Abstract
In this brief overview of a large and complex subject, as presented at the 2018 Surfactants in Solution conference, the need for, and impact of, hard surface antimicrobial products is demonstrated. The composition of the interfaces of three common classes of pathological microbes, bacteria, viruses, and fungi, is discussed so that surfactant and cleaning product development scientists better understand their interfacial characteristics. Studies of antimicrobial efficacy from the four major classes of surfactants (cationic, anionic, amphoteric, and nonionic) are shown. The need for preservatives in surfactants is elucidated. The regulatory aspects of antimicrobials in cleaning products to make antimicrobial claims are stressed.
Keywords: Anionic surfactants, Cationic surfactants, Microbiology, Nonionic surfactants, Switchable surfactants
Impact of Antimicrobial Cleaning Products
Antimicrobial cleaning products are of high importance to public health, the economy, and society. Recent trends have increased their importance and relevance. Annually, 1.7 million hospital‐acquired infections (HAI) are reported in the United States, resulting in 99,000 deaths and an increased cost to the national health care system of US$20 billion (CDC, 2018a). Many of these can be prevented by compliance with recommended disinfecting protocols. Disease outbreaks in recent years are more severe due to global travel, large scale food processing, adaptation of microorganisms, and reduced vaccination rates (CDC, 2018b). Natural disasters in recent years have had a more severe impact, particularly when large metropolitan areas are affected. These events may disrupt water, sanitation, healthcare access, and hygiene practices; in addition, those who require sheltering during and after such events are at higher risk for illness due to crowded conditions (Watson et al., 2007).
Higher health care costs and limited access to health care may cause some individuals to avoid treatment, thus causing the spread of illness; in the US, 4.4% of persons failed to get health care due to cost, and 12% did not have a regular place to obtain health care (CDC, 2018c). It is estimated that illnesses cost the US economy $273B in lost producitivity (Forbes, 2018). The average school age child in the US misses 4.5 days of school per year, impacting schools where funding is based on school attendance (Deb Group, 2018).
Chronic illnesses, particularly respiratory, have been attributed to exposure to mycotoxins and allergenic proteins resulting from mold growth in built environments (Pestka et al., 2008). Effective remediation of infected spaces is difficult and expensive. Mold requires the presence of both moisture and an organic food source (many building materials will suffice) to thrive; mold growth in buildings thus typically results from plumbing leaks in buildings, poor humidity control, poor building design, appliance breakdown, and disasters such as floods.
Many clinical studies have found benefits to the use of hard surface antimicrobials to prevent exposure to pathogens that cause illness. For example, antimicrobials help prevent exposure to Escherichia coli in household cleaning sponges (Gerba et al., 2017; Rossi et al., 2012) and frequent washing helps lower levels of coliform bacteria in kitchen towels (Gerba et al., 2014). Children in classrooms where disinfecting wipes were used were over two times less likely to report absenteeism due to illness than children in classrooms where no disinfectant was used (Bright et al., 2010). Significantly lower populations of norovirus were found in classrooms where disinfectants and hand sanitizers were used than where they were not (Sandora et al., 2008). The United States Centers for Disease Control recommend the use of disinfectants in health care settings as part of an infection control program (CDC, 2018d).
Surfactants and detergents, which lift soils from surfaces, are a recommended part of disinfection and sterilization in healthcare to work in concert with other antimicrobial agents (CDC, 2019a). In this brief overview of a complex and dynamic field of research, the effect of surfactants as antimicrobials themselves will be explored. First, to set the context for the interfaces to be studied with surfactants, the chemical composition of microbial interfaces is discussed in the next section.
Overview of Chemical Compositions of Interfaces in Bacteria, Viruses, and Fungi
Bacterial Interfaces
Gram‐Positive Bacteria
Gram positive bacteria are detected by their ability to respond to a Gram stain, generally crystal violet, which detects peptidoglycan, a polymer of sugars and proteins, in the cell wall of these species. A high‐level diagram of the interface of a gram positive bacterium is shown in Fig. 1. The key features that distinguish gram‐positive bacteria from gram‐negative bacteria are: a thick peptidoglycan layer; the presence of teichoic acids (copolymers of glycerol phosphate and carbohydrates) and lipids (together, these form lipoteichoic acids, which serve as chelators and also aid in adherence to the cell wall) (Brown et al., 2013; Percy et al., 2014); the crosslinking by DD‐transpeptidase of peptidoglycan chains to form rigid cell walls; and a much smaller volume of periplasm than that in Gram‐negative bacteria (Madigan et al., 2006). Some gram‐positive bacteria, such as Clostridium difficile, form spores. The stabilized spore coat proteins have been studied by Permpoonpattana et al. (2013); this coat makes it more difficult for antimicrobials to penetrate and kill.
Figure 1.
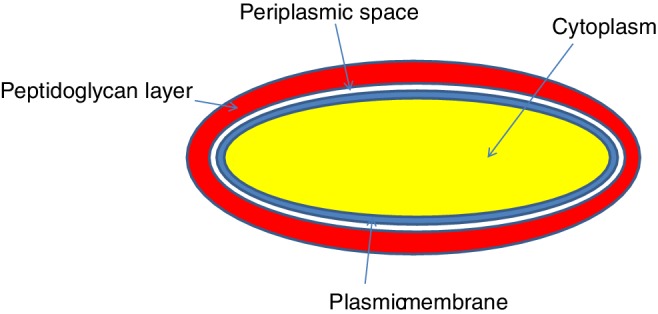
Simplified structure of gram‐positive bacterial cell interface
Many gram‐positive species are known to cause illness, including Staphylcoccus aureus (skin, eye, and upper respiratory tract infections), C. difficile (intestinal illness), Mycobacterium (tuberculosis, leprosy), and Listeria (food poisoning) (Buchanan and Gibbons, 1974).
Gram‐Negative Bacteria
A simple schematic of a gram‐negative bacterial cell wall is shown in Fig. 2. Gram‐negative bacteria display these characteristics (Baron et al., 1996): a thin peptidoglycan layer is present (this is much thicker in gram‐positive bacteria); an outer membrane containing lipopolysaccharides (LPS, which consists of lipid A (a phospholipid consisting of two glucosamine units, with phosphonates and acyl chains attached to each; this links the core and antigen components to the inner leaflet), core polysaccharide (comprised of oligosaccharides, linking O antigen with lipid A), and O antigen (strain‐specific repetitive polysaccharide on the outermost surface of the cell) in its outer leaflet, and phospholipids in the inner leaflet (Raetz and Whitfield, 2002)) and porins (proteins in arrangements like pores to enable transport of particular molecules) (University of Hamburg, 2019); between the outer membrane and the cytoplasmic membrane, there is a space filled with periplasm, a concentrated gel‐like substance comprised of proteins and ions; the S‐layer is directly attached to the outer membrane rather than to the peptidoglycan; flagella (if present) have four supporting rings instead of two; there are no teichoic acids or lipoteichoic acids; lipoproteins are attached to the polysaccharide backbone; and most, with very few exceptions, do not form spores.
Figure 2.
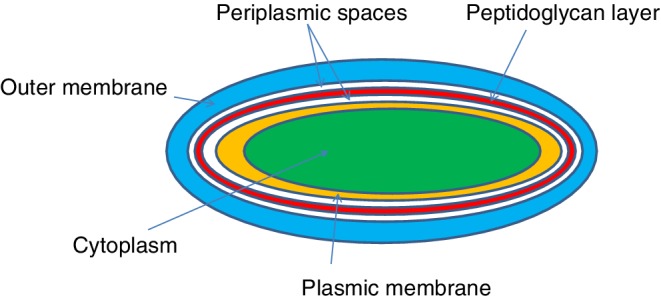
Simplified structure of gram‐negative bacterial cell interface
Medically relevant gram‐negative species include types that cause sexually transmitted disease (e. g., Neisseria gonorrhoeae), meningitis (Neisseria meningitidis), respiratory symptoms (Moraxella catarrhalis, Haemophilus influenza, Klebsiella pneumoniae, Legionella pneumophila, and Pseudomonas aeruginosa), urinary tract infections (E. coli, Proteus mirabilis, Enterobacter cloacae, and Serratia marcescens), and gastrointestinal disease (Helicobacter pylori, Salmonella enteritidis, and Salmonella typhi).
Biofilm Composition
Some bacteria excrete extracellular polymeric substances after adherence to a surface, in which the bacteria grow and colonize, typically coating a surface. The resulting film is called a biofilm. These polymeric substances typically consist of polysaccharides, DNA, proteins, and lipids (Aggarwal et al., 2016; Hall‐Stoodley et al., 2004; López et al., 2010). The resulting polymeric matrix protects the microbes from attack, including that of antimicrobials, and can enable survival of bacteria outside a host for extended periods of time (Hokkanson, 2018). Penetration of this matrix is key to killing. It can be done through additional chemical attack and/or physical disruption. Biofilm contamination can be a key issue with plumbing, food and water handling systems, and medical devices, and can cause health issues such as gingivitis, eye infections from contaminated contact lenses, and middle‐ear and urinary tract infections (Rogers, 2008).
To prevent biofilm formation on a surface, surface charge can be modified by the adsorption of polymers, or the surface can be made hydrophobic to lessen the affinity for polar and ionic groups in cells and biofilms for the surface (Guo, 2013). Small‐scale surface roughness, also known as the Lotus effect, can also be effective in repelling the adherence of bacteria and the generation of films (Jansen and Kohnen, 1995). Antimicrobial ingredients may be incorporated therein (Contreras‐Garcia et al., 2011; Stobie et al., 2008).
Viral Interfaces
Viruses exist in particles that consist of RNA or DNA genetic material, surrounded by a protein coat, or capsid, that protects the genetic material (Clinical Gate, 2018). Virus particles take on a variety of shape, including helical, icosahedral, or more complex structures. In some cases, the capsid is further surrounded by a lipid membrane, or envelope. Those viruses that possess the lipid membrane are referred to as “enveloped” viruses; those that do not are “non‐enveloped” viruses. A simple rendering of the structure of a virus is shown in Fig. 3.
Figure 3.
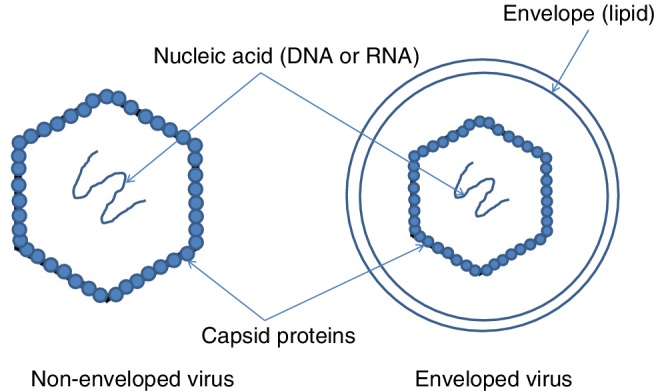
Simplified structure of nonenveloped and enveloped viruses
Enveloped viruses include groupings such as Herpesviridae (e. g., herpes infections, chickenpox), Poxviridae (e.g., smallpox), Hepadnaviridae (e. g., Hepatitus B), Flavivirus (e.g., West Nile, Zika), Togavirus (e.g., rubella, encephalitis), Coronavirus (SARS, colds, and MERS), Hepatitis D, Orthomyxoviridae (influenza types A‐D), Paramyxoviridae (measles, mumps, and respiratory infections), Rhabdoviridae (e. g. rabies, encephalitis), Bunyavirales (Hantavirus, Crimean‐Congo hemorrhagic fever), Filoviridae (Ebola, Marburg), and retroviruses (viruses that change viral genomes, used in gene therapy, but also causative of some types of cancer). To remain active, the lipid membrane envelope must remain wet. The envelope can also be readily disrupted by cleaning agents and antimicrobials, thus making them more dependent on direct human contact or bodily fluid contact to spread.
Nonenveloped groupings include Adenoviridae (colds, pneumonia, tonsillitis, gastroenteritis, conjunctivitis, and meningitis), Papillomavirus (HPV, which can cause cancer), and Picornavirus (polio, Coxsackie, and colds). The capsids for these species are more robust than with enveloped viruses. The UK National Health Service (2018) notes that Norovirus, a nonenveloped virus that causes gastrointestinal illness, can survive on, and transmit illness from, hard surfaces for days to weeks.
Fungal Interfaces
A simple schematic of a fungal cell is shown in Fig. 4. The outermost surface of the cell is comprised of glucans and cellulose; further there is the chitin layer, adjacent to the phospholipid membrane, which is adjacent to the cytoplasm. Additional detail is described below.
Figure 4.
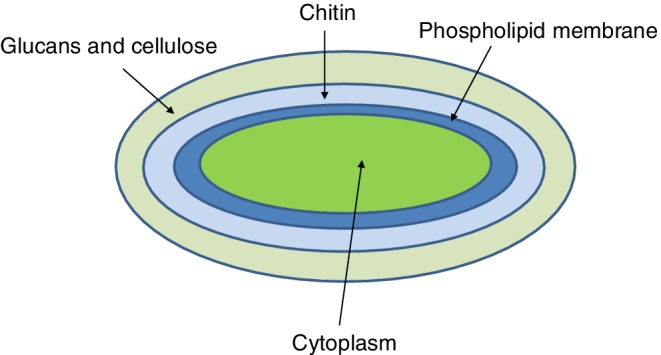
Simplified structure of the fungal cell interface
Mold
Molds are fungal organisms that grow in multicellular filaments (hyphae). Mold support structures consist predominantly of the carbohydrate polymers chitin and beta‐1,3‐glucan (Munro, 2013). A high‐level schematic of a mold structure is shown in Fig. 5. The external structure is rigid and difficult to penetrate. In addition, the allergenic proteins and mycotoxins emitted by mold species, particularly Stachyborys, can be a problem even after the mold organism has been killed, unless those proteins and toxins are deactivated (Yang, 1972). Chlorine bleach kills mold (Reynolds et al., 2012); additional use of surfactants helps the bleach wet and penetrate through the mold and building materials to be more effective (Wisconsin DHS, 2018).
Figure 5.
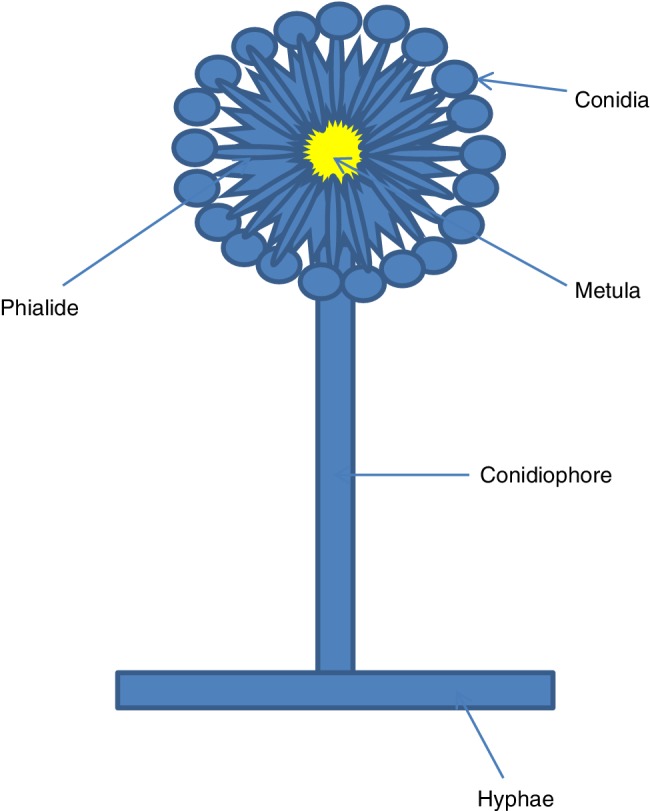
Simplified structure of mold organism
Yeast
Yeast fungal organisms, in contrast to the multicellular structures of molds, are single‐celled. There are many types of yeast, including some that are used in fermentation and baking processes. For this discussion, the focus will be on two pathogenic yeast species, those of the Candida and Cryptococcus types. The cell wall of Candida albicans, the most commonly isolated yeast, and one attributed to common yeast infections in humans, consists of glucans, chitin (to a lesser extent than the molds discussed earlier), and mannoproteins (glycoproteins containing mannose, attributed to the immune response in host organisms) (Chaffin et al., 1998). Cryptococcus yeasts have a shell comprised of polysaccharides that help to transport nutrients from their soil habitat (Bose et al., 2003). The best‐known pathogenic species, C. neoformans, can cause serious illness, such as respiratory illness or meningitis in immunocompromised individuals (CDC, 2019b).
Now that the chemical compositions of various microbial species have been shown, the next section will document studies of the antimicrobial action of different surfactant types.
Studies of Surfactants as Antimicrobials
Cationics
Cationic surfactants are the best known class of antimicrobial surfactants, due to the widespread use of selected quaternary ammonium compounds (quats). In particular, alkyldimethylbenzylammonium chloride (ADBAC), alkyldimethylethylbenzylammonium chloride (ADEBAC), and didecylammonium chloride (DDAC), shown in Fig. 6, have found common use in hard‐surface disinfection applications. These distinguish themselves from quaternary compounds used in antistatic or conditioning applications with a shorter alkyl chain length opposed with a benzyl substitution (typically C10–C16, versus the C16–C18 dialkyl structures found in conditioning products), resulting in higher water solubility.
Figure 6.
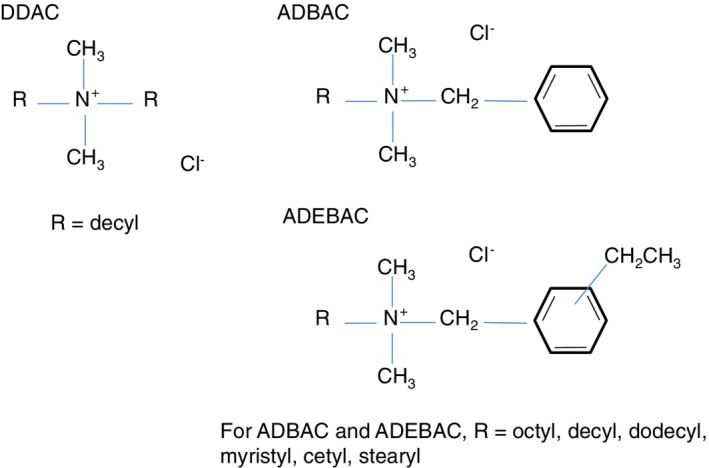
Molecular structures of the most common antimicrobial quaternary ammonium compounds
Cationic surfactants, particularly when applied at alkaline to neutral pH, show a high affinity for the interfaces of all three microbial classes of interest, due to the negative charge of the microbial interfaces. Vieira and Carmona‐Ribeiro (2006) found that charge reversal from negative to positive that occurred for C. albicans exposure to dioctadecyldimethyl ammonium bromide and hexadecyltrimethyl ammonium bromide was correlated with viability. However, a proposed mechanism (McDonnell, 2007) for the antimicrobial action of quats demonstrates the effect beyond adsorption to the interface.
First, adsorb and penetrate cell wall
Second, react with cytoplasmic membrane to cause disorder
Third, leakage of lower‐MW intracellular material
Fourth, degradation of proteins and nucleic acids,
Fifth, cell lysis and death
Quats are often referenced by “generations,” which are defined as (Gerba, 2015):
1st Benzalkonium, alkyl chains, C12–C18.
2nd Aromatic rings with hydrogen and chlorine, methyl and ethyl groups.
3rd Dual quaternary ammonium compound(s) (QAC); mixture of alkyl dimethyl benzyl ammonium chloride (lower toxicity).
4th Dialkylmethyl aminos with twin chains.
5th Synergistic combinations of dual QAC.
6th Polymeric QAC.
7th Bis‐QAC with polymeric QAC.
Succeeding generations demonstrated increased efficacy. Ioannou (2007) shows that equilibrium DDAC adsorption (3rd generation) on microorganisms is significantly higher than that for ADBAC (first generation); DDAC appears to form multiple layers, or perhaps accumulation in the cells begins to happen at a lower concentration than for ADBAC. Kill rates for ADBAC were also demonstrated to be significantly faster for DDAC than ADBAC. Notably, a plateau was achieved in the kill/time curve, indicating that kill effectively stopped at a certain time point. This time was independent of concentration for DDAC in the study, but decreased with increasing concentration of ADBAC.
Quats are most effective in the monomer form, where they can readily interact with cell interfaces, thus higher CMC values yield a higher monomer concentration. When formulating with quats, Rosen (1989) notes the effect of the alkyl chain length on CMC, where the addition of one methylene group is estimated to decrease the CMC by half. Corrin and Harkins (1947) indicates that the increase of salinity can impact the CMC of an ionic surfactant; a 1 M NaCl solution is estimated to decrease the CMC of cetylpyridinium chloride by 100×, again reducing the monomer concentration. In addition, Walker et al. (2004) found that the zeta potential of bacteria can be affected by the presence of salts, potentially lowering the affinity of quats to cell interfaces. Should one seek to thicken a quat‐containing formulation with the use of salt, microefficacy of the formula should be evaluated.
Anionics
Anionic surfactants have been leveraged as antimicrobials. Block (2001) notes that acid/anionic formulas were first leveraged in the 1930s; acidic pH of 2–3, particularly an anionic surfactant with polyvalent cations and alkyl chain length with a good match to the cell wall lipid structure (C12–C16) aids in efficacy.
With acidic pH values of 2–3, Nelson et al. (2017) show that isoelectric points of many proteins are in this range, thus causing a switch in cell charge from negative to positive. This results in an improved affinity for anionic surfactants, particularly those sourced from strong acids that maintain negative charge in this range, such as sulfates and sulfonates.
Anionic surfactants without acids can also demonstrate antimicrobial activity. Tsujimura et al. (2015) and Inácio et al. (2011) showed that viruses can be deactivated with linear alkylbenzene sulfonate (LAS) and sodium lauryl sulfate (SLS), respectively; Johnston et al. (2000) show disinfection of S. aureus with an SDS solution. SLS is well known to denature proteins and is a useful part of cell lysing solutions for assays. LAS is used as a food contact sanitizer (EPA, 2018a) and was shown to have antimicrobial efficacy in laundry; however, some LAS resistant strains were identified (Maehara, 2017). LAS acid neutralized with sodium carbonate showed inhibition of several microorganisms recovered from jewelry; the LAS ammonium salt was less effective (Isitua, 2016). Sodium dodecyl benzene sulfonate (SDBS) is registered as an antimicrobial active that can be used in organic food processing (USDA, 2018).
Amphoterics
The best known materials in this class are amine oxides and betaines. As amphoteric materials, at acidic pH, they can take on a positive charge, thus yielding stronger affinity for cell membranes provided the cell membrane isoelectric point is not achieved. Birnie et al. (2000) notes efficacy against S. aureus and E. coli, with best performance at myristic or palmitic (C14–C16) chain lengths. Krasowska et al. (2012) notes that a synergy in antimicrobial performance was found between cocoamidopropyl betaine and alkyl dimethyl amine oxide; however, the minimum inhibitory concentration of the blend was still much higher than that of benzalkonium chloride. Subik et al. (1977) found that heterocyclic amine oxides demonstrated antimicrobial activity against Bacillus, yeast, and molds; the inhibitory concentration decreased with increasing alkyl chain length, indicating that the longer chain lengths appear to interact more strongly with the cell membrane.
Glycine‐based surfactants, such as dodicin (dodecyl‐di(aminoethyl)‐glycine) (Block, 2001; Copello et al., 2008), show efficacy on bacteria and some viruses; optimal pH depends on the structure, as added aminoethyl functionality demonstrates peak efficacy at higher pH values. In Copello's work, dodicin was formulated into an antimicrobial coating that demonstrated efficacy.
Nonionics
Moore (1997) reported that C10E6 and C12E6 surfactants showed efficacy against E. coli. The C10 material adsorbed on the cell wall, whereas the C12 material entered the membrane bilayer; both pathways interfered with viability. C14 and C16 materials had no microbial effect. In Maehara's studies noted earlier, polyethoxylated lauryl ether had only a minor antimicrobial effect in laundry applications.
Surface‐active glycolipids, such as sophorolipids and rhamnolipids, are derived biologically and have demonstrated antimicrobial activity (Sleiman et al., 2009; Magalhães and Nitschke, 2013). Kim et al., (2002) found that a sophorolipid caused the release of intercellular enzymes in Gram‐positive bacteria, indicating membrane damage, and showed inhibition of mold growth, but was not effective against E. coli (Gram‐negative). Efficacy thus varies by the type of microorganism—thus if you seek to target killing a particular type of microbe, a glycolipid may be a good choice (Cortes‐Sanchez et al., 2013).
Surfactin, a cyclic polypeptide derived from species of Bacillus, has surface‐active properties (Heerklotz and Seelig, 2001). Antibacterial (Sabate, 2013) and antiviral (Jung et al., 2000) activity have been demonstrated. Its ability to lyze many types of cell membranes makes it a potential candidate for cancer treatment; however, to avoid lysis of noncancer cells, targeting cancer cell receptors is needed (Wu, 2017).
Kabara (1972) found that fatty acids demonstrated efficacy against bacteria and yeast. Lauric acid was found to be the most effective of the saturated fatty acids, indicating that optimal hydrophobicity plays a role in microefficacy. Monoenoic and dienoic (cis‐configuration) acids showed improved efficacy over saturated acids; trans‐configurations had no microefficacy. Among derivatives of fatty acids, esterification reduces microefficacy, but derivatization to aldehyde, alcohol, or amine forms improves it. Desbois and Smith (2010) indicates that free fatty acids target cell membranes and disrupt electron transport and oxidative phosphorylation, and may play a role in cell membrane lysis and interference in other cellular metabolic pathways.
Surfactant Preservation
Surfactant raw materials, even those that demonstrate some antimicrobial activity, may require preservation. This is because surfactants may not be effective against contaminating species (for example, they may be effective only against Gram‐negative bacteria, or not against molds). Susceptible raw materials have high water activity and pH between 3.0 and 10. If the surfactant can tolerate pH values above 10 or below 3.0, pH adjustment may be an effective way to preserve the raw material; vendors of preservatives can be consulted to identify the best preservative for the chemistry, the processing requirements and equipment, the regulations in the jurisdiction of sale, and the final use of the raw material (Shaw, 2014; Srikanth, 2018).
Making Antimicrobial Claims on Disinfecting Products
Antimicrobial chemistry and advertising claims related to their efficacy are regulated in many countries (e. g., EPA (2018b); HSE (2018), CIRS (2018), EC (2019)). Active ingredients and formulations must be registered with proof of efficacy and demonstration of safety/environmental impact; inert ingredients must be registered as well. Testing protocols/regulations vary by jurisdiction and final product application; these protocols require complete or nearly complete kill in a limited amount of time with a significant inocula population. Significant lead time may be required to process registrations for both actives and formulations, and chemical efficacy and safety testing may be extensive and require significant financial investment. This registration is above and beyond that for chemical registries such as the United States Toxic Substances Control Act (TSCA) and the European Inventory of Existing Commercial Chemical Substances (EINICS).
Antimicrobial activity as described in this article or the references therein is not a guarantee of passing antimicrobial tests required for claims substantiation. Those who wish to make such claims for products they intend to market need to consult regulatory agencies in their jurisdictions for specific requirements.
Summary
In this brief overview of a large and complex topic presented at the 2018 Surfactants in Solution conference, the chemical nature of microbial interfaces was shown. Through case studies, it was demonstrated that surfactants aid in the killing of microbes that cause illness. Examples of microefficacy have been demonstrated in all major surfactant classes, but not all surfactants will demonstrate microefficacy, not all microbes will be equally susceptible to surfactants, and structural differences in a particular surfactant type can have an impact on antimicrobial efficacy. Microefficacy may also vary with formulation variables such as pH and ionic strength. Formulators who seek antimicrobial claims on disinfecting products should consult local regulations for the appropriate testing methodologies and requirements for making claims. This article, as it was derived from a conference presentation, is not intended to be a full review of this topic, but an overview to guide those formulating antimicrobial cleaning products for inanimate surfaces.
Conflict of Interest
The author declares no conflict of interest.
Acknowledgements
The author thanks The Clorox Company for supporting this work (note that views expressed in this article are those of the author) and its presentation at the 2018 Surfactants in Solution Conference, and conference chair Brian Grady for the invitation to speak.
References
- Aggarwal, S., Stewart, P. S., & Hozalski, R.M. (2016) Biofilm cohesive strength as a basis for biofilm recalcitrance: Are bacterial biofilms overdesigned? Microbiology Insights, 8:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron,S., & Schuenke, S. (1996) Structure In Baron S. (Eds.), Baron's medical microbiology (4th ed.). Galveston, TX:University of Texas Medical Branch. [PubMed] [Google Scholar]
- Birnie, C. R. , Malamud, D. , & Schnaare, R. L. (2000) Antimicrobial evaluation of N‐alkyl betaines and N‐alkyl‐N,N‐dimethylamine oxides with variations in chain length. Antimicrobial Agents and Chemotherapy, 44:2514–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, S. (2001) Block's disinfection, sterilization, and preservation (Vol. 63, 4th ed., pp. 256–263). Philadelpha PA: Lippincott, Williams, and Wilkins. [Google Scholar]
- Bose, I. , Reese, A. J. , Ory, J. J. , Janbon, G. , & Doering, T. L. (2003) A yeast under cover: The capsule of Cryptococcus neoformans . Eukaryotic Cell, 2:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright, K. R. , Boone, S. A. , & Gerba, C. P. (2010) Occurrence of bacteria and viruses on elementary classroom surfaces and the potential role of classroom hygiene in the spread of infectious diseases. The Journal of School Nursing, 26:33–41. [DOI] [PubMed] [Google Scholar]
- Brown, S. , Santa Maria Jr., J. P. , & Walker, S. (2013) Wall teichoic acids of gram‐positive bacteria. Annual Review of Microbiology, 67:313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. E., & Gibbons N. R. (Eds.) (1974) Bergey's manual of determinative bacteriology (8th ed.). Baltimore, MD: Williams & Wilkins. [Google Scholar]
- Chaffin,W. L., López‐Ribot, J. L., Casanova, M., Gozalbo, D., & Martínez, J. P. (1998) Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiology and Molecular Biology Reviews, 62:130–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRS . (2018, May 29) Retrieved from http://www.cirs-reach.com/China_Chemical_Regulation/Disinfectant_Registration_in_China.html
- Clinical Gate . (2018, May 29). Viral structure classification and replication Retrieved from https://clinicalgate.com/viral-structure-classification-and-replication
- Contreras‐García, A., Bucio, E., Brackman, G., Coenye, T., Conchiero, A., & Alvarez‐Lorenzo, C. (2011) Biofilm inhibition and drug‐eluting properties of novel DMAEMA‐modified polyethylene and silicone rubber surfaces. Biofouling, 27:123–135. [DOI] [PubMed] [Google Scholar]
- Copello, G. J. , Teves, S. , Degrossi, J. , D'Aquino, M. , Desimone, M. F. , & Díaz, L. E. (2008) Proving the antimicrobial spectrum of an amphoteric surfactant‐sol‐gel coating: A food‐borne pathogen study. Journal of Industrial Microbiology & Biotechnology, 35:1041–1048. [DOI] [PubMed] [Google Scholar]
- Corrin, M. L. , & Harkins, W. D. (1947) The effect of salts on the critical concentration for the formation of micelles in colloidal electrolytes. Journal of the American Chemical Society, 69:683–688. [DOI] [PubMed] [Google Scholar]
- Cortes‐Sanchez, A. , et al. (2013) Biological activity of glycolipids produced by microorganisms: new trends and possible therapeutic alternatives. Microbiological Research, 168:22–32. [DOI] [PubMed] [Google Scholar]
- Deb Group . (2018, May 23). School absenteeism due to illness: Fact or Fiction Retrieved from http://info.debgroup.com/blog/bid/314366/school-absenteeism-due-to-illness-fact-or-fiction
- Desbois, A. P. , & Smith, V. J. (2010) Antibacterial free fatty acids: Activities, mechanisms of action, and biotechnological potential. Applied Microbiology and Biotechnology, 85:1629–1642. [DOI] [PubMed] [Google Scholar]
- European Commission (EC) . (2019, April 14) Retrieved from https://ec.europa.eu/health/biocides/regulation_en
- Forbes . (2018, May 23). US workforce illness costs $576B annually from Sick Days to Workers' compensation Retrieved from https://www.forbes.com/sites/brucejapsen/2012/09/12/u-s-workforce-illness-costs-576b-annually-from-sick-days-to-workers-compensation/#46206dc85db0
- Gerba, C. P. (2015) Quaternary ammonium biocides: Efficacy in application. Applied and Environmental Microbiology, 81:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba,C. P., Tamimi, A. H., Maxwell, S., Sifuentes, L. Y., Hoffman, D.R., & Koenig,D. W. (2014) Bacterial occurrence in kitchen hand towels. Food Protection Trends, 34:312–331. [Google Scholar]
- Gerba, C. P., Sifuentes, L. Y., & Tamimi, A. H. (2017) A comparison of urethane and cellulose sponges as cleaning tools in household kitchens. Food Protection Trends, 37:170–175. [Google Scholar]
- Guo, K. (2013) Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environmental Science & Technology, 47:7563–7570. [DOI] [PubMed] [Google Scholar]
- Hall‐Stoodley, L. , Costerton, J. W. , & Stoodley, P. (2004) Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews Microbiology, 2:95–108. [DOI] [PubMed] [Google Scholar]
- Heerklotz, H. , & Seelig, J. (2001) Detergent‐like action of the antibiotic peptide surfactin on lipid membranes. Biophysical Journal, 81:1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokkanson, A. (2018, September 30). Toys, books, cribs can harbor bacteria for long periods, study finds Retrieved from http://www.buffalo.edu/news/releases/2013/12/030.html
- Inácio, A. S. , Mesquita, K. A. , Baptista, M. , Ramalho‐Santos, J. , Vaz, W. L. C. , & Vieira, O. V. (2011) In Vitro surfactant structure‐toxicity relationships: Implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One, 6:e19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou, C. J. , Hanlon, G. W. , & Denyer, S. P. (2007) Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 51:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isitua, C. C. (2016) Effects of surfactants on microorganisms isolated from gold jewelry. International Journal of Scientific Research, 5:637–640. [Google Scholar]
- Jansen, B. , & Kohnen, W. (1995) Prevention of biofilm formation by polymer modification. Journal of Industrial Microbiology, 15:391–396. [DOI] [PubMed] [Google Scholar]
- Johnston, M. D. , Simons, E. A. , & Lambert, R. J. W. (2000) One explanation for the variability of the bacterial suspension test. Journal of Applied Microbiology, 88:237–242. [DOI] [PubMed] [Google Scholar]
- Jung, M. , Lee, S. , Kim, H. , & Kim, H. (2000) Recent studies on natural products as anti‐HIV agents. Current Medicinal Chemistry, 7:649–661. [DOI] [PubMed] [Google Scholar]
- Kabara, J. J. (1972) Fatty acids and derivatives as antimicrobial agents. Antimicrobial Agents and Chemotherapy, 2:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. J., Yoo, D. S., Kim, Y. B., Lee, B. S., Shin, D. H., & Kim, E. I., (2002) Characteristics of sophorolipid as an antimicrobial agent. J Microbiol Biotechnol, 12:235–241. [Google Scholar]
- Krasowska, A., Biegalska, A., & Lukaszewicz, M. (2012) Comparison of antimicrobial activity of three commercially used quaternary ammonium surfactants. Sepsis, 5:170–174. [Google Scholar]
- López, D., Vlamakis, H., & Kolter, R. (2010) Biofilms. Cold Spring Harbor Perspectives in Biology, 2:a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan, M. T., & Martinko, J. T. (2006) Brock biology of microorganisms (11th ed.). Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- Maehara, Y. (2017) Antibacterial activities of surfactants in the laundry detergents. Biocontrol Science, 22:229–232. [DOI] [PubMed] [Google Scholar]
- Magalhães, L. , & Nitschke, M. (2013) Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control, 29:138–142. [Google Scholar]
- McDonnell, G. E. (2007) Antisepsis, disinfection, and sterilization. Washington, DC: ASM Press. [Google Scholar]
- Moore, S. L. (1997) The mechanisms of antibacterial action of some nonionic surfactants (Dissertation). University of Brighton.
- Munro, C. A. (2013) Chitin and glucan, the ying and yang of the fungal cell wall, implications for antifungal drug discovery and therapy. Advances in Applied Microbiology, 83:145–172. [DOI] [PubMed] [Google Scholar]
- Nelson, D. L., & Cox, M. M. (2017) Lehninger principles of biochemistry (7th ed.). New York, NY: Macmillan. [Google Scholar]
- Percy, M. G., & Gründling, A. (2014) Lipotechoic acid synthesis and function in gram‐positive bacteria. Annual Review of Microbiology, 68:81–100. [DOI] [PubMed] [Google Scholar]
- Permpoonpattana, P. , Phetcharaburanin, J. , Mikelsone, A. , Dembek, M. , Tan, S. , Brisson, M. C. , … Cutting, S. M. (2013) Functional characterization of Clostridium difficile spore coat proteins. Journal of Bacteriology, 195:1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka, J. J. , Yike, I. , Dearborn, D. G. , Ward, M. D. W. , & Harkema, J. R. (2008) Stachybotrys chartarum, trichothecene mycotoxins, and damp building‐related illness: New insights into a public health enigma. Toxicological Sciences, 104:4–26. [DOI] [PubMed] [Google Scholar]
- Raetz, C. R. H. , & Whitfield, C. (2002) Lipopolysaccharide Endotoxins. Annual Review of Biochemistry, 71:635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds,K. A. , Boone, S. , Bright, K. R. , & Gerba, C. P. (2012) Occurrence of household mold and efficacy of sodium hypochlorite disinfectant. Journal of Occupational and Environmental Hygiene, 9:663–669. [DOI] [PubMed] [Google Scholar]
- Rogers, A. (2008) Molecular oral microbiology (pp. 88–91). Norfolk, UK: Caister Academic Press. [Google Scholar]
- Rosen, M. J. (1989) Surfactants and interfacial phenomena. New York, NY: Wiley‐Interface. [Google Scholar]
- Rossi, E. M. , Scapin, D. , Grando, W. F. , & Tondo, E. C. (2012) Microbiological contamination and disinfection procedures of kitchen sponges used in food services. Food and Nutrition Sciences, 3:975–980. [Google Scholar]
- Sabate, D. C., & Audisio, M. C. (2013) Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin‐resistant strains. Microbiol Res. 168:125–129. [DOI] [PubMed] [Google Scholar]
- Sandora, T. J., Shih, M. C., & Goldmann, D. A. (2008) Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: A randomized, controlled trial of an infection‐control intervention. Pediatrics, 121:1555–1562. [DOI] [PubMed] [Google Scholar]
- Shaw, D. S. (2014). Critical elements of household product preservation Retrieved from https://www.happi.com/issues/2014-05-01/view_features/critical-elements-of-household-product-preservation
- Sleiman, J. N.,Kohlhoff, S. A., Roblin, P. M., Wallner, S., Gross, R., Hammerschlag, M. R., … Bluth, M. H. (2009) Sophorolipids as antibacterial agents. Annals of Clinical and Laboratory Science, 39:60–63. [PubMed] [Google Scholar]
- Srikanth, M. (2018) Preservation: Manufacturing and finished goods. Paper presented at the AOCS Annual Meeting.
- Stobie, N. , Duffy, B. , McCormack, D. E. , Colreavy, J. , Hidalgo, M. , McHale, P. , & Hinder, S. J. (2008) Prevention of Staphylococcus epidermidis biofilm formation using a low‐temperature processed silver‐doped Phenyltriethoxysilane sol–gel coating. Biomaterials, 29:963–969. [DOI] [PubMed] [Google Scholar]
- Subik, J. , Takacsova, G. , Psenak, M. , & Devinsky, F. (1977) Antimicrobial activity of amine oxides: Mode of action and structure‐activity correlation. Antimicrobial Agents and Chemotherapy, 12:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura, K., Murase, H., Bannai, H., Nemoto, M., Yamanaka, T., & Kondo, T. (2015) Efficacy of five commercial disinfectants and one anionic surfactant against equine herpesvirus type 1. The Journal of Veterinary Medical Science, 77:1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Health and Safety Executive . (2018, May 29). UK health and safety executive: The EU biocides regulation 528/2012 (EU BPR) Retrieved from http://www.hse.gov.uk/biocides/eu-bpr/
- United Kingdom National Health Service . (2018, October 9). UK national health service: How long do bacteria and viruses live outside thebody? Retrieved from https://www.nhs.uk/common-health-questions/infections/how-long-do-bacteria-and-viruses-live-outside-the-body/
- United States Centers for Disease Control . (2018a, May 23). Infections Retrieved from https://www.cdc.gov/washington/~cdcatwork/pdf/infections.pdf
- United States Centers for Disease Control . (2018b, May 23). Current outbreak list Retrieved from https://www.cdc.gov/outbreaks/index.html
- United States Centers for Disease Control . (2018c, May 23). Accessto health care Retrieved from https://www.cdc.gov/nchs/fastats/access-to-health-care.htm
- United States Centers for Disease Control . (2018d, May 23). Prevention strategies for seasonal influenza in healthcare settings Retrieved from https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm
- United States Centers for Disease Control . (2019a, April 4). Cleaning Retrieved from https://www.cdc.gov/infectioncontrol/guidelines/disinfection/cleaning.html
- United States Centers for Disease Control . (2019b, April 4). C. Neoformans infection Retrieved from https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/index.html
- United States Department of Agriculture . (2018, October 9). NationalOrganic Standards Board, Handling Subcommittee, petitioned material proposal,sodium dodecylbenzene sulfonate (SDBS) Retrieved from https://www.ams.usda.gov/sites/default/files/media/HSSDBSpetitionedApril2018.pdf
- United States Environmental Protection Agency . (2018a, May 29). Summaryof federal insecticide, fungicide, and rodenticide act Retrieved from https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act
- United States Environmental Protection Agency . (2018b, October 4). Registrationeligibility documents for alkylbenzene sulfonates Retrieved from https://archive.epa.gov/pesticides/reregistration/web/pdf/alkylbenzene_red.pdf
- University of Hamburg, “Porins” . (2019, April 14) Retrieved from http://www.biologie.uni-hamburg.de/lehre/bza/kanal/porin/eporin.htm
- Vieira, D. B. , & Carmona‐Ribeiro, A. M. (2006) Cationic lipids and surfactants as antifungal agents: Mode of action. The Journal of Antimicrobial Chemotherapy, 58:760–767. [DOI] [PubMed] [Google Scholar]
- Walker, S. L. , Redman, J. A. , & Elimelech, M. (2004) Role of cell surface lipopolysaccharides in Escherichia coli K12 adhesion and transport. Langmuir, 20:7736–7746. [DOI] [PubMed] [Google Scholar]
- Watson, J. T. , Gayer, M. , & Connolly, M. A. (2007) Epidemics after natural disasters. Emerging Infectious Diseases, 13:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisconsin State Department of Health Services . (2018, May 4). Cleaningmold in your home Retrieved from https://www.dhs.wisconsin.gov/mold/clean.htm
- Wu, Y. S. (2017) Anticancer activities of Surfactin and potential application of nanotechnology assisted Surfactin delivery. Frontiers in Pharmacology, 8:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. (1972) Comparative studies on the detoxification of aflatoxins by sodium hypochlorite and commercial bleaches. Applied Microbiology, 24:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]


