 185: 253–259. [DOI] [PubMed] [Google Scholar]
- Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. 2005. Angiotensin‐converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 280: 39353–39362. [DOI] [PubMed] [Google Scholar]
- Wei CC, Tian B, Perry G, Meng QC, Chen YF, Oparil S, Dell'Italia LJ. 2002. Differential ANG II generation in plasma and tissue of mice with decreased expression of the ACE gene. Am J Physiol Heart Circ Physiol 282: H2254–H2258. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2004. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.
- Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. 2006. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139. [DOI] [PubMed] [Google Scholar]
- Yamada T, Horiuchi M, Dzau VJ. 1996. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA 93: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. 2004. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension 43: 1120–1125. [DOI] [PubMed] [Google Scholar]


